Abstract
Understanding cell-specific transcriptome responses following intracerebral hemorrhage (ICH) and ischemic stroke (IS) will improve knowledge of the immune response to brain injury. Transcriptomic profiles of 141 samples from 48 subjects with ICH, different IS etiologies, and vascular risk factor controls were characterized using RNA-seq in isolated neutrophils, monocytes and whole blood. In both IS and ICH, monocyte genes were down-regulated, whereas neutrophil gene expression changes were generally up-regulated. The monocyte down-regulated response to ICH included innate, adaptive immune, dendritic, NK cell and atherosclerosis signaling. Neutrophil responses to ICH included tRNA charging, mitochondrial dysfunction, and ER stress pathways. Common monocyte and neutrophil responses to ICH included interferon signaling, neuroinflammation, death receptor signaling, and NFAT pathways. Suppressed monocyte responses to IS included interferon and dendritic cell maturation signaling, phagosome formation, and IL-15 signaling. Activated neutrophil responses to IS included oxidative phosphorylation, mTOR, BMP, growth factor signaling, and calpain proteases-mediated blood–brain barrier (BBB) dysfunction. Common monocyte and neutrophil responses to IS included JAK1, JAK3, STAT3, and thrombopoietin signaling. Cell-type and cause-specific approaches will assist the search for future IS and ICH biomarkers and treatments.
Keywords: Intracerebral hemorrhage, ischemic stroke, gene expression, monocytes, neutrophils
Introduction
Of the peripheral leukocytes that respond to cerebrovascular injury, monocytes and neutrophils are major cell types that respond to intracerebral hemorrhage (ICH) and ischemic stroke (IS).1–3 They infiltrate injured vessels and brain, induce an inflammatory response, and participate in complex multiphasic damage and repair mechanisms.4,5
Monocytes account for ∼2–8% of peripheral leukocytes and can be pro-inflammatory or anti-inflammatory.6 Since monocytes transform into macrophages in injured tissue, gene expression changes in monocytes could provide a better understanding of this transformation. Monocyte recruitment to injured areas depends on cytokines and other factors. Pro-inflammatory monocytes express the C-C Motif Chemokine Receptor 2 protein (CCR2) important for monocyte mobilization, while anti-inflammatory monocytes express CX3CR1.3 In the acute phase following IS, increased pro-inflammatory monocytes and reduced anti-inflammatory monocytes maintain vascular stability, eliminate necrotic cells, and induce macrophage/microglia M2 polarization.7 A lower lymphocyte-to-monocyte ratio at admission is associated with worse stroke outcome.8 In chronic IS pro-inflammatory, monocytes can convert to anti-inflammatory ones, mediating repair, and debris clearance.7
Neutrophils are the most numerous peripheral blood leukocyte. Their counts increase a few hours after stroke and remain high for a week. Higher white blood cell counts and neutrophil-to-lymphocyte ratios are associated with worse stroke outcome.9,10 Neutrophils may initiate stroke, by mediating thrombosis via interaction with platelets and atherosclerotic plaque.11 They interact with the endothelium by binding adhesion molecules like ICAM-1 and interact with chemokines through their plasma membrane chemokine receptors. Neutrophils release proteases (i.e. elastase and proteinase 3) and peptidases such as MMP-9 which disrupt the BBB; and neutrophils release cytokines which attract other leukocytes and injure brain.3,11 Neutrophils can polarize to subtypes, like N1, which produces IFNγ and TNFα, or to the N2 subtype, induced by TGFβ. The N2 subtype contributes to the resolution of inflammation and promotes clearance of neutrophils by macrophages.12–14
In ICH, infiltration of circulating leukocytes peaks between one and three days and persists for weeks.1 Monocyte recruitment is mediated partly by CCR2 and CCR2 expressing cells are involved in hematoma clearance and recovery.15 By 12-h post-ICH, monocytes outnumber neutrophils in the perihematomal area.16 Activation of monocytes, monocyte-derived macrophages, and neutrophils occurs via cytokines and other inflammatory factors that further promote tissue damage.1,17,18 Neutrophils produce reactive oxygen species (ROS) and attract monocytes and macrophages,18 but at later stages produce lactoferrin to bind iron and contribute to hematoma resolution.19
Though the immune response is important in stroke, the transcriptional changes in specific blood cell types in humans have not been studied. Our previous microarray studies following IS and ICH showed altered expression for some genes in whole blood that were monocyte- or neutrophil-specific.20,21 For IS, innate immune responses were enriched in PMN-specific genes, including TREM1, interleukins, and interleukin receptors. Differentially expressed transcripts found in whole blood showed enrichment of neutrophil and monocyte specific genes in ICH and for neutrophil genes in IS.21 We have previously shown that different IS causes have a specific gene response in whole blood.22–24 Thus, here we analyzed the transcriptomic responses of isolated monocytes and neutrophils from ischemic strokes of cardioembolic (CE), large vessel (LV), and small vessel (SV) etiologies, and ICH when compared to controls with vascular risk factors (VRFC).
We show, for the first time in humans, that there are cell type-specific gene expression profiles in monocytes and neutrophils following ICH and IS and its different etiologies. These cell-specific human data will provide novel biomarkers and therapeutic targets, and will help inform future experimental stroke model studies as to whether the immune responses in animal models are similar to those seen in human ICH and IS.
Materials and methods
Patients and sample preparation
The protocol was approved by the University of California-Davis Institutional Review Board (IRB-ID 248994-41) and adheres to all federal and state regulations related to the protection of human research subjects, including The Common Rule, The Belmont Report, and Institutional policies and procedures. Written informed consent was obtained from ischemic stroke (IS), spontaneous intracerebral hemorrhage (ICH), and vascular risk factor control (VRFC) subjects at the UC Davis Medical Center from all participants or their proxy. Stroke diagnoses were made by history, exam, and CT or MRI by two neurologists. Ischemic strokes included three etiologies: cardioembolic (CE), large vessel (LV), and small vessel (SV). Three ischemic stroke patients of 33 received thrombolytic therapy (recombinant tissue plasminogen activator, rtPA). None of the IS cases developed hemorrhagic transformation. Subjects were excluded from the study with current or recent (two weeks) infection, anticoagulation, immunosuppressive therapy, or blood malignancies. Control subjects had vascular risk factors (hypertension, hypercholesterolemia, diabetes mellitus type 2, and/or smoking) (VRFC) but no history of stroke, myocardial infarction, or peripheral vascular disease. Blood was collected by venipuncture in PAXgene whole blood RNA tubes and K2 EDTA tubes (BD Biosciences). Monocytes and neutrophils were isolated using the RoboSep Cell Isolation Protocol, with the EasySep human buffy coat CD14+ selection for monocytes, and isolation kits which negatively selected neutrophils (Stemcell Technologies Inc.). Cell purity was validated using flow cytometry on a BD LSR II (Becton Dickinson) and fluorescent antibodies directed to cell-type specific markers not used for cell sorting. Monocytes were detected by anti-CD36-APC (Biolegend) with purity >93%; neutrophils were detected with dual labels for anti-CD16-PE and anti-CD66b-FITC (Biolegend) with purity >99% (Supplementary Figure 1(a) and (b)).
RNA isolation and library preparation
Total RNA was extracted from monocyte and neutrophil samples using Zymo Direct-zol RNA mini-prep kit (Zymo Research), as per manufacturer’s protocol, followed by DNase treatment (QIAgen). RNA from whole blood samples was extracted using Qiagen QIAcube with PAXgene Blood mRNA Kit (QIAgen). Libraries were prepared using NuGEN Ovation Universal RNA-Seq system (Tecan). Ribosomal RNA and globin transcripts were depleted using InDA-C (Tecan).
RNA-seq analyses
RNA sequencing was performed on Illumina HiSeq 4000 platform with an average of 200 M ± 10M PE (paired-end) reads per sample. Raw reads were processed with expHTS25 to trim low-quality sequences and adapter contamination, and to remove PCR duplicates. Trimmed reads were aligned using STAR v.2.5.2b aligner26 to the human GRCh38 primary assembly genome with GENCODE v25 annotation. Raw counts/genes were generated using featureCounts of the Subread software v.1.6.0.27 Raw counts were normalized (transcripts per million, TPM) per sample. Partek Genomics suite was used to analyze differentially expressed genes using analysis of covariance (ANCOVA) models and REML variance estimate.28 The statistical models are outlined in the results below. Fisher's least significant difference (LSD) was used for individual contrasts.29 Principal component analyses were performed using Partek Genomics suite. Gene ontology enrichment was explored using DAVID.30,31
Pathway and upstream regulation analyses
Pathway enrichment analyses were performed using ingenuity pathway analysis (IPA, Ingenuity Systems®, QIAgen).32 Gene lists analyzed in IPA used uncorrected p < 0.05 and FC > |1.2| to provide more genes per pathway. Pathways with Fisher’s exact test p < 0.05 were considered statistically over-represented. In the figures, pathways with Benjamini–Hochberg corrected p < 0.05 are labeled with an asterisk. IPA also predicted significant pathway activation (Z ≥ 2.0) or significant suppression (Z ≤ −2). The Z-score is based on an algorithm that compares the input dataset with pathway patterns, accounting for the activation state of molecules related to the pathway, causal relationships, and curated literature. Upstream transcriptional regulators were also predicted.
Results
Subject demographics and samples analyzed
The cohort demographics and clinical characteristics are presented in Table 1. Sex was not significant. Age was significantly different for IS versus control and IS versus ICH comparisons; thus, age was included in the statistical models. Time from symptom onset to the blood collection time was significantly different between IS and ICH, but not for the main comparisons between IS and VRFC, and ICH and VRFC. Given the broad range of times in both groups, we did not include this variable in our gene expression model in order to analyze the consistent aspect of the immune response irrespective of time. Thus, the ICH data encompass a period after ICH of ∼ 0.5 to 3.5 days, and the IS data encompass a period of ∼0.5 to 2.0 days. Hypertension was not significantly different between the groups, while diabetes and hypercholesterolemia were for IS versus VRFC and ICH versus VRFC, but not for IS versus ICH (Table 1). Smoking status was not significantly different between groups. We investigated the differentially expressed gene (DEGs) lists by hierarchical clustering to make sure the differential responses were not driven by these vascular risk factor differences (not shown). Most subjects (n = 44 of 48) had three sample types – neutrophils, monocytes, and whole blood. Analysis at the whole transcriptome level showed a clear separation of transcriptomes based on sample type (Supplementary Figure 1(c)), which is consistent with the presence of two distinct cell populations (monocytes and neutrophils) and whole blood.
Table 1.
Cohort demographics and relevant clinical characteristics.
|
ICH |
IS |
VRFC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| M | N | WB | M | N | WB | M | N | WB | |
| Subjects | 6 | 6 | 5 | 32 | 30 | 33 | 10 | 9 | 10 |
| Subjects with 3 sample types (all 3) | 5 | 30 | 9 | ||||||
| NIHSS all 3, at admission/blood draw (mean ± SD) | 9.2 ± 13.6/8.2 ± 14.1 | 3.5 ± 4.3/2.2 ± 3.4a | N/A | ||||||
| Sex (male/female) | 5/1 | 5/1 | 4/1 | 15/17 | 14/16 | 15/18 | 5/5 | 4/5 | 5/5 |
| Smoking status (no, current, past) | 2, 2, 2 | 14, 9, 10 | 4, 4, 2 | ||||||
| Age, years (mean ± SD) | 57.7 ± 20.1 | 55.7 ± 20 | 55.2 ± 6.3 | 66.4 ± 11.7 | 66.8 ± 11.9 | 66.2 ± 6.3 | 60.1 ± 6.3 | 60.2 ± 6.7 | 60.1 ± 6.3 |
| Time since event, h (mean ± SD) | 49.2 ± 29.6 | 49.2 ± 29.6 | 55.5 ± 6.3 | 28.2 ± 15.7 | 27.9 ± 16 | 29.7 ± 6.3 | N/A | N/A | N/A |
| Diabetes | 0 | 0 | 0 | 5 | 4 | 5 | 6 | 6 | 6 |
| Hyperlipidemia | 2 | 2 | 2 | 14 | 13 | 15 | 10 | 9 | 10 |
| Hypertension | 6 | 6 | 5 | 26 | 24 | 27 | 7 | 6 | 7 |
aNIHSS available on 32/33 IS subjects.
Specific monocyte, neutrophil, and whole blood responses following ICH and ischemic stroke
For analyses of the genomic changes associated with IS and ICH, differentially expressed genes were identified using a mixed effects regression model including Sample_Type (monocytes (M), neutrophils (N), whole blood (WB)), Diagnosis (IS, ICH, VRFC), Subject_ID (random effect), age, and an interaction Sample_Type * Diagnosis. Genes with FDR (False Discovery Rate)-corrected p(Sample_Type*Diagnosis) < 0.2, which also had p < 0.05 and fold change (FC) > |1.2| for individual contrasts, were considered DEGs.
ICH versus VRFC
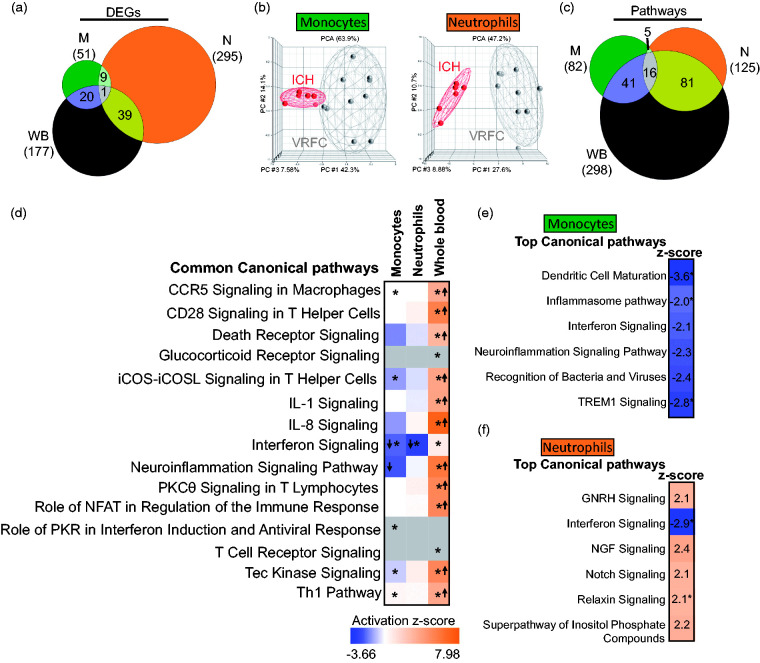
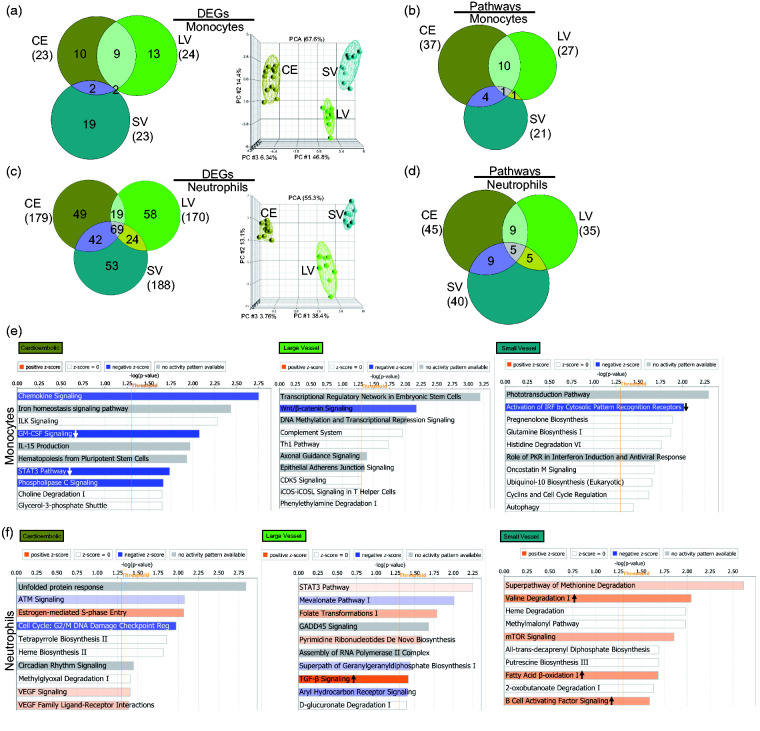
Using the above criteria, a total of 51, 295, and 177 DEGs were found for ICH versus VRFC in monocytes, neutrophils and whole blood, respectively (FDR p(Dx)<0.2 and p < 0.05) (Figure 1(a)). Only the GBP5 gene (guanylate binding protein 5, an activator of NLRP3 inflammasome assembly) was shared between all three sample types. There was significant overlap of DEGs between monocytes and WB (p < 10e-16), neutrophils and WB (p < 10e-16), and between M and N (p = 5.2e-10) (Figure 1(a)). The ICH signatures revealed group separation (ICH vs. VRFC) in both monocytes and neutrophils on principal components analysis (PCA) (Figure 1(b)). Since blood samples’ collection time from onset was within 3.5 days for ICH patients and within 2 days for IS patients, we investigated whether ICH subjects with earlier blood draws would group together and separately from ICH subjects with later blood draws based on the DEG signatures. No subgrouping was observed after performing unsupervised hierarchical clustering of monocyte and neutrophil DEGs between ICH and VRFC (Supplementary Figure 2).
Figure 1.
Molecular signatures for ICH versus VRFC in the sample types analyzed. (a) Venn diagram of the numbers of DEGs in monocytes (green), neutrophils (orange), and whole blood (brown); (b) principal component analyses (PCA) using the DEGs in ICH-monocytes versus VRFC-monocytes (left PCA) and ICH-neutrophils versus VRFC-neutrophils (right PCA); (c) Venn diagram of the numbers of over-represented significant canonical pathways (p < 0.05) for ICH; (d) common pathways in all three sample types – monocytes, neutrophils, and whole blood. The asterisk depicts significant pathways that passed additional stringency criteria (Benjamini–Hochberg corrected p-value < 0.05). In the plot, orange cells indicate predicted activation of the pathway, blue ones show predicted inhibition, grey cells – no prediction can be performed, and white depicts no direction can be inferred. The shades for every colored cell represent z-scores values. Arrows represent the direction of the change for significant activation (Z ≥ 2= activation, up arrow); or significant inhibition (Z ≤ −2, down arrow). Top enriched pathways are shown for monocytes (e) and neutrophils (f). Role of pattern recognition receptors in recognition of bacteria and viruses is abbreviated.
Pathway enrichment analysis functionally characterized the immune response per cell type: 573, 3250, and 2550 DEG for ICH in monocytes, neutrophils, and whole blood, respectively, were considered for the analysis (p < 0.05) (Table S2). The monocyte ICH response included significant suppression (Z ≤ −2) of pathways including dendritic cell maturation, TREM1 signaling, role of pattern recognition receptors, neuroinflammation signaling, interferon signaling, and Inflammasome (Table S3). None of the 82 monocyte ICH pathways were predicted to be significantly activated (Z ≥ 2). The neutrophil activated ICH response included relaxin signaling, notch signaling, GnRH (gonadotropin-releasing hormone) signaling, inositol phosphate compounds, and NGF signaling, and one significantly suppressed pathway – interferon signaling (Table S3). Most overrepresented pathways in monocytes and neutrophils were shared with those enriched in whole blood (69.5% and 77.6%, respectively) (Figure 1(c)). Notably, some of the common pathways between monocytes and whole blood have opposite activation/suppression status (Figure 1(d)) like neuroinflammation signaling, predicted to be inhibited in monocytes and activated in whole blood. Considering the cell-specific responses in monocytes (Figure 1(e) and Table S3) to ICH, the top pathways included communication between innate and adaptive immune cells, crosstalk between dendritic and natural killer cells, and atherosclerosis. In neutrophils, the top ICH pathways (Figure 1(f) and Table S3) included tRNA charging, linolenate, cholesterol, and nucleotide synthesis.
IS versus VRFC
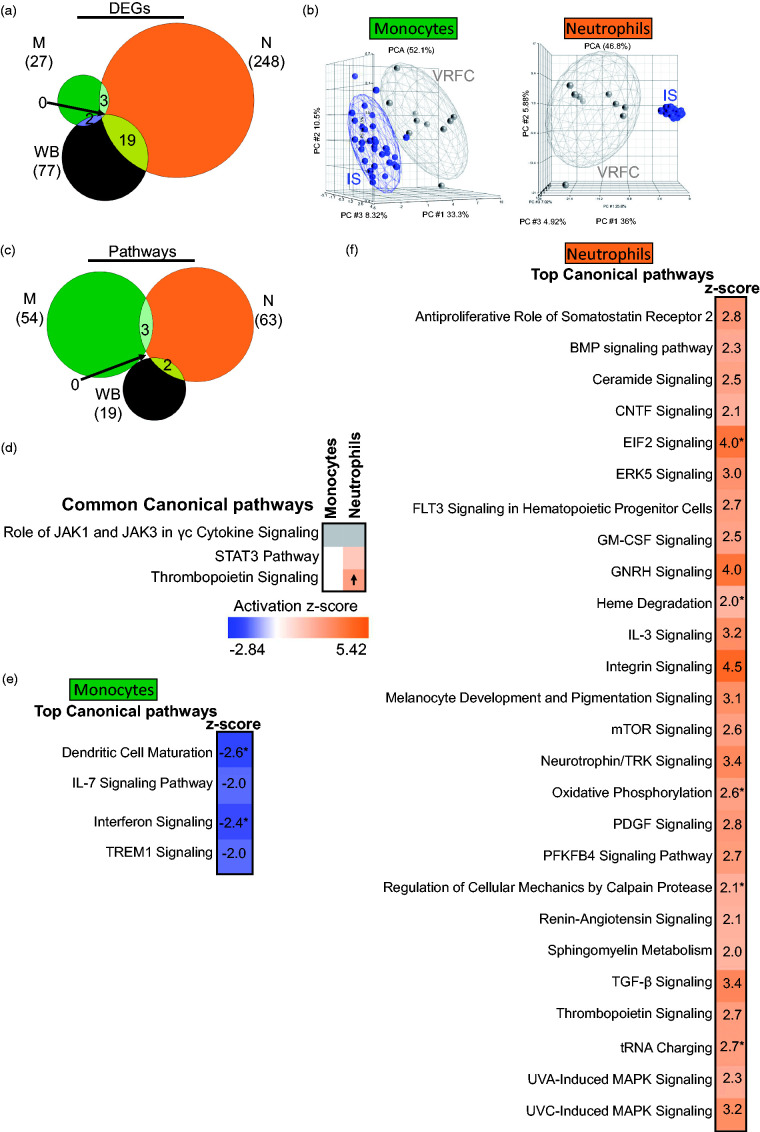
Analysis of IS subjects irrespective of IS cause yielded 27, 248, and 77 DEGs in IS versus VRFC (monocytes, neutrophils, and whole blood, respectively) (FDR p(Dx) <0.2 and p < 0.05 for each individual contrast) (Figure 2(a)) (Supplementary Table S1). There was significant overlap of DEGs between monocytes and WB (p = 0.003), neutrophils and WB (p < 10e-16), and between M and N (p = 0.002) (Figure 2(a)). The IS signatures revealed separation of monocytes and neutrophils on PCA (Figure 2(b)). rtPA administration in three IS patients did not affect the differential expression results, as no subgrouping was observed after performing unsupervised hierarchical clustering of the monocytes’ and neutrophils’ DEGs between IS and VRFC (Supplementary Figure 3).
Figure 2.
Molecular signatures for IS versus VRFC in the sample types analyzed. (a) Venn diagram of the numbers of DEGs in monocytes (green), neutrophils (orange), and whole blood (brown); (b) principal component analyses (PCA) using the DEGs in IS-monocytes versus VRFC-monocytes (left PCA) and IS-neutrophils versus VRFC-neutrophils (right PCA), the effects of additional factors were removed before plotting. (c) Venn diagram of the numbers of over-represented significant canonical pathways (p < 0.05) for IS; (d) common pathways in monocytes and neutrophils. Top enriched pathways are shown for monocytes (e) and neutrophils (f). The asterisk depicts significant pathways that passed additional stringency criteria (Benjamini–Hochberg corrected p-value < 0.05). In the plot, orange cells indicate predicted activation of the pathway, blue ones show predicted inhibition, grey cells – no prediction can be performed, and white depicts no direction can be inferred. The shades for every colored cell represent z-scores values. Arrows represent the direction of the change for significant activation (Z ≥ 2 = activation, up arrow); or significant inhibition (Z ≤ −2, down arrow).
DEGs subjected to pathway analysis for IS included 365, 3366, and 1855 genes (monocytes, neutrophils, and whole blood, respectively) with p < 0.05 for each individual contrast. The monocyte IS response included significant suppression (Z ≤ −2) of dendritic cell maturation, interferon, TREM1, and IL-7 signaling pathways (Table S3). None of the 54 monocyte IS pathways were significantly activated (Z ≥ 2). The neutrophil IS response included significantly activated pathways including TGF-β, mTOR, Integrin, BMP, GM-CSF, and IL-3 signaling (Table S3). No neutrophil IS pathway was significantly suppressed. There was little overlap of enriched canonical pathways (Figure 2(c)). Pathways related to cytokine signaling, STAT3, and thrombopoietin signaling were common between monocytes and neutrophils, with significant activation of thrombopoietin signaling in neutrophils (Figure 2(d)). Pathway analysis of IS monocytes showed significantly inhibited pathways (Z ≤ −2) including interferon and dendritic cell maturation (Figure 2(e)). IS neutrophils had activated oxidative phosphorylation, mTOR signaling, and calpain protease pathways (Figure 2(f)).
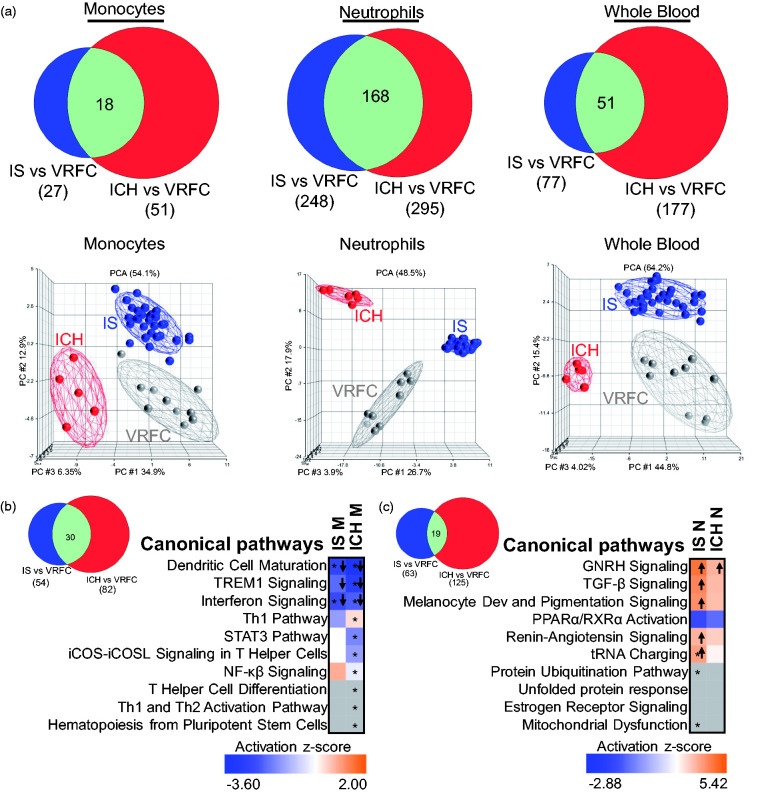
ICH versus IS
ICH was compared to IS to identify common and specific responses to ICH and IS. Overlaps between the DEGs in the transcriptional response of IS_M and ICH_M, IS_N and ICH_N and IS_WB and ICH_WB were all highly significant (p < e-16 for each of the overlaps) (Figure 3(a)). For IS, the shared genes with ICH were fairly constant – in monocytes (67%), neutrophils (68%), and whole blood (66%), while for ICH there was a more variable response – the largest proportion of genes shared with IS was in ICH neutrophils (57%), followed by ICH monocytes (35%) and ICH whole blood (29%). PCAs using all DEGs in IS and ICH showed good separation of ICH and IS from VRFC for the monocyte and neutrophil genes (Figure 3(a), bottom row). At the pathway level (Supplementary Table S3 and Figure 3(b) and (c)), when comparing between IS and ICH, the latter has a higher number of significant over-represented pathways in both monocytes and neutrophils. In monocytes, signaling pathways (i.e. STAT3, NF-κβ) and immune pathways, such as interferon, dendritic cell maturation and TREM1 signaling, were enriched and predicted to be suppressed in both IS and ICH. For neutrophils, over-represented pathways seen in both groups included mitochondrial dysfunction, TGF-β, protein regulation, and PPARα. GNRH signaling was predicted to be activated in both ICH and IS, and was previously identified in our study of ruptured versus non-ruptured brain arteriovenous malformations.33
Figure 3.
Comparison of the transcriptional changes in ICH and IS (irrespective of IS-cause). (a) Venn diagrams are shown for the genes found DE in IS and ICH according to the criteria used (FC > |1.2|, p < 0.05 for the individual contrasts and having Sample_Type * Diagnosis FDR (step up) < 0.2), in monocytes, neutrophils, and whole blood. The overlap of DEGs between ICH and IS is significant (hypergeometric test). From all the gene lists included in the Venn diagrams, below are the corresponding PCAs, where grey circles indicate VRFC, red depicts ICH, and blue, IS. Ellipsoids represent two standard deviations from the centroid. Top 10 canonical pathways for IS and ICH (Fisher’s p-value < 0.05) in (b) monocytes and (c) neutrophils are presented. The asterisk depicts pathways with Benjamini–Hochberg corrected p-value < 0.05. In the plot, orange cells indicate predicted activation of the pathway, blue ones show predicted inhibition, white cells – no direction can be predicted, and grey depicts no prediction can be performed. The shades for every colored cell represent z-scores values. Arrows indicate predicted significant activation (Z ≥ 2, up arrow) or significant inhibition (Z ≤ −2, down arrow) of the pathway. Melanocyte development and pigmentation signaling is abbreviated. The Venn diagrams next to the heatmaps represent the number of pathways with p-value < 0.05 for both diagnoses in monocytes (b) and neutrophils (c).
For pathways exclusively enriched in either IS or ICH (Supplementary Figure 4 and Table S3), monocyte cytokine signaling, phagosome formation, and suppressed IL-7 signaling were among the top 10 pathways in IS, while ICH monocyte pathways included innate and adaptive immune cell communication, crosstalk between dendritic and NK cells, CCR5 and atherosclerosis signaling. In neutrophils, IS-specific activated pathways included heme degradation, EIF2, oxidative phosphorylation, mTOR, and calpain protease signaling. In ICH neutrophils, interferon signaling was significantly suppressed, while Relaxin signaling, involved in vasodilation and angiogenesis, was significantly activated along with fMLP signaling which regulates cytokines (Supplementary Figure 4(b) and Table S3).
Predicted upstream transcriptional regulators, which can identify drivers of the differential expression in IS or ICH, are presented in Supplementary Table S4. ICH has more unique regulators than IS, while 37–42% of the IS’s upstream regulators were shared with ICH upstream regulators in both cell types. Interferon-related proteins are common regulators in ICH and IS for both monocytes and neutrophils (Supplementary Figure 5).
Specific monocyte, neutrophil, and whole blood responses following ischemic stroke of different etiologies
For the genomic changes associated with IS causes, DEGs were identified using a mixed effects regression model including Sample_Type (M, N, WB), diagnosis (IS-CE, IS-LV, IS-SV, ICH, VRFC), Subject_ID (random effect), age, and an interaction Sample_Type*Diagnosis. Genes with FDR-corrected p (Sample_Type*Diagnosis) < 0.2 (535 genes), which also had p < 0.05 and FC>|1.2| for individual contrasts, were considered DEGs.
Transcriptome differences between different cell types per IS cause
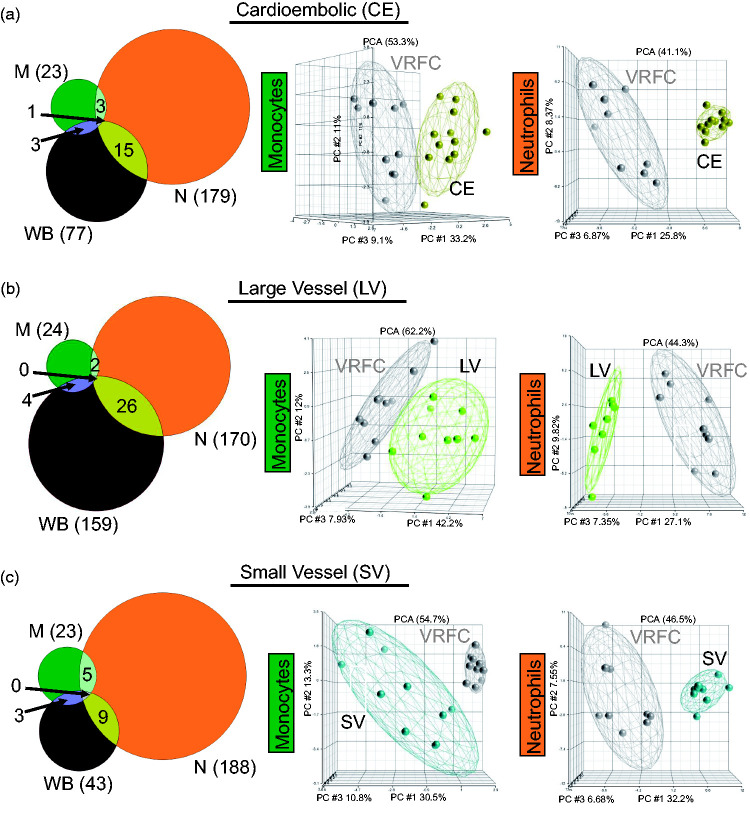
In cardioembolic IS, there were 23, 179, and 77 DEGs in monocytes, neutrophils, and whole blood, respectively, when compared to VRFC (Figure 4(a), Supplementary Table 5). In large vessel IS, there were 24, 170, and 159 DEGs in monocytes, neutrophils, and whole blood, respectively, when compared to VRFC (Figure 4(b), Supplementary Table 5). In small vessel IS, there were 23, 188, and 43 DEGs in monocytes, neutrophils, and whole blood, respectively, when compared to VRFC (Figure 4(c), Supplementary Table 5). Based on all DEGs for each IS cause, PCAs (Figure 4) showed separation of the sample types per diagnostic group (whole blood, not shown). Figure 4 shows a high percentage of unique DEGs per sample type, demonstrating cell-type specific response for monocytes and neutrophils. A role for other cell types is supported by the many specific DEGs in whole blood.
Figure 4.
Comparison of the transcriptional changes in IS in the sample types analyzed in monocytes (green), neutrophils (orange), and whole blood (brown) considering IS cause. Venn diagrams and principal component analyses are shown for all the DEGs (fold change ±1.2 plus overlap of FDR (step up) < 0.2, with p-value < 0.05 for the ANOVA interactions assessed against VRFC: (a) CE stroke, (b) LV stroke, and (c) SV stroke). Ellipsoids represent two standard deviations from the centroid.
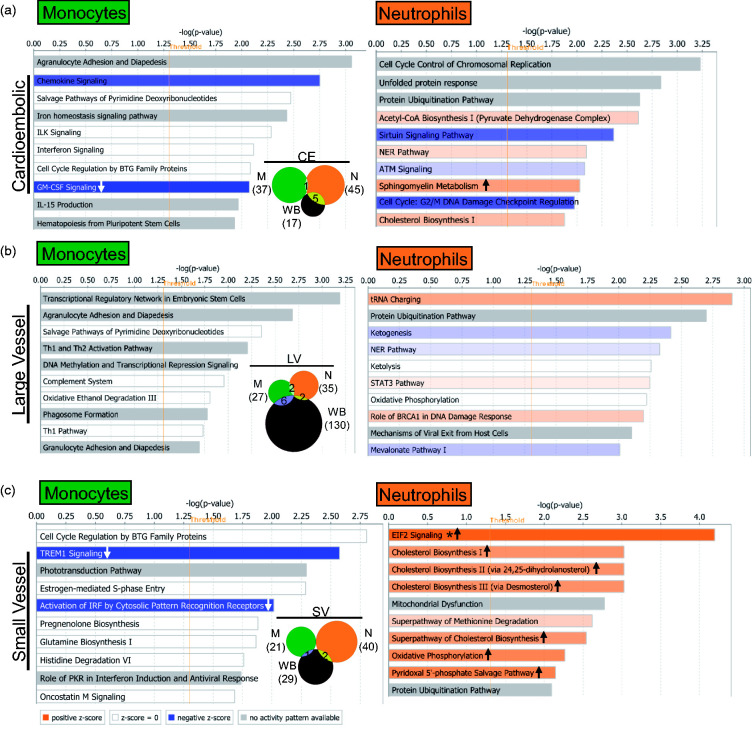
The number of significant pathways in all three causes of stroke was similar in the three sample types (p < 0.05) (Figure 5), except for LV in whole blood, for which there were ∼5 times as many pathways compared to CE and SV stroke. This suggests a greater involvement of other blood cells in the response to LV stroke (Figure 5 and Table S7). The monocyte response of IS-CE subjects included GM-CSF and STAT3 signaling which were significantly suppressed, while in IS-SV, interferon and TREM1 signaling were significantly suppressed (Figure 5, Table S7). The neutrophil response in all IS causes included many activated pathways, i.e. oxidative phosphorylation in IS-CE, ERK5 signaling in IS-LV, and EIF2 signaling in IS-SV (Figure 5, Table S7). Notably, the neutrophil IS-SV response included several activated metabolic pathways, including four cholesterol biosynthesis pathways implicated in experimental SV stroke.34 Monocytes display many pathways related to immune response regulation (Figure 5) and showed a larger number of upstream regulators than neutrophils (Supplementary Figure 6 and Table S8). Interferon pathway regulators were over-represented in monocytes for all IS etiologies. The predicted upstream neutrophil pathways were mediated more by cell and molecular regulation pathways (Supplementary Figure 6 and Table S8).
Figure 5.
Top 10 IS cause-specific significantly over-represented (p < 0.05) pathways in monocytes and neutrophils in the different IS etiologies: (a) cardioembolic stroke; (b) large vessel stroke; and (c) small vessel stroke. The asterisk depicts pathways with Benjamini–Hochberg corrected p-value < 0.05. In the plots, arrows in the orange or blue bars indicate predicted significant activation (Z ≥ 2, up arrow) or significant inhibition (Z ≤ −2, down arrow) of the pathway. White bars – no direction can be predicted, and grey depicts no prediction can be performed. The vertical orange line marks the −log(p-value) > 1.3 threshold. The Venn diagrams represent all the pathways with a Fisher’s p-value < 0.05 in every IS cause.
Transcriptome differences between different IS causes per cell type
Transcriptional responses for the three IS etiologies for each cell type were compared since we have previously shown that different IS causes produce a specific transcriptome response in whole blood.22,23 In monocytes, there were 23, 24, and 23 DEGs between IS-CE, IS-LV, and IS-SV, respectively, versus VRFC (Figure 6(a)). In neutrophils there were 179, 170, and 188 DEGs between IS-CE, IS-LV, and IS-SV, respectively, versus VRFC (Figure 6(c), Supplementary Table S5). In monocytes, there was significant overlap between CE and LV stroke (p < 10e-16), CE and SV (p = 5.4e–9), and SV and LV stroke (p = 2.3e–4). Neutrophils responded with a much larger number of genes (Figure 6(c)). In neutrophils, the overlaps between the causes were also significant (p < 10e-16 for CE vs. SV, CE vs. LV, and SV vs. LV). Based on the unique genes for each IS cause, PCAs showed clear group separation in monocytes and neutrophils (Figure 6(a) and (c)).
Figure 6.
Comparison of the transcriptional changes in different IS etiologies versus VRFC in monocytes and neutrophils. Venn diagrams for the genes that meet the defined criteria (FC > |1.2|, p < 0.05 for the individual contrasts and having Sample_Type * Diagnosis FDR (step up) < 0.2). From the unique genes included in the Venn diagrams (not overlapped regions), the corresponding PCA is shown for (a) monocytes and (c) neutrophils. The effects of additional factors were removed before plotting. Ellipsoids represent 2 standard deviations from the centroid. Venn diagrams of the numbers of over-represented (Fisher’s p-value < 0.05) canonical pathways in (b) monocytes and (d) neutrophils. Top 10 over-represented canonical pathways exclusively found in CE, LV, and SV stroke in (e) monocytes and (f) neutrophils. In the plot, orange bars indicate predicted activation of the pathway, blue ones show predicted inhibition, white bars – no direction can be predicted, and grey depicts no prediction can be performed. The shades for every colored cell represent z-scores values. Arrows indicate predicted significant activation (Z ≥ 2, up arrow) or significant inhibition (Z ≤ −2, down arrow) of the pathway. The vertical orange line marks the −log(p-value)>1.3 threshold. In neutrophils, superpathway of geranylgeranyl diphosphate biosynthesis i (via mevalonate) and regulation (reg) are abbreviated.
Pathway analyses were performed for each stroke cause and cell type including monocytes (Figure 6(b) and (e)) and neutrophils (Figure 6(d) and (f)). Most enriched pathways (Fisher’s p-value < 0.05) were IS cause-specific. For monocytes, most pathways were unique for every IS cause, with the greatest overlap between CE and LV strokes (Figure 6(b)). Among the top 10 cause-specific pathways in monocytes, CE stroke showed significant suppression of STAT3 and GM-CSF signaling, LV stroke had a trend towards suppression of Wnt/β-catenin signaling, and SV showed suppression of cytosolic recognition receptors for IRF (Figure 6(e)). In neutrophils, for the top enriched pathways, many were predicted to be activated, including TGF-β – unique to LV, and fatty acid β-oxidation, B cell activating factor signaling, and valin degradation, which were unique for SV (Figure 6(f)). Notably, though neutrophils and monocytes had common pathways across the IS causes per cell type, they were represented by unique molecules that were exclusive for every IS etiology (Table S7).
Upstream regulator analyses in monocytes and neutrophils showed that most predicted transcriptional regulators were IS-cause specific, with neutrophils displaying more differences (Supplementary Figure 7). Several interferon regulators were exclusively represented in SV in neutrophils, along with T cell receptors which were predicted to be activated (Supplementary Table S8).
Discussion
Ischemic stroke (IS) and intracerebral hemorrhage (ICH) induce a complex multiphasic immune response that contributes to damage and repair.1,3,18 Though previous studies show leukocyte subtypes have cell-specific transcriptional profiles,35 this is the first to show cell-specific, whole-genome transcriptional profiles that change in neutrophils and monocytes and are specific for ICH and different IS causes. Building on previous whole blood studies that have shown the whole genome response to IS and ICH,20,21,24,36 these studies show that gene expression is generally activated in neutrophils and suppressed in monocytes in the first few days following ICH and all causes of IS. Thus, the discussion will focus on these individual cellular responses that have never been investigated before in human IS or ICH.
Monocyte gene response to ischemic stroke is down-regulated
Communication between injured ischemic brain and peripheral blood leukocytes drives changes of the peripheral immune system that affect stroke pathophysiology and outcome.7 Monocytes change phenotypes in response to their microenvironment, differentiating into different subtypes and to macrophages.37 However, relatively few genes were dysregulated exclusively in IS monocytes, and all were down-regulated. Most were associated with the innate immune response and regulation of steroid biosynthesis.30,31 We interrogated the DEGs for markers of monocytes subsets or polarization,38–41 but there was no evidence of predominance. Moreover, the gene markers showed high variability of expression, suggesting mixed monocyte populations, possibly due to the broad time window of the samples.
Amongst the most significantly over-represented suppressed pathways in IS monocytes were interferon signaling, dendritic cell maturation, and phagosome formation. The interferon pathway and its components, from cytokines, to receptors and associated transcription factors are involved in the immune response post-stroke (such as IFNγ, STAT1, and IRF-9),42–45 and were dysregulated in our previous studies.21 In this study, in the subacute phase (28.2 ± 15.7 h), the interferon pathway was suppressed in monocytes, possibly by down-regulation of pro-inflammatory pathways, such as TREM1 signaling and/or by reducing the pro-inflammatory monocyte subtypes.7 In addition, IFNγ, predicted to be a suppressed upstream regulator, has been associated with stroke-induced neurodegeneration since it is pro-inflammatory.46 The down-regulation of these upstream regulators and pro-inflammatory pathways may suggest peripheral monocytes switch to more anti-inflammatory functions. Dendritic cells, whose maturation was predicted to be suppressed, are antigen-presenting cells that migrate to the injury site and regulate inflammation.42,47–49 Monocytes can differentiate into dendritic cells50–52 and the monocyte profile we observed may reflect suppression of this transition. Phagocytosis signaling, which was over-represented in IS monocytes but not IS neutrophils, is essential in the stroke resolution phase, and monocytes and monocyte-derived macrophages participate in this stage.53
There was a similar number of monocyte DEGs across IS causes that were mostly cause-specific; 17/23 DEGs in SV stroke were up-regulated. CA4 (carbonic anhydrase 4), which is associated with the BBB54 was down-regulated in all IS causes. The only up-regulated monocyte genes in CE and LV stroke were SRCIN1 and DEFA3 (defensin alpha 3), respectively. SRCIN1 (SRC kinase signaling inhibitor 1) is a Src inhibitor.55 Inhibition of Src-kinases has been associated with improved outcomes in experimental IS and ICH models.56,57 DEFA3, an antimicrobial peptide found to be up-regulated in hypercholesterolemia,58 has a role in thrombosis59 and belongs to the family of defensins. Another gene from the defensins family, DEFA1B, was down-regulated in cardioembolic stroke.
Other monocyte DEGs in SV stroke are GLP2R, which is involved in glucose homeostasis and has been linked to risk of type 2 diabetes60,61; and LRP4 which responds to ischemia in astrocytes, and in peripheral axons promotes nerve regeneration.62,63 Interestingly, under normal conditions, there is no expression of this gene in monocytes.64 The same was true for FGD5, a neovascularization inhibitor65; HHIP, which is associated with prediabetes,66 and PERM1, a gene involved in mitochondrial biogenesis whose expression pattern is altered with exercise.67
CE, LV, and SV strokes had similar numbers of unique monocyte pathways per cause. Only the estrogen-mediated-S-phase entry pathway was shared between IS etiologies though most of the differentially expressed molecules from this pathway differed amongst IS causes. CCND1 (cyclin D1), the only pathway molecule down-regulated in all three IS causes, regulates the cell cycle which has an important role in stroke.68 In a rat stroke model, bone marrow stromal cells (BMSCs) modulated cell cycle progression via down-regulation of the CCD1/cdk4/pRb pathway which appeared to contribute to stroke recovery.69 For SV, the estrogen-mediated-S-phase entry pathway molecules E2F7 (E2F Transcription Factor 7) and CCNE2 (cyclin E2) were up-regulated, while E2F5 (E2F Transcription Factor 5) and CDK1 (Cyclin Dependent Kinase 1) were down-regulated in CE and LV stroke, respectively. E2F7 and E2F5 are E2F transcription factor family members which also regulate cell cycle progression.70 E2F7 promotes angiogenesis by associating with HIF1A to activate VEGFA.71 Absence of another family member, E2F1, attenuates brain injury and improves behavior following focal ischemia.72 CCEN2 and CDK1 are involved in cell cycle regulation. Cell cycle machinery promotes angiogenesis, gliosis, and neuronal apoptosis and has been a therapeutic target for stroke.68,73 Other unique monocyte pathways for IS causes included chemokine signaling, T helper regulation, TREM1 signaling, and GM-CSF. The last two were inhibited, suggesting a self-regulatory loop for monocytes to progress into the resolving phase of the injury.74,75
Peripheral neutrophil response following IS
Neutrophils are key for the innate immune response and post-stroke neuroinflammation. Ischemia induces release of pro-inflammatory cytokines, matrix metalloproteinases (MMPs), and other molecules that lead to neutrophil recruitment to the damaged area and the breakdown of the BBB.37 It has recently been suggested that neutrophils also have beneficial roles in later stages,19 in particular the N2 phenotype.13,14 No enrichment of gene markers of a particular neutrophil subset was observed in the DEGs.
The majority of the DEGs in neutrophils (197/248, 79%) were up-regulated in IS versus VRFC. Of these, only three were shared with IS monocytes. IS neutrophils had a large number of activated pathways (n = 33), many previously implicated in stroke, including integrin,76 ERK5,77 thrombopoietin,78 calpain, and mTOR79 signaling. ERK5 signaling, a MAP kinase family member, mediates inflammation and when activated, it induces PPARδ which is neuroprotective.77,80,81 mTOR, activated after cerebral ischemia via Akt (protein kinase B), mediates gene transcription, autophagy, and apoptosis.79,82 Integrins interact with extracellular matrix components to alter BBB permeability after stroke.76 Calpain proteases dysregulate synapses,83 and cause BBB breakdown and hyperpermeability following cerebral ischemia.84
Neutrophil activation included BMP signaling, a TGFβ super-family member, which mediates glial scar formation after stroke.85 Additional neutrophil activated pathways included: GM-CSF signaling which activates cell survival and differentiation75,86; neutrophin/TRK signaling which activates hematopoietic cell survival, neurite outgrowth, and neurite differentiation87; and PDGF signaling which activates chemotaxis and membrane ruffling which precedes cell migration and is crucial for leukocyte function.88,89 Activation of TGFβ signaling, including TGFB3, and predicted suppression on IFN suggests some IS neutrophils may switch from N1 to N2 type neutrophils involved in the resolution of inflammation.13,37
Though approximately 38% of neutrophil DEGs were shared between IS causes, there were unique signatures for every IS etiology. For example, HSPA2 (Heat Shock Protein Family A (Hsp70) Member 2) was up-regulated in lacunar stroke neutrophils.90 EIF3J-AS1 (hypoxia induced lncRNA)91 and PMM2 (phosphomannomutase 2) were differentially expressed in neutrophils of LV IS subjects. PMM2 is responsible for congenital disorder of glycosylation type IA with stroke-like and hemorrhagic events.92
Among the neutrophil pathways in CE stroke were VEGF signaling and cell cycle regulation/DNA damage pathways. VEGF is associated with atherosclerosis, edema, neuroprotection, and angiogenesis.93 High VEGF plasma levels correlate with CE stroke severity.94 Estrogen-mediated-S-phase entry pathway was associated with all IS etiologies in monocytes, but only associated with CE in neutrophils. TGF-β signaling was activated in neutrophils in LV IS, and has a neuroprotective role in stroke.95 B cell activating factor signaling was associated with SV IS, which is essential for B lymphocyte homeostasis. B cells help mediate adaptive immune responses to stroke and repair,96 and appear to be activated by neutrophils which also produce the B cell activating factor.97
There were five common neutrophil pathways among the three IS etiologies including mitochondrial dysfunction, oxidative phosphorylation, protein ubiquitination, UVC-induced MAPK signaling, and tRNA charging. However, nearly 33% of the molecules in these pathways were unique for CE, LV, or SV strokes, further underscoring the unique neutrophil response to each IS cause.
Monocyte response following ICH
Monocytes infiltrate ICH and can damage as well repair brain.98 Our whole blood human studies have shown many differentially expressed monocyte-specific transcripts in ICH versus VRFC,21 as well as monocyte ICH co-expression modules.99
Most monocyte genes were suppressed after ICH. Only 3/51 monocyte DEG were up-regulated: MEOX1, not usually detected in monocytes64; RN7SL546P (RNA, 7SL, cytoplasmic 546, pseudogene), and RP11-104G3.2, a pseudogene. Forty-one DEGs were unique to the ICH monocyte response, playing roles in the inflammatory/innate immune response, cytokine production, and cell migration.30,31
Most ICH monocyte pathways were predicted to be significantly suppressed (Z ≤ −2), including interferon, dendritic cell maturation, TREM1, inflammasome, role of pattern recognition receptors, and neuroinflammation signaling pathway. PPAR signaling was predicted to be significantly activated, and PPARγ in macrophages has been shown to promote hematoma resolution and improve experimental ICH.100–102
Peripheral neutrophil response following ICH
Neutrophils infiltrate perihematomal tissue after ICH.102 Our previous studies in whole blood of ICH patients pointed to many neutrophil-specific DEG and co-expression modules.21,99 The neutrophil response after ICH in this study was much more robust than for ICH monocytes. The neutrophil unique DEGs regulate gene expression, enzymatic activity, and noncoding RNA. Many ICH DEGs are transcriptional regulators (41/295), such as TGFBIF1, IRF1, and several kinases (12/295), including up-regulated Fgr, which is a Src family tyrosine kinase which modulates ICH outcomes.56
Neutrophils showed many activated pathways. These included: Relaxin signaling, involved in vasodilation and angiogenesis103 with predicted activation of VEGF-A, MMP9, and angiogenesis; GNRH signaling, which has a role in neurodegeneration104; NGF signaling which may be neuroprotective after ICH105; and Notch signaling which modulates neuronal injury, apoptosis, angiogenesis, and BBB function following experimental ICH.106,107 As in monocytes, the interferon pathway was significantly suppressed in ICH neutrophils along with IL-1 and α-adrenergic signaling pathways in our previous study.21 IL-1 is a proinflammatory neutrophil mediator in ICH and represents a promising ICH treatment target108–110 (clinical trial NCT03737344). ICH causes activation of the α-adrenergic system which also has been an ICH treatment target.111,112
Atypical gene expression in ICH, IS, and VRFC monocytes and neutrophils
There were many genes differentially expressed in monocytes and neutrophils following IS and ICH in this study which are typically expressed by other cell types. For example, immunoglobulin gene expression, typically expressed by B cells, was present in neutrophil and monocyte DEGs for both IS and ICH. Notably, they were all down-regulated in ICH and IS monocytes, up- and down-regulated in ICH neutrophils, and only up-regulated in IS neutrophils. However, this is not surprising since non-lymphoid cells can produce immunoglobulins,113,114 including immunoglobulins from macrophages.115 This atypical expression pattern shows fine-tuning of genes by IS and ICH, as has been described for systemic inflammation and cardiovascular disease.116
To underscore the utility of the transcriptome findings in this study, we estimated the Pearson’s correlation coefficient between the expression of the DEGs and the NIHSS (NIH Stroke Scale), a measure of severity, at the time of the blood draw. We found FFAR2 (r=−0.98, FDR-corrected p = 0.011) and WASF3 (r = 0.96, FDR-corrected p = 0.038) correlated with NIHSS in ICH monocytes. FFAR2, a short chain free fatty acids receptor, is associated to chronic inflammatory diseases and modulates the monocyte inflammatory response.117–120 WASF3 has a role in cell migration in cancer, acts as a cytoskeleton regulator, and is a target for miR-200b during monocyte-derived dendritic cell differentiation.121–124 DHX58, a regulator of innate immune genes,125 negatively correlated with the NIHSS (r=−0.96, FDR-p = 0.047) in ICH neutrophils. In IS neutrophils, up-regulation of TTC31, which has been associated to atrial fibrillation126 and down-regulation of IATPR (ITGB1 adjacent tumor promoting lncRNA) correlated with NIHSS (r=−0.65, FDR-p = 0.025, and r=−0.63, FDR-p = 0.025, respectively). Thus, these DEGs may be potential therapeutic targets since they correlated with the NIHSS.
There was relatively little overlap of monocyte DEGs with whole blood DEGs. This could be accounted for in part by counteracting expression from other cell types in whole blood (i.e. some genes may increase in some cell types but decrease in other cell types with little net change in whole blood). In addition, monocytes were isolated by positive selection in IS, ICH, and control samples, which might have blunted the expression differences observed in isolated monocytes compared to whole blood where cells are immediately lysed and mRNA stabilized just after the blood draw. In addition, blood samples were taken over the first 3.5–4 days following symptom onset which likely dampened expression patterns that can occur within half a day.21 Time-dependent ICH and IS responses in the peripheral whole blood transcriptome have been reported in our previous studies.21,127 Thus, future time-dependent trajectory analysis of the monocyte and neutrophil ICH and IS responses would help delineate the dynamic time-dependent peripheral response over hours to days.
Though many studies have shown extremely high correlation between RNA-Seq results and qRT-PCR128–133 and our analysis pipeline and very high sequencing coverage of our samples allow for confident transcript quantitation,134–138 future studies will need to validate findings in separate/larger cohorts, and with alternative RNA quantification methods like PCR. In addition, there was a significant difference in some vascular risk factors (VRF) between ICH and IS and controls. Therefore, some differences of gene expression could be due to these VRF. However, examination of the DEGs using unsupervised hierarchical clustering showed grouping by diagnosis only, and not by diabetes, hyperlipidemia, or other VRFs (not shown).
Overall, the identified monocyte and neutrophil dysregulated genes and pathways in ICH and IS and its different etiologies will help us understand the immune responses to each, to better distinguish among IS etiologies, and eventually may guide biomarker and treatment development. These data will also allow for comparison of the immune responses in experimental IS and ICH models to the human immune responses to each and to potentially improve the models.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-3-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-4-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-5-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-6-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-7-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-8-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-9-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We thank the patients who participated in this study and appreciate the support of the UCD Department of Neurology and MIND Institute. Sequencing was performed by the UCD DNA Technologies and Expression Analysis Core (NIH 1S10OD010786-01). We thank Dr. Paul Ashwood, Dr. Milo Careaga, Dr. Destanie R. Rose and Mr. Houa Yang for help with flow cytometry.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were supported by NIH grants NS106950, NS101718, and NS097000 (FRS, BSS, BPA).
Data availability: The data generated for this study will be available upon written request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: Conceived and designed the experiments, and edited the manuscript: BS, FRS, BPA, PC-M. Performed the experiments and/or reviewed data: BPA, FH, HH, XZ, CD-A, EF, HA, PC-M. Cell purity characterization and analysis: BPA, CD-A. Clinical characterization: GJ, FRS. Analyzed the gene expression data and wrote the first draft: PC-M.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Paulina Carmona-Mora https://orcid.org/0000-0001-8560-1232
References
- 1.Askenase MH, Sansing LH.Stages of the inflammatory response in pathology and tissue repair after intracerebral hemorrhage. Semin Neurol 2016; 36: 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jian Z, Liu R, Zhu X, et al. The involvement and therapy target of immune cells after ischemic stroke. Front Immunol 2019; 10: 2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JY, Park J, Chang JY, et al. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol 2016; 25: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shichita T, Sakaguchi R, Suzuki M, et al. Post-ischemic inflammation in the brain. Front Immunol 2012; 3: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin R, Yang G, Li G.Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87: 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atri C, Guerfali FZ, Laouini D.Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci 2018; 19: 1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ElAli A, LeBlanc NJ.The role of monocytes in ischemic stroke pathobiology: new avenues to explore. Front Aging Neurosci 2016; 8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren H, Liu X, Wang L, et al. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis 2017; 26: 2595–2602. [DOI] [PubMed] [Google Scholar]
- 9.Xue J, Huang W, Chen X, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis 2017; 26: 650–657. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Song TJ, Park JH, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis 2012; 222: 464–467. [DOI] [PubMed] [Google Scholar]
- 11.Jickling GC, Liu DZ, Ander BP, et al. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 2015; 35: 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermann DM, Kleinschnitz C, Gunzer M.Implications of polymorphonuclear neutrophils for ischemic stroke and intracerebral hemorrhage: predictive value, pathophysiological consequences and utility as therapeutic target. J Neuroimmunol 2018; 321: 138–143. [DOI] [PubMed] [Google Scholar]
- 13.Cuartero MI, Ballesteros I, Moraga A, et al. N2 Neutrophils, novel players in brain inflammation after stroke. Stroke 2013; 44: 3498–3508. [DOI] [PubMed] [Google Scholar]
- 14.Cai W, Liu S, Hu M, et al. Functional dynamics of neutrophils after ischemic stroke. Transl Stroke Res 2020; 11: 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y, Tsirka SE.The CCL2-CCR2 system affects the progression and clearance of intracerebral hemorrhage. Glia 2012; 60: 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond MD, Ai Y, Sansing LH.Gr1+ macrophages and dendritic cells dominate the inflammatory infiltrate 12 h after experimental intracerebral hemorrhage. Transl Stroke Res 2012; 3: s125–s131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond MD, Taylor RA, Mullen MT, et al. CCR2+Ly6Chi inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci 2014; 34: 3901–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mracsko E, Veltkamp R.Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci 2014; 34: 3901–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Ting SM, Sun G, et al. Beneficial role of neutrophils through function of lactoferrin after intracerebral hemorrhage. Stroke 2018; 49: 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y, Xu H, Du X, et al. Gene expression in blood changes rapidly in neutrophils and monocytes after ischemic stroke in humans: a microarray study. J Cereb Blood Flow Metab 2006; 26: 1089–1102. [DOI] [PubMed] [Google Scholar]
- 21.Stamova B, Ander BP, Jickling G, et al. The intracerebral hemorrhage blood transcriptome in humans differs from the ischemic stroke and vascular risk factor control blood transcriptomes. J Cereb Blood Flow Metab 2019; 39: 1818–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Tang Y, Liu DZ, et al. in peripheral blood differs after cardioembolic compared with large-vessel atherosclerotic stroke: biomarkers for the etiology of ischemic stroke. J Cereb Blood Flow Metab 2008; 28: 1320–1328. [DOI] [PubMed] [Google Scholar]
- 23.Jickling GC, Stamova B, Ander BP, et al. Profiles of lacunar and nonlacunar stroke. Ann Neurol 2011; 70: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dykstra-Aiello C, Jickling GC, Ander BP, et al. Intracerebral hemorrhage and ischemic stroke of different etiologies have distinct alternatively spliced mRNA profiles in the blood: a pilot RNA-seq study. Transl Stroke Res 2015; 6: 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streett DA, Petersen KR, Gerritsen AT, et al. ExpHTS: analysis of high throughput sequence data in an experimental framework. In: BCB 2015 – 6th ACM conference on bioinformatics, computational biology, and health informatics, Atlanta, GA, USA, 9–12 September 2015.
- 26.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013; 29: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Y, Smyth GK, Shi W.FeatureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014; 30: 923–930. [DOI] [PubMed] [Google Scholar]
- 28.Thompson WA.The problem of negative estimates of variance components. Ann Math Stat 1962; 33: 273–289. [Google Scholar]
- 29.Tamhane AC and Dunlop DD. Statistics and data analysis from elementary to intermediate. Upper Saddle River, NJ: Prentice Hall, 2000. [Google Scholar]
- 30.Huang DW, Sherman BT, Lempicki RA.Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57. [DOI] [PubMed] [Google Scholar]
- 31.Huang DW, Sherman BT, Lempicki RA.Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krämer A, Green J, Pollard J, et al. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014; 30: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinsheimer SM, Xu H, Achrol AS, et al. Gene expression profiling of blood in brain arteriovenous malformation patients. Transl Stroke Res 2011; 2: 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraft P, Schuhmann MK, Garz C, et al. Hypercholesterolemia induced cerebral small vessel disease. PLoS One 2017; 12: e0182822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watkins NA, Gusnanto A, De Bono B, et al. A HaemAtlas: characterizing gene expression in differentiated human blood cells. Blood 2009; 113: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jickling GC, Ander BP, Zhan X, et al. microRNA expression in peripheral blood cells following acute ischemic stroke and their predicted gene targets. PLoS One 2014; 9: e99283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García-Culebras A, Durán-Laforet V, Peña-Martínez C, et al. Myeloid cells as therapeutic targets in neuroinflammation after stroke: specific roles of neutrophils and neutrophil–platelet interactions. J Cereb Blood Flow Metab 2018; 38: 2150–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Italiani P, Boraschi D.From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 2014; 5: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanazawa M, Ninomiya I, Hatakeyama M, et al. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci 2017; 18: 2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon S, Taylor PR.Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5: 953–964. [DOI] [PubMed] [Google Scholar]
- 41.Miklasz P, Idzkowska E, Moniuszko M, et al. The role of different monocyte subsets in the pathogenesis of atherosclerosis and acute coronary syndromes. Scand J Immunol 2015; 82: 163–173. [DOI] [PubMed] [Google Scholar]
- 42.Rayasam A, Hsu M, Kijak JA, et al. Immune responses in stroke: how the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology 2018; 154: 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seifert HA, Pennypacker KR.Molecular and cellular immune responses to ischemic brain injury. Transl Stroke Res 2014; 5: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takagi Y, Harada J, Chiarugi A, et al. STAT1 is activated in neurons after ischemia and contributes to ischemic brain injury. J Cereb Blood Flow Metab 2002; 22: 1311–1318. [DOI] [PubMed] [Google Scholar]
- 45.Chen HZ, Guo S, Li ZZ, et al. A critical role for interferon regulatory factor 9 in cerebral ischemic stroke. J Neurosci 2014; 34: 11897–11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seifert HA, Collier LA, Chapman CB, et al. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J Neuroimmune Pharmacol 2014; 9: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelderblom M, Gallizioli M, Ludewig P, et al. IL-23 (Interleukin-23)-producing conventional dendritic cells control the detrimental IL-17 (Interleukin-17) response in stroke. Stroke 2018; 49: 155–164. [DOI] [PubMed] [Google Scholar]
- 48.Yilmaz A, Fuchs T, Dietel B, et al. Transient decrease in circulating dendritic cell precursors after acute stroke: potential recruitment into the brain. Clin Sci 2009; 118: 147–157. [DOI] [PubMed] [Google Scholar]
- 49.Felger JC, Abe T, Kaunzner UW, et al. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun 2010; 24: 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ludewig P, Gallizioli M, Urra X, et al. Dendritic cells in brain diseases. Biochim Biophys Acta 2016; 1862: 352–367. [DOI] [PubMed] [Google Scholar]
- 51.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science 2010; 327: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mildner A, Yona S, Jung S. A close encounter of the third kind. Monocyte-derived cells. In: Kenneth MM and Miriam M (eds) Advances in immunology. Elsevier, 2013, pp. 69–103. [DOI] [PubMed]
- 53.Woo M-S, Yang J, Beltran C, et al. Cell-surface CD36 in monocyte/macrophage contributes to phagocytosis during the resolution phase of ischemic stroke in mice. J Biol Chem 2016; 291: 23654–23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghandour MS, Langley OK, Zhu XL, et al. Carbonic anhydrase IV on brain capillary endothelial cells: a marker associated with the blood-brain barrier. Proc Natl Acad Sci U S A 1992; 89: 6823–6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao M, Hou D, Liang H, et al. MiR-150 promotes the proliferation and migration of lung cancer cells by targeting SRC kinase signalling inhibitor 1. Eur J Cancer 2014; 50: 1013– 1024. [DOI] [PubMed] [Google Scholar]
- 56.Ardizzone TD, Zhan X, Ander BP, et al. Src kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke 2007; 38: 1621– 1625. [DOI] [PubMed] [Google Scholar]
- 57.Liu DZ, Sharp FR, Van KC, et al. Inhibition of Src family kinases protects hippocampal neurons and improves cognitive function after traumatic brain injury. J Neurotrauma 2014; 31: 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li YX, Lin CQ, Shi DY, et al. Upregulated expression of human alpha-defensins 1, 2 and 3 in hypercholesteremia and its relationship with serum lipid levels. Hum Immunol 2014; 75: 1104–1109. [DOI] [PubMed] [Google Scholar]
- 59.Horn M, Bertling A, Brodde MF, et al. Human neutrophil alpha-defensins induce formation of fibrinogen and thrombospondin-1 amyloid-like structures and activate platelets via glycoprotein IIb/IIIa. J Thromb Haemost 2012; 10: 647–661. [DOI] [PubMed] [Google Scholar]
- 60.Guan X.The CNS glucagon-like peptide-2 receptor in the control of energy balance and glucose homeostasis. Am J Physiol Regul Integr Comp Physiol 2014; 307: R585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scott RA, Scott LJ, Mägi R, et al. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes 2017; 66: 2888–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye XC, Hu JX, Li L, et al. Astrocytic Lrp4 (Low-density lipoprotein receptor–related protein 4) contributes to ischemia-induced brain injury by regulating ATP release and adenosine-A2AR (Adenosine A2A Receptor) signaling. Stroke 2018; 49: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gribble KD, Walker LJ, Saint-Amant L, et al. The synaptic receptor Lrp4 promotes peripheral nerve regeneration. Nat Commun 2018; 9(1): 2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uhlén M, Fagerberg L, Hallström BM, et al. Tissue-based map of the human proteome. Science 2015; 347: 1260419-1-9. [DOI] [PubMed] [Google Scholar]
- 65.Cheng C, Haasdijk R, Tempel D, et al. Endothelial cell-specific fgd5 involvement in vascular pruning defines neovessel fate in mice. Circulation 2012; 125: 3142–3158. [DOI] [PubMed] [Google Scholar]
- 66.Lin A-C Hung H-C, Chen Y-W, et al. Elevated Hedgehog-interacting protein levels in subjects with prediabetes and type 2 diabetes. J Clin Med 2019; 8: 1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cho Y, Hazen BC, Russell AP, et al. Peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1)- and estrogen-related receptor (ERR)-induced regulator in muscle 1 (PERM1) is a tissue-specific regulator of oxidative capacity in skeletal muscle cells. J Biol Chem 2013; 288: 25207–25218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rashidian J, Iyirhiaro GO and, Park DS.Cell cycle machinery and stroke. Biochim Biophys Acta 2007; 1772: 484–493. [DOI] [PubMed] [Google Scholar]
- 69.Cai K, Di Q, Shi J, et al. Dynamic changes of cell cycle elements in the ischemic brain after bone marrow stromal cells transplantation in rats. Neurosci Lett 2009; 467: 15–19. [DOI] [PubMed] [Google Scholar]
- 70.Johnson DG, Schneider-Broussard R.Role of E2F in cell cycle control and cancer. Front Biosci 1998; 3: d447–448. [DOI] [PubMed] [Google Scholar]
- 71.Weijts BGMW, Bakker WJ, Cornelissen PWA, et al. E2F7 and E2F8 promote angiogenesis through transcriptional activation of VEGFA in cooperation with HIF1. EMBO J 2012; 31: 3871–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacManus JP, Jian M, Preston E, et al. Absence of the transcription factor E2F1 attenuates brain injury and improves behavior after focal ischemia in mice. J Cereb Blood Flow Metab 2003; 23: 1020–1028. [DOI] [PubMed] [Google Scholar]
- 73.Osuga H, Osuga S, Wang F, et al. Cyclin-dependent kinases as a therapeutic target for stroke. Proc Natl Acad Sci U S A 2000; 97: 10254–10259.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bouchon A, Dietrich J, Colonna M.Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol 2000; 164: 4991–4995. [DOI] [PubMed] [Google Scholar]
- 75.Becher B, Tugues S, Greter M.GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity 2016; 45: 963–973. [DOI] [PubMed] [Google Scholar]
- 76.Edwards DN, Bix GJ.Roles of blood-brain barrier integrins and extracellular matrix in stroke. Am J Physiol Cell Physiol 2019; 316: C252–C236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin R, Liu L, Zhang S, et al. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res 2013; 6: 834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rama R, Garcia Rodriguez JC.Excitotoxicity and oxidative stress in acute ischemic stroke. In: Acute Ischemic Stroke 2012, pp. 29–58. [Google Scholar]
- 79.Maiese K.Cutting through the complexities of mTOR for the treatment of stroke. Curr Neurovasc Res 2014; 11: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woo CH, Massett MP, Shishido T, et al. ERK5 activation inhibits inflammatory responses via peroxisome proliferator-activated receptor δ (PPARδ) stimulation. J Biol Chem 2006; 281: 32164–32174. [DOI] [PubMed] [Google Scholar]
- 81.Su C, Sun F, Cunningham RL, et al. ERK5/KLF4 signaling as a common mediator of the neuroprotective effects of both nerve growth factor and hydrogen peroxide preconditioning. Age 2014; 36: 9685. [DOI] [PMC free article] [PubMed]
- 82.Hadley G, Beard DJ, Couch Y, et al. Rapamycin in ischemic stroke: old drug, new tricks? J Cereb Blood Flow Metab 2019; 39: 20–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curcio M, Salazar IL, Mele M, et al. Calpains and neuronal damage in the ischemic brain: the Swiss knife in synaptic injury. Prog Neurobiol 2016; 143: 1–35. [DOI] [PubMed] [Google Scholar]
- 84.Alluri H, Grimsley M, Shaji CA, et al. Attenuation of blood-brain barrier breakdown and hyperpermeability by calpain inhibition. J Biol Chem 2016; 291: 26958–26969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin JA, Kang JL, Lee KE, et al. Different temporal patterns in the expressions of bone morphogenetic proteins and noggin during astroglial scar formation after ischemic stroke. Cell Mol Neurobiol 2012; 32: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cox G, Gauldie J, Jordana M.Bronchial epithelial cell-derived cytokines (G-CSF and GM-CSF) promote the survival of peripheral blood neutrophils in vitro. Am J Respir Cell Mol Biol 1992; 7: 507–513. [DOI] [PubMed] [Google Scholar]
- 87.Reichardt LF.Neurotrophin-regulated signalling pathways. Philos Transac R Soc B 2006; 361: 1545–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deuel TF, Senior RM, Huang JS, et al. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest 1982; 69: 1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee C, Li X.Platelet-derived growth factor-C and -D in the cardiovascular system and diseases. Mol Aspects Med 2018; 62: 12–21. [DOI] [PubMed] [Google Scholar]
- 90.Giacconi R, Caruso C, Lio D, et al. 1267 HSP70-2 polymorphism as a risk factor for carotid plaque rupture and cerebral ischaemia in old type 2 diabetes-atherosclerotic patients. Mech Ageing Dev 2005; 126: 866–873. [DOI] [PubMed] [Google Scholar]
- 91.Yang X, Yao B, Niu Y, et al. Hypoxia-induced lncRNA EIF3J-AS1 accelerates hepatocellular carcinoma progression via targeting miR-122–5p/CTNND2 axis. Biochem Biophys Res Commun 2019; 518: 239–245. [DOI] [PubMed] [Google Scholar]
- 92.Mizugishi K, Yamanaka K, Kuwajima K, et al. Missense mutations in the phosphomannomutase 2 gene of two Japanese siblings with carbohydrate-deficient glycoprotein syndrome type I. Brain Dev 1999; 21: 223–228. [DOI] [PubMed] [Google Scholar]
- 93.Greenberg DA, Jin K.Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sc 2013; 70: 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsuo R, Ago T, Kamouchi M, et al. Clinical significance of plasma VEGF value in ischemic stroke – research for biomarkers in ischemic stroke (REBIOS) study. BMC Neurol 2013; 13: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dobolyi A, Vincze C, Pál G, et al. The neuroprotective functions of transforming growth factor beta proteins. Int J Mol Sci 2012; 13: 8219–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Selvaraj UM, Poinsatte K, Torres V, et al. Heterogeneity of B cell functions in stroke-related risk, prevention, injury, and repair. Neurotherapeutics 2016; 13: 729–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scapini P, Bazzoni F, Cassatella MA.Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol Lett 2008; 116: 1-6. [DOI] [PubMed] [Google Scholar]
- 98.Keep RF, Zhou N, Xiang J, et al. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS 2014; 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Durocher M, Ander BP, Jickling G, et al. Inflammatory, regulatory, and autophagy co-expression modules and hub genes underlie the peripheral immune response to human intracerebral hemorrhage. J Neuroinflammation 2019; 4: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu R, Wang S, Li W, et al. Activation of peroxisome proliferator-activated receptor-γ by a 12/15-lipoxygenase product of arachidonic acid: a possible neuroprotective effect in the brain after experimental intracerebral hemorrhage. J Neurosurg 2017; 127: 522–531. [DOI] [PubMed] [Google Scholar]
- 101.Zhao X, Guanghua S, Jie Z, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol 2007; 61: 325–362. [DOI] [PubMed] [Google Scholar]
- 102.Tschoe C, Bushnell CD, Duncan PW, et al. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke 2020; 22; 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jeyabalan A, Shroff SG, Novak J, et al. The vascular actions of relaxin. Adv Exp Med Biol 2007; 612: 65–87. [DOI] [PubMed] [Google Scholar]
- 104.Wang L, Chadwick W, Park S-S, et al. Gonadotropin-releasing hormone receptor system: modulatory role in aging and neurodegeneration. CNS Neurol Disord 2010; 9: 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shi W, Wang Z, Pu J, et al. Changes of blood-brain barrier permeability following intracerebral hemorrhage and the therapeutic effect of minocycline in rats. Acta Neurochir Suppl 2011; 110: 61–67. [DOI] [PubMed] [Google Scholar]
- 106.Chen M, Sun J, Lu C, et al. The impact of neuronal Notch-1/JNK pathway on intracerebral hemorrhage-induced neuronal injury of rat model. Oncotarget 2016; 7: 73903–73911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cai Z, Zhao B, Deng Y, et al. Notch signaling in cerebrovascular diseases (Review). Mol Med Rep 2016; 14: 2883–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galea J, Brough D.The role of inflammation and interleukin-1 in acute cerebrovascular disease. J Inflamm Re 2013; 6: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murray KN, Parry-Jones AR, Allan SM.Interleukin-1 and acute brain injury. Front Cell Neurosci 2015; 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clancy DM, Sullivan GP, Moran HBT, et al. Extracellular neutrophil proteases are efficient regulators of IL-1, IL-33, and IL-36 cytokine activity but poor effectors of microbial killing. Cell Rep 2018; 221: 2937–2950. [DOI] [PubMed] [Google Scholar]
- 111.Mittal MK, LacKamp A.Intracerebral hemorrhage: perihemorrhagic edema and secondary hematoma expansion: from bench work to ongoing controversies. Front Neurol 2016; 7: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sansing LH, Messe SR, Cucchiara BL, et al. Anti-adrenergic medications and edema development after intracerebral hemorrhage. Neurocrit Care 2011; 14: 395–400. [DOI] [PubMed] [Google Scholar]
- 113.Duan Z, Zheng H, Xu S, et al. Activation of the Ig Iα1 promoter by the transcription factor Ets-1 triggers Ig Iα1-Cα1 germline transcription in epithelial cancer cells. Cell Mol Immunol 2014; 11: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Z, Qiu X, Gu J.Immunoglobulin expression in non-lymphoid lineage and neoplastic cells. Am J Pathol 2009; 174: 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fuchs T, Hahn M, Ries L, et al. Expression of combinatorial immunoglobulins in macrophages in the tumor microenvironment. PLoS One 2018; 13: e0204108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee TI, Young RA.Transcriptional regulation and its misregulation in disease. Cell 2013; 152: 1237–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009; 461: 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang C, Chang J, Wu W, et al. Activation of GPR43 suppresses TNF-α-induced inflammatory response in human fibroblast-like synoviocytes. Arch Biochem Biophys 2020; 684: 108297. [DOI] [PubMed] [Google Scholar]
- 119.Tang C, Ahmed K, Gille A, et al. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med 2015; 21: 173–177. [DOI] [PubMed] [Google Scholar]
- 120.Ang Z, Er JZ, Tan NS, et al. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci Rep 2016; 6: 34145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Y, Li J, Xia W, et al. MiR-200b modulates the properties of human monocyte-derived dendritic cells by targeting WASF3. Life Sci 2015; 122: 26–36. [DOI] [PubMed] [Google Scholar]
- 122.Sossey-Alaoui K, Ranalli TA, Li X, et al. WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res 2005; 308: 135–145. [DOI] [PubMed] [Google Scholar]
- 123.Sossey-Alaoui K, Li X, Cowell JK.c-Abl-mediated phosphorylation of WAVE3 is required for lamellipodia formation and cell migration. J Biol Chem 2007; 282: 26257–23265. [DOI] [PubMed] [Google Scholar]
- 124.Sossey-Alaoui K, Safina A, Li X, et al. Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am J Pathol 2007; 170: 2112–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Y, Qu L, Liu Y, et al. PUM1 is a biphasic negative regulator of innate immunity genes by suppressing LGP2. Proc Natl Acad Sci U S A 2017; 114: E6902–E6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tan N, Chung MK, Smith JD, et al. Weighted gene coexpression network analysis of human left atrial tissue identifies gene modules associated with atrial fibrillation. Circ Cardiovasc Genet 2013; 6: 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng X, Ander BP, Jickling GC, et al. MicroRNA and their target mRNAs change expression in whole blood of patients after intracerebral hemorrhage. J Cereb Blood Flow Metab 2020; 40: 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang CM, Pan YY, Liu MH, et al. RNA-seq expression profiling of rat MCAO model following reperfusion Orexin-A. Oncotarget 2017; 8: 113066–113081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Logan JG, Yun S, Bao Y, et al. RNA-sequencing analysis of differential gene expression associated with arterial stiffness. Vascular. Epub ahead of print May 2020. DOI: 10.1177/1708538120922650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet 2015; 47: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pan Y, Yu C, Huang J, et al. Bioinformatics analysis of vascular RNA-seq data revealed hub genes and pathways in a novel Tibetan minipig atherosclerosis model induced by a high fat/cholesterol diet. Lipids Health Dis 2020; 19: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao P, Wu H, Zhong Z, et al. Expression profiles of long noncoding RNAs and mRNAs in peripheral blood mononuclear cells of patients with acute myocardial infarction. Medicine 2018; ; 97: e12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li P, Piao Y, Shon HS, et al. Comparing the normalization methods for the differential analysis of Illumina high-throughput RNA-Seq data. BMC Bioinform 2015; 16: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Everaert C, Luypaert M, Maag JLV, et al. Benchmarking of RNA-sequencing analysis workflows using whole-transcriptome RT-qPCR expression data. Sci Rep 2017; 7: 1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lonsdale J, Thomas J, Salvatore M, et al. The genotype-tissue expression (GTEx) project. Nat Genet 2013; 45: 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ardlie KG, DeLuca DS, Segrè AV, et al. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 2015; 348: 648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sims D, Sudbery I, Ilott NE, et al. Sequencing depth and coverage: key considerations in genomic analyses. Nat Rev Genet 2014; 15: 121–132. [DOI] [PubMed] [Google Scholar]
- 138.Conesa A, Madrigal P, Tarazona S, et al. A survey of best practices for RNA-seq data analysis. Genome Biol 2016; 17: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-3-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-4-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-5-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-6-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-7-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-8-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-9-jcb-10.1177_0271678X20953912 for Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke by Paulina Carmona-Mora, Bradley P Ander, Glen C Jickling, Cheryl Dykstra-Aiello, Xinhua Zhan, Eva Ferino, Farah Hamade, Hajar Amini, Heather Hull, Frank R Sharp and Boryana Stamova in Journal of Cerebral Blood Flow & Metabolism