Abstract
The long-term functional outcome of acute basilar artery occlusion (BAO) patients who received modern endovascular therapy (EVT) is unclear. We sought to assess the long-term functional outcome of BAO patients treated with EVT and determine the prognostic factors associated with favorable outcome. We enrolled consecutive BAO patients who received EVT between December 2012 and December 2018 in this observational study. Baseline characteristics and outcomes were presented. Multivariable logistic regression analysis was performed to identify the prognostic factors associated with long-term outcome. Among the 177 BAO patients included in this study, 80 patients (45.2%) obtained favorable outcome and 97 patients (54.8%) had unfavorable outcome at long-term follow-up with a median observation time of 12 months (interquartile range, 3–19). A total of 67 patients (37.9%) died. National Institutes of Health Stroke Scale (NIHSS), posterior circulation Alberta Stroke Program Early Computed Tomography Score (pc-ASPECTS), time from stroke onset to recanalization, and recanalization condition were identified as independent predictors for long-term outcome. Over 40% of BAO patients who were treated with modern EVT achieved favorable outcome at long-term follow-up. NIHSS, pc-ASPECTS, time from stroke onset to recanalization, and recanalization condition were identified as independent prognostic factors of long-term outcome.
Keywords: Basilar artery occlusion, endovascular therapy, long-term outcome, prognostic factor, stroke
Introduction
Acute basilar artery occlusion (BAO) is rare, accounting for approximately 1% of all ischemic strokes and 5–10% of large vessel occlusion strokes.1,2 It always represents a catastrophe as severe disability and mortality remain high in BAO patients even with active treatments.3 Previous studies have indicated that BAO patients may benefit from endovascular therapy (EVT); however, the small sample sizes, patient heterogeneity, and older reperfusion technologies limit the generalization of these findings.4–6 The most recent study, Acute Basilar Artery Occlusion: Endovascular Interventions vs Standard Medical Treatment, was the only multicenter randomized controlled clinical trial that investigated the efficacy and safety of EVT in patients with BAO. However, it was terminated prematurely due to the excessive crossovers and progressive drop in the average recruitment rate, and failed to support the superiority of EVT over standard medical therapy in these patients.7 More evidence on EVT in BAO patients seems to be needed.
Additionally, only few studies have evaluated the long-term functional outcome of BAO patients who were treated with EVT.8,9 Given the relatively old technologies used in these studies (i.e. intra-arterial thrombolysis and obsolete endovascular devices), these results may not guide the current clinical practice. In recent years, modern approaches of EVT, represented by the second-generation stent retrievers and thromboaspiration devices, have been greatly improved, resulting in a significant increase in the recanalization rate.10,11 Understanding the long-term functional outcome of these patients in the modern era will also add evidence about EVT in BAO patients.
In this study, we sought to assess the long-term functional outcome among BAO patients who underwent EVT and to investigate the prognostic factors associated with favorable outcome.
Material and methods
Data availability statement
All supporting data for this analysis are available from the corresponding author upon reasonable request.
Registration and patient consent
This observational study was based on a prospective registry at Xuanwu Hospital, Capital Medical University in Beijing, China. As a comprehensive stroke center in North China, our institution receives stroke patients from both primary and tertiary referral populations. Since 2012, we prospectively collected data of consecutive acute ischemic stroke patients undergoing recanalization therapy (i.e. intravenous thrombolysis and EVT). The institutional review board at Xuanwu Hospital of Capital Medical University approved this study. Verbal and written informed consent was obtained at the time of admission from patients or their legal authorized representatives according to the Declaration of Helsinki.
Patient selection
In the present study, all acute ischemic stroke patients admitted to our institution and underwent EVT from December 2012 to December 2018 were selected. Patients were eligible for inclusion in this study if they had an acute ischemic stroke resulted from acute BAO which was confirmed by computed tomographic angiography, magnetic resonance angiography, or digital subtraction angiography. Patients with bilateral vertebral artery V4 segment occlusion resulting in no flow to the basilar artery were also included in this study. Another inclusion criterion was EVT initiated (groin puncture) within 24 h of stroke onset (or last proof of good health). The exclusion criteria of the present study included: (1) patients with anterior circulation stroke, (2) patients with posterior cerebral artery occlusion, and (3) patients who were lost to follow-up.
Procedures
In our institution, acute ischemic stroke patients caused by large vessel occlusion receive EVT on the basis of the recommendations in the guidelines.12 Local anesthesia during the procedure is more favored if feasible. However, for those patients with uncontrollable agitation, respiratory abnormality, etc., general anesthesia is more likely to be selected.13 Intravenous thrombolysis administration for patients within 4.5 h of stroke onset is permitted in accordance with the current management guidelines.12
Per institutional protocol, all EVTs for stroke patients are performed by neuro-interventionalists with extensive experience in neurovascular intervention. All patients undergo four-vessel diagnostic digital subtraction angiography to identify the occluded artery and assess the collateral flow in the affected territory. The first-line approach of EVT consists of mechanical thrombectomy with stent retriever or thrombo-aspiration devices. Intra-arterial thrombolysis, stenting, and balloon angioplasty are used as rescue therapy when the first-line procedure fails to achieve reperfusion. The specific strategies and devices during the procedure are chosen at the treating neuro-interventionalist’s discretion based on the clinical conditions and imaging results. At the end of the procedure, recanalization condition is classified using the modified Thrombolysis in Cerebral Infarction (mTICI) perfusion score with a score of 2 b or more indicating successful recanalization and a score of 3 indicating complete recanalization.14,15
Data collection
The following data were retrieved from the database: age, gender, body mass index, stroke severity as assessed by the National Institutes of Health Stroke Scale (NIHSS) score, non-contrast computed tomography as assessed by posterior circulation Alberta Stroke Program Early Computed Tomography Score (pc-ASPECTS), level of consciousness as assessed by Glasgow Coma Scale (GCS) score, intravenous thrombolysis, baseline blood pressure and heart rate, comorbidities, pathogenesis, time intervals, details of the procedure, collateral status as assessed by the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) scale, recanalization condition, and outcomes. All the neuroimaging data were analyzed independently by two experienced neuro-radiologists not knowing the clinical information. For cases with disagreement, the final evaluation outcome was reached by consensus.
Follow-up
Clinical assessment was performed via routinely scheduled clinical visits or standardized telephone interviews with the patients or their relatives by an independent investigator blinded to the baseline performance of the patients and the details of the procedure. Clinical status of EVT-treated patients was documented using the modified Rankin Scale (mRS).16 Favorable outcome was defined as the mRS score of 0–3, while unfavorable outcome was defined as the mRS score of 4–6. The mRS score of 3 was still categorized into the favorable outcome group considering the often poor outcome in BAO patients.
Outcome assessment
Patient outcomes were measured at 90 days and beyond for long-term outcome determination. Primary outcome was the score on the mRS at long-term follow-up. Secondary outcomes consisted of clinical outcomes and safety outcomes. Clinical outcomes included the NIHSS score at 24 h and 5–7 days post procedure and the rates of mRS 0–2, mRS 0–3, and mortality at 90-day follow-up as well as at long-term follow-up. Safety outcomes consisted of procedure-related complications and serious adverse events. Procedure-related complications included vessel perforation, dissection, vasospasm, new clot, distal thrombus, and any hemorrhage. Serious adverse events included symptomatic intracranial hemorrhage, pneumonia, other infections, and myocardial ischemia. Symptomatic intracranial hemorrhage was defined according to the definition of the European Cooperative Acute Stroke Study III (any apparently extravascular blood in the brain or within the cranium that was associated with clinical deterioration, as defined by an increase of four points or more in the score on the NIHSS, or that led to death and that was identified as the predominant cause of the neurological deterioration).17
Statistical analysis
Baseline characteristics and outcomes of all patients were reported. Normally distributed continuous variables were presented by their mean and standard deviation (SD) as mean (SD), while non-normally distributed continuous variables were presented by their median and interquartile range (IQR) as median (IQR). The normality of distributions was tested using visual inspection. Categorical variables were reported as absolute numbers and percentages. Only body mass index was missing in about 5% of observations and was replaced with mean value to make efficient use of the data. Additionally, to adjust for confounders and identify independent prognostic factors associated with favorable outcome at long-term follow-up, a multivariable logistic regression analysis was conducted including age, NIHSS, pc-ASPECTS, GCS, time from stroke onset to recanalization, collateral status, recanalization condition, and symptomatic intracranial hemorrhage as covariates. Kaplan–Meier survival analysis was used to reflect the long-term survival probability of BAO patients treated with EVT.
A two-sided P value of 0.05 or less was considered statistically significant and all statistical analyses were performed using IBM SPSS Statistics 26 (IBM Corp, Armonk, NY, USA).
Results
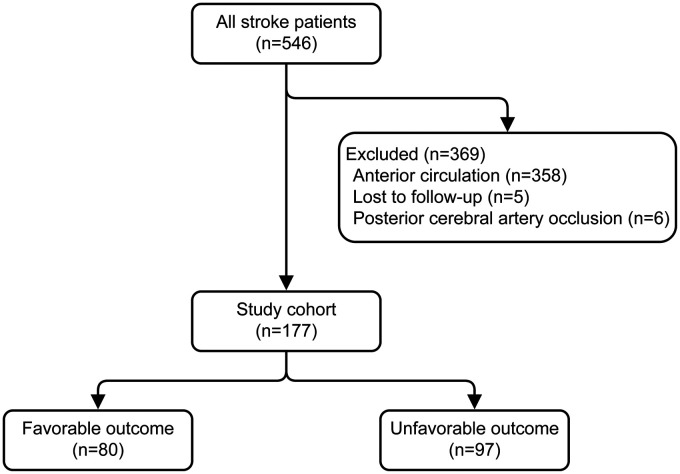
A total of 546 consecutive ischemic stroke patients were admitted to our institution and underwent EVT during the observation period between December 2012 and December 2018. Three hundred and sixty-nine of these patients did not fulfill the predefined inclusion criteria and were excluded, and thus 177 patients underwent primary analysis. Enrollment information including the reasons for exclusion of this study is shown in Figure 1.
Figure 1.
Flow diagram. This figure shows the enrollment information of patients in the present study, including the reasons for exclusion.
Baseline characteristics
Baseline characteristics are presented in Table 1. Of the 177 enrolled patients, the mean age was 59.7 years (SD, 11.8) and 144 patients (81.4%) were male. The median NIHSS score, pc-ASPECTS, and GCS score were 22 (IQR, 14–32), 8 (IQR, 7–10), and 8 (IQR, 5–12), respectively. Thirty-four patients (19.2%) received intravenous thrombolysis.
Table 1.
Baseline characteristics in the study cohort.
| Overall (n = 177) | Favorable outcome (n = 80) | Unfavorable outcome (n = 97) | |
|---|---|---|---|
| Patient characteristics | |||
| Age, y, mean (SD) | 59.7 (11.8) | 57.2 (11.7) | 61.8 (11.5) |
| Male, n (%) | 144 (81.4) | 66 (82.5) | 78 (80.4) |
| BMI, mean (SD)a | 25.9 (3.4) | 25.9 (3.2) | 26.0 (3.5) |
| NIHSS, median (IQR) | 22 (14–32) | 16 (10–27) | 26 (19–36) |
| pc-ASPECTS, median (IQR) | 8 (7–10) | 9 (8–10) | 8 (6–9) |
| GCS, median (IQR) | 8 (5–12) | 10 (5–13) | 6 (4–11) |
| Intravenous alteplase, n (%) | 34 (19.2) | 14 (17.5) | 20 (20.6) |
| Systolic BP, median (IQR) | 150 (133–169) | 145 (132–169) | 150 (133–169) |
| Diastolic BP, median (IQR) | 86 (76–95) | 85 (75–95) | 87 (78–95) |
| Heart rate, median (IQR) | 80 (74–96) | 80 (73–95) | 85 (75–99) |
| Comorbidities, n (%) | |||
| Hypertension | 137 (77.4) | 60 (75) | 77 (79.4) |
| Diabetes mellitus | 53 (29.9) | 22 (27.5) | 31 (32.0) |
| Hyperlipidemia | 41 (23.2) | 21 (26.3) | 20 (20.6) |
| Atrial fibrillation | 30 (16.9) | 13 (16.3) | 17 (17.5) |
| Smoking | 83 (46.9) | 40 (50) | 43 (44.3) |
| Pathogenesis, n (%) | |||
| Large vessel atherosclerosis | 105 (59.3) | 48 (60) | 57 (58.8) |
| Cardioembolic | 40 (22.6) | 14 (17.5) | 26 (26.8) |
| Other | 10 (5.6) | 7 (8.8) | 3 (3.1) |
| Undetermined | 22 (12.4) | 11 (13.8) | 11 (11.3) |
| Time intervals, min | |||
| From stroke onset to groin puncture, median (IQR) | 549 (365–777) | 484 (336–679) | 662 (393–854) |
| From groin puncture to recanalization, median (IQR) | 60 (46–80) | 59 (45–72) | 70 (50–86) |
| From stroke onset to recanalization, median (IQR) | 621 (419.5–833) | 530 (392–715) | 708 (455–906) |
| General anesthesia, n (%) | 108 (61.0) | 48 (60) | 60 (61.9) |
| Collateral status, n (%) | |||
| ASITN/SIR grade 0–2 | 129 (72.9) | 52 (65) | 77 (79.4) |
| ASITN/SIR grade 3–4 | 48 (27.1) | 28 (35) | 20 (20.6) |
| Interventional procedures, n (%) | |||
| Stent retriever | 141 (79.7) | 62 (77.5) | 79 (81.4) |
| Aspiration | 48 (27.1) | 22 (27.5) | 26 (26.8) |
| Intra-arterial thrombolysis | 32 (18.1) | 15 (18.8) | 17 (17.5) |
| Stenting | 59 (33.3) | 25 (31.3) | 34 (35.1) |
| Balloon angioplasty | 33 (18.6) | 16 (20) | 17 (17.5) |
| Recanalization, n (%) | |||
| mTICI≥2b | 157 (88.7) | 77 (96.3) | 80 (82.5) |
| mTICI 3 | 84 (47.5) | 46 (57.5) | 38 (39.2) |
aSix data were missing.
SD: standard deviation; BMI: body mass index; NIHSS: National Institutes of Health Stroke Scale; IQR: interquartile range; pc-ASPECTS: posterior circulation Alberta Stroke Program Early Computed Tomography Score; GCS: Glasgow Coma Scale; BP: blood pressure; ASITN/SIR: American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; mTICI: modified thrombolysis in cerebral infarction.
In terms of time intervals, the median time from stroke onset to groin puncture, time from groin puncture to recanalization, and time from stroke onset to recanalization were 549 min (IQR, 365–777), 60 min (IQR, 46–80), and 621 min (IQR, 419.5–833), respectively. Forty-eight patients (27.1%) had good collateral status (ASITN/SIR grade 3–4). During the procedure, stent retrievers were more frequently used (79.7%) than other approaches. Overall, successful recanalization was achieved in 157 cases (88.7%), while complete recanalization was achieved in 84 cases (47.5%).
Outcomes
The median NIHSS score at 24 h and 5–7 days post procedure was 20 (IQR, 10–35) and 16 (IQR, 8–30), with a median decrease of 1 (IQR, –4 to 2) and 3 (IQR, –8 to 0) compared to the baseline NIHSS score, respectively. At 90 days, 76 patients (42.9%) had a favorable outcome and 54 patients (30.5%) died. After long-term follow-up with a median observation period of 12 months (IQR, 3–19; range, 1–46), 80 patients (45.2%) had a favorable outcome and 97 patients (54.8%) had an unfavorable outcome. The median mRS score at long-term post EVT was 4 (IQR, 1–6).
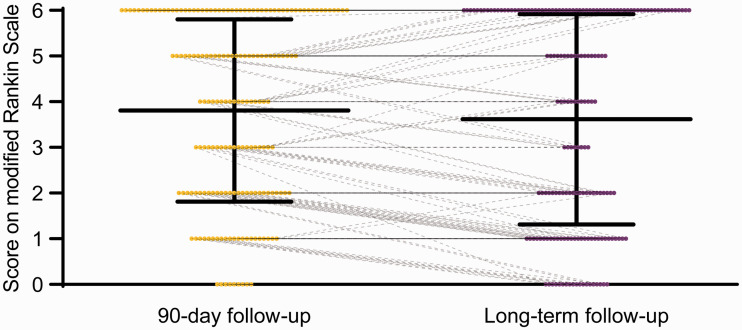
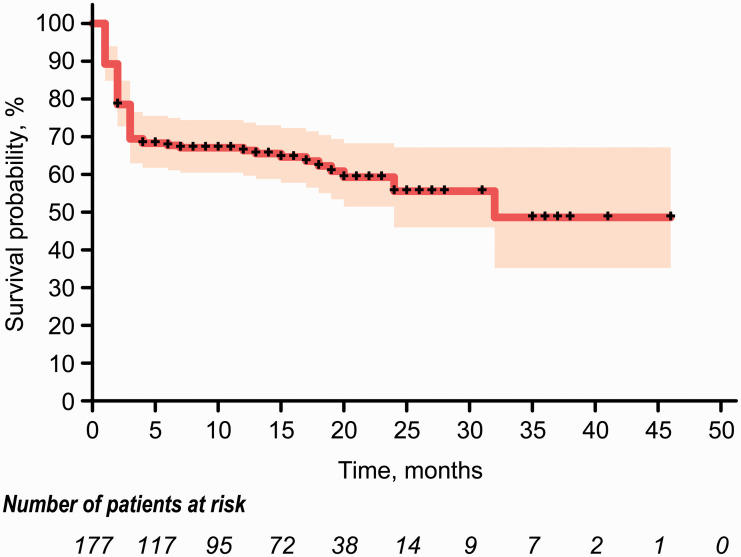
From 90-day to long-term follow-up, four additional patients (2.3%) achieved favorable outcome and 13 additional patients (7.3%) died. Meanwhile, 48 patients (27.1%) displayed an improvement in functional outcomes of at least one point on the mRS score, 108 (61%) patients were unchanged, while 21 patients (11.9%) worsened. Detailed distributions of the mRS score at 90-day and long-term follow-up are shown in Table 2 and Figure 2. Kaplan–Meier curve for the long-term follow-up showed a survival probability of 69.4% at 3 months, 65.5% at 1 year, and 55.6% at 2 years post EVT (Figure 3). In the present study, death was always related to stroke, and no patient died from other causes.
Table 2.
Primary and secondary outcomes in the study cohort.
| Overall(n = 177) | Favorable outcome (n = 80) | Unfavorable outcome (n = 97) | |
|---|---|---|---|
| Primary outcome, median (IQR) | |||
| mRS at long-term | 4 (1–6) | 1 (1–2) | 6 (5–6) |
| Secondary outcomes, clinical | |||
| NIHSS at 24 h, median (IQR) | 20 (10–35) | 14 (8–25) | 25 (17–37) |
| Change in NIHSS at 24 h, median (IQR) | –1 (–4 to 2) | –2 (–3 to 0) | –1 (–4 to 3) |
| NIHSS at 5–7 days, median (IQR) | 16 (8–30) | 12 (6–18) | 22 (10–38) |
| Change in NIHSS at 5–7 days, median (IQR) | –3 (–8 to 0) | –3 (–9 to 0) | –2 (–8 to 0) |
| mRS 0–2 at 90 days, n (%) | 57 (32.2) | 57 (71.3) | 0 (0) |
| mRS 0–3 at 90 days, n (%) | 76 (42.9) | 72 (90) | 4 (4.1) |
| Mortality at 90 days, n (%) | 54 (30.5) | 0 (0) | 54 (55.7) |
| mRS 0–2 at long-term, n (%) | 72 (40.7) | 72 (90) | 0 (0) |
| mRS 0–3 at long-term, n (%) | 80 (45.2) | 80 (100) | 0 (0) |
| Mortality at long-term, n (%) | 67 (37.9) | 0 (0) | 67 (69.1) |
| Secondary outcomes, safety, n (%) | |||
| Procedure-related complications | 37 (20.9) | 17 (21.3) | 20 (20.6) |
| Serious adverse events | |||
| Symptomatic intracranial hemorrhage | 11 (6.2) | 2 (2.5) | 9 (9.3) |
| Pneumonia | 48 (27.1) | 19 (23.8) | 29 (29.9) |
| Other infections | 10 (5.6) | 4 (5) | 6 (6.2) |
| Myocardial ischemia | 6 (3.4) | 2 (2.5) | 4 (4.1) |
IQR: interquartile range; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale.
Figure 2.
90-day and long-term functional outcomes according to the modified Rankin Scale. The median observation period for long-term follow-up was 12 months (interquartile range, 3 –19; range, 1–46).
Figure 3.
Kaplan–Meier curve for the long-term survival probability of acute basilar artery occlusion patients treated with endovascular therapy. The curve for long-term follow-up shows a survival probability of 69.4% at three months, 65.5% at one year, and 55.6% at two years post endovascular therapy. Censored events are represented by tick marks on this curve. The shaded region represents the 95% confidence interval.
In terms of safety outcomes, 37 patients (20.9%) experienced procedure-related complications and 11 patients (6.2%) had symptomatic intracranial hemorrhage. Further details regarding safety outcomes are given in Table 2.
Prognostic factors
Multivariable logistic regression analysis identified NIHSS (OR, 0.933; 95% CI, 0.891–0.977), pc-ASPECTS (OR, 1.336; 95% CI, 1.023–1.746), time from stroke onset to recanalization (OR, 0.998; 95% CI, 0.997–1.000), and recanalization condition (OR, 6.385; 95% CI, 1.495–27.266) as prognostic factors of favorable outcome at long-term follow-up (Table 3). We further categorized these identified prognostic factors into subgroups (NIHSS: 0–10, 10–20, 20–42; pc-ASPECTS: 0–8; 8–10; time from stroke onset to recanalization: 0–6 h, 6–12 h, 12–24 h; recanalization condition: mTICI 2a, mTICI 2 b-3) and described their distributions of the mRS score at long-term follow-up, respectively. These results are presented in Supplemental Table 1.
Table 3.
Predictors of favorable outcome at long-term follow-up (multivariable analysis).
| Odds ratio | 95% Confidence interval | |
|---|---|---|
| Age, per 1-y increase | 0.970 | 0.941–1.001 |
| NIHSS, per one-point increase | 0.933 | 0.891–0.977 |
| pc-ASPECTS, per one-point increase | 1.336 | 1.023–1.746 |
| GCS, per one-point increase | 0.994 | 0.881–1.121 |
| Time from stroke onset to recanalization, per one-minute increase | 0.998 | 0.997–1.000 |
| Collateral status | 1.945 | 0.874–4.327 |
| Recanalization condition | 6.385 | 1.495–27.266 |
| Symptomatic intracranial hemorrhage | 0.296 | 0.046–1.917 |
NIHSS: National Institutes of Health Stroke Scale; pc-ASPECTS: posterior circulation Alberta Stroke Program Early Computed Tomography Score; GCS: Glasgow Coma Scale.
Discussion
In this observational study, we report the long-term functional outcome of acute ischemic stroke patients caused by BAO who were treated with EVT. After a median long-term follow-up period of 12 months, 45.2% patients achieved favorable outcome. Over 60% of patients survived the long-term follow-up, while nearly 40% of patients died. Furthermore, we found that NIHSS, pc-ASPECTS, time from stroke onset to recanalization, and recanalization condition were the independent prognostic factors of functional outcome at long-term follow-up among BAO patients underwent EVT.
To our knowledge, this is by far the first systematic study to evaluate the long-term functional outcome of BAO patients who underwent EVT with modern devices and techniques. We found that both the proportions of favorable outcome and mortality in these patients further increased during long-term follow-up compared to 90-day follow-up. This trend is consistent with previously reported long-term outcome of BAO patients who underwent recanalization therapy.8,9,18 In fact, compared to the previous reports, a higher proportion of favorable outcome and a lower mortality at long-term follow-up were observed in this study. The main explanation for these differences is the implementation of modern endovascular devices and techniques, which has significantly improved the recanalization rate.19,20 Approximately 90% of our patients achieved successful recanalization with modern EVT, significantly higher than the rate of 52% for intravenous thrombolysis18 and 69.8% for intra-arterial thrombolysis.8 Another study investigating long-term outcome of BAO patients who were treated with multimodal recanalization therapy (i.e. intravenous thrombolysis, intra-arterial thrombolysis, and endovascular mechanical recanalization) also reported a recanalization rate (Thrombolysis in Myocardial Infarction (TIMI) grades 2–3) of up to 89%.9 However, given the limitations of the TIMI scoring system,21,22 defining successful recanalization as TIMI 2–3 may lead to an overestimation of the actual recanalization rate. Prior studies have revealed the importance of successful recanalization in the three-month prognosis of BAO patients.23,24 We showed an over six-fold increased odds of long-term favorable outcome if successful recanalization was attained, which provided further evidence for the use of EVT in BAO patients. Another explanation for the different proportion of favorable outcome and mortality is that, the median follow-up period of 12 months in this study was still relatively short compared to the 2.8–4.2-year follow-up periods in the previous studies. Recently, Bouslama et al analyzed 214 BAO patients who were treated with EVT and reported that 26.6% of cases achieved good outcome (mRS 0–2) with a mortality of 46.7% at 90 days.25 These results also have some discrepancies with our findings, probably due to the differences in the specific definition of favorable outcome and the period of long-term follow-up. A comparison of the present study with previous studies exploring the outcome of BAO patients who underwent recanalization therapy is summarized in Supplemental Table 2. Additionally, the results from the Kaplan–Meier curve indicated that the bulk of the mortality in BAO patients occurred at 90 days and leveled off thereafter. In other words, long-term survival after EVT for BAO patients depends mainly on survival at 90 days post procedure. This finding is consistent with a previous report of anterior circulation stroke.26
We found that stroke severity as assessed by NIHSS and early ischemic changes as assessed by pc-ASPECTS were independent predictors of long-term functional outcome in EVT treated patients with BAO. Specifically, every one-point increase in NIHSS was associated with a 6.7% decreased odds of attaining favorable outcome, while the odds of long-term favorable outcome would increase by 33.6% for every one-point increase in pc-ASPECTS. The association between NIHSS and prognosis is easy to understand because NIHSS is the most commonly used scale to evaluate the neurologic deficit in stroke patients. Numerous studies have also confirmed the predictive effect of NIHSS on three-month prognosis of BAO patients.23,25,27 However, studies examining whether pc-ASPECTS can predict the prognosis in BAO patients have yielded conflicting results. A recent study suggested that pc-ASPECTS ≤8 was independently associated with poor outcome in EVT-treated patients, which is consistent with our findings.28 In contrast, a retrospective study failed to confirm the predictive value of pc-ASPECTS for three-month outcome of BAO patients.29 Additionally, another study also failed to detect a significant association between pc-ASPECTS and outcome after adjusting for related confounders.30 These discrepancies may be due to the differences in patient characteristics, recanalization therapies, and recanalization conditions among these studies. Further studies are needed to clarify this issue.
Time was also identified as a predictor for long-term outcome in this study. According to the results of multivariable analysis, the probability of achieving favorable outcome at long-term decreased by 0.2% for each minute of delay between stroke onset to recanalization. Subgroup analysis of time from stroke onset to recanalization indicated that in 0–6 h, 6–12 h, and 12–24 h groups, percentages of patients with long-term favorable outcome were 55.8%, 50.6%, and 29.1%, while mortalities were 32.6%, 36.7%, and 43.6%, respectively. The robust impact of time on the prognosis of stroke patients has been widely recognized. According to the Basilar Artery International Cooperation Study, none of the patients with severe deficit treated beyond 9 h after stroke onset achieved favorable outcome.2 Another multicenter retrospective analysis found that the proportion of good outcome was twice as high in patients who received EVT within the first 6 h than those received beyond 6 h.31 However, despite a longer time always implies a worse prognosis, it is inappropriate to exclude these patients from EVT directly. According to a cohort of 184 patients, in the absence of extensive baseline ischemia, recanalization of BAO up to 48 h after onset still produced good outcomes in 50% of cases.32 In view of the dismal natural course of BAO patients, in our institution, those patients with stroke onset over 6 h would still receive EVT as long as they are considered to benefit from recanalization after in-depth evaluation.
Our study has several limitations, most of which are inherent to the single-center and non-randomized design. We also lacked a control group of patients who did not receive EVT for comparison. Furthermore, we only evaluated the long-term functional outcome of BAO patients, but lacked the evaluation of quality of life. Overall, however, considering the absence of a clear conclusion on the optimal treatment for BAO patients, this study improves our understanding of the long-term functional outcome of BAO patients undergoing EVT with modern devices and techniques, and could at least provide some clinical evidence to guide the daily clinical practice.
In conclusion, over 40% of BAO patients who were treated with modern EVT achieved favorable outcome at long-term follow-up. NIHSS, pc-ASPECTS, time from stroke onset to recanalization, and recanalization condition were identified as independent prognostic factors of long-term outcome.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20958587 for Long-term outcome of endovascular therapy for acute basilar artery occlusion by Longfei Wu, Da Zhang, Jian Chen, Chenghe Sun, Kangxiang Ji, Weili Li, Wenbo Zhao, Chuanhui Li, Chuanjie Wu, Ming Li, Di Wu and Xunming Ji in Journal of Cerebral Blood Flow & Metabolism
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Key Research and Development Program of China (2016YFC1301502 and 2017YFC1308401) and National Science Foundation of China (81771260, 81601006, and 81620108011).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author’s contributions: LW, DZ, and XJ conceptualized and designed the study. JC, CS, KJ, WL, WZ, CL, CW, ML, and DW collected the data. LW analyzed the data. LW drafted the manuscript. LW, DZ, WZ, and XJ revised the manuscript.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Longfei Wu https://orcid.org/0000-0002-0954-9126
Wenbo Zhao https://orcid.org/0000-0001-7141-6394
Xunming Ji https://orcid.org/0000-0003-0293-2744
References
- 1.Mattle HP, Arnold M, Lindsberg PJ, et al. Basilar artery occlusion. Lancet Neurol 2011; 10: 1002–1014. [DOI] [PubMed] [Google Scholar]
- 2.Schonewille WJ, Wijman CA, Michel P, et al. ; BASICS Study Group. Treatment and outcomes of acute basilar artery occlusion in the basilar artery international cooperation study (BASICS): a prospective registry study. Lancet Neurol 2009; 8: 724–730. [DOI] [PubMed] [Google Scholar]
- 3.Schonewille WJ, Algra A, Serena J, et al. Outcome in patients with basilar artery occlusion treated conventionally. J Neurol Neurosurg Psychiatry 2005; 76: 1238–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hacke W, Zeumer H, Ferbert A, et al. Intra-arterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke 1988; 19: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 5.Van Houwelingen RC, Luijckx GJ, Mazuri A, et al. Safety and outcome of intra-arterial treatment for basilar artery occlusion. JAMA Neurol 2016; 73: 1225–1230. [DOI] [PubMed] [Google Scholar]
- 6.Gory B, Eldesouky I, Sivan-Hoffmann R, et al. Outcomes of stent retriever thrombectomy in basilar artery occlusion: an observational study and systematic review. J Neurol Neurosurg Psychiatry 2016; 87: 520–525. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Dai Q, Ye R, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol 2020; 19: 115–122. [DOI] [PubMed] [Google Scholar]
- 8.Jung S, Mono ML, Fischer U, et al. Three-month and long-term outcomes and their predictors in acute basilar artery occlusion treated with intra-arterial thrombolysis. Stroke 2011; 42: 1946–1951. [DOI] [PubMed] [Google Scholar]
- 9.Ottomeyer C, Zeller J, Fesl G, et al. Multimodal recanalization therapy in acute basilar artery occlusion: long-term functional outcome and quality of life. Stroke 2012; 43: 2130–2135. [DOI] [PubMed] [Google Scholar]
- 10.Goyal M, Menon BK, Van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Wu D, Yang T, et al. Hypothermic neuroprotection against acute ischemic stroke: the 2019 update. J Cereb Blood Flow Metab 2020; 40: 461–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, Jadhav AP, Zhao W, et al. General anesthesia vs local anesthesia during mechanical thrombectomy in acute ischemic stroke. J Neurol Sci 2019; 403: 13–18. [DOI] [PubMed] [Google Scholar]
- 14.Zaidat OO, Yoo AJ, Khatri P, et al. ; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L, Zhao W, Rajah GB, et al. Postinterventional sedation worsens functional outcomes in patients with acute ischemic stroke treated with endovascular therapy. World Neurosurg 2019; 130: e794–e803. [DOI] [PubMed] [Google Scholar]
- 16.Van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 17.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 18.Lindsberg PJ, Soinne L, Tatlisumak T, et al. Long-term outcome after intravenous thrombolysis of basilar artery occlusion. JAMA 2004; 292: 1862–1866. [DOI] [PubMed] [Google Scholar]
- 19.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012; 380: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 20.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 2012; 380: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saver JL, Albers GW, Dunn B, et al. ; STAIR VI Consortium. Stroke therapy academic industry roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke 2009; 40: 2594–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo AJ, Simonsen CZ, Prabhakaran S, et al. Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke 2013; 44: 2509–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gory B, Mazighi M, Labreuche J, et al. ; ETIS (Endovascular Treatment in Ischemic Stroke) Investigators. Predictors for mortality after mechanical thrombectomy of acute basilar artery occlusion. Cerebrovasc Dis 2018; 45: 61–67. [DOI] [PubMed] [Google Scholar]
- 24.Gory B, Mazighi M, Blanc R, et al. Mechanical thrombectomy in basilar artery occlusion: influence of reperfusion on clinical outcome and impact of the first-line strategy (ADAPT vs stent retriever). J Neurosurg 2018; 129: 1482–1491. [DOI] [PubMed] [Google Scholar]
- 25.Bouslama M, Haussen DC, Aghaebrahim A, et al. Predictors of good outcome after endovascular therapy for vertebrobasilar occlusion stroke. Stroke 2017; 48: 3252–3257. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Shang S, Li C, et al. Long-term outcomes of acute ischemic stroke patients treated with endovascular thrombectomy: a real-world experience. J Neurol Sci 2018; 390: 77–83. [DOI] [PubMed] [Google Scholar]
- 27.Yoon W, Kim SK, Heo TW, et al. Predictors of good outcome after stent-retriever thrombectomy in acute basilar artery occlusion. Stroke 2015; 46: 2972–2975. [DOI] [PubMed] [Google Scholar]
- 28.Alemseged F, Shah DG, Bivard A, et al. Cerebral blood volume lesion extent predicts functional outcome in patients with vertebral and basilar artery occlusion. Int J Stroke 2019; 14: 540–547. [DOI] [PubMed] [Google Scholar]
- 29.Gilberti N, Gamba M, Premi E, et al. Endovascular mechanical thrombectomy in basilar artery occlusion: variables affecting recanalization and outcome. J Neurol 2016; 263: 707–713. [DOI] [PubMed] [Google Scholar]
- 30.Karameshev A, Arnold M, Schroth G, et al. Diffusion-weighted MRI helps predict outcome in basilar artery occlusion patients treated with intra-arterial thrombolysis. Cerebrovasc Dis 2011; 32: 393–400. [DOI] [PubMed] [Google Scholar]
- 31.Mokin M, Sonig A, Sivakanthan S, et al. Clinical and procedural predictors of outcomes from the endovascular treatment of posterior circulation strokes. Stroke 2016; 47: 782–788. [DOI] [PubMed] [Google Scholar]
- 32.Strbian D, Sairanen T, Silvennoinen H, et al. Thrombolysis of basilar artery occlusion: impact of baseline ischemia and time. Ann Neurol 2013; 73: 688–694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20958587 for Long-term outcome of endovascular therapy for acute basilar artery occlusion by Longfei Wu, Da Zhang, Jian Chen, Chenghe Sun, Kangxiang Ji, Weili Li, Wenbo Zhao, Chuanhui Li, Chuanjie Wu, Ming Li, Di Wu and Xunming Ji in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
All supporting data for this analysis are available from the corresponding author upon reasonable request.