Abstract
The positron emission tomography (PET) tracer [18F]GE-179 binds to the phencyclidine (PCP) site in the open N-methyl-D-aspartate receptor ion channel (NMDAR-IC). To demonstrate that PET can visualise increased [18F]GE-179 uptake by active NMDAR-ICs and that this can be blocked by the PCP antagonist S-ketamine, 15 rats had an electrode unilaterally implanted in their ventral hippocampus. Seven rats had no stimulation, five received pulsed 400 µA supra-threshold 60 Hz stimulation alone, and three received intravenous S-ketamine injection prior to stimulation. Six other rats were not implanted. Each rat had a 90 min [18F]GE-179 PET scan. Stimulated rats had simultaneous depth-EEG recordings of induced seizure activity. [18F]GE-179 uptake (volume of distribution, VT) was compared between hemispheres and between groups. Electrical stimulation induced a significant increase in [18F]GE-179 uptake at the electrode site compared to the contralateral hippocampus (mean 22% increase in VT, p = 0.0014) and to non-stimulated comparator groups. Rats injected with S-ketamine prior to stimulation maintained non-stimulated levels of [18F]GE-179 uptake during stimulation. In conclusion, PET visualisation of focal [18F]GE-179 uptake during electrically activated NMDAR-ICs and the demonstration of specificity for PCP sites by blockade with S-ketamine support the in vivo utility of [18F]GE-179 PET as a use-dependent marker of NMDAR-IC activation.
Keywords: Epilepsy, seizure, [18F]GE-179, glutamate, NMDA ion channel, positron emission tomography
Introduction
Glutamate N-methyl-D-aspartate receptors (NMDARs) regulate excitatory synaptic transmission and are widely expressed in the central nervous system. Most NMDARs assemble as tetramer complexes forming a ligand and voltage gated ion channel (IC) responsible for the synaptic plasticity involved in encoding memory and learning.1 Activation of NMDARs is complex, requiring simultaneous binding of the agonists L-glutamate and L-glycine. This leads to opening of the NMDAR-IC at a voltage which is determined by AMPA receptor activity and expulsion of the Mg2+ cation blocking its intra-channel site so allowing inward and outward flow of Na+, Ca2+ and K+ ions.2–5 The NMDAR-IC contains a phencyclidine (PCP) binding site accessible when opened on activation to non-competitive antagonists such as S-ketamine.3
Excessive glutamate release following cellular injury causes abnormal activation of NMDAR-ICs leading to excitotoxicity6 due to the associated Ca2+ influx into cells as Mg2+ is expelled. Such excitotoxicity is seen after stroke and brain trauma,7–9 can lead to epileptogenesis10,11 and may also be a feature of paroxysmal neurological disorders.5
The use of positron emission tomography (PET) to localise in vivo overactive NMDAR-ICs associated with epileptogenic foci has proved difficult. To date, no PET tracer has convincingly shown a capacity to map an epileptogenic zone in-vivo, despite development of around 50 radioligands targeting different sites on the NMDAR complex.12 Several PET ligands for the intra-channel PCP site13 have been evaluated for human use but have produced inconclusive results due to (a) rapid metabolism and washout ([11C]S-Ketamine),13,14 (b) low specific binding ([11C]CNS-5161)13 or (c) high background binding ([18F]MK-801).15 The radioligand [18F]GE-179, a fluorinated analogue of the compound CNS-5161, has been developed by GE Healthcare. It labels the PCP site in open NMDAR-ICs with an in vitro affinity (Ki) of 2.4 nM in rats and shows little cross-reactivity for over 60 other CNS receptors including glutamate ionotropic and metabotropic receptor subtypes.16,17 Rats and humans share 87%–95% sequence homology of the NR2 receptor subunit, the intramembranous PCP site-containing region being almost identical and having a nanomolar affinity for [18F]GE-179.16 A pre-clinical autoradiography study using lateral fluid-percussion to induce superficial traumatic brain injury in rats detected a focal increase in [18F]GE-179 binding to NMDAR-IC followed by progression of the increased [18F]GE-179 binding from the initial lesion site to encompass subcortical structures over the following weeks.18
In a human [18F]GE-179 PET study, 10 healthy volunteers and 11 patients with frequent interictal epileptiform discharges were scanned. Four of the patients showed increased [18F]GE-179 uptake in regions concordant with foci found on EEG.19,20 The authors suggested that failure to detect focal increases in the other 7 of the 11 patients might be due to chronic spiking leading to widespread NMDAR-IC activation extending beyond the epileptogenic focus.
In a parallel study to this current one, we used unilateral deep brain stimulation (DBS) of the hippocampus in minipigs to induce NMDAR-IC activation. We detected a significant mean 48% global increase in brain [18F]GE-179 uptake with PET during the focal stimulation.21 However, in that study, a challenge with the PCP inhibitor MK-801 did not attenuate or block [18F]GE-179 uptake despite this agent being a potent antagonist. This failure to see an in vivo blocking effect of MK-801 at the PCP site of NMDAR-ICs has also been reported in an animal study involving non-human primates.22
An earlier study by van der Doef et al. demonstrated a significant reduction in whole brain tracer influx rate and accumulation in humans of the NMDAR ligand [11C]GMOM after an S-ketamine challenge.23 This suggests that S-ketamine and [11C]GMOM bind to overlapping sites in the NMDAR-IC whereas the MK-801 site may be separate. Given this, we hypothesised that S-ketamine might be better suited for a pre-clinical blocking study to demonstrate the specificity of [18F]GE-179 to the NMDAR-IC when opened using electrical stimulation.
In this cross-sectional PET study we measured brain [18F]GE-179 uptake in rats during repetitive seizure activity induced by pulsed 60 Hz electrical stimulation of the ventral hippocampus. We hypothesised that [18F]GE-179 PET would visualise electrically induced focal NMDAR-IC activation as increased tracer uptake and that S-ketamine administration ahead of DBS would block the signal confirming [18F]GE-179 specificity for the NMDAR-IC. Our primary outcome measure was detection of focally increased [18F]GE-179 uptake around the stimulating electrode tip. The secondary outcome measure was demonstration of blocked uptake at the electrode tip during stimulation following a prior injection of S-ketamine.
Materials and methods
Animals
Twenty-one adult male Wistar rats (12–14 weeks old/350–400 g, Taconic) were housed individually (12/12-hour dark/light cycle) with free access to food and water. All experiments followed a protocol approved and regulated by the Danish Animal Experiment Inspectorate (2013-15-2934-00934) and regulations of the European Legislation for the protection of vertebrate animals and Directive 2010/63/EU-EUR-Lex. All studies were carried out in accordance with ARRIVE guidelines.
Groups and experimental design
We randomised the 21 rats into four groups: 6 intact rats were not implanted with an electrode (baseline_no_implant), 7 control rats were unilaterally implanted but not electrically stimulated (implant_no_stim_ctrl), 5 rats were unilaterally implanted and electrically stimulated (implant_and_stim) and 3 rats were pre-dosed with the PCP antagonist S-ketamine before electrical stimulation (implant_KET_and_stim). To minimise the number of animals used, the right hippocampus was always implanted and stimulated and the left hippocampus was used as an internal control. We used a cross-sectional study design to avoid repeated catheter placement in the femoral artery used to obtain the blood input function for the kinetic modelling of PET time–activity curves (TACs).
Surgery and implantation
Fifteen rats were anesthetised with isoflurane, placed in a stereotactic frame (Leica Biosystems) and implanted in the right ventral hippocampus with a tripolar MRI compatible polyimide insulated tungsten electrode (total diameter in tissue: 0.02 mm: Plastics One) held in place with superglue (Loctite) and dental cement (Fuji), under sterile conditions. Trajectory coordinates relative to bregma (AP –5.2; ML; –4.6; DV 8.0, from brain) were used to target the ventral hippocampus (Paxinos and Watson, 6th edition). After surgery, rats were inspected daily, treated with analgesia and antibiotics for 4 days and allowed at least 1 week of recovery before stimulation and PET.
Stimulation thresholding
In a pilot study, the current threshold which induced afterdischarges (AD) in the ventral hippocampus of individual rats (n = 8) was determined by increasing the 60 Hz electrical stimulation current by 25 µA until ictal ADs were detected by the depth EEG. Simultaneous video allowed the seizure duration and intensity to be documented using Racine staging.24 Based on that data, we used a supra-threshold current of 400 µA in all the subsequent cases to induce ADs arising from the ventral hippocampus without causing generalised seizures.
Stimulation paradigm
We modified the single evoked epileptic AD model25 by using repeated stimulations with inter-stimuli intervals of 5 min which avoided inducing status epilepticus.26 This pulsed 400 µA supra-threshold stimulation resulted in AD (spikes or spike-wave) after each stimulation train.26 Based on our pilot study (where we combined candidate excitatory paradigms26–28) the most suitable AD inducing stimulation paradigm consisted of a repetitive series of 10 s trains with 60 Hz monophasic square waves, 1 ms pulse width, 5 V pulse height and 400 µA. A bioamplifier (Powerlab 8/30, ADInstruments) was used to control whether the electrode was in the stimulating or recording mode, and the stimulation paradigm was programmed in Labchart (ADInstruments v.7).
EEG and afterdischarge recording
The EEG signals were amplified and recorded through an analogue differential pre-amplifier (NL104, Digitimer) 2000 × gain, low-pass filtered at 1–500 Hz (NL125/6 Filter, Digitimer), then digitalised by the Powerlab at 1 kHz frequency sampling and low-pass filtered at 40 Hz (mains in DK is 50 Hz). Calibration and validation of EEG discharge detection sensitivity was performed with a function generator (PM 5138, Phillips) and tested with a digital oscilloscope (DS1042C, RIGOL). ADs were identified as high-amplitude EEG waves appearing in a train of low-frequency (1–12 Hz) spikes and spike-waves lasting for at least 5 s or voltage amplitude twice the baseline.29–31 On longer EEGs we could see delayed AD, spiking or sporadic epileptiform discharges.
Radiotracer preparation
[18F]GE-179 was synthesised by adapting the method of Robins et al.15 The thiol precursor (15 mg AH113184, GE) was reacted with the fluorotosylate in the presence of 25 µL pentamethylpiperidine base in 300 µL DMSO (Sigma Aldrich, Denmark, Batch: STBD7807V+STBF2442V) and 700 µl MeCN (ABX, Germany; Batch: TM-A-141104404) (3:7) for 30 min at 95°C.
Experiments
Imaging was performed with a hybrid microPET/1 T MRI scanner (nanoScan® PM, Mediso Ltd). Animals were initially anaesthetised with an isoflurane mixture (4%–5% isoflurane and medical O2). After placement of a heparinised femoral arterial catheter, the rats were positioned prone in a stereotaxic frame, and then connected to the stimulation equipment. A short tail pinch resulting in a single spike on the EEG confirmed connection. Anaesthesia was maintained with isoflurane (2.5%–3.5% and medical O2) through a mask and body temperature was kept stable.
Animals from the implant_and_stim group received the first 10 s 60 Hz stimulus train and EEG recording 5 min prior to intravenous [18F]GE-179 injection into a tail vein with simultaneous onset of PET acquisition. [18F]GE-179 was intravenously injected as a slow bolus over 10–15 s, and arterial blood sampling was performed. Ten-second stimulus trains followed every 5 min resulting in 19–20 trains throughout the 90 min PET. The EEG recording continued uninterrupted during the 5 min intervals between stimulations. The intact baseline_no_implant as well as the implant_no_stim_ctrl groups were not connected to the stimulation equipment, but were otherwise handled exactly as the implant_and_stim group. The implant_KET_and_stim group were administered S-ketamine (0.5 mg/kg i.v. T1/2:2h) 5 min prior to the first stimulus train, followed by the exact same stimulation paradigm as the implant_and_stim group (see timeline, Figure 1). Pilot studies had shown that this S-ketamine dose was the highest blocking dose achievable without inducing respiratory arrest.
Figure 1.
Experimental timeline. After isoflurane induction, arterial catheter insertion and preparation in scanner bed, the first 10 s electrical stimulus train was given 5 min before [18F]GE-179 tracer injection. In three of the eight stimulated rats, S-ketamine was administered 5 min prior to the first stimulus train.
EEG: electroencephalogram; MRI: magnetic resonance imaging; PET: positron emission tomography.
After the 90 min dynamic PET acquisition in list mode, a 12 min transmission scan was performed to correct for tissue attenuation of emitted radiation and a 20 min structural T1-weighted MRI (0.5 mm3 voxel) was acquired to allow co-registration of PET images. After the scan sequence had ended, anesthetised animals were killed by decapitation using an approved protocol. Electrodes were carefully taken out and the brain was then immersed in 4% formaldehyde to enable histology.
Blood processing
[18F]GE-179 was injected (20–40 MBq) as a slow intravenous bolus over 10–15 s, and arterial blood sampling was successfully performed in 18 of the 21 rats (in 3 of the 7 implant_no_stim_ctrl rats there was difficulty in obtaining femoral access). Four drops of blood (200 µL) were collected in heparinised tubes every 10 s during the first 2 min of the scan and then at 3, 5, 10, 20, 30, 45, 60, 75 and 88 min after tracer injection and replaced with equivalent volumes of saline. The tubes were split in two unequal portions: 150 µL to acquire plasma (centrifuged 12 min, 1.000 g, 4°C) and 50 µL with whole blood. A well counter (Packard Cobra Gamma Counter D5003, Packard Biosciences) was used to count and decay-correct plasma and whole blood activity to determine the input functions for data analysis.
To determine the tracer metabolite and parent fractions in plasma, 100 µL plasma from the time points 2, 5, 20, 30 and 45 min was mixed with 100 µL perchloric acid to precipitate plasma proteins. After centrifugation (5 min × 13.000 r/min) the supernatant was analysed and fractionated by HPLC (Phenomenex Luna C18(2) 10 µm 250 × 10 mm, ACN:70 mM NaH2PO4, 50:50, 5 ml/min). Peaks were identified by ultraviolet (λ = 254 nm) and gamma detection. 18 F radioactivity concentrations were measured in a well counter Packard Cobra Gamma Counter D5003, Packard Biosciences.
Image processing and co-registration
Dynamic PET images were reconstructed using a 3-D TeraTomo (OSEM algorithm) providing a spatial resolution of 1 mm3. The reconstruction generated a 255 × 255×236 matrix of voxels (0.4 mm in size) using four iterations, six subsets and 400–600 keV energy window after applying attenuation and scatter corrections. List mode data were then re-binned into 26 time frames with increasing duration from 15 s to 10 min.
We co-registered the 90 min dynamic PET images to the T1 MRI of each individual rat using rigid transformations with mutual information-based methodology32 (PMOD, v 3.607). Images of each 90 min PET were also co-registered with elastic transformations33–35 to an average rat MRI brain atlas36 to allow interrogation at a voxel level using statistical parametric mapping (SPM12).
Volumes of interest
A spherical volume of interest (VOI) was defined (2 mm radius) on each individual’s MRI anterior to the electrode tip (right side) in the implant_and_stim group using PMOD, see Supplementary Figure 1. Then the summed and matched standard uptake values (SUV) image was overlaid, and the position of the VOI was visually adjusted to lie over the clearly delineated zones of increased tracer uptake surrounding the electrode in the ventral hippocampus. This right side VOI was then mirrored with a VOI on the contralateral non-stimulated left side. This resulted in corresponding VOIs on the stimulated right side (R_VOI) and on the left control side (L_VOI). Placements of VOIs in the baseline_no_implant and in the implant_no_stim control animals were then matched to the locations of VOIs in the implant_and_stim group.
[18F]GE-179 SUV for R_VOI and L_VOI were computed and the complete SUV dataset was statistically interrogated. The SUV (g/mL) was calculated as measured activity (kBq/mL) divided by the injected dose (MBq/mL) at injection time and multiplied by the body mass (kg). SUV images were generated for interrogation at a voxel level by statistical parametric mapping (SPM). Parametric VT images were computed using Logan graphical analysis with metabolite corrected arterial plasma input functions.37,38
Statistical analysis
First, the hippocampal [18F]GE-179 uptake in the left control side VOI (L_VOI) and its corresponding active right side VOI (R_VOI) was determined for each individual animal in order to calculate the inter-hemispheric difference in uptake (ΔSUV) by subtracting L_VOI SUV from the R_VOI SUV. This was performed in all four groups in order to determine the effects of implantation, electrical stimulation and S-ketamine injection on the active and control hemisphere within each group.
Second, the effect (at a group level) of electrical stimulation on hippocampal [18F]GE-179 uptake was interrogated by comparing the SUVs and ΔSUVs of equivalent right and left VOIs of the implant_and_stim group with the implant_no_stim_ctrl group.
Third, the effect of S-ketamine injection on [18F]GE-179 uptake was determined at a group level by comparing mean SUVs and ΔSUVs (from corresponding R_VOI and L_VOIs) of the implant_KET_and_stim group with their equivalent mean SUV and ΔSUV in the implant_and_stim group.
Last, the effect of surgery and electrode implantation per se on [18F]GE-179 uptake was determined at a group level, by comparing mean SUVs and ΔSUVs (from corresponding R_VOI and L_VOIs) of the intact animals that were not implanted (baseline_no_implant) with their equivalent mean SUVs and ΔSUVs in the animals that were implanted but not stimulated (implant_no_stim_ctrl).
Mean inter-hemispheric SUV differences in [18F]GE-179 uptake at a group level between the three implanted groups (implant_no_stim_ctrl, implant_and_stim and implant_KET_and_stim) were analysed with one-way analysis of variance (ANOVA) and a Bonferroni correction for multiple comparisons post-hoc.
The inter-hemispheric difference in [18F]GE-179 uptake in rats from the implant_and_stim group was interrogated with a paired one-tailed Student t-test applied on the mean SUV between the R_VOIs and L_VOIs.
To determine whether there was an accompanying difference in [18F]GE-179 uptake between the left reference sides of the implant_no_stim_ctrl group compared to the left sides of the implant_and_stim group, we interrogated their mean SUV differences with an unpaired one-tailed t-test. One-way ANOVA, Bonferroni correction and t-tests were performed with Prism (v.7, Graphpad).
Last, we tested differences in injected dose, specific activity and activity in whole blood and plasma input functions of the four groups using one-way ANOVA.
Normal distribution of data was confirmed using Q,Q plot and Shapiro–Wilk test in STATA (v. 13, StataCorp LP). Inter-hemispheric changes (ΔSUV) in mean SUV within individual rats or between-group changes in ΔSUV or in mean R_VOI and L_VOI were expressed as both absolute and percentage SUV changes. Where applicable, results are presented as mean ± standard deviation (SD).
Statistical parametric mapping
To confirm the findings from our visually placed VOIs, we then used exploratory SPM12 software to localise clusters of voxels exhibiting significant NMDAR-IC activation surrounding the electrode. In order to detect focal [18F]GE-179 uptake increases that exceeded any induced changes in the global uptake, we corrected for global variance as a nuisance covariate using analysis of covariance. Summed SUV images were spatially normalised into standard atlas space with implemented Paxinos coordinates.36 Individual masks were set as explicit and co-aligned with the corresponding binary mask in atlas space. We compared the means of implant_and_stim with implant_no_stim_ctrl groups with a two-sample t-test applying an FWE correction at cluster level with an extent threshold of 15 voxels. We then compared voxel values of each individual stimulated rat with the mean voxel value and SD of the combined implant_no_stim_ctrl group.
EEG analysis
EEG recordings were analysed using Labchart to digitally low-pass filter and visualise the baseline and stimulation amplitude. In addition, mechanical gating signs on starting and stopping the stimulation equipment as well as spikes upon connection testing (tail pinch) were documented. ADs were identified as 1–12 Hz spikes and spike-waves lasting for at least 5 s with an amplitude at least twice the baseline by visual inspection.29–31 In a pilot study we video-documented behaviour during seizures and rated their severity using Racine staging.24 As this pilot study in both awake and anesthetised rats provided convincing video and EEG documentation of AD at a 400 µA stimulating current, we omitted video documentation and Racine behaviour evaluation in the subsequent experiments prior and during the PET scan of anesthetised rats.
Post mortem slicing and histology
Formaldehyde fixated brains were immersed in 30% sucrose for 2 days. Tissue blocks were then embedded in Tissue-Tek® OCT and frozen prior to coronal sectioning at 40 µm. Sections containing the electrode were directly mounted on the microscopic slides and stained with Toluidine Blue (Nissl).
Results
VOI analysis: Individual level
[18F]GE-179 PET detected a mean 22% increase in [18F]GE-179 uptake (p = 0.0014) at the site of the hippocampal electrode due to the electrical stimulation in the implant_and_stim rats compared to the non-stimulated side, see Figure 2(a) . The mean SUV of the electrically stimulated right VOI was 1.18 ± 0.18 compared to 0.97 ± 0.13 for the non-stimulated left VOI.
Figure 2.
Mean SUV ± SD of [18F]GE-179 uptake. (a) Within group analysis: Right VOI and left VOI uptake in all rats. The implant_and_stim group showed 22% increase in uptake during stimulation. Comparing left VOI from implant_no_stim_ctrl to left VOI from implant_and_stim shows 18.71% increased uptake in the stimulated group (0.82 ± 0.13 vs 0.97 ± 0.13). *p<0.05, ****p<0.0001. (b) Between-group analysis: Inter-hemispheric difference in uptake (ΔSUV = right VOI – left VOI). The analysis between the implanted and stimulated animals and the control animals that were implanted but not stimulated showed a clear increase in ΔSUV (0.01 ± 0.03 vs 0.21 ± 0.07). Preliminary injection with S-ketamine led to baseline uptake levels in stimulated rats. Surgery and implantation alone did not affect [18F]GE-179 uptake. (A and B) No significant alteration in SUV as a result of surgery was found.
SUV: standard uptake values.
The control animals (implant_no_stim_ctrl) that were implanted but not stimulated showed no significant inter-hemispheric difference in [18F]GE-179 uptake (SUV in R_VOI: 0.83 ± 0.11 vs L_VOI: 0.82 ± 0.13; p = 0.395). No significant differences in inter-hemispheric [18F]GE-179 uptake was found within each of the other three subgroups when comparing mean R_VOIs to their corresponding L_VOIs, see Figure 2(a) and Table 1.
Table 1.
Mean SUV ± SD of uptake in the volumes of interest.
| Left VOI | Right VOI | ΔSUV | % change | |
|---|---|---|---|---|
| Baseline_no_implant (n=6) | 0.84 ± 0.13 | 0.83 ± 0.12 | –0.01 ± 0.02 | −1 |
| Implant_no_stim_ctrl (n=7) | 0.82 ± 0.13 | 0.83 ± 0.11 | 0.01 ± 0.03 | 1.18 |
| Implant_and_stim (n=5) | 0.97 ± 0.13 | 1.18 ± 0.18 | 0.21 ± 0.07 | 21.76 |
| Implant_KET_and_stim (n=3) | 0.80 ± 0.16 | 0.83 ± 0.16 | 0.03 ± 0.02 | 4.00 |
Note: Δ SUV = the right VOI – the left VOI.
KET: S-Ketamine; SUV: standard uptake values; VOI: volume of interest.
VOI analysis: Group level
When comparing (at a group level) the mean R-L inter-hemispheric [18F]GE-179 uptake difference (ΔSUV) between the implant_no_stim and the implant_and_stim groups, we found that PET detected a clear increase in ΔSUV (0.01 ± 0.03 vs 0.21 ± 0.07) due to the increased [18F]GE-179 uptake in the right VOI surrounding the stimulating electrode during stimulation, see Figures 2(b) and 3 and Table 1.
When comparing the mean SUV of the left hemisphere VOIs from the implant_no_stim_ctrl group with the mean SUV of their equivalent left side VOIs from the implant_and_stim group, we found an 18.71% increase in [18F]GE-179 uptake (p = 0.0354) in the non-electrode side of the stimulated animals (0.97 ± 0.13) compared to the equivalent side in the non-stimulated control group (0.82 ± 0.13), see Figure 2(b) and Table 1.
Comparing the two non-stimulated groups (baseline_no_implant and implant_no_stim_ctrl) to measure the effect of surgery (implant) per se, we found no significant alteration in mean SUV, see Figure 2(a) and (b).
Pre-dosing before stimulation with an S-ketamine injection led to significantly reduced SUV levels after stimulation compared to the implant_and_stim group (p = 0.0001). These low SUV uptake values (mean SUV: R_VOI: 0.83 ± 0.16; L_VOI: 0.80 ± 0.16, ΔSUV: 0.03 ± 0.02) were at the level of the intact baseline_no_implant and the implant_no_stim_ctrl groups (mean SUV: R_VOI: 0.83 ± 0.11, L_VOI: 0.82 ± 0.13 and Δ SUV: 0.01 ± 0.03), see Figures 2 and 3 and Table 1.
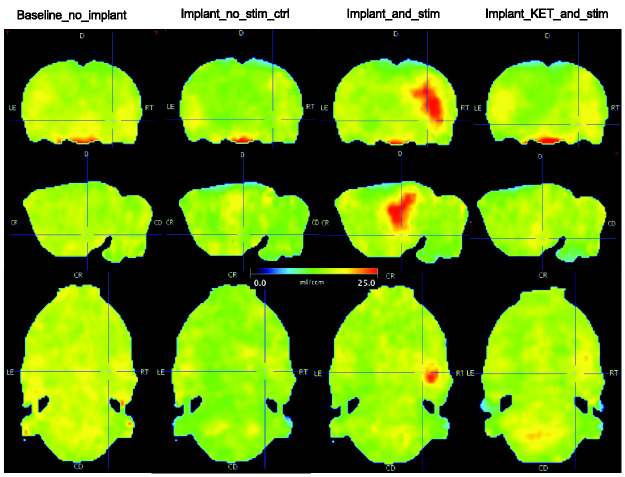
Figure 3.
Parametric VT images. A representative rat from each of the four groups is shown in each column. Stimulation-induced unilateral elevated ventral hippocampus binding of [18F]GE-179 by NMDAR-IC next to the electrode (cross-hair) by both direct conduction and possible indirect capacitive stimulation. The last column shows the blocking effect of S-Ketamine.
In the implant_and_stim group the shape of the TAC for the right stimulated VOI showed retention with a slower washout following stimulation compared with controls (see Figure 4), while TAC curves from right and left VOIs showed that the S-ketamine block acting at the PCP site reduced the retention of [18F]GE-179 resulting in the same washout kinetics as for the non-stimulated side, see Figure 4. S-ketamine also reduced the number of ADs recorded on the EEG, see below and Figure 5.
Figure 4.
Time–activity curve. Right and left volume of interest (VOI) in representative rats from all four groups. Upper row: (a) Rat from the implant_and_stim group: The shape of the right (red) stimulated side (ventral hippocampus) shows retention with reduced tracer washout on stimulation. (b) Blocking doses of S-ketamine (T1/2: 2 h) acting at the PCP site reduced the binding and trapping of [18F]GE-179 resulting in the same washout kinetics as for the non-stimulated side (blue). Lower row: TACs from control groups baseline_no_implant (c) and implant_no_stimulation (d) showing no side difference in uptake. The control left side of the stimulated animal (a) and all control VOIs (non-implanted and implanted) showed similar washout and steady state levels at 1 h.
SUV: standard uptake values; VOI: volume of interest.
Figure 5.
Depth EEG epileptiform afterdischarge. (a) Stimulated rat, showing clear AD consisting of low frequency spikes and waves. (b) Stimulated animal injected with S-ketamine 5 min prior to first stimulation. S-ketamine did not abolish stimulation or generation of AD, but showed a small reduction in the number of spikes.
Statistical parametric mapping
The between group interrogation of the implant_and_stim group (n = 5) and the implant_no_stim_ctrl group (n = 7) with Student’s t statistic localised a cluster with significant focal increase (p = 0.003, FWEcorr) of [18F]GE-179 uptake in the stimulated group surrounding the electrode tip in the targeted ventral hippocampus with peak activation at the tip of the electrode, see Figure 6. Comparing each individual implant_and_stim rat against the entire implant_no_stim_ctrl group showed similar significant clusters of activation adjacent to the stimulating electrodes at an individual level.
Figure 6.
Focal increase in [18F]GE-179 uptake. Group-wise SPM localisation of increased SUV. The cluster (FWEcorr p = 0.003) is overlaid on the MRI from an implanted rat and shows the voxels with increased SUV around the electrode tip within ventral hippocampus.
Blood, metabolism and modelling
There were no significant differences in injected radioactivity dose (20–40 MBq) or levels of injected cold tracer between groups (ANOVA), see Supplementary Table 1. No difference was found in whole blood or plasma activity (AUC) within or between groups (ANOVA), see Supplementary Figures 2 to 6 and Tables 1 and 2. Unmetabolised [18F]GE-179 accounted for a mean of 25% of the plasma radioactivity at 5 min and 14% at 20 min post injection. While the 2TCM model has been reported to best describe human brain [18F]GE-179 uptake kinetics,19 total volumes of distribution (VT) obtained from the gradients of Logan plots best modelled the data in our rodent study. VT values correlated closely with SUVs (Supplementary Figure 7A and B), but were not available for three animals due to failed femoral artery cannulations.
Electroencephalogram
All stimulated animals showed ADs on EEG, see Figure 5. Long duration EEGs post stimulation showed both delayed ADs and sporadic epileptiform discharges. EEG recordings showed that S-ketamine did not abolish the generation of ADs by stimulation, but reduced the number of spikes counted during the 90 min PET scan, see Figure 5 and ‘Discussion’ Section.
Histology
Histological analysis revealed a consistent relationship between the position of the activated focus of increased [18F]GE-179 uptake, the electrode tip on brain slices and the corresponding MRI, see Supplementary Figure 1. The focus always lay adjacent to the electrode tip within the predicted induced field potential radius of 0.5–1 cm.39
Discussion
Here, we show that [18F]GE-179 PET can detect in vivo focally activated NMDAR-ICs following pulsed 60 Hz electrical stimulation in rats and that tracer uptake can be blocked by pre-dosing with an injection of S-ketamine confirming that [18F]GE-179 is binding specifically to PCP sites in open NMDAR-ICs. We found far lower elevation of [18F]GE-179 uptake in the group of rats implanted with an electrode but receiving no stimulation. This suggests that any trauma or blood brain barrier disruption from the surgical procedure had only a mild effect on levels of NMDAR-IC activation compared with electrical stimulation.
We decided to perform our study of in vivo imaging NMDAR-IC activation with [18F]GE-179 PET using acute supra-threshold stimulation inducing evoked field potentials and epileptiform spiking rather than using sustained kindling to induce chronic epileptogenesis. Our aim was to activate NMDAR-ICs in a small well-delineated zone and demonstrate that pre-dosing with S-ketamine could block the tracer uptake.
A recent study by Schoenberger et al.40 and the related commentary letters by McGinnity et al.41 and Sander et al.42 have discussed the difficulties of determining the in vivo specificity of [18F]GE-179 to the NMDAR using pharmacological challenges. Schoenberger et al. used several PCP-antagonists (including MK-801 and S-ketamine) to block [18F]GE-179 binding in rats and non-human primates, but the [18F]GE-179 PET signal was not convincingly modulated by these agents. It is likely that in their study there was low basal IC activity and so little specific [18F]GE-179 binding to block. In contrast, our DBS paradigm led to significant IC activation allowing blockade of [18F]GE-179 binding by S-ketamine.
Our recent minipig study using hippocampal DBS revealed globally increased brain [18F]GE-179 uptake, and [15O]H2O PET confirmed this was in the absence of accompanying cerebral blood flow changes.21 However, a bolus injection of MK-801 during active DBS failed to block this [18F]GE-179 uptake. Although MK-801 is a potent antagonist of the PCP site it may have a different binding site to [18F]GE-179. Here, we chose to block NMDAR-IC with S-ketamine in line with a human study where S-ketamine pre-injection demonstrated a significant reduction in whole brain net influx rate and tracer accumulation of another putative NMDAR ligand ([11C]GMOM).23
We believe that the combination of 60 Hz electrical stimulation to open and activate the NMDAR-ICs so allowing [18F]GE-179 to enter and access the PCP site and blockade with S-ketamine during and after electrical stimulation effectively blocked these sites to [18F]GE-179.
The rats in our current study received a bolus injection of 0.5 mg/kg S-ketamine i.v. which proved to give an adequate NMDAR-IC blocking effect without inducing respiratory arrest. Its brain levels can be easily controlled by altering the rate of intravenous infusion. S-ketamine has complex pharmacodynamics and exerts its action on several receptor systems in addition to NMDAR-IC PCP sites. Recent studies have shown that S-ketamine decreases the frequency of NMDAR-IC opening by binding to the allosteric site on the NMDA-IC complex where it acts as an antagonist as well as binding to the PCP site.43 In a recent rat study, ketamine inhibited AMPA receptor opening44 and this could, in theory, reduce the AMPA-dependent voltage gating of NMDARs. Finally, high blocking doses of ketamine lead to increased GABAergic activity and decreased glutamate levels45 – another non-NMDAR pathway of ketamine action.46
Wherever else S-ketamine might bind, its dominating and primary action is still non-competitive open IC blocking via binding to the NMDAR-IC PCP site when the IC is activated.47,48
After pre-blocking unilaterally stimulated animals with S-ketamine we saw no difference between the stimulated R_VOI and control L_VOI TACs, suggesting retention of [18F]GE-179 by NMDAR-ICs was abolished during and after electrical stimulation despite active discharges still being evident on the EEG, see Figures 4 and 5. While EEG recordings showed that S-ketamine did not abolish ADs after stimulation, counting spikes over 90 min suggested that S-ketamine led to a reduction in the number of spikes. This could be due to both NMDAR-IC blockade and non-NMDAR dependent mechanisms, but we believe that the highly efficient S-ketamine blockade of the signal is mainly due to its PCP site antagonism with only a minority of the lowering of the signal due to secondary NMDAR-dependent and independent actions.
It could be argued that the stimulation-induced increase in [18F]GE-179 uptake in part reflected increased tracer delivery due to a rise in blood flow rather than raised NMDAR-IC availability. However, the shape of the [18F]GE-179 TAC (Figure 4) is not consistent with a rise in blood flow as there was no increase in amplitude of the early peak in brain concentration, and the shape of the washout phase indicates greater retention rather than faster washout.
The blocking effect of S-ketamine on increased focal uptake of [18F]GE-179 post stimulation confirms that this ligand binds to NMDAR-ICs. Neither the baseline_no_implant, the implant_no_stim group, nor the implant_KET_and_stim group showed right side tracer retention kinetics excluding significant [18F]GE-179 influx across a disrupted blood brain barrier, see Figures 2 and 4.
A limitation of this study lies in the low numbers of rats in each group, which reduced our power to detect subtle effects of electrical stimulation. We conducted a pilot study ahead of this study in order to optimise our protocol and minimise the number of animals used. Our design using supra-threshold stimulation resulted in large effect sizes of [18F]GE-179 uptake that were highly significant despite the low number of rats. We also used the left hemisphere as the control side in paired analyses. We could not perform paired studies using intact rats as their own controls although that would have been optimal, as the animals had an arterial catheter inserted for blood sampling and were euthanised after each scan. Additionally, we experienced electrode displacement during attempted successive paired experiments in our pilot study. For this reason, we used the left side as the internal paired reference and non-stimulated animals as controls. An additional ‘control + S-ketamine’ group would have been relevant to our study but we decided to minimise the number of animals required by using the left hemisphere as a paired internal reference. Finally, our visually placed VOI findings were confirmed using an exploratory SPM12 voxel level analysis which also localised clear voxel clusters of raised [18F]GE-179 uptake after stimulation.
Conclusion
In this study, we have validated the in vivo specificity of [18F]GE-179 binding to the PCP site and the use of [18F]GE-179 PET as an in vivo imaging marker of NMDAR-IC activation. Electrical stimulation of the ventral hippocampus revealed a 22% focal elevation in the mean SUV in a spherical VOI sited at the tip of the stimulating electrode. SPM localised a cluster of voxels with increased SUV at the same site while pre-dosing with an S-ketamine injection blocked this rise and restored SUV uptake levels to those of the non-stimulated groups despite continuing spike activity. Our study opens the door for exploring the use of [18F]GE-179 PET for mapping and localising regional or focal differences in abnormal NMDAR distribution. [18F]GE-179 PET has already been shown to detect epileptic foci in human subjects suffering from recurrent focal seizures. It could potentially be used to examine local and distant DBS effects on the glutamatergic system in larger animal models or in human patients.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20954928 for NMDA receptor ion channel activation detected in vivo with [18F]GE-179 PET after electrical stimulation of rat hippocampus by Ali K Vibholm, Anne M Landau, Arne Møller, Jan Jacobsen, Kim Vang, Ole L Munk, Dariusz Orlowski, Jens CH Sørensen and David J Brooks in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
Special thanks to staff at the PET Centre, CFIN and CENSE at Aarhus University for technical support, handling animals, tracer synthesis, histology and EEG. GE Healthcare supported the study by supplying the precursor for [18F]GE-179.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Lundbeck Foundation grant to DJB: R108-2012-10831.
Authors’ contributions: AKV: Study design, surgery, PET, MRI and EEG experiments, data acquisition and analysis, interpretation, imaging and modelling, drafting and final approval of the paper. AML: Supervision, study design, analysis and interpretation, reviewing and final approval of the paper. AM: Supervision, interpretation, reviewing and final approval of the paper. JJ: Tracer production, experiments, analysis of blood and metabolism data and final approval of the paper. KV: Data analysis, imaging, review and final approval of the paper. OLM: Data analysis, Logan modelling and final approval of the paper. DO: Histology and final approval of the paper. JCHS: Study design, surgery, histology, review and final approval of the paper. DJB: Conceptualisation, study design, analysis and interpretation, reviewing and final approval of the paper.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DJB is a consultant for Biogen and has been a consultant for GE Healthcare, Plexxikon, and GenePod. DJB has stock options in GE. The funders had no role in the study design, data analyses, data interpretation or writing of the article. All other authors declare that they have no conflict of interest.
Supplementary material: Supplemental material for this article is available online.
ORCID iDs
Ali K Vibholm https://orcid.org/0000-0002-4316-6540
Anne M Landau https://orcid.org/0000-0002-7371-8713
References
- 1.Paoletti P, Neyton J.NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 2007; 7: 39–47. [DOI] [PubMed] [Google Scholar]
- 2.Paoletti P.Molecular basis of NMDA receptor functional diversity. Eur J Neurosci 2011; 33: 1351–1365. [DOI] [PubMed] [Google Scholar]
- 3.Dingledine R, Borges K, Bowie D, et al. The glutamate receptor ion channels. Pharmacol Rev 1999; 51: 7–61. [PubMed] [Google Scholar]
- 4.Wollmuth LP, Sobolevsky AI.Structure and gating of the glutamate receptor ion channel. Trends Neurosci 2004; 27: 321–328. [DOI] [PubMed] [Google Scholar]
- 5.Lau A, Tymianski M.Glutamate receptors, neurotoxicity and neurodegeneration. Pflug Arch 2010; 460: 525–542. [DOI] [PubMed] [Google Scholar]
- 6.Syntichaki P, Tavernarakis N.The biochemistry of neuronal necrosis: rogue biology? Nat Rev Neurosci 2003; 4: 672–684. [DOI] [PubMed] [Google Scholar]
- 7.Wroge CM, Hogins J, Eisenman L, et al. Synaptic NMDA receptors mediate hypoxic excitotoxic death. J Neurosci 2012; 32: 6732–6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothman S.Synaptic release of excitatory amino acid neurotransmitter mediates anoxic neuronal death. J Neurosci 1984; 4: 1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi DW, Rothman SM.The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci 1990; 13: 171–182. [DOI] [PubMed] [Google Scholar]
- 10.Bush PC, Prince DA, Miller KD.Increased pyramidal excitability and NMDA conductance can explain posttraumatic epileptogenesis without disinhibition: a model. J Neurophysiol 1999; 82: 1748–1758. [DOI] [PubMed] [Google Scholar]
- 11.de Curtis M, Avanzini G.Interictal spikes in focal epileptogenesis. Prog Neurobiol 2001; 63: 541–567. [DOI] [PubMed] [Google Scholar]
- 12.Sobrio F, Gilbert G, Perrio C, et al. PET and SPECT imaging of the NMDA receptor system: an overview of radiotracer development. Mini Rev Med Chem 2010; 10: 870–886. [DOI] [PubMed] [Google Scholar]
- 13.Waterhouse RN, Waterhouse RN.Imaging the PCP site of the NMDA ion channel. Nucl Med Biol 2003; 30: 869–878. [DOI] [PubMed] [Google Scholar]
- 14.Kumlien E, Hartvig P, Valind S, et al. NMDA-receptor activity visualized with (S)-[N-methyl-11C]ketamine and positron emission tomography in patients with medial temporal lobe epilepsy. Epilepsia 1999; 40: 30–37. [DOI] [PubMed] [Google Scholar]
- 15.Robins EG, Zhao Y, Khan I, et al. Synthesis and in vitro evaluation of 18F-labelled S-fluoroalkyl diarylguanidines: novel high-affinity NMDA receptor antagonists for imaging with PET. Bioorg Med Chem Lett 2010; 20: 1749–1751. [DOI] [PubMed] [Google Scholar]
- 16.Healthcare GE. Internal Communication IB. 1–120.
- 17.GE Healthcare. Supplemental Data. 1–26.
- 18.López-Picón F, Snellman A, Shatillo O, et al. Ex vivo tracing of NMDA and GABA-A receptors in rat brain after traumatic brain injury using 18F-GE-179 and 18F-GE-194 autoradiography. J Nucl Med 2016; 57: 1442–1447. [DOI] [PubMed] [Google Scholar]
- 19.McGinnity CJ, Hammers A, Riano Barros DA, et al. Initial evaluation of 18F-GE-179, a putative PET tracer for activated N-methyl D-aspartate receptors. J Nucl Med 2014; 55: 423–430. [DOI] [PubMed] [Google Scholar]
- 20.McGinnity CJ, Koepp MJ, Hammers A, et al. NMDA receptor binding in focal epilepsies. J Neurol Neurosurg Psychiatry 2015; 86: 1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vibholm AK, Landau AM, Alstrup AKO, et al. Activation of NMDA receptor ion channels by deep brain stimulation in the pig visualised with [18F]GE-179 PET. Brain Stimul 2020; 13: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 22.Golla SSV, Klein PJ, Bakker J, et al. Preclinical evaluation of [18F]PK-209, a new PET ligand for imaging the ion-channel site of NMDA receptors. Nucl Med Biol 2015; 42: 205–212. [DOI] [PubMed] [Google Scholar]
- 23.van der Doef TF, Golla SS, Klein PJ, et al. Quantification of the novel N-methyl-d-aspartate receptor ligand [11C]GMOM in man. J Cereb Blood Flow Metab 2016; 36: 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimoto K, Fahnestock M, Racine RJ.Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol 2004; 73: 1–60. [DOI] [PubMed] [Google Scholar]
- 25.Kandratavicius L, Balista P, Lopes-Aguiar C, et al. Animal models of epilepsy: use and limitations. Neuropsychiatry Dis Treat 2014; 10: 1693–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandt C, Glien M, Potschka H, et al. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res 2003; 55: 83–103. [DOI] [PubMed] [Google Scholar]
- 27.Van Den Berge N, Keereman V, Vanhove C, et al. Hippocampal deep brain stimulation reduces glucose utilization in the healthy rat brain. Mol Imaging Biol 2015; 17: 373–383. [DOI] [PubMed] [Google Scholar]
- 28.Morales JC, Alvarez-Ferradas C, Roncagliolo M, et al. A new rapid kindling variant for induction of cortical epileptogenesis in freely moving rats. Front Cell Neurosci 2014; 8: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rempe DA, Bertram EH, Williamson JM, et al. Interneurons in area CA1 stratum radiatum and stratum oriens remain functionally connected to excitatory synaptic input in chronically epileptic animals. J Neurophysiol 1997; 78: 1504–1515. [DOI] [PubMed] [Google Scholar]
- 30.Musto AE, Samii MS, Hayes JF.Different phases of afterdischarge during rapid kindling procedure in mice. Epilepsy Res 2009; 85: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuchiya K, Kogure S.Fast Fourier transformation analysis of kindling-induced afterdischarge in the rabbit hippocampus. Epilepsy Res 2011; 95: 144–151. [DOI] [PubMed] [Google Scholar]
- 32.Viola P, Wells WM., III.Alignment by maximization of mutual information. Int J Comput Vis 1997; 24: 16–23. [Google Scholar]
- 33.Woods RP, Mazziotta JC, Cherry SR.MRI-PET registration with automated algorithm. J Comput Assist Tomogr 1993; 17: 536–546. [DOI] [PubMed] [Google Scholar]
- 34.Maes F, Collignon A, Vandermeulen D, et al. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 1997; 16: 187–198. [DOI] [PubMed] [Google Scholar]
- 35.Technologies P. PMOD Fuse It Tool (PFUSEIT). 1–91.
- 36.Schiffer WK, Mirrione MM, Biegon A, et al. Serial microPET measures of the metabolic reaction to a microdialysis probe implant. J Neurosci Methods 2006; 155: 272–284. [DOI] [PubMed] [Google Scholar]
- 37.Logan J.Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol 2000; 27: 661–670. [DOI] [PubMed] [Google Scholar]
- 38.Logan J, Fowler JS, Volkow ND, et al. Graphical analysis of reversible radioligand binding from time–activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990; 10: 740–747. [DOI] [PubMed] [Google Scholar]
- 39.Buzsáki G, Anastassiou CA, Koch C.The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat Rev Neurosci 2012; 13: 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoenberger M, Schroeder FA, Placzek MS, et al. In vivo [18F]GE-179 brain signal does not show NMDA-specific modulation with drug challenges in rodents and nonhuman primates. ACS Chem Neurosci 2018; 9: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGinnity CJ, Årstad E, Beck K, et al. Comment on ‘In Vivo [18F]GE-179 Brain Signal Does Not Show NMDA-Specific Modulation with Drug Challenges in Rodents and Nonhuman Primates’. ACS Chem Neurosci 2019; 10: 768–772. [DOI] [PubMed] [Google Scholar]
- 42.Sander CY, Schoenberger M, Hooker JM.Response to comment on ‘In Vivo [18F]GE-179 Brain Signal Does Not Show NMDA-Specific Modulation with Drug Challenges in Rodents and Nonhuman Primates’. ACS Chem Neurosci 2018; 10: 773–775. [DOI] [PubMed] [Google Scholar]
- 43.Mion G, Villevieille T.Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 2013; 19: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyler MW, Yourish HB, Ionescu DF, et al. Classics in chemical neuroscience: ketamine. ACS Chem Neurosci 2017; 8: 1122–1134. [DOI] [PubMed] [Google Scholar]
- 45.Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kavalali ET, Monteggia LM.Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry 2012; 169: 1150–1156. [DOI] [PubMed] [Google Scholar]
- 47.Kohrs R, Durieux ME. Ketamine. Anesth Analg 1998; 87: 1186–1193. [DOI] [PubMed]
- 48.Sleigh J, Harvey M, Voss L, et al. Ketamine – more mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care 2014; 4: 76–81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20954928 for NMDA receptor ion channel activation detected in vivo with [18F]GE-179 PET after electrical stimulation of rat hippocampus by Ali K Vibholm, Anne M Landau, Arne Møller, Jan Jacobsen, Kim Vang, Ole L Munk, Dariusz Orlowski, Jens CH Sørensen and David J Brooks in Journal of Cerebral Blood Flow & Metabolism