Abstract
Glibenclamide inhibits sulfonylurea receptor (SUR), which regulates several ion channels including SUR1-transient receptor potential melastatin 4 (SUR1-TRPM4) channel and ATP-sensitive potassium (KATP) channel. Stroke upregulates SURl-TRPM4 channel, which causes a rapid edema formation and brain swelling. Glibenclamide may antagonize the formation of cerebral edema during stroke. Preclinical studies showed that glibenclamide inhibits KATP channel-induced vasodilation without altering the basal vascular tone. The in vivo human cerebrovascular effects of glibenclamide have not previously been investigated.
In a randomized, double-blind, placebo-controlled, three-way cross-over study, we used advanced 3 T MRI methods to investigate the effects of glibenclamide and KATP channel opener levcromakalim on mean global cerebral blood flow (CBF) and intra- and extracranial artery circumferences in 15 healthy volunteers. Glibenclamide administration did not alter the mean global CBF and the basal vascular tone. Following levcromakalim infusion, we observed a 14% increase of the mean global CBF and an 8% increase of middle cerebral artery (MCA) circumference, and glibenclamide did not attenuate levcromakalim-induced vascular changes. Collectively, the findings demonstrate the vital role of KATP channels in cerebrovascular hemodynamic and indicate that glibenclamide does not inhibit the protective effects of KATP channel activation during hypoxia and ischemia-induced brain injury.
Keywords: Human models, stroke, migraine, KATP channel, levcromakalim
Introduction
Cerebral edema after stroke or trauma worsens neurological function and causes a high mortality. Although, molecular mechanisms of cerebral edema are poorly understood, sulfonylurea receptor (SUR) seems to be involved.1 Acute cerebral ischemia upregulates SUR1-transient receptor potential melastatin 4 (SUR1-TRPM4) channel in all CNS cell types, leading to an inward flow of Na+ and cell depolarization.2–4 Persistent unopposed activation of SUR1–TRPM4 channel causes blebbing, characteristic of cytotoxic edema and thus necrotic cell death.1 Glibenclamide is a widely used antidiabetic drug that belongs to the second-generation sulfonylurea.5 It targets SUR and inhibits SUR-regulated channel activity in several ion channels.5 Cumulative evidence suggests that glibenclamide may be a promising drug for treating stroke-associated edema.6,7
Apart from involvement with TRPM4 channel, SUR subunits also regulate activity of ATP-sensitive potassium (KATP) channel expressed in several tissues, including pancreatic β cells, vascular smooth muscle cells (VSMCs), neurons and glial cells.8 Local as well as systemic administration of KATP channel opener (KCO) levcromakalim causes vasodilation.9–11 Preclinical studies implicate KATP channel in the regulation of cerebral blood flow (CBF),12,13 and demonstrate that glibenclamide inhibits KATP channel-induced vasodilation without altering the basal vascular tone.14–18 KATP channel activation may have inherent protective effects during hypoxia and ischemia-induced brain injury.19,20 Effect of glibenclamide on cerebral hemodynamics and on KATP channel-induced vasodilation in humans is not elucidated.
Here, we investigated the effect of systemic glibenclamide and levcromakalim on total brain perfusion and on circumference of intracranial and extracranial arteries in healthy volunteers by high-resolution 3T time-of-flight magnetic resonance angiography. We hypothesized that glibenclamide would increase the basal vascular tone, reduce the CBF, and inhibit levcromakalim-induced vascular changes.
Materials and methods
We recruited 15 healthy volunteers through the Danish test subject website (www.forsøgsperson.dk). All participants gave written informed consent before inclusion. The female participants were required to have sufficient contraception (contraceptive pill or intrauterine device/system (IUD/IUS)). Exclusion criteria were (1) history of cardiovascular or cerebrovascular disease, diabetes mellitus, or hypercholesterolemia, (2) abnormal electrocardiogram (ECG), (3) a history of and arterial hypertension at baseline on an experimental day (defined as a systolic blood pressure above 150 mmHg or a diastolic blood pressure above 100 mmHg), (4) current pregnancy or breastfeeding, (5) daily intake of medication (except oral contraceptives), (6) daily smoking within last five years, (7) first-degree relatives with a history of diabetes mellitus, (8) contraindications for magnetic resonance imaging (MRI), and (9) history of any primary headache disorders (except episodic tension-type headache more than two days per month during the last year).21 A full medical examination and ECG were performed on the day of recruitment. The study was approved by the Ethics Committee of Capital Region of Denmark (H-18052188) and the Danish Data Protection Agency and was conducted according to the Declaration of Helsinki of 1964 with later revisions. The study was registered at ClinicalTrials.gov (NCT03886922).
Experimental design
In a double-blind, placebo-controlled, three-way crossover design, the participants were in a balanced order randomly allocated to receive a continuous intravenous infusion of 0.05 mg/min (20 mL of 50 µg/mL) levcromakalim (manufactured by Sigma-Aldrich) or 20 mL placebo (isotonic saline) over 20 min after oral administration of 10 mg glibenclamide (Hexaglucon, Sandoz) or placebo (multivitamin pill) on three different days separated by at least one week (Figure 1): oral glibenclamide followed by infusion of levcromakalim (day 1), oral glibenclamide followed by infusion of placebo (day 2), and oral placebo (multivitamin pill) followed by infusion of placebo (day 3). The levcromakalim or placebo infusion was administered 120 min after the pretreatment with glibenclamide or placebo. Randomization and preparation of the study drug were performed by the Capital Region Central Pharmacy. The randomization code remained in the hospital during the study and was not available to the investigators until the study was completed.
Figure 1.
Overview of the study design. Fifteen healthy volunteers were randomly allocated to receive glibenclamide–levcromakalim in one day, glibenclamide–placebo in second day, and placebo–placebo in third day. The washout period between the days is at least one week.
All participants arrived at the clinic after 2-h fasting. The participants were placed in the supine position and a venous catheter was inserted into the left and right antecubital vein for drug (levcromakalim/placebo) and 20% glucose infusion. Then, the participants rested for at least 30 min before baseline measurements of vital signs were performed. All participants underwent five MRI sessions: baseline, 60, 120, 160, and 200 min (Figure 2). The infusion started using a time and volume-controlled infusion pump.
Figure 2.
Timeline of the study. The participants underwent five scans (time points: −20, 60, 120, 160, and 200 min, angio: MR angiography; PCM: phase-contrast mapping for global mean CBF measurement). At time 0 min, the participants received oral glibenclamide/placebo. At time 140 min, levcromakalim/placebo was infused over 20 min.
Vital signs were monitored during the experiments using an MR-compatible system (Veris Monitor, Medrad, Warrendale, PA). Blood pressure (mean arterial blood pressure (MABP)), heart rate (HR), respiratory rate, blood oxygen saturation, nasal end-tidal CO2 tension (water trap and gas sample line, Medrad, Warrendale, PA), were continuously monitored and recorded every 10 min. Room temperature was continuously monitored and recorded every 5 min. For data analyses, all vital signs data were time-shifted for each participant relative to infusion start.
Plasma glucose
Plasma glucose concentration was monitored during a 20-min baseline period before the administration of oral glibenclamide/placebo by ABL800 FLEX blood gas analyzer. After the start and when initial fasting glycaemia had declined by 10%, blood glucose concentrations were clamped around this level (4–6 mmol/L) by 20% glucose infusion. Infusion rates necessary to maintain blood glucose after drug intake were registered throughout the ensuing 220 min. The following standard formula was used to calculate glucose infusion rate (GIR);22,23
Measurements of plasma glucose, pH, bicarbonate concentration (HCO3−), and lactate were drawn at 10-min intervals between −20 to 150 min and 20-min intervals thereafter (Figure 3).
Figure 3.
Glucose concentration. After oral glibenclamide/placebo administration, blood glucose concentration was clamped by intravenous infusion of 20% glucose.
MRI data acquisition and data analysis
All MRI scans were performed on a 3.0 T Philips Achieva dStream MRI scanner (Philips Medical Systems, Best, The Netherlands) using a 32-channel phase array receive head coil. High-resolution anatomical scans were obtained with a 3D T1-weighted turbo field echo sequence (field of view (FOV) = 240 × 240 × 170 mm3; voxel size = 1.00 × 1.08 × 1.10 mm3; echo time (TE) = 3.7 ms; repetition time (TR) = 8.0 ms; flip angle = 8°). For all analyses, data analysts were blinded with respect to whether data were acquired on glibenclamide-levcromakalim day, glibenclamide-placebo day, or placebo-placebo day.
MR angiography
A three-dimensional time-of-flight MR angiography (TOF-MRA) sequence was used for vessel imaging as described previously.24–26 Measurements were performed bilaterally on the following vessels: middle cerebral artery (MCA), middle meningeal artery (MMA), and superficial temporal artery (STA) (Figure 4). The MMA was identified by marking the branch from the main trunk of the maxillary artery, the STA by marking the lateral terminal branch of the external carotid artery and the MCA by marking the branch from the main trunk of the internal carotid artery.
Figure 4.
Arterial segments of interest. The yellow circle is the middle cerebral artery (MCA). The red circle is the middle meningeal artery (MMA). The blue circle is the superficial temporal artery (STA). KATP channel opener levcromakalim increased the circumferences of MCA (at 160 min (P = 0.004) and at 200 min (P < 0.001)), MMA (at 160 min (P < 0.001) at 200 min (P < 0.001)) and STA (at 160 min (P < 0.001) and 6 at 200 min (P < 0.001)) compared to placebo on glibenclamide days.
Phase-contrast mapping (PCM)
Quantitative global CBF values was measured in using PCM.27,28 The technique directly measures the blood velocity and cross-sectional area of the arteries feeding the brain (the carotid arteries and basilar artery) using a turbo field echo MRI-sequence. By multiplying the blood velocity and cross-sectional area quantitative CBF measurements in mL/min can be calculated. The blood flow is then normalized to brain size to get the CBF per brain weight (). Brain weight was estimated from the anatomical scan using FSL BET and FAST software parts of FSL 5.0.10 (FMRIB Software Library, Functional Magnetic Resonance Imaging of the Brain Centre, Department of Clinical Neurology, University of Oxford, Oxford, UK)29 and assuming a brain density of 1.05 g/mL.30
Data analysis and statistics
All values are presented as mean values ± SD, except age and weight, which are presented as mean values with range. The normality of data was assessed using Shapiro–Wilk test. The differences in mean global CBF are presented as ratio with 95% confidence interval (CI). Baseline was defined as T0 before the start of administration of oral glibenclamide/placebo. For glucose measurement, the baseline was defined as T-20 − 0 before the start. Calculation of sample size was based on previous similar studies.9,31,32 The primary endpoints were to compare the vascular changes between the different days. To compare the vascular outcome from the glibenclamide–placebo day and placebo–placebo day, we used a mixed model with a covariance structure induced by random effects of person, person × day, person × part 1 measurements (part 1: measurements at 60 and 120) or × part 2 measurements (part 2: measurements at 160 and 200). To compare the vascular outcome from the glibenclamide–placebo day and glibenclamide–levcromakalim day, we used a mixed model with a covariance structure induced by random effects of person, person × day, person × treatment (treatments are placebo or levcromakalim). We tested for period and carryover effects for all variables with Mann–Whitney test and independent t-test. All analyses were performed with SAS Statistics version 9.4 for Windows and a two-sided probability level of <0.05 was considered statistically significant. We did not adjust for multiple comparisons, as our primary endpoints, hypotheses and statistical tests were not many, and they all were predefined and clearly stated in study protocol.
Data availability
The data supporting the findings in the present study are available from the corresponding author upon reasonable request.
Results
Fifteen healthy volunteers (nine women and six men) completed the study (Figure 1). The mean age was 24 years (range 20–39) and mean weight 68.5 kg (range 65–90). Three participants completed the placebo–placebo day without scanning due to technical problems. There was no carryover or period effect for values of vital signs, MRA, or global CBF. There was no difference between the right and left-sided vessels, and we, therefore, calculated the average of the two sides and found no difference in baseline arterial circumference.
We found no changes in global CBF or any other vascular parameters (i.e., arterial circumferences, MABP, and HR) during part 1 measurements (0–120 min), P > 0.05. No differences were found for plasma glucose, pH, bicarbonate concentration (HCO3−), and lactate between the three days.
PCM and TOF-MRA
We found no difference in the mean global CBF between glibenclamide–placebo day and placebo–placebo day at 160 min (the ratio was 4.1%, 95% CI = (−2.7%, 11.3%); P = 0.11) and at 200 min (2.1%, CI = (−5.5%, 9.2%); P = 0.06).
The global mean CBF increased significantly after levcromakalim compared to placebo on glibenclamide days at 160 min (14.2%, CI = (7.9%, 20.8%); P < 0.001) and at 200 min (12.2%, CI = (6.0%, 18.7%); P < 0.001). The increased CBF remained significant after correction for MABP at 160 min (11.6%, CI = (5.1%, 18.5%); P < 0.001) and at 200 min (9.7%, CI= (3.4%, 16.5%); P = 0.002) (Figure 5).
Figure 5.
Arterial circumferences and cerebral blood flow (CBF). Administration of glibenclamide or placebo did not alter the circumferences of the MMA, STA or MCA. Levcromakalim increased the circumferences of the MMA, STA, or MCA significantly. CBF remained largely unchanged after glibenclamide or placebo. Whereas, levcromakalim increased CBF significantly. MMA: middle meningeal artery, STA: superficial temporal artery, MCA: middle cerebral artery, CBF: cerebral blood flow.
No difference (P > 0.05) in the circumferences of cranial arteries was found in glibenclamide–placebo day; MCA (7.95 ± 0.92 at 160 min and 7.74 ± 0.89 at 200 min), MMA (4.49 ± 0.78 at 160 min and 4.34 ± 1.14 at 200 min), and STA (5.81 ± 0.81 at 160 min and 5.82 ± 0.78 at 200 min) compared to placebo–placebo day; MCA (7.9 ± 0.72 at 160 min and 7.98 ± 0.69 at 200 min), MMA (4.56 ± 0.62 at 160 min and 4.54 ± 0.68 at 200 min), and STA (5.89 ± 0.60 at 160 min and 5.98 ± 0.55 at 200 min).
The circumferences increased significantly after levcromakalim, MCA (8.52 ± 1.30 at 160 min (P = 0.004) and 8.68 ± 1.34 at 200 min (P < 0.001)), MMA (5.24 ± 0.86 at 160 min (P < 0.001) and 5.14 ± 0.84 at 200 min (P < 0.001)) and STA (7.04 ± 1.27 at 160 min (P < 0.001) and 6.82 ± 1.32 at 200 min (P < 0.001)) compared to placebo on glibenclamide days (Figure 5).
Systemic hemodynamics
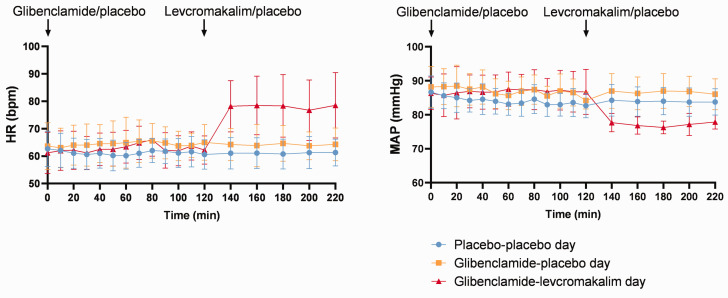
We found no difference in MABP between glibenclamide–placebo day and placebo–placebo day over time (P > 0.05). MABP decreased significantly after levcromakalim (78.76 ± 5.1 at 160 min and 79.42 ± 4.8 at 200 min) compared to placebo (86.16 ± 4.32 at 160 min (P < 0.001) and 86.69 ± 4.11 at 200 min (P = 0.001)) in glibenclamide days and to placebo–placebo day (84.0 ± 4.11 at 160 min (P = 0.03) and 84.35 ± 4.69 at 200 min (P = 0.02)) (Figure 6).
Figure 6.
Vital parameters. Mean heart rate (HR) and mean arterial blood pressure (MABP) after administration of oral glibenclamide/placebo and infusion of levcromakalim/placebo. Glibenclamide did not alter or HR or MABP. Levcromakalim increased HR and decreased MABP, whereas, glibenclamide did not inhibit levcromakalim-induced changes.
We found no difference in HR between glibenclamide–placebo day and placebo–placebo day over time (P > 0.05). HR increased significantly after levcromakalim (78.47 ± 10.68 at 160 min and 76.73 ± 11.0 at 200 min) compared to placebo (63.87 ± 7.43 at 160 min (P = 0.001) and 63.73 ± 4.94 at 200 min (P = 0.001)) in glibenclamide days and to placebo–placebo day (61.08 ± 5.58 at 160 min (P < 0.001) and 61.33 ± 5.45 at 200 min (P < 0.001)) (Figure 6).
No differences were found for respiratory rate, blood oxygen saturation, and nasal end-tidal CO2 tension. Room temperatures did not differ between glibenclamide–placebo day (mean 20.6°C ± 0.5°C), glibenclamide–levcromakalim day (mean 20.7°C ± 0.4°C), and placebo–placebo day (mean 20.6°C ± 0.4°C).
Discussion
We conducted the first in vivo human study of the effects of systemic glibenclamide on CBF and circumference of cranial arteries, and its effect on KATP channel-induced vascular changes. The novel finding was that glibenclamide did not alter the cerebral hemodynamics and did not inhibit levcromakalim-induced vascular changes. Another important finding was that levcromakalim increased the global CBF and dilated cerebral arteries. Collectively, these data suggest that KATP channel regulates the cerebral hemodynamics and indicate that the use of glibenclamide during stroke is unlikely to affect the favorable action of KATP channel.
Under ischemic conditions, the entire neurovascular unit, including neurons, astrocytes, microglia, oligodendrocytes, and endothelial cells, upregulates SUR1–TRPM4 channels.2–4 The rapid and irreversible brain injury under stroke might be caused, at least partly, by activation of SUR1–TRPM4 channels. Glibenclamide targets SUR1 subunits and reduces edema, leading to better outcomes in clinical and preclinical stroke models.6,33,34 In human studies, glibenclamide attenuated peripheral artery dilation.35–39 These studies used doses between 5 and 10 mg and reported no alternation in MAP and HR. To date, no studies have explored glibenclamide effect on CBF and cranial arteries. Although glibenclamide is a highly lipophilic drug, the results of whether it crosses blood brain barrier (BBB) are conflicting.40,41 Recent studies in patients with brain swelling after large hemispheric infarction did not investigated effect of glibenclamide on cerebral hemodynamics.33,34
In preclinical experiments, glibenclamide attenuated KCOs-induced vasodilation without altering the basal vascular tone.14 Few studies reported that glibenclamide increased the basal tone in rat dural artery,16,17 and one study reported that glibenclamide had no effect on brain perfusion signals in a rat model of severe ischemia with delayed reperfusion.6 In the present study, glibenclamide did not alter the in vivo global mean CBF and arterial circumferences. For stroke patients, the dose used was intravenous 0.13 mg bolus injection for the first 2 min, followed by an infusion of 0.16 mg/h for the first 6 h and then 0.11 mg/h for the remaining 66 h.33,34,42 Thus, the collective dose was 8.35 mg over 72 h. In the present study, we used 10 mg glibenclamide. As the oral bioavailability of glibenclamide is >90%,43 the dose we used corresponds to the dose used in stroke studies. The highest plasma concentration of glibenclamide was reached 2 h after oral administration and lasted for 3–4 h.43 During this time, we observed no change in cerebrovascular hemodynamics. Given that glibenclamide did not increase the basal vascular tone, glibenclamide should not aggravate brain injury during stroke.
KATP channels are present in several tissues including neurons, vascular endothelia, and smooth muscle cells. Additionally, KATP channel current has been detected in micro- and macrovascular complexes.44 Ischemia causes depletion of intracellular ATP, which, in turn, activates KATP channels in VSMCs and neurons.45 KATP channel activation permits potassium efflux and leads to hyperpolarisering, resulting in vasodilation and reduced neuronal activity. In a mouse model of ischemia, mice undergoing permanent middle cerebral artery occlusion (MCAo), had much smaller infarct volumes when they were pretreated with KCO, than mice pretreated with KATP channel inhibitor.46 Also, KATP channel knockout in the brain aggravates the neuron death after acute hypoxic–ischemic insults.47,48 Given a protective role of KATP channels during stroke46–48 the use of its blocker glibenclamide may reverse this action. In the present study, however, we found that glibenclamide did not prevent vascular effect of KCO. These data suggest that glibenclamide might exert its effect against stroke-associated edema without inhibiting KATP channel activity.
Three isoforms of SUR (SUR1, SUR2A, and SUR2B) subunits are expressed in the KATP channels.49 SUR1–KATP channels are expressed in brain and pancreas, SUR2A–KATP channels are expressed in cardiac and skeletal muscle, whereas, SUR2B–KATP channels are mainly found in smooth muscle cells including VSMCs.8 Levcromakalim is a selective KCO with high affinity to SUR2B subunit. Intravenous infusion of levcromakalim dilates cranial arteries and the time to have maximum effect is 20 min after the start of infusion. In the present study, the participants were scanned 20 and 40 min after the start of the infusion.10,11 In a previous study, we reported that levcromakalim dilated extracerebral but not cerebral arteries.9 In contrast, here, we demonstrated that levcromakalim dilated MCA. Of note, the MCA circumference increased with approximately 8% in both studies but failed to reach a significant level in the previous one. Different design of the studies might explain the different findings. In the present study, we use crossover design with 15 participants, which is more powerful than the previous study. In support, we also find increase in the global CBF, and previous preclinical studies implicate KATP channels in the cerebrovascular system.12,13 Since KATP channels are expressed in the MCA,50 the MCA dilation observed in the present study might be a direct effect of KATP channel activation. In support, infusion of vasoactive substances including calcitonin gene-related peptide (CGRP) caused peripheral vasodilation, while the diameter of MCA remained unchanged.24,51 Change in cerebral metabolic rate of oxygen (CMRO2) might affect CBF and investigation of possible effects from levcromakalim on brain metabolism would be very interesting. However, in the present study, we found no differences in plasma glucose, pH, (HCO3−), and lactate between the three days. Also, the average brain oxygen consumption is remarkably stable outside brain disease or change in consciousness (e.g., sleep or sedation), and change in CMRO2 of ∼14%, as we observe in CBF, is unlikely.
In contrast to previous preclinical findings, we report that glibenclamide did not inhibit levcromakalim-induced vascular changes. Different effects of glibenclamide between pre- and posttreatment cannot be applied as both approaches inhibited KCOs-induced vasodilation in preclinical studies.14 One possible explanation for the lack of glibenclamide effect on vascular changes could be that glibenclamide has less efficacy on SUR2B subunit expressed in the VSMCs compared to SUR1. Another explanation is that the preclinical studies used a high dose which is not applicable for clinical use.
In conclusion, systemic glibenclamide administration did not alter the global CBF or the basal vascular tone and it did not inhibit or attenuate KCO-induced vascular changes. Levcromakalim infusion increased the global CBF and MCA circumference. These findings demonstrate an important role of the KATP channels in cerebral hemodynamics in humans.
Acknowledgements
The authors thank all participating subjects. Further thanks to Lundbeck Foundation and Aase and Ejnar Danielsen Foundation for their support.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The research leading to these results has received funding from Lundbeck Foundation (R155–2014–171) and Aase & Ejnar Danielsen Foundation.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MMK has acted as an invited speaker for Novartis and received travel grant from ElectroCore, LLC. MA is a consultant, speaker, or scientific advisor for Allergan, Amgen, Alder, ATI, Eli Lilly, Novartis, and Teva, primary investigator for Alder, Amgen, Allergan, Eli Lilly, Novartis, and Teva trials. MA has no ownership interest and does not own stocks of any pharmaceutical company. MA serves as associate editor of Cephalalgia, associate editor of Headache, coeditor of the Journal of Headache and Pain. MA is President of the International Headache Society. FMA is a consultant, speaker, or scientific advisor for Teva, Novartis, and Eli Lilly and primary investigator for Teva and Novartis. HG, CAWN, AA, CG, SY, MBV, HBWL, and LTS declare no conflict of interest.
Authors’ contributions: MMK and HG initiated the study and contributed to study design; protocol development; participant enrolment; data acquisition, data processing, analysis, statistics, and interpretation; and drafting and revision of the paper. CAWN, AA, and CG contributed to participant enrolment, data acquisition, statistical analyses, and critical review of the paper. SY, MBV, HBWL, and FMA contributed to data acquisition and critical review of the paper. LTS contributed to statistical analyses and critical review of the paper. MA initiated the study and contributed to study design, protocol development, statistics, data interpretation, and drafting and revision of the paper.
ORCID iDs
Mohammad Al-Mahdi Al-Karagholi https://orcid.org/0000-0003-1118-9665
Hashmat Ghanizada https://orcid.org/0000-0001-7954-8519
Samaria Younis https://orcid.org/0000-0002-9750-690X
Mark B Vestergaard https://orcid.org/0000-0002-3510-9204
References
- 1.Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NCCa-ATP channel mediates cerebral edema after ischemic stroke. Nat Med 2006; 12: 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo SK, Kwon MS, Ivanov A, et al. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem 2013; 288: 3655–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simard JM, Woo SK, Schwartzbauer GT, et al. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab 2012; 32: 1699–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta RI, Ivanova S, Tosun C, et al. Sulfonylurea receptor 1 expression in human cerebral infarcts. J Neuropathol Exp Neurol 2013; 72: 871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubaiy HN.The therapeutic agents that target ATP-sensitive potassium channels. Acta Pharm 2016; 66: 23–34. [DOI] [PubMed] [Google Scholar]
- 6.Simard JM, Tsymbalyuk N, Tsymbalyuk O, et al. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke 2010; 41: 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caffes N, Kurland DB, Gerzanich V, et al. Glibenclamide for the treatment of ischemic and hemorrhagic stroke. Int J Mol Sci 2015; 16: 4973–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Karagholi MA-M, Hansen JM, Severinsen J, et al. The KATP channel in migraine pathophysiology: a novel therapeutic target for migraine. J Headache Pain 2017; 18: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al‐Karagholi MA, Ghanizada H, Hansen JM, et al. Levcromakalim, an adenosine triphosphate‐sensitive potassium channel opener, dilates extracerebral but not cerebral arteries. Headache J Head Face Pain 2019; 59: 1468–1480. [DOI] [PubMed] [Google Scholar]
- 10.Al-Karagholi MA-M, Ghanizada H, Aghazadeh S, et al. Extracranial activation of ATP-sensitive potassium channels induces vasodilation without nociceptive effects. Cephalalgia 2019; 39: 1789–1789. [DOI] [PubMed] [Google Scholar]
- 11.Al-Karagholi MA-M, Hansen JM, Guo S, et al. Opening of ATP-sensitive potassium channels causes migraine attacks: a new target for the treatment of migraine. Brain 2019; 142: 2644–2654. [DOI] [PubMed] [Google Scholar]
- 12.Faraci FM, Heistad DD.Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiol Rev 1998; 78: 53–97. [DOI] [PubMed] [Google Scholar]
- 13.Faraci FM, Sobey CG.Role of potassium channels in regulation of cerebral vascular tone. J Cereb Blood Flow Metab 1998; 18: 1047–1063. [DOI] [PubMed] [Google Scholar]
- 14.Al-Karagholi MA, Sode M, Gozalov A, et al. The vascular effect of glibenclamide : a systematic review. Cephalalgia Reports Int J Headache 2019; 2: 251581631988493–251581631988413. [Google Scholar]
- 15.Hosford PS, Christie IN, Niranjan A, et al. A critical role for the ATP-sensitive potassium channel subunit K IR 6. 1 in the control of cerebral blood flow. J Cereb Blood Flow Metab 2019; 39: 2089–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syed AU, Koide M, Brayden JE, et al. Tonic regulation of middle meningeal artery diameter by ATP-sensitive potassium channels. J Cereb Blood Flow Metab 2019; 39: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gozalov A, Petersen KA, Mortensen C, et al. Role of KATP channels in the regulation of rat dura and pia artery diameter. Cephalalgia 2005; 25: 249–260. [DOI] [PubMed] [Google Scholar]
- 18.Foster MN, Coetzee WA.KATP channels in the cardiovascular system. Physiol Rev 2016; 96: 177–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wind T, Prehn JHM, Peruche B, et al. Activation of ATP-sensitive potassium channels decreases neuronal injury caused by chemical hypoxia. Brain Res 1997; 751: 295–299. [DOI] [PubMed] [Google Scholar]
- 20.Zhu HL, Luo WQ, Wang H.Iptakalim protects against hypoxic brain injury through multiple pathways associated with ATP-sensitive potassium channels. Neuroscience 2008; 157: 884–894. [DOI] [PubMed] [Google Scholar]
- 21.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 22.Guenst JM, Nelson LD.Predictors of total parenteral nutrition-induced lipogenesis. Chest 1994; 105: 553–559. [DOI] [PubMed] [Google Scholar]
- 23.Heinemann L, Bender R, Schmidt A, et al. Comparative dose-related time-action profiles of glibenclamide and a new non-sulphonylurea drug, AG-EE 623 ZW, during euglycaemic clamp in healthy subjects. Diabetologia 1994; 37: 703–707. [DOI] [PubMed] [Google Scholar]
- 24.Amin FM, Hougaard A, Schytz HW, et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 2014; 137: 779–794. [DOI] [PubMed] [Google Scholar]
- 25.Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol 2013; 12: 454–461. [DOI] [PubMed] [Google Scholar]
- 26.Amin F, Lundholm E, Hougaard A, et al. Measurement precision and biological variation of cranial arteries using automated analysis of 3 T magnetic resonance angiography. J Headache Pain 2014; 15: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakker CJG, Hartkamp MJ, Mali WPTM.Measuring blood flow by nontriggered 2D phase-contrast MR angiography. Magn Reson Imaging 1996; 14: 609–614. [DOI] [PubMed] [Google Scholar]
- 28.Vestergaard MB, Lindberg U, Aachmann-Andersen NJ, et al. Comparison of global cerebral blood flow measured by phase-contrast mapping MRI with 15O-H2O positron emission tomography. J Magn Reson Imaging 2017; 45: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkinson M, Beckmann CF, Behrens TEJ, et al. FSL. Neuroimage 2012; 62: 782–790. [DOI] [PubMed] [Google Scholar]
- 30.Torack RM, Alcala H, Gado M, et al. Correlative assay of computerized cranial tomography CCT, water content and specific gravity in normal and pathological postmor- tem brain. J Neuropathol Exp Neurol 1976; 35: 385–392. [DOI] [PubMed] [Google Scholar]
- 31.Hougaard A, Younis S, Iljazi A, et al. Cerebrovascular effects of endothelin-1 investigated using high-resolution magnetic resonance imaging in healthy volunteers. J Cereb Blood Flow Metab 2020; 40: 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amin FM, Asghar MS, Guo S, et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia 2012; 32: 140–149. [DOI] [PubMed] [Google Scholar]
- 33.Sheth KN, Petersen NH, Cheung K, et al. Long-term outcomes in patients aged ≤70 years with intravenous glyburide from the phase II GAMES-RP study of large hemispheric infarction. Stroke 2018; 49: 1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheth KN, Elm JJ, Molyneaux BJ, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2016; 15: 1160–1169. [DOI] [PubMed] [Google Scholar]
- 35.Gori T, Sicuro S, Dragoni S, et al. Sildenafil prevents endothelial dysfunction induced by ischemia and reperfusion via opening of adenosine triphosphate-sensitive potassium channels: a human in vivo study. Circulation 2005; 111: 742–746. [DOI] [PubMed] [Google Scholar]
- 36.Fujii N, Louie JC, McNeely BD, et al. K + channel mechanisms underlying cholinergic cutaneous vasodilation and sweating in young humans: roles of K Ca, K ATP, and K V channels.? Am J Physiol Regul Integr Comp Physiol 2016; 311: R600–R606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hojs N, Strucl M, Cankar K.The effect of glibenclamide on acetylcholine and sodium nitroprusside induced vasodilatation in human cutaneous microcirculation. Clin Physiol Funct Imag 2009; 29: 38–44. [DOI] [PubMed] [Google Scholar]
- 38.Bayerle-Eder M, Wolzt M, Polska E, et al. Hypercapnia-induced cerebral and ocular vasodilation is not altered by glibenclamide in humans. Am J Physiol Regul Integr Comp Physiol 2000; 278: R1667–73. [DOI] [PubMed] [Google Scholar]
- 39.Williams S, Abbott D, Morfis L, et al. Effects of glibenclamide on blood pressure and cardiovascular responsiveness in non-insulin dependent diabetes mellitus. J Hypertens 1998; 16: 705–711. [DOI] [PubMed] [Google Scholar]
- 40.Lahmann C, Kramer HB, Ashcroft FM.Systemic administration of glibenclamide fails to achieve therapeutic levels in the brain and cerebrospinal fluid of rodents. PLoS One 2015; 10: e0134476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen F, Dong RR, Zhong KL, et al. Antidiabetic drugs restore abnormal transport of amyloid-β across the blood-brain barrier and memory impairment in db/db mice. Neuropharmacology 2016; 101: 123–136. [DOI] [PubMed] [Google Scholar]
- 42.Vorasayan P, Bevers MB, Beslow LA, et al. Intravenous glibenclamide reduces lesional water uptake in large hemispheric infarction. Stroke 2019; 50: 3021–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coppack S, Lant A, McIntosh C, et al. Pharmacokinetic and pharmacodynamic studies of glibenclamide in non‐insulin dependent diabetes mellitus. Br J Clin Pharmacol 1990; 29: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishizaki E, Fukumoto M, Puro DG.Functional KATP channels in the rat retinal microvasculature: topographical distribution, redox regulation, spermine modulation and diabetic alteration. J Physiol 2009; 587: 2233–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King ZA, Sheth KN, Kimberly WT, et al. Profile of intravenous glyburide for the prevention of cerebral edema following large hemispheric infarction: evidence to date. Drug Des Devel Ther 2018; 12: 2539–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu R, Wang H, Xu B, et al. Cerebrovascular safety of sulfonylureas: the role of KATP channels in neuroprotection and the risk of stroke in patients with type 2 diabetes. Diabetes 2016; 65: 2795–2738. [DOI] [PubMed] [Google Scholar]
- 47.Sun HS, Xu B, Chen W, et al. Neuronal KATP channels mediate hypoxic preconditioning and reduce subsequent neonatal hypoxic-ischemic brain injury. Exp Neurol 2015; 263: 161–171. [DOI] [PubMed] [Google Scholar]
- 48.Zhong CJ, Chen MM, Lu M, et al. Astrocyte-specific deletion of Kir6.1/K-ATP channel aggravates cerebral ischemia/reperfusion injury through endoplasmic reticulum stress in mice. Exp Neurol 2019; 311: 225–233. [DOI] [PubMed] [Google Scholar]
- 49.Clement JP, Kunjilwar K, Gonzalez G, et al. Association and stoichiometry of K(ATP) channel subunits. Neuron 1997; 18: 827–838. [DOI] [PubMed] [Google Scholar]
- 50.Ploug KB, Baun M, Hay-Schmidt A, et al. Presence and vascular pharmacology of KATP channel subtypes in rat central and peripheral tissues. Eur J Pharmacol 2010; 637: 109–117. [DOI] [PubMed] [Google Scholar]
- 51.Christensen CE, Amin FM, Younis S, et al. Sildenafil and calcitonin gene-related peptide dilate intradural arteries: a 3T MR angiography study in healthy volunteers. Cephalalgia 2019; 39: 264–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings in the present study are available from the corresponding author upon reasonable request.