Abstract
Background
This is the first case report demonstrating the use of a smartphone device, enabling the diagnosis of an arrhythmia in the sports cardiology literature.
Case summary
A 17-year-old semi-professional rugby player presented with recurrent episodes of palpitations terminated by vagal manoeuvres. The rugby player’s resting 12-lead electrocardiogram (ECG), echocardiogram, and exercise stress test were normal. Due to his suggestive history and an ECG trace from a smartphone device, demonstrating a narrow complex tachycardia, an electrophysiological study was arranged. The study demonstrated a slow-fast atrioventricular nodal re-entrant tachycardia which was successfully ablated.
Discussion
The ambulatory use of a smartphone ECG device assisted in the timely diagnosis and management of an undiagnosed paroxysmal arrhythmia in a rugby player. This resulted in an expedited return to play.
Keywords: Sports cardiology, Electrophysiology, Digital, Case report, Arrhythmia
Learning points
Smartphone electrocardiogram (ECG) devices can assist in the diagnosis of culprit arrhythmias.
These devices may assist sports cardiologists/physicians in expediting diagnosis and return to play for athletes.
Smartphone ECG devices are not appropriate for preparticipation screening. However, they may assist in event monitoring in symptomatic athletes who have been assessed to be at low risk for sudden cardiac death.
Introduction
Smartphones are a significant feature of modern-day life with approximately two-thirds of the European/North American population owning at least one device.1 Smartphone applications now have the ability to record high-quality single-lead electrocardiogram (ECG) traces, making event-monitoring more accessible, whilst also being accurate in detecting arrhythmias such as atrial fibrillation (AF).2 Smartphones are both user-friendly and accessible 24 h a day, making them, potentially, highly effective event monitors. The 2015 American College/American Heart Association/Heart Rhythm Society (HRS) guidelines call for more literature observing the impact of shared decision-making with regards to monitoring patients with devices such as smartphone-enabled ECG monitoring.3 Therefore, the potential use of smartphone applications is very much in the forefront of modern-day cardiology. Tachyarrhythmias can be problematic for athletes since discordance between atrial and ventricular activity may significantly impair cardiac performance.4 Ambulatory cardiac monitoring is considered an important investigation for athletes who present with symptoms of a suspected paroxysmal arrhythmia.4 We report the case of a young semi-professional athlete who had his arrhythmia diagnosis aided by the use of smartphone technology.
Timeline
Case presentation
A 17-year-old semi-professional rugby player was referred to our institution with recurrent episodes of sudden onset, rapid, regular palpitations during exercise. His last episode had occurred during a training session when his radial pulse rate was recorded manually to be 220 b.p.m., shortly after commencing exercise. This episode eventually terminated after repeated vagal manoeuvres. The rugby player denied experiencing any chest pain or syncope during these episodes. He had no significant past medical history and denied use of recreational drugs, alcohol, or tobacco. The rugby player participated in 8–10 h of vigorous training per week, with up to 3–4 additional gym sessions.
On examination, the athlete’s body mass index was 27, blood pressure was 115/58, and the radial pulse was regular at 52 b.p.m. Heart sounds were normal with no murmurs. There were no peripheral stigmata of heart failure on examination. All routine laboratory blood tests were normal.
A 12-lead ECG was recorded (Figure 1) demonstrating sinus rhythm, ventricular rate 59 b.p.m., with a normal frontal axis. All conduction intervals were within normal limits, with no repolarization abnormalities and no pathological Q waves. There was no evidence of ventricular pre-excitation, QT prolongation, or the Brugada phenotype at rest. This ECG would be considered normal for an athlete, in line with current international expert consensus.4
Figure 1.
The rugby player’s resting 12-lead electrocardiogram, which is considered to be normal electrocardiogram for an athlete.
Echocardiography demonstrated a structurally normal heart with normal biventricular systolic and diastolic function, and normal left ventricular (LV) wall thickness (LV ejection fraction: >55%, LV end-diastolic dimension 55 mm, MV E/A ratio 1.9, maximal LV wall thickness 9 mm, right ventricular basal dimension 42 mm). During treadmill exercise stress testing, he completed 13 min of the Bruce protocol, achieving 93% of his maximal predicted heart rate (Figure 2). He was asymptomatic throughout, with appropriate heart rate and blood pressure increases. There were no induced arrhythmias.
Figure 2.
Results of the exercise stress test, which revealed a normal blood pressure and heart rate increase with exercise. There were no inducible arrhythmias.
The clinical history was suggestive of a paroxysmal tachyarrhythmia, most likely atrioventricular (AV) node dependent, given the history of termination of symptoms with vagal manoeuvres. Attempts to document the arrhythmia had been unsuccessful with Holter monitoring for periods ranging from 24 h to a maximum of one week. During these periods, the rugby player still engaged in normal rugby training sessions.
The athlete therefore installed the AliveCor® Kardia application on his smartphone in an attempt to document an arrhythmia. The athlete subsequently experienced an episode of palpitations during training which again were terminated by vagal manoeuvres. This episode was captured on his smartphone. The heart trace demonstrated a regular narrow complex tachycardia at a rate of 182 b.p.m. (Figure 3). The athlete’s history and AliveCor® Kardia traces were highly suggestive of a paroxysmal AV node dependent tachycardia. He was therefore scheduled for an invasive electrophysiological (EP) study.
Figure 3.

Single lead trace from the AliveCor® Kardia application, demonstrating a narrow complex tachycardia at 182 b.p.m.
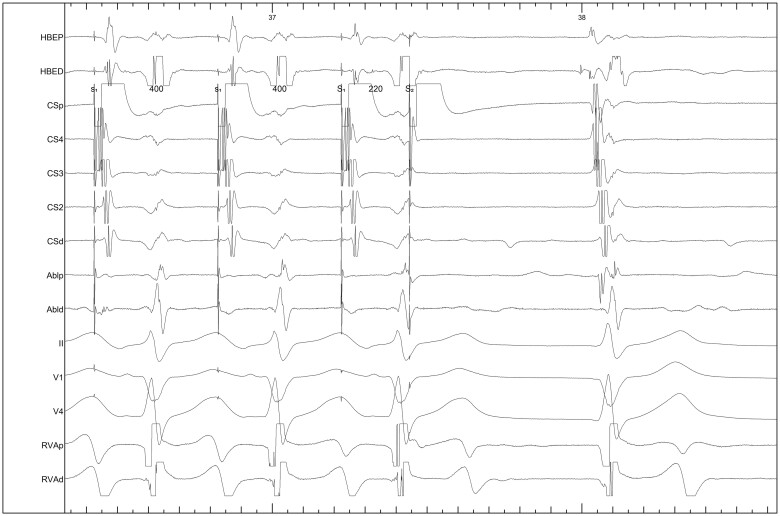
The EP study demonstrated normal baseline conduction intervals. There was ventriculo-atrial block at 460 ms. There was a short RP narrow complex tachycardia induced by atrial extrastimuli blocking the fast pathway, resulting in conduction down the slow pathway (Figures 4 and5). The ventriculo-atrial cycle length was 0 ms, with no reset. The study was consistent with a slow-fast AV nodal re-entrant tachycardia (AVNRT). We therefore proceeded to radiofrequency ablation. After ablation there was no jump, echo, crossover or inducible tachycardia by atrial extrastimuli with the same coupling intervals (Figure 6); this confirmed a successful slow pathway modification. The athlete was counselled not to play any competitive sport for 4 weeks, in order to reduce femoral vein complications. He has subsequently made a full return to competition without any recurrent symptoms at 6 months follow-up. This is in line with European Society of Cardiology (ESC) guidelines5; athletes diagnosed with paroxysmal supraventricular arrhythmias who undergo successful catheter ablation may return to full competition.
Figure 4.
Short RP narrow complex tachycardia at a paper speed of 25 mm/s.
Figure 5.
Tachycardia induction by atrial extrastimuli. The intracardiac and precordial electrocardiogram traces, at a paper speed of 100 mm/s, show a pacing beat and jump due to block in the fast pathway and conduction down the slow pathway. This results in a short RP narrow complex tachycardia, consistent with AVNRT.
Figure 6.
Post-ablation atrial extrastimuli at the same coupling intervals as Figure 5 no longer induced a tachycardia, demonstrating a successful ablation.
Discussion
The utility of smartphone ECG applications has previously been reported in the general population.6 Previously, smartphone ECG devices have been used to rule out sinister pathology in athletes presenting with palpitations.7 This case is novel, however, as there are no known previous reports in the literature where smartphone ECG monitoring has been successfully used to diagnose arrhythmias in a competitive athlete. This was of significant benefit for the athlete as he made a safe and timely return to sport. Supraventricular arrhythmias are not commonly seen in ECGs of athletes and require thorough investigation if suspected.4 Interestingly, the diagnostic accuracy of the AliveCor® Kardia in detecting supraventricular tachycardias has been investigated by placing the device in different chest orientations in one study.8 This led to a reasonable accuracy of 76% compared to an electrophysiology study; although, the specific device positioning described in this study could be challenging for patients to adopt during symptoms.8 The AliveCor® Kardia device’s major area of efficacy is detecting AF when evaluated by systematic review.1 The device demonstrates high sensitivity, specificity and accuracy with the added benefit of user convenience, potentially creating a future role for this device as an adjunct in standard AF diagnostic investigations.1
The Apple Watch is an increasingly popular device. Recently, the Apple Heart study9 highlighted the ability of the device’s irregular rhythm algorithm to diagnose AF with a positive predictive value of 0.84. The study cautioned users to be aware that other significant arrhythmias may not cause notification. In addition, the study stated it was not designed to validate it as a screening tool, discrediting its potential use in population screening.9 The Apple Watch is an optical heart rate monitor which works through photoplethysmography.10 Optical heart rate monitors are popular with athletes as they are convenient and easy to wear, compared to strap-based heart rate monitoring.10 These devices allow athletes to monitor performance measures which are deemed important to athletes and coaching staff; they also have the ability to record an ECG which is important to the sports medical team.10 When an arrhythmia is suspected in an athlete, continuous ECG monitoring becomes a more desirable function for athletes.10 Continuous ECG monitoring can be achieved by devices such as QARDIO MD, a strap device that records and transmits continuous ECG recordings to an athlete’s smartphone during exercise.10 In addition to smartphone and smartwatch technology, mid/long-term monitoring can also be achieved by means of wearable devices such as the Zio® Patch, and Nuubo ECG vest.
The implications for screening athletes with wearable devices such as AliveCor® Kardia are less clear. Conditions predisposing to sudden cardiac death are rare. It may be difficult and unreliable to detect pathologies such as pre-excitation, high-degree heart block, Brugada syndrome, and cardiomyopathies with software such as AliveCor® Kardia.11 A significant limitation of this device is that it only captures lead 1 equivalent traces.11 This device, therefore, is not appropriate for pre-participation screening but may be considered for ambulatory monitoring in athletes diagnosed with a potentially arrhythmogenic condition. In addition to our investigations, one should also consider using a different stress protocol in young athletes with suspected arrhythmias, for example, sprinting alternating with gentle jogging. This may more closely reproduce the physiological conditions giving rise to training-induced arrhythmias and may have been superior to a standard Bruce protocol in this case.
There is cause for concern with respect to wearable ECG devices as there is the potential to create large datasets which could be misused by third parties, or be susceptible to cyber-attacks.12 There is also a risk of increased patient anxiety from false positive results, which could also lead to inappropriate demand on health services.12,13 In addition, there are medico-legal concerns with regards to the impact of these devices on clinical decision-making, since current evidence-based practice has not been informed by the use of this technology.14
A recent randomized controlled trial has demonstrated utility in the acute medical setting, when allocating an AliveCor® Kardia device to patients presenting with presyncope or palpitations.15. The intervention was found to result in a shorter time to diagnosis and was more cost-effective than standard investigations.15 This highlights a potential role for these devices in the primary/acute care setting, which may be most suitable in patients deemed to be at low risk for sudden death.
In conclusion, smartphone-based ECG technology may facilitate earlier arrhythmia diagnosis and management, expediting the return to competition.
Lead author biography

Dr Daniel Phillips is a Clinical Teaching Fellow and Honorary Lecturer in Cardiology/Medical Education at the University Hospital of Wales. Daniel has a particular interest in Sports Cardiology. He is currently an MSc student, reading Sports & Exercise Medicine at Cardiff Metropolitan University.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report, including the text and associated images, was obtained from the patient’s guardians, in line with the COPE guidelines.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1. Giebel GD, Gissel C.. Accuracy of mHealth devices for atrial fibrillation screening: systematic review. JMIR mHealth uHealth 2019;7:e13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garabell P, Stavrakis S, Po S.. Smartphone-based arrhythmia monitoring. Curr Opin Cardiol 2017;32:53–57. [DOI] [PubMed] [Google Scholar]
- 3. Page R, Joglar J, Caldwell M, Calkins H, Conti JB, Deal BJ. et al. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients with Supraventricular Tachycardia: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm. Circulation 2016;133:e471–505. [DOI] [PubMed] [Google Scholar]
- 4. Drezner JA, Sharma S, Baggish A, Papadakis M, Wilson MG, Prutkin JM. et al. International criteria for electrocardiographic interpretation in athletes: consensus statement. Br J Sports Med 2017;51:704–731. [DOI] [PubMed] [Google Scholar]
- 5. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S. et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: the Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur Heart J 2021;42:17–96. [DOI] [PubMed] [Google Scholar]
- 6. Tabing A, Harrell TE, Romero S, Francisco G.. Supraventricular tachycardia diagnosed by smartphone ECG. BMJ Case Rep 2017;2017:bcr-2016-217197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peritz DC, Howard A, Ciocca M, Chung EH. et al. Smartphone ECG aids real time diagnosis of palpitations in the competitive college athlete. J Electrocardiol 2015;48:896–899. [DOI] [PubMed] [Google Scholar]
- 8. Ferdman DJ, Liberman L, Silver ES.. A smartphone application to diagnose the mechanism of pediatric supraventricular tachycardia. Pediatr Cardiol 2015;36:1452–1457. [DOI] [PubMed] [Google Scholar]
- 9. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T. et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gajda R. Is continuous ECG recording on heart rate monitors the most expected function by endurance athletes, coaches, and doctors? Diagnostics (Basel ). 2020;10: 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haberman ZC, Jahn RT, Bose R, Tun H, Shinbane JS, Doshi RN. et al. Wireless Smartphone ECG enables large-scale screening in diverse populations. J Cardiovasc Electrophysiol 2015;26:520–526. [DOI] [PubMed] [Google Scholar]
- 12. Schukat M, McCaldin D, Wang K, Schreier G, Lovell NH, Marschollek M. et al. Unintended consequences of wearable sensor use in healthcare. Yearb Med Inform 2016;25:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tieleman RG, Hemels M.. Mobile health: solution or a threat? Neth Heart J 2019;27:16–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charani E, Castro-Sánchez E, Moore LS, Holmes A.. Do smartphone applications in healthcare require a governance and legal framework? It depends on the application! BMC Med 2014;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reed MJGrubb NRLang CCO'Brien RSimpson KPadarenga Met al. . Multi-centre Randomised Controlled Trial of a Smartphone-based Event Recorder Alongside Standard Care Versus Standard Care for Patients Presenting to the Emergency Department with Palpitations and Pre-syncope: The IPED (Investigation of Palpitations in the ED) study. EClinicalMedicine 2019;8:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.