Abstract
The aging of our population has been accompanied by increasing concerns about older adults’ vulnerability to violations of trust and a growing interest in normative age-related changes to decision making involving social partners. This inter-section has spurred research on age-related neurocognitive and affective changes underlying social decision making. Based on our review and synthesis of this literature, we propose a specification that targets social decision making in aging to the recently proposed Affect-Integration-Motivation (AIM) framework. Our framework specification, Changes in Integration for Social Decisions in Aging (CISDA), emphasizes three key components of value integration with particular relevance for social decisions in aging: theory of mind, emotion regulation, and memory for past experience. CISDA builds on converging research from economic decision making, cognitive neuroscience, and lifespan development to outline how age-related changes to neurocognition and behavior impact social decision making. We conclude with recommendations for future research based on CISDA’s predictions, including implications for the development of interventions to enhance social decision outcomes in older adults.
Keywords: affect, aging, neurocognition, social decision making
1 |. INTRODUCTION
Individuals 65 years and older make up the fastest growing population segment in developed countries. Recent estimates suggest that by 2050 over 20% of the U.S. population will be over the age of 65 (Pew Research Center, 2014). With the aging of our society, there is increasing interest in how this demographic group thinks and behaves, especially when their decisions involve others. Understanding how older adults make social decisions holds implications for public health, marketing, and political campaigns. There are growing concerns that older adults may be susceptible to fraud perpetrated by strangers or close social partners (Ebner et al., 2018; Lachs & Han, 2015; Oliveira et al., 2017; Spreng et al., 2017), especially as age is associated with wealth accumulation (Li & Tsang, 2016). Most instances of elder abuse involve an individual, usually a family member, gaining an older adult’s trust for later exploitation (Metlife, 2011; Peterson et al., 2014). Estimates of loss by elderly victims of financial abuse in the United States vary between $2.9 (Metlife, 2011) and $36.5 (True Link Financial, 2015) billion per year. Even these dire assessments may be low, as experts estimate that for every case of elderly financial exploitation that is reported another approximately 44 remain unreported (Huang & Lawitz, 2016). Despite the urgency of this issue and a large body of research on social decision making in younger adults (Rilling & Sanfey, 2011; Sanfey, 2007), there have been few investigations of changes to social decision making in advanced age. Specifically addressing this knowledge gap, our paper provides a review and a synthesis of the currently existing literature on social decision making and aging and develops a specification—which we termed Changes in Integration for Social Decisions in Aging (CISDA)—to the recently proposed Affect-Integration-Motivation (AIM) framework for an application to social decisions in aging.
Social decision making involves identifying and weighing the merits of multiple alternatives and choosing the best course of action in a social context (Rilling & Sanfey, 2011). With age, people increasingly experience losses in physical ability, cognitive sharpness, and social support leading to a shift in goals from a focus on growth to a focus on loss prevention and maintenance (Ebner, Freund, & Baltes, 2006). Concurrently, older adults typically become more reliant on others for healthcare, financial stability, and other aspects of daily functioning (Nielson & Mather, 2011). Thus, older adults are faced with many important and complex decisions that involve others and hold major implications for their (and others’) wellbeing, health, and finances.
One of the gaps in our understanding of how aging affects social decision making concerns changes in the “integration” stage of decision making. According to Samanez-Larkin and Knutson’s (2015) proposed AIM framework, it is at the integration stage that the values of various decision attributes are determined within and across decision options. Attribute evaluation can involve integration of standard choice-related features, including outcome probabilities, delays in expected outcomes, and effort required by a decision. This decision stage is particularly important in social contexts as it guides choice selection in complex decisions. Social decision making is multifaceted, involves not only integration of standard, nonsocial choice features but also features associated with social contexts and concerns (e.g., inferring what others are thinking and feeling, regulating affective reactions to others, recalling applicable social experiences, etc.). In this way, social contexts and concerns add layers of complexity to value-based decision making that necessitate integration. This added complexity leads to the recruitment of additional neurocognitive resources in the form of integration functions. These conditions make it clear that understanding social decision processing in aging requires research targeting the integration phase of decision making; however, theories and research have given little attention to this topic to date. In particular, the impact of age-related changes in integrative functions on the quality of social decisions is presently unclear. To address this issue, the present review offers a focused discussion of three processes that are involved in choice valuation through the integration of social-decision features: theory of mind, emotion regulation, and memory for past experience. While other integrative processes, such as those supporting subjective valuation of nonsocial decision options (Seaman et al., 2018), appear to remain stable in aging, these three integrative processes show unique and significant patterns of change with age. This diverse pattern of change trajectories for integration-related functions holds important implications for social decision outcomes among older adults. From our focused review on these three integrative functions, we offer the framework specification CISDA: Changes in Integration for Social Decisions in Aging.
This paper sets out with a summary of the AIM framework from which we draw the concept of integration. We then describe CISDA, our social decision-making specification of the AIM model. Like AIM, CISDA adopts a biobehavioral approach to conceptualize age-related changes in social decision making. By understanding how neurobiological correlates affect behavior, we can describe and contextualize social decision making in aging. Next, we discuss current evidence of age-related changes to the integrative components highlighted by CISDA: theory of mind, emotion regulation, and memory for past experience. We conclude with a discussion of challenges that must be addressed in order to understand how aging affects social decision processing and suggestions for future directions toward theory refinement and research efforts.
2 |. AFFECT-INTEGRATION-MOTIVATION FRAMEWORK
AIM (Samanez-Larkin & Knutson, 2015) provides a framework for understanding and testing predictions about value-based decision processing in aging without a specific focus on social decisions. AIM has some clear advantages over other broader models of decision making, like Brunswik’s Lens Model (Brunswik, 1939), because AIM specifically targets the neurological underpinnings of decision making and aging. The proposed three stages to the decision-making process in AIM are: affect, integration, and motivation.
2.1 |. Affect
The affect stage of decision making is thoroughly described in the AIM framework. According to AIM, decision processing begins with parallel impulses to approach and/or avoid a stimulus, as regulated via affective neural circuits. Dopaminergic projections from the ventral tegmental area to the nucleus accumbens (NAc) have been primarily associated with approach behavior and gain valuation, while noradrenergic projections from the locus coeruleus to the anterior insula (AI) and the NAc have been associated with avoidance behavior and loss valuation (Knutson, Katovich, & Suri, 2014; Palminteri et al., 2012; Pessiglione, Seymour, Flandin, Dolan, & Frith, 2006).
2.2 |. Integration
Relative to the affect stage, the integration stage is described rather broadly in the AIM framework. For complex choices, AIM posits a value integration stage in which approach and avoidance impulses are weighed against other considerations with inputs from previous experience (Samanez-Larkin & Knutson, 2015). The medial prefrontal cortex (mPFC) receives direct and indirect inputs from network components that contribute to the integration of choice-option valuation, while the dorsolateral prefrontal cortex (dlPFC) supports comparison of choice-relevant information (Alexander & Brown, 2012; Knutson, Scott, Wimmer, Prelec, & Loewenstein, 2007; Levy & Glimcher, 2012; Samanez-Larkin, 2015). Memory-related information from the medial temporal lobe (MTL) is predicted to enter the integration stage through the MTL-dlPFC circuit (Samanez-Larkin & Knutson, 2015).
2.3 |. Motivation
The AIM framework predicts that glutamatergic outputs potentiate resulting choice actions though connections to the dorsal striatum and presupplementary motor area (pSMA; Samanez-Larkin, 2015). AIM hypothesizes that motivational component processes cannot operate without input from affective component processes, and motivated actions may be initiated by any number of integration components. Thus, the influence of specific integrative processes on decision making is highly variable and context dependent.
3 |. TAKING AIM: CHANGES IN INTEGRATION FOR SOCIAL DECISIONS IN AGING
We propose CISDA as a specification to AIM (Figure 1a). CISDA offers a framework for understanding mechanisms of age-related changes in decision making that are specific to choices in the social domain. Such a specification is required for several reasons: (a) Understanding social decision making across the adult lifespan requires an increased focus on age-related changes to integration functions because social contexts and concerns add dimensions of complexity to choice valuation that necessitate value integration. (b) Social decision making often involves recruitment of higher-level functions that are not required for nonsocial decisions—with some processing components observed only in social decision making. Thus, the mechanisms of social decision making overlap with, but are distinct from, those of nonsocial decision making. (c) Of the processes that are likely to be recruited during social decision making, we have identified three that undergo significant age-related changes: theory of mind, emotion regulation, and memory for past experience. These processes are all involved during the integration stage of value-based decision making. (d) CISDA provides hypotheses about specific mechanisms of resilience and vulnerability in social decision making with age that can be used to guide future research. We propose that CISDA’s specific focus on social decision making can also help to inform interventions targeted at improving older adults’ social decision outcomes and, in the long-term possibly, reducing rates of elder abuse victimization. In the following, we present the conceptualization of the AIM framework components in the context of our novel CISDA specification.
FIGURE 1.
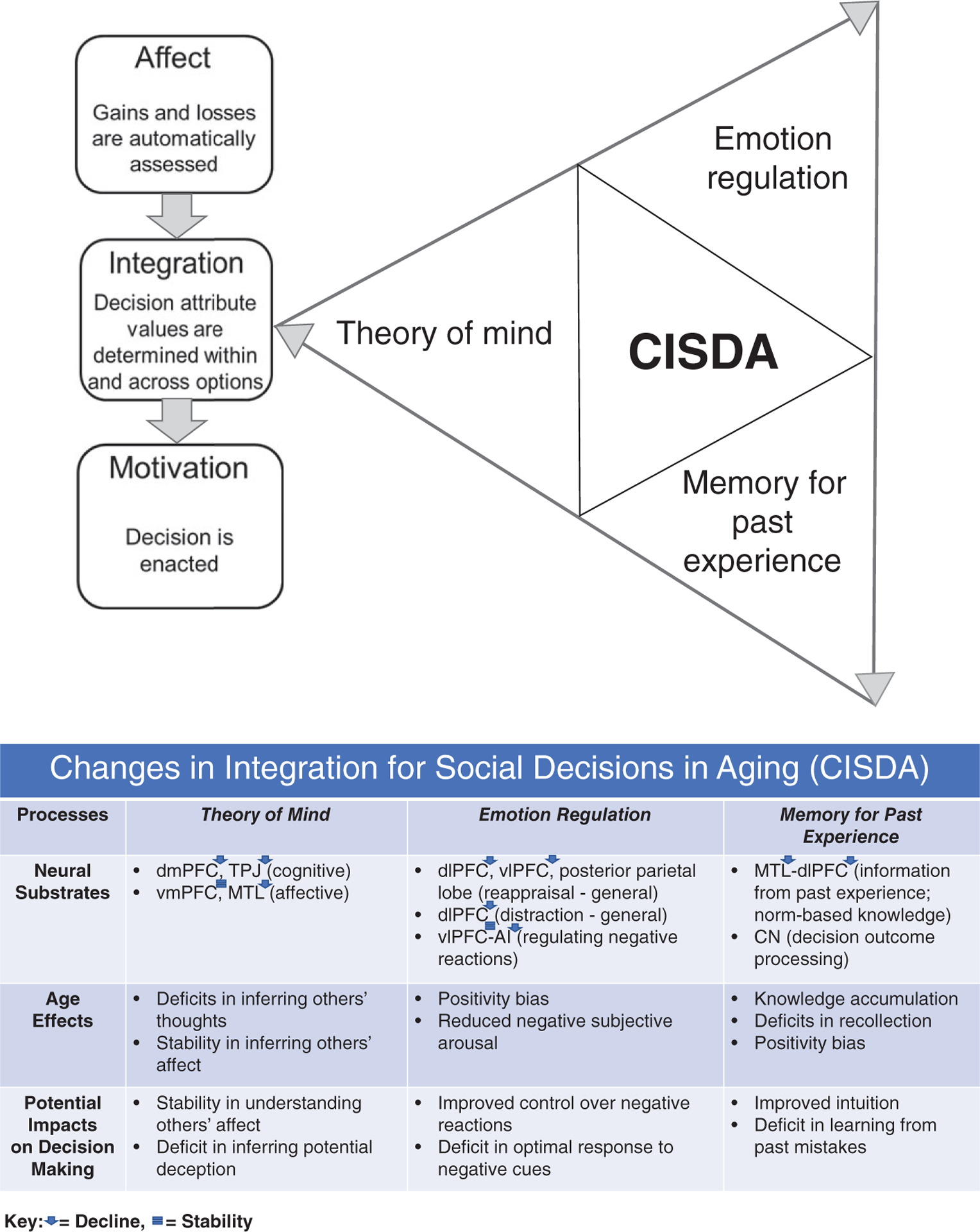
(a) Changes in Integration for Social for Social Decisions in Aging (CISDA) provides a theoretical framework for understanding mechanisms of age-related changes in decision making in the social domain. CISDA focuses on neurobiological and experiential changes underlying three key interacting integrative functions: Theory of mind, emotion regulation, and memory for past experience. (b) Neural substrates, age effects, and potential impacts on decision making in the three integrative processes proposed in CISDA. dmPFC = dorsomedial prefrontal cortex; TPJ = temporal–parietal junction; vmPFC = ventromedial prefrontal cortex; MTL = medial temporal lobe; dlPFC = dorsolateral prefrontal cortex; vlPFC = ventrolateral prefrontal cortex; AI = anterior insula; CN = caudate nucleus
3.1 |. Affect
As in AIM, the first step of the social decision-making process involves the automatic evaluation of a stimulus via affective neural components to anticipate potential gains and losses. For this stage of social decision making, evidence suggests that older compared to younger adults are more likely to under-anticipate loss-predictive cues (e.g., untrustworthy faces; Castle et al., 2012).
3.2 |. Integration
Output from initial affective reactions then enter the integration phase where evaluative processes involving theory of mind, emotion regulation, and memory for past experience (among potential other processes) occur. The features of the decision context (e.g., amount of time to deliberate, perceived costs and benefits) will determine the number of integrative components employed and how they interact. Each of these three select integrative processes will be reflected on in the context of CISDA more closely next.
Theory of mind
Theory of mind is a neurocognitive process unique to social decisions, as other forms of decision making do not require reasoning about the state of another’s mind. This process involves making inferences about another’s thoughts, emotions, and intentions (Lecce et al., 2017; Premack & Woodruff, 1978). In social decisions, we consider the thoughts and feelings of others when determining the value of our choice options. For example, decisions to trust require inferences about the intentions and actions of others. Such inferences become inputs (value weights) in expected value calculations for decision options.
Theory of mind is predicted to affect the valuation of decision options via inferences about a social partner’s mental and affective states. Its neural correlates depend on whether cognitive (dorsomedial prefrontal cortex [dmPFC], temporoparietal junction [TPJ]) or affective (ventromedial prefrontal cortex [vmPFC], MTL) theory of mind are employed (Li, Mai, & Liu, 2014). Age-related declines in ability and neural mechanisms of cognitive theory of mind (Bottiroli, Cavallini, Ceccato, Vecchi, & Lecce, 2016; Charlton, Barrick, Markus, & Morris, 2009; Moran, Jolly, & Mitchell, 2012) but relative stability in affective theory of mind (Bottiroli et al., 2016; Ebner, Bailey, Horta, Joiner, & Chang, 2016; Mather, 2016), lead to older adults’ deficits in mentalizing others’ thoughts but not in mentalizing their affective states. These predicted asymmetrical age-related trajectories for cognitive and affective theory of mind and their subsequent effects on social-decision making performance are illustrated in Figure 2a. CISDA predicts that this asymmetrical change in theory of mind domains contributes to inaccurate valuation of social decision options among older adults when inferring the mental states of others is more challenging, particularly when social partners’ mental states conflict with their emotional display (e.g., friendly con artist).
FIGURE 2.

Predicted trajectories of integration functions in CISDA. CISDA proposes stability or change in decision-making performance as a function of aspects of theory of mind, emotion regulation, and memory for past experience. (a) Performance reliant on affective theory of mind is predicted to remain stable across adulthood while performance reliant on cognitive theory of mind is predicted to decline. (b) Performance reliant on the ability to regulate negative affective impulses is predicted to improve across adulthood while performance reliant on negative subjective arousal is predicted to decline. (c) Performance reliant on semantic or implicit memory is predicted to improve over adulthood while performance reliant on working or episodic memory is predicted to decline
Emotion regulation
Emotion regulation constitutes another key process in value integration for social decisions. This process involves selectively attending to stimuli based on their affective qualities or changing the interpretation of affective stimuli and contexts to alter one’s affective state (Braunstein, Gross, & Ochsner, 2017; Eisenberg & Spinrad, 2004). Emotion regulation often becomes necessary when making decisions in the social domain because signals from, and behavior of, others are relatively salient and can trigger affective responses (e.g., anger, excitement, etc.) that need to be modulated to achieve one’s goals (e.g., reducing anger due to a business partner’s selfish behavior). Emotion regulation can play a role in integration as it can alter both the magnitude and valence of choice-option values.
Emotion regulation is predicted to impact valuation of social decision options through alteration of affective inputs in the service of one’s goals. Affective reappraisal is supported by the dlPFC, ventrolateral prefrontal cortex (vlPFC), and posterior parietal lobe, while distraction is primarily associated with the dlPFC (Buhle et al., 2013). In social decision making, the vlPFC has been specifically implicated in regulating negative affective reactions from the AI to increase financial gains (Tabibnia, Satpute, & Lieberman, 2008). With age, response to negative stimuli decreases (Mather, 2016; Mather et al., 2004), negative subjective arousal decreases (Samanez-Larkin et al., 2007), downregulation of negative emotions increases (Mather & Carstensen, 2005), self-directed information processing becomes positively biased (Charles, Mather, & Carstensen, 2003; Mather & Carstensen, 2005), and older adults become more motivated to pursue emotionally regulatory social goals (Lang & Carstensen, 2002). These predicted age-related trajectories for negative affect impulse regulation, positivity bias, and negative subjective arousal as well as their subsequent effects on social decision-making performance are illustrated in Figure 2b. CISDA proposes that these changes will lead to an under-weighting of negative choice features in social decisions (e.g., social partner’s past breach) and generally greater trust in others, especially close others.
Memory for past experience
Memory for past experience comprises yet another process that we propose is critical for value integration in social decisions. Social decisions frequently involve valuation inputs based on past experiences with others. Such inputs may enter our conscious awareness (explicit memory) or influence option valuation without our awareness (implicit memory). These forms of memory have different age trajectories (Hoyer & Verhaeghen, 2006) and the value older adults place on social-decision options is affected by integration of choice-relevant memories.
Memory for past experience is predicted to affect valuation of social decision options via the usage of stored information about social partners and contexts. The neural correlates underlying memory for past experience involve the MTL-dlPFC circuitry (Bailey et al., 2013; Maillet & Rajah, 2013; Samanez-Larkin & Knutson, 2015). Usage of such memories affects decision outcome processing in the caudate nucleus (CN; Delgado, Frank, & Phelps, 2005), potentially through an iterative loop within the affective-integration-motivation system. CISDA proposes that decision making contexts that rely on semantic and/or implicit memory can lead to equal or better performance in older relative to younger adults due to the accumulation of previous experience in older adults or the ability to rely on intuition in familiar contexts. However, older adults are expected to perform more poorly when social decisions require working and/or episodic memory abilities such as recollection accuracy, particularly recollection of past negative outcomes given an age-related positivity bias, or error detection in recent events. These predicted age-related trajectories for semantic, implicit, working, and episodic memory as well as their subsequent effects on social decision-making performance are illustrated in Figure 2c.
As depicted in Figure 1a, CISDA predicts that the processes of theory of mind, emotion regulation, and memory for past experience can interact with one another during the value integration phase of decision making. CISDA also predicts that one does not need to engage in all three integrative functions in order to make a decision. For example, the value of a decision to trust your spouse in everyday social decisions can be strongly influenced by prior knowledge that s/he has been consistently trustworthy in the past (semantic memory). Here, the valuation phase can be carried out without regulation of emotions or concern over the spouse’s intentions. However, if you recently learned that she/he had taken out a large loan in secret, you would likely weigh the value of your decisions to trust her/him against your inferences about her/his intentions and affective state in their clandestine actions. We propose that the ways in which these three components interact are variable depending on internal (e.g., goals, preferences, etc.) and external factors (e.g., time to make the decision, people involved, etc.).
We acknowledge, that other nonsocial factors (outcomes delays, risk, decision effort; Seaman et al., 2018) may also affect subjective value integration in social decision making. We contend, however, that theory of mind, emotion regulation, and memory for past experience represent key processes that impact value integration in aging because they predict social decision-making quality (Tabibnia et al., 2008; Beadle et al., 2012; Rilling & Sanfey, 2011) and change over the aging process (Mather, 2016; Ebner et al., 2016; Scheibe & Carstensen, 2010; Agarwal, Driscoll, Gabaix, & Laibson, 2009).
3.3 |. Motivation
As in AIM, the last step of the social decision-making process involves motor processes necessary to enact the decision via glutamatergic projections to the dorsal striatum and pSMA (Samanez-Larkin & Knutson, 2015).
Taken together, we propose that the CISDA framework specification targets important neurobehavioral functions of social decision making to aid both research efforts and the development of tailored interventions for the aging decision maker. CISDA is based on the notion that the complexity added to the decision-making process caused by reasoning about thoughts and feelings of others distinguishes social decision making from other forms of decision making. Our CISDA specification focuses on three select integrative processes: theory of mind, emotion regulation, and memory for past experience, which predict social decision-making task performance and change with age. We do not suggest that CISDA predicts that older adults will be universally worse at making social decisions compared to younger adults. Rather, depending on the demands of a decision context, older adults will perform more or less optimally than younger adults. To fill these predictions with more content, we will next synthesize the current theory and research from behavioral and brain sciences which have informed our CISDA framework specification.
4 |. KEY INTEGRATIVE COMPONENTS IN CISDA
The following sections present research on the three integrative processes that form the basis of CISDA. The present review builds on foundational behavioral economics and neuroeconomics research that set the stage for understanding mechanisms of age-related changes in social decision making (see Box 1). Our review advances this area of study by synthesizing emerging evidence that age-related changes in the neural correlates of theory of mind, emotion regulation, and memory for past experience yield specific changes to processes that promote social decision making. In this context, we reflect on the extent to which the age-related changes in these processes interact with social contexts to confer beneficial or detrimental effects on social decision making (see Figure 1b for a summary of neural substrates, age effects, and potential impacts on decision making in the three integrative processes proposed in CISDA).
BOX 1. LABORATORY SOCIAL DECISION TASKS: DIRECT AGE COMPARISONS.
Social decision making has classically been studied in the context of game theory, which economists use to model financial decision making (Sanfey, 2007). Associated paradigms include the Dictator Game, Ultimatum Game, Trust Game, and Prisoner’s Dilemma (Sanfey, 2007). Game theory typically measures participants’ behavior against the strategy a rational, self-interested person would adopt to maximize their profit in a social situation. Research utilizing these experimental economic games indicates that people are generally more concerned with fairness and reciprocity than with the maximization of personal profit (Sanfey, 2007). These paradigms are useful in laboratory studies of social decision making as there is a broad history of work using these experimental games, including some of the first laboratory-controlled experiments allowing for precise predictions and measurements of factors that influence social decision behavior (von Neumann & Morgenstern, 1947). Further, the high degree of control afforded by these tasks is especially useful for isolating neurological correlates of behavior in neuroimaging studies of social decision making (e.g., NAc activity in response to manipulating reward delay and AI activity in response to manipulating risk; Samanez-Larkin & Knutson, 2015). The neural correlates of behavior in these economic games have been examined in a substantial number of studies, particularly in younger adults (Rilling & Sanfey, 2011, for review). While the literature base is still small, these experimental games have recently been applied to investigate age differences in social decision making (e.g., Bailey et al., 2015; Bailey, Zacks, et al., 2013; Beadle et al., 2012; Harlé & Sanfey, 2012; Roalf, Mitchell, Harbaugh, & Janowsky, 2012; Suzuki, 2016; Webb, Hine, & Bailey, 2016). The majority of studies in this emerging area of research have investigated effects of age on generosity, trust, and empathy.
Research examining generosity in social decision making has shown that when older adults are placed in a role where they can give money to a partner (i.e., investor), they are typically more generous than their younger counterparts. In multi-round and one-shot variants of the Ultimatum and Dictator Games, older adults have exhibited greater generosity when in the investor role than younger adults (Bailey, Ruffman, & Rendell, 2013; Bailey, Zacks, et al., 2013; Beadle et al., 2012; Roalf et al., 2012). In a meta-analysis of 133 Dictator Game studies between 1992 and 2010, older adults from developed countries had a greater likelihood of offering more to their partners than other age groups, and the single most common amount older adults offered was their entire initial endowment (Engel, 2011). Furthermore, learning empathy-inducing information about an investee in a one-shot version of the Dictator Game increased investments by older adults compared with younger adults (Beadle, Sheehan, Dahlben, & Gutchess, 2013). With respect to trust, one-shot Trust Game studies have indicated that age is associated with stable or increased investments in game partners (Bailey et al., 2015; Josef et al., 2016; Sutter & Kocher, 2007), increased likelihood to fairly return as an anonymous investee (Bailey et al., 2015), and increased proportional returns (Sutter & Kocher, 2007). Research using a multi-round trust game has found that older adults are more likely than younger adults to invest with all trustees regardless of experimentally manipulated trustworthiness of the trustees and their social closeness to their partners (except for the most trustworthy and socially close trustees; Webb et al., 2016). Thus, increased generosity in aging may be mediated by age-related increases in trust.
These experimental economic games can also be played with participants in the opposite role. When older adults are placed in a role where they receive money from a partner (i.e., investee), however, they are more likely to be concerned with fairness and emotion regulation than younger adults. For instance, in the role of investee during a one-shot Ultimatum Game, older adults were more likely than younger adults to reject moderately unfair offers (30% splits) and displayed brain activation patterns suggesting downregulation of negative emotions in response to unfair offers (Harlé & Sanfey, 2012; Roalf et al., 2012; but see Girardi, Sala, & MacPherson, 2018). While older adults may be more concerned with fairness than younger adults, their ability to monitor fairness over multiple interactions maybe hindered. Correspondingly, findings from a multi-round Trust Game indicated that older adults had a bias to trust those with “trustworthy faces” regardless of the trustworthiness of their past behavior (Suzuki, 2016). The latter finding suggests that older adults may inflexibly rely on facial cues when making social decisions.
Together, these findings converge on several age-related differences in social decision making: compared to their younger adults, older adults give money more generously, are more concerned with being treated fairly and down-regulate negative affect. Notably, however, there are concerns that the generalizability of these experimental economic games is limited because they cannot represent the full complexity of real-world social decision making. For example, a study using cross cultural methods did not replicate findings showing age-related increases in generous, trusting behavior (Rieger & Mata, 2013). Issues of ecological validity are not isolated to research on social decision making in aging. For example, in a primarily college-age sample, Galizzi and Navarro-Martínez (2018) examined altruistic behaviors in a select set of field experiments (e.g., helping a confederate carry boxes, donating to a children’s charity) and found that most altruistic actions were not predicted by participants’ prior behavior in experimental economic games. Future research should examine factors that affect the generalizability of experimental economic games, which may include individual differences in reward valuation, numeracy, and task ambiguity. Identification of significant barriers to external validity can be used to improve both task design and analytic approaches in research on social decision making, including research utilizing CISDA.
5 |. AGE-RELATED CHANGES IN THEORY OF MIND
Theory of mind is involved in inferences about others’ mental perspectives (cognitive theory of mind; Beadle et al., 2012) and emotions (affective theory of mind; Shamay-Tsoory, 2011). The mPFC is involved in both cognitive and affective theory of mind. However, while cognitive theory of mind is associated with greater engagement of the dmPFC and TPJ, affective theory of mind involves greater engagement of the vmPFC and medial temporal lobe (MTL; Kalbe et al., 2010; Li et al., 2014; Shamay-Tsoory, 2011).
Current research findings on older adults’ theory of mind abilities is somewhat mixed. The tasks generally employed in these studies involve presenting older adults with stories, static scenes, or movies in which they are asked to make inferences about the mental states of those depicted (Henry, Phillips, Ruffman, & Bailey, 2013; Moran, 2013). Generally, age-related declines of a moderate magnitude are seen in cognitive theory of mind ability, and these changes are at least partially mediated by declines in executive functioning and fluid intelligence (Bottiroli et al., 2016; Ebner et al., 2016; Henry et al., 2013; Johansson Nolaker, Murray, Happé, & Charlton, 2018; Moran, 2013). A recent study looking at cognitive theory of mind ability utilized the Movie for the Assessment of Social Cognition (MASC) task to examine both accuracy and typology of errors in mental states inferences in younger and older adults (Lecce, Ceccato, & Cavallini, 2018). In this study, older adults were less accurate than younger adults in mental states inferences and were more likely to make errors in which they insufficiently mentalized, or completely neglected to mentalize, those depicted in the MASC. Comparatively, affective theory of mind ability appears to be relatively preserved with age (Bottiroli et al., 2016; Johansson Nolaker et al., 2018; but see Bailey & Henry, 2008). A study utilizing both cognitive and affective theory of mind versions of the Faux Pas task found that younger and older adults did not differ in the affective task version while older adults performed worse than younger adults in the cognitive version (Bottiroli et al., 2016). Additionally, older adults’ performance in the affective version of the Faux Pas task was unrelated to measures of executive functioning whereas performance in the cognitive version was mediated by at least one aspect of executive functioning (Bottiroli et al., 2016).
Further, similar but distinct neural substrates of cognitive and affective theory of mind are differentially impacted by age. While the dmPFC shows age-related decline (Moran et al., 2012; but see Cassidy, Hedden, Yoon, & Gutchess, 2014), the vmPFC remains relatively stable with age (Mather, 2016). In accordance with these regional differences, older adults have performed more poorly on cognitive than affective theory of mind tasks (Bottiroli et al., 2016). Global age-related white matter integrity decline has also been linked to worse theory of mind ability (Charlton et al., 2009). This effect may be especially pronounced for cognitive theory of mind. The neural correlates of cognitive theory of mind (e.g., dmPFC and TPJ) are activated more as social decision-making processes become less automatic, more complex, and require greater transfer of information, processes which are all dependent on healthy white matter tracts (Li et al., 2014).
Little research to date has examined age-related change in theory of mind abilities in the context of social decision making. One experiment investigated the relationship between measurements of cognitive theory of mind abilities and behavior on a multi-round Ultimatum Game (Beadle et al., 2012). When playing with age-matched partners, older adult “investors” with high cognitive theory of mind ability rejected more unfair offers than younger adults. Additionally, while older compared to younger adults had lower cognitive theory of mind scores, overall payoffs were higher in older adults with high versus low cognitive theory of mind ability. These results suggest that for older adults, utilization of cognitive theory of mind in value integration can lead to more optimal social decision making, especially when fairness and finances are involved. These findings underline the importance of developing ways to preserve and/or recover cognitive theory of mind functions in older adults.
6 |. AGE-RELATED CHANGES IN EMOTION REGULATION
Attributes of decision options may evoke negative emotions which may hinder optimal decision making. When this is the case, individuals may benefit from emotion-regulation strategies such as cognitive reappraisal, which involves changing one’s reaction to an affective stimulus (Gross, 1998), or distraction, which involves disengaging with an affective stimulus (Shafir, Schwartz, Blechert, & Sheppes, 2015). Reappraisal is a cognitively demanding strategy that predicts better long-term adaption to affective stimuli and is more effortful than strategies such as distraction (Shafir et al., 2015). Cognitive reappraisal involves recruitment of the dlPFC, vlPFC, and the posterior parietal lobe (Buhle et al, 2013). In comparison, distraction appears to rely primarily on the dlPFC (Buhle et al, 2013) and effectively modulates short-term negative experiences involving stimuli with high affective intensity (Shafir et al., 2015).
Improvements in affective control capacity appear in late life despite age-related declines in cognitive control capacities (Mather & Carstensen, 2005). This paradox may be explained by several factors, including older adults’ greater self-reported focus on emotion regulation (Lawton, Kleban, Rajagopal, & Dean, 1992), better ability to predict affective arousal (Nielsen, Knutson, & Carstensen, 2008; Urry & Gross, 2010), and a preference for the less cognitively demanding strategy of distraction over cognitive reappraisal (Mather, 2016; Opitz, Rauch, Terry, & Urry, 2012). Older adults typically rate themselves as having better affective control than younger adults and report avoiding affectively maladaptive conflict resolution techniques such as shouting and insult throwing (Birditt & Fingerman, 2005; Gross et al., 1997). Correspondingly, negative mood appears to dissipate more quickly in older than younger adults (Carstensen, Pasupathi, Mayr, & Nesselroade, 2000).
Older adults also exhibit a “positivity bias” when processing affective stimuli such that they show less attention for, and encoding of, negative relative to positive stimuli (Mather, 2016; Mather et al., 2004). For example, older compared to younger adults were faster at identifying the presence of visual stimuli that appeared after a face with a positive versus a negative expression (Mather & Carstensen, 2005). Older adults were also more likely to remember positive compared to negative scenes, whereas younger and middle-aged adults showed no difference in recall for positive versus negative scenes (Charles et al., 2003).
It is theorized that the positivity bias influences the affect stage of decision making based on research supporting an automatic influence of the positivity bias on attention and early affective processing (Mather, 2016). However, the positivity bias in aging also appears to be at work at the integration stage; for example, in the context of evaluating trustworthiness cues in social decisions (Castle et al., 2012; Petrican et al., 2013; Zebrowitz, Ward, Boshyan, Gutchess, & Hadjikhani, 2017). Perceptions and judgments of facial cues are particularly important as they are both automatic and heavily weighted in the decision to trust (Castle et al., 2012; Todorov, Pakrashi, & Oosterhof, 2009; van ‘t Wout & Sanfey, 2008; Winston, Strange, O’Doherty, & Dolan, 2002). There is evidence that older adults rated untrustworthy-looking faces as more trustworthy and approachable than did younger adults (Castle et al., 2012; Zebrowitz et al., 2017). Further, in a directed gaze-following task, older compared to younger adults showed more gaze following when viewing self-rated trustworthy compared to untrustworthy faces (Petrican et al., 2013). Thus, the positivity bias may affect both automatic and goal-directed processing for facial trustworthiness in older adults. Effects of the positivity bias appear to extend to the processing of reputational information as well. In one-shot Trust Games, older adults were more likely than younger adults to invest with trustees who had untrustworthy reputations (Bailey et al., 2015).
One possible mechanism that could contribute to older adults’ positivity bias is their reduced sensitivity to negative subjective arousal cues (i.e., cues that trigger subjective feelings of threat and loss). Activation in the AI has been implicated in negative subjective arousal (Knutson et al., 2014) and was attenuated in older adults during anticipation of losses but not gains (Samanez-Larkin et al., 2007). Further, there is evidence of age-related volume decline and reduced projection strength of AI to frontal regions (Muller, Mérillat, & Jäncke, 2016). The AI generally has been associated with conscious experience of recognizing one’s own and others’ affective states (Gu, Hof, Friston, & Fan, 2013). This functional decline has relevance for perception of facial trustworthiness cues as older compared to younger adults display less activation of the AI in response to untrustworthy faces (Castle et al., 2012). It is currently unclear whether emotion regulation in social decision making involves other interrelated projections of the AI, such as those to the NAc (Leong, Pestilli, Wu, Samanez-Larkin, & Knutson, 2016).
The vmPFC and vlPFC appear to undergo little age-related decline relative to other frontal lobe regions regarding volume and structural connectivity (Fjell & Walhovd, 2010; Mather, 2016). For example, a large-scale meta-analysis found that cortical thickness in the vmPFC and vlPFC was uncorrelated with age, whereas cortical thickness in other frontal areas was highly negatively correlated with age (Fjell & Walhovd, 2010). Damage to the vmPFC and vlPFC is associated with loss of impulse control, anger, violent outbursts, and deficits in attachment processes—behaviors that are not generally present in healthy aging (Charles & Carstensen, 2009; Chopik, Edelstein, & Fraley, 2013; Damasio et al., 1990; Grafman et al., 1996). Also, there is evidence that both vmPFC and vlPFC exert a regulatory effect on AI activation in response to cues related to untrustworthiness such as in unfair monetary divisions in the Ultimatum Game (Tabibnia et al., 2008). These findings suggest a possible neurological mechanism for older adults’ apparent surfeit of control over negative affective impulses: the functional decline of the AI and its projections to the vmPFC and vlPFC as well as the functional stability of these frontal regions across adulthood.
Taken together, this research suggests the possibility that age-related changes in emotion regulation processes and associated neural correlates may predispose older adults to affectively “cooler” reactions to cues of untrustworthiness. This may for example result in a more positively biased valuation by older adults of social partners who display untrustworthiness.
7 |. AGE-RELATED CHANGES IN MEMORY FOR PAST EXPERIENCE
Memory for past experience with social partners can strongly influence social decision making. For example, someone might hesitate to loan a friend money if this friend failed to pay back a previous loan. Despite the breadth of possible influence on social decision making, there is surprisingly little work directly assessing effects of previous experience and knowledge about social partners on the social decision-making process. In one of the few existing studies, partners who violated initial expectations of trustworthiness in a reciprocal Trust Game were more memorable than those who simply did not reciprocate in a subsequent memory test (Chang, 2009). These finding suggest a role for memory processes in forming and maintaining reciprocal trusting relationships.
In social decisions, older adults are likely to have more experience from which to draw given their longer lifespan. Explicit memory for facts and knowledge (semantic memory) is well maintained with age (Hoyer & Verhaeghen, 2006), allowing older adults to perform as well, or even better, than younger adults on decision tasks that benefit from semantic memory (Mata et al., 2012). Research on financial and health-related decision contexts support these age-related benefits for accumulated knowledge (Agarwal et al., 2009; Li et al., 2015; Löckenhoff & Carstensen, 2007), but little work has directly examined how semantic memory affects social decision making.
In contrast to semantic memory, episodic memory declines significantly with age (Hoyer & Verhaeghen, 2006; Maillet & Rajah, 2014). Studies examining age-related deficits in episodic memory have consistently found evidence of older adults’ under-recruitment in brain regions associated with successful encoding in younger adults, such as the bilateral occipital cortex and bilateral parahippocampal gyrus (Gutchess et al., 2005; Maillet & Rajah, 2014). Many of these same studies on age-related deficits in episodic memory have also found evidence of age-related over-recruitment in the PFC during encoding (Gutchess et al., 2005; Maillet & Rajah, 2014). This PFC over-recruitment may reflect age-related compensation for the under-recruitment in more posterior brain regions (Davis, Dennis, Daselaar, Fleck, & Cabeza, May 1, 2008; Dennis & Cabeza, 2008; Gutchess et al., 2005) and may constitute an age-related shift in focus from perceptual details to personal thoughts and feelings during encoding (Maillet & Rajah, 2014).
In addition to deficits in encoding episodic memories, there is evidence of age-related deficits in the explicit recollection of episodic and other types of memories (Bailey, Zacks, et al., 2013; Maillet & Rajah, 2013). This may be due to age-related declines in the dlPFC and the MTL which are both involved in the retrieval of previous events (Bailey, Zacks, et al., 2013; Maillet & Rajah, 2013). Additionally, older adults’ positivity bias can extend to past choices because of poorer recollection, memory encoding, and executive control in aging (Mather & Johnson, 2000, 2003). Recent findings also indicate that older adults display deficits in error awareness reflected by both autonomic response (i.e., error related pupil dilation) and self-report during tasks (Wessel, Dolan, & Hollingworth, 2018). There is also evidence of older adults being unable to remember previous mistakes from a study on spear phishing (i.e., targeted email scams), in which younger and older Internet users were exposed to simulated spear-phishing emails and their susceptibility to fall for these scams was assessed (Oliveira et al., 2017). Results from this study showed that many younger and older adults were susceptible to fall for the spear-phishing attacks, with no significant age difference in this effect. However, older adults were significantly less aware than younger adults of their high susceptibility to these deceptive attacks (Oliveira et al., 2017). As a net result, older adults may have less accurate episodic memory, feel more positively about previous choices, and are unaware of errors that lead to negative consequences, which in turn reduces their ability to learn from past mistakes (Del Missier et al., 2013).
Implicit forms of memory are generally well preserved in normal aging (Hoyer & Verhaeghen, 2006). Likely, as a result, intuitive social decision-making abilities remain relatively intact in older adults (Peters, Hess, Västfjäll, Auman, & Vastfjall, 2007; Queen & Hess, 2010). Due to the links between implicit memory processes and judgments utilizing intuition, previous work has referred to intuition as the subjective experience of using knowledge gained via implicit learning (Lieberman, 2000). Reliance on intuitive judgments, however, can lead older adults to become susceptible to framing effects. These framing effects constitute shifts in preferences for acceptable amounts of risk depending on if a given decision is phrased in terms of gains (less risk preferred) versus losses (more risk preferred; Kim, Goldstein, Hasher, & Zacks, 2005; but see also Reyna & Brainerd, 2011; Rönnlund, Karlsson, Laggnäs, Larsson, & Lindström, 2005). Reliance on intuition may also be a liability when decision contexts become more difficult to navigate such as when optimal social decision making relies on integrating multiple pieces of information (Mata et al., 2012).
8 |. SYNTHESIS OF AGE-RELATED CHANGES IN THE THREE INTEGRATIVE PROCESSES
From synthesizing the interdisciplinary research on the three integration processes which comprise CISDA, we draw the following conclusions. Older adults’ cognitive theory of mind abilities and the integrity of neurological correlates associated with cognitive theory of mind decline with age. Affective theory of mind abilities and their neurological correlates, in contrast, remain stable. Furthermore, emotion-regulation abilities improve in older adults due to a mixture of stability and decline in the neurological correlates of negative subjective arousal, development of a positivity bias, and a greater focus on emotional control. Finally, older adults can benefit from having accumulated knowledge and experience to draw from, both directly and indirectly via intuition. However, declines in the neurological correlates of episodic memory and explicit recollection leave older adults increasingly vulnerable, especially in decision contexts that are less familiar to them and more difficult to navigate.
We acknowledge that the specific ways in which these age-related changes in integrative processes will affect social decision making in older adults also depend on the nature of the decision. Broadly, however, CISDA predicts that older adults will perform better when a social decision involves accurately inferring others’ affective state, keeping affectively cool, having applicable experience, and/or when the decision context is relatively simple. In contrast, CISDA predicts that older adults will perform worse when a social decision involves accurately inferring what others are thinking, identifying and avoiding threat or loss, being in an unfamiliar situation, learning from the past, and/or when the decision context is highly complex.
9 |. REMAINING CHALLENGES FOR CISDA IN IMPROVING OUR UNDERSTANDING OF SOCIAL DECISION MAKING IN AGING
To conclude our proposal of CISDA, we will reflect on the remaining challenges that CISDA faces in theory refinement and generation of knowledge about social decision making in aging.
In this article, we focused on theory of mind, emotion regulation, and memory for past experience. This emphasis is not meant to imply that these three processes are the only integration functions relevant in social decision making. Rather, our goal in this review paper was to highlight the wealth of findings on specifically age-related changes in integration functions and consider the implications of such changes on social decision making in late life. We acknowledge that there are other integrative processes (e.g., subjective valuation across choice options). These processes, however, do not seem to be affected by aging (Seaman et al., 2018) and thus were less informative for our reflection about social decision making and aging in the context of CISDA.
Some of the brain regions involved in the three integration functions specified in CISDA are shared between processes, and it is currently unknown to what extent these neurologically overlapping mechanisms are interacting. For example, the vmPFC both converts choice options into a neural common currency (Levy & Glimcher, 2012) and is implicated in affective theory of mind ability (Shamay-Tsoory, 2011). Also, functioning in the dlPFC is implicated in comparing values across choice options (Alexander & Brown, 2012), the emotion-regulation strategy of distraction (Buhle et al., 2013), and memory for past experience (Maillet & Rajah, 2013). Further, the MTL is implicated in conjunction with both the vmPFC (in affective theory of mind; Shamay-Tsoory, 2011) and the dlPFC in memory for past experience (Maillet & Rajah, 2013). While it has been shown that older adults’ decision-making ability declines when they must integrate multiple pieces of information (Mata et al., 2012), it is currently not known yet to what degree these overlapping neurological processes interfere with or facilitate one another. Future research determining these interactions will further inform CISDA.
In this article, we mention the “affect” or the “motivation” stages of AIM, but the focus is on the “integration” stage. There is a large body of research on age-related changes in the affective stage, coverage of which was beyond the scope of our paper (see Mather, 2016; for an extensive review). Also, determining how physical decision parameters, such as the amount of physical effort required to enact a choice or whether the choice affects oneself or another person, influence social decision making (e.g., quality, enaction and/or inaction, etc.) in older adults constitutes a fruitful future research avenue that will allow further specification of the affective and motivational stages of AIM. However, this line of inquiry was not possible in the context of the current focused review.
Interindividual and contextual factors impact social decision making in aging but are currently not considered in CISDA. Influential interindividual characteristics include risk preferences, general intelligence (Frey, Pedroni, Mata, Rieskamp, & Hertwig, 2017), wisdom (Lim & Yu, 2015), hormone activity (Ebner, Kamin, Diaz, Cohen, & MacDonald, 2015), and sensory processes (Marsiske, Klumb, & Baltes, 1997). Contextual factors of relevance in this context are culture (Rieger & Mata, 2013), socioeconomic status (Webb et al., 2016), stress exposure (Birn, Roeber, & Pollak, 2017), and cohort effects (Mayhorn, Fisk, & Whittle, 2002). A comprehensive coverage of all these additional factors that impact the social decision-making process in aging was too extensive for the current paper. Finally, the select focus and brevity of this review also did not permit a full description of the large body of research on the aging brain (for review see Fjell & Walhovd, 2010, Mather, 2016, & Samanez-Larkin & Knutson, 2015) in its effects on social decision making.
10 |. POTENTIAL APPLICATIONS OF CISDA
We conclude this theoretical paper with a brief discussion of potential applications of CISDA. In this context, we formulate specific predictions that can direct future research toward enhanced understanding of social decision making in aging.
With respect to theory of mind, CISDA contends that reasoning about thoughts and feelings of others is one major aspect of social decision making that differentiates it from other forms of decision making. Given greater age-related decline in cognitive relative to affective theory of mind abilities (Beadle et al., 2012) and their structural correlates (Kalbe et al., 2010; Li et al., 2014; Shamay-Tsoory, 2011), future research should directly compare how cognitive and affective theory of mind impact older adults’ social decisions and determine the extent to which these components may interact during decision processing. CISDA specifically proposes that (a) aging will be associated with poorer performance in social decision contexts where cognitive theory of mind ability leads to better quality decisions; (b) older adults will perform similar to younger adults in social decision contexts where affective theory of mind ability leads to better quality decisions (see Figure 2a); (c) older adults will rely more on affective than cognitive theory of mind in social decision making; and (d) the underuse of cognitive theory of mind in older adults will be mediated by age-related decline in the dmPFC and white matter connections between the dmPFC and the TPJ.
Regarding emotion regulation, CISDA predicts that older adults possess a surfeit of emotion-regulation ability (Birditt & Fingerman, 2005; Carstensen et al., 2000; Mather, 2016), which leads to relatively better social decision-making quality when control of negative affective impulses is required. However, age-related decline in negative subjective arousal (Samanez-Larkin et al., 2007) may lead to worse social decision making when untrustworthy individuals attempt to exploit older adults. Functional declines in the AI and its frontal projections have been identified as neural substrates associated with reduced negative subjective arousal in aging (Muller et al., 2016). Accordingly, CISDA hypothesizes that (a) older adults will perform better in social decision making contexts where controlling negative affective impulses leads to better decision-making quality; (b) older adults will perform worse in social decision making contexts where negative subjective arousal is required for optimal decision making, such as decisions that require detection of untrustworthiness and deceitfulness in others (see Figure 2b); (c) age-related greater emotional control ability and negative subjective arousal decline will be mediated in part by age-related decline of the AI and its projections to the vmPFC and vlPFC; (d) age-related greater emotional control ability and negative subjective arousal decline will also be mediated in part by age-related functional stability of the vmPFC and vlPFC and their projections to the AI.
Pertaining to memory for past experience, CISDA predicts that age-related accumulation of experience and knowledge will lead to better social decision quality when performance relies on semantic and/or implicit memory. However, age-related declines in episodic memory, recollection, and working memory will lead to worse social decision quality when performance relies on accurate recollection, learning from past mistakes, or integration of multiple pieces of information. Correspondingly, CISDA hypothesizes that (a) older adults will perform better in social decision contexts that rely on semantic and implicit memory ability, such as drawing on familiar social norms and knowledge; (b) older adults will perform worse in social decision making contexts that rely on working and episodic memory ability, such as learning from recent or unfamiliar social experiences and navigating multiple options (see Figure 2c); and (c) age-related declines in the dlPFC, MTL, and projections between these structures will mediate older adults’ ability to adaptively react to unfamiliar and/or complex social decision-making contexts, especially in response to making errors.
The application of CISDA to future research endeavors has great potential to spur theory refinement and promote empirical characterization of social decision-making processes in aging. Research hypotheses derived from CISDA can also inform practical applications including addressing vulnerability to fraud and exploitation among older adults. Also, CISDA can be applied to examining decisions to trust in contexts such as advertising, news media, or phone or email solicitations. In this way, CISDA holds relevance for policy, products, and protecting older adults from targeted scams.
11 |. CONCLUSION
CISDA’s proposition of three key integrative components central in social decision making in late life fills a theoretical gap in the literature and compliments a growing body of work that argues against the notion that older adults suffer from uniformly decreasing decision-making abilities. Informed by interdisciplinary research on economic decision making, cognitive neuroscience, and lifespan development, CISDA outlines specific age-related changes in theory of mind, emotion regulation, and memory for past experience that affect the integration of choice valuation and how such changes can impact older adults’ decision making in social contexts. CISDA aims to stimulate further theory development and empirical work on applications that can optimize the decision-making process and align older adults’ social decision-making tendencies with their goals of wellbeing and independence.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
REFERENCES
- Agarwal S, Driscoll JC, Gabaix X, & Laibson DI (2009). The age of reason: Financial decisions over the life-cycle with implications for regulation. Brookings Papers on Economic Activity, 40, 51–117. 10.1353/eca.0.0067 [DOI] [Google Scholar]
- Alexander WH, & Brown JW (2012). NIH public access. Brain, 14(10), 1338–1344. 10.1038/nn.2921.Medial [DOI] [Google Scholar]
- Bailey HR, Zacks JM, Hambrick DZ, Zacks RT, Head D, Kurby CA, & Sargent JQ (2013). Medial temporal lobe volume predicts elders’ everyday memory. Psychological Science, 24(7), 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey PE, & Henry JD (2008). Growing less empathic with age: Disinhibition of the self-perspective. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 63(4), P219–P226. [DOI] [PubMed] [Google Scholar]
- Bailey PE, Ruffman T, & Rendell PG (2013). Age-related differences in social economic decision making: The ultimatum game. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 68(3), 356–363. 10.1093/geronb/gbs073 [DOI] [PubMed] [Google Scholar]
- Bailey PE, Slessor G, Rieger M, Rendell PG, Moustafa AA, & Ruffman T (2015). Trust and trustworthiness in young and older adults. Psychology and Aging, 30(4), 977–986. [DOI] [PubMed] [Google Scholar]
- Beadle JN, Paradiso S, Kovach C, Polgreen L, Denburg NL, & Tranel D (2012). Effects of age-related differences in empathy on social economic decision-making. International Psychogeriatrics, 24(5), 822–833. 10.1017/S1041610211002547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle JN, Sheehan AH, Dahlben B, & Gutchess AH (2013). Aging, empathy, and prosociality. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 70(2), 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birditt KS, & Fingerman KL (2005). Do we get better at picking our battles? Age group differences in descriptions of behavioral reactions to interpersonal tensions. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60, 121–128. [DOI] [PubMed] [Google Scholar]
- Birn RM, Roeber BJ, & Pollak SD (2017). Early childhood stress exposure, reward pathways, and adult decision making. Proceedings of the National Academy of Sciences, 114(51), 13549–13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottiroli S, Cavallini E, Ceccato I, Vecchi T, & Lecce S (2016). Theory of mind in aging: Comparing cognitive and affective components in the faux pas test. Archives of Gerontology and Geriatrics, 62, 152–162. 10.1016/j.archger.2015.09.009 [DOI] [PubMed] [Google Scholar]
- Braunstein LM, Gross JJ, & Ochsner KN (2017). Explicit and implicit emotion regulation: A multi-level framework. Social Cognitive and Affective Neuroscience, 12(10), 1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswik E (1939). The conceptual focus of some psychological systems. The Journal of Unified Science, 8(1), 36–49. [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Ochsner KN (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, & Nesselroade JR (2000). Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology, 79(4), 644–655. [PubMed] [Google Scholar]
- Cassidy BS, Hedden T, Yoon C, & Gutchess AH (2014). Age differences in medial prefrontal activity for subsequent memory of truth value. Frontiers in Psychology, 5(February), 87. 10.3389/fpsyg.2014.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E, Eisenberger NI, Seeman TE, Moons WG, Boggero IA, Grinblatt MS, & Taylor SE (2012). Neural and behavioral bases of age differences in perceptions of trust. Proceedings of the National Academy of Sciences, 109(51), 20848–20852. 10.1073/pnas.1218518109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ (2009). Unforgettable ultimatums? Expectation violations promote enhanced social memory following economic bargaining. Frontiers in Behavioral Neuroscience, 3, 1–12. 10.3389/neuro.08.036.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, & Carstensen LL (2009). NIH public access. Psychology, 23(3), 495–504. 10.1037/a0013284.Unpleasant [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, & Carstensen LL (2003). Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General, 132, 310–324. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, Markus HS, & Morris RG (2009). Theory of mind associations with other cognitive functions and brain imaging in normal aging. Psychology and Aging, 24(2), 338–348. 10.1037/a0015225 [DOI] [PubMed] [Google Scholar]
- Chopik WJ, Edelstein RS, & Fraley RC (2013). From the cradle to the grave: Age differences in attachment from early adulthood to old age. Journal of Personality, 81(2), 171–183. 10.1111/j.1467-6494.2012.00793.x [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, & Damasio H (1990). Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research, 41(2), 81–94. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, & Cabeza R (May 1, 2008). Qué PASA? The posterior–anterior shift in aging. Cerebral Cortex, 18(5), 1201–1209. 10.1093/cercor/bhm155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Missier F, Mäntylä T, Hansson P, Bruine de Bruin W, Parker AM, & Nilsson L-G (2013). The multifold relationship between memory and decision making: An individual-differences study. Journal of Experimental Psychology. Learning, Memory, and Cognition, 39, 1344–1364. 10.1037/a0032379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Frank RH, & Phelps EA (2005). Perceptions of moral character modulate the neural systems of reward during the trust game. Nature Neuroscience, 8(11), 1611–1618. 10.1038/nn1575 [DOI] [PubMed] [Google Scholar]
- Dennis NA, & Cabeza R (2008). Neuroimaging of healthy cognitive aging. In The handbook of aging and cognition (Vol. 3, pp. 1–54). New York, NY: Psychology Press. [Google Scholar]
- Ebner NC, Bailey PE, Horta M, Joiner J, & Chang SWC (2016). Multidisciplinary perspective on prosociality in aging. In Sommerville JA & Decety J (Eds.), Social cognition, for the Frontiers in developmental science series (pp. 303–325). New York, NY: Psychology Press, Taylor and Francis Group. [Google Scholar]
- Ebner NC, Ellis DM, Lin T, Rocha HA, Yang H, Dommaraju S, … Oliveira DS (2018). Uncovering susceptibility risk to online deception in aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, gby036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Freund AM, & Baltes PB (2006). Developmental changes in personal goal orientation from young to late adulthood: From striving for gains to maintenance and prevention of losses. Psychology and Aging, 21, 664–678. 10.1037/0882-7974.21.4.664 [DOI] [PubMed] [Google Scholar]
- Ebner NC, Kamin H, Diaz V, Cohen RA, & MacDonald K (2015). Hormones as “difference makers” in cognitive and socioemotional aging processes. Frontiers in Psychology, 5, 1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, & Spinrad TL (2004). Emotion-related regulation: Sharpening the definition. Child Development, 75(2), 334–339. [DOI] [PubMed] [Google Scholar]
- Engel C (2011). Dictator games: A meta study. Experimental Economics, 14(4), 583–610. [Google Scholar]
- Fjell AM, & Walhovd KB (2010). Structural brain changes in aging: Courses, causes and cognitive consequences. Reviews in the Neurosciences, 21, 187–221. [DOI] [PubMed] [Google Scholar]
- Frey R, Pedroni A, Mata R, Rieskamp J, & Hertwig R (2017). Risk preference shares the psychometric structure of major psychological traits. Science Advances, 3(10), e1701381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizzi MM, & Navarro-Martínez D (2018). On the external validity of social preference games: A systematic lab-field study. Management Science. [Google Scholar]
- Girardi A, Sala SD, & MacPherson SE (2018). Theory of mind and the ultimatum game in healthy adult aging. Experimental Aging Research, 44(3), 246–257. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown H, & Salazar A (1996). Frontal lobe injuries, violence, and aggression: A report of the Vietnam Head Injury Study. Neurology, 46, 1231–1238. [DOI] [PubMed] [Google Scholar]
- Gross JJ (1998). Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74, 224–237. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Götestam Skorpen C, & Hsu AY (1997). Emotion and aging: Experience, expression, and control. Psychology and Aging, 12(4), 590–599. [DOI] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, & Fan J (2013). Anterior insular cortex and emotional awareness. Journal of Comparative Neurology, 521(15), 3371–3388. 10.1002/cne.23368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, & Park DC (2005). Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial-temporal activity. Journal of Cognitive Neuroscience, 17(1), 84–96. [DOI] [PubMed] [Google Scholar]
- Harlé KM, & Sanfey AG (2012). Social economic decision-making across the lifespan: An fMRI investigation. Neuropsychologia, 50(7), 1416–1424. 10.1016/j.neuropsychologia.2012.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, Ruffman T, & Bailey PE (2013). A meta-analytic review of age differences in theory of mind. Psychology and Aging, 28(3), 826–839. 10.1037/a0030677 [DOI] [PubMed] [Google Scholar]
- Hoyer WJ, & Verhaeghen P (2006). Memory aging. In Handbook of the psychology of aging (6th ed., pp. 209–232). [Google Scholar]
- Huang Y, & Lawitz A (2016). The New York state cost of financial exploitation study. New York State Office of Family and Child Services. Retrieved from https://ocfs.ny.gov/main/reports/Cost%20of%20Financial%20Exploitation%20Study%20FINAL%20May%202016.pdf [Google Scholar]
- Johansson Nolaker E, Murray K, Happé F, & Charlton RA (2018). Cognitive and affective associations with an ecologically valid test of theory of mind across the lifespan. Neuropsychology, 32, 754–763. [DOI] [PubMed] [Google Scholar]
- Josef AK, Richter D, Samanez-Larkin GR, Wagner GG, Hertwig R, & Mata R (2016). Stability and change in risk-taking propensity across the adult life span. Journal of Personality and Social Psychology, 111(3), 430–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe E, Schlegel M, Sack AT, Nowak DA, Dafotakis M, Bangard C, … Kessler J (2010). Dissociating cognitive from affective theory of mind: A TMS study. Cortex, 46(6), 769–780. [DOI] [PubMed] [Google Scholar]
- Kim S, Goldstein D, Hasher L, & Zacks RT (2005). Framing effects in younger and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60(4), 215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Katovich K, & Suri G (2014). Inferring affect from fMRI data. Trends in Cognitive Sciences, 18(8), 422–428. 10.1016/j.tics.2014.04.006 [DOI] [PubMed] [Google Scholar]
- Knutson B, Scott R, Wimmer GE, Prelec D, & Loewenstein G (2007). Neural predictors of energy-efficient purchases. Neuron, 53(1), 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachs MS, & Han SD (2015). Age-associated financial vulnerability: An emerging public health issue. Annals of Internal Medicine, 163(11), 877–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang FR, & Carstensen LL (2002). Time counts: Future time perspective, goals, and social relationships. Psychology and Aging, 17(1), 125–139. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Kleban MH, Rajagopal D, & Dean J (1992). Dimensions of affective experience in three age groups. Psychology and Aging, 7(2), 171–184. [DOI] [PubMed] [Google Scholar]
- Lecce S, Ceccato I, Bianco F, Rosi A, Bottiroli S, & Cavallini E (2017). Theory of mind and social relationships in older adults: The role of social motivation. Aging & Mental Health, 21(3), 253–258. [DOI] [PubMed] [Google Scholar]
- Lecce S, Ceccato I, & Cavallini E (2018). Investigating theory of mind in aging with the MASC: From accuracy to error type. Aging, Neuropsychology, and Cognition, 1–17. [DOI] [PubMed] [Google Scholar]
- Leong JK, Pestilli F, Wu CC, Samanez-Larkin GR, & Knutson B (2016). White-matter tract connecting anterior insula to nucleus accumbens correlates with reduced preference for positively skewed gambles. Neuron, 89(1), 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, & Glimcher PW (2012). The root of all value: A neural common currency for choice. Current Opinion in Neurobiology, 22(6), 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, & Tsang H-LV (2016). Age moderates the relationship between generativity concern and understanding of wealth. Current Aging Science, 9(3), 210–216. [DOI] [PubMed] [Google Scholar]
- Li W, Mai X, & Liu C (2014). The default mode network and social understanding of others: What do brain connectivity studies tell us. Frontiers in Human Neuroscience, 8, 1–15. 10.3389/fnhum.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao J, Enkavi AZ, Zaval L, Weber EU, & Johnson EJ (2015). Sound credit scores and financial decisions despite cognitive aging. Proceedings of the National Academy of Sciences of the United States of America, 112(1), 65–69. 10.1073/pnas.1413570112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD (2000). Intuition: A social cognitive neuroscience approach. Psychological Bulletin, 126(1), 109–137. 10.1037//0033-2909.126.1.109 [DOI] [PubMed] [Google Scholar]
- Lim KTK, & Yu R (2015). Aging and wisdom: Age-related changes in economic and social decision making. Frontiers in Aging Neuroscience, 7, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löckenhoff CE, & Carstensen LL (2007). Aging, emotion, and health-related decision strategies: Motivational manipulations can reduce age differences. Psychology and Aging, 22(1), 134–146. 10.1037/0882-7974.22.1.134 [DOI] [PubMed] [Google Scholar]
- Maillet D, & Rajah MN (2013). Association between prefrontal activity and volume change in prefrontal and medial temporal lobes in aging and dementia: A review. Ageing Research Reviews, 12(2), 479–489. [DOI] [PubMed] [Google Scholar]
- Maillet D, & Rajah MN (2014). Age-related differences in brain activity in the subsequent memory paradigm: A meta-analysis. Neuroscience & Biobehavioral Reviews, 45, 246–257. [DOI] [PubMed] [Google Scholar]
- Marsiske M, Klumb P, & Baltes MM (1997). Everyday activity patterns and sensory functioning in old age. Psychology and Aging, 12(3), 444–457. [DOI] [PubMed] [Google Scholar]
- Mata R, Pachur T, Von Helversen B, Hertwig R, Rieskamp J, & Schooler L (2012). Ecological rationality: A framework for understanding and aiding the aging decision maker. Frontiers in Neuroscience, 6, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M (2016). The affective neuroscience of aging. Annual Review of Psychology, 67(1), 213–238. 10.1146/annurev-psych-122414-033540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, … Carstensen LL (2004). Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science, 15(4), 259–263. [DOI] [PubMed] [Google Scholar]
- Mather M, & Carstensen LL (2005). Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences, 9(10), 496–502. 10.1016/j.tics.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Mather M, & Johnson MK (2000). Choice-supportive source monitoring: Do our decisions seem better to us as we age? Psychology and Aging, 15(4), 596–606. [DOI] [PubMed] [Google Scholar]
- Mather M, & Johnson MK (2003). Affective review and schema reliance in memory in older and younger adults. American Journal of Psychology, 116, 169–189. [PubMed] [Google Scholar]
- Mayhorn CB, Fisk AD, & Whittle JD (2002). Decisions, decisions: Analysis of age, cohort, and time of testing on framing of risky decision options. Human Factors, 44(4), 515–521. [DOI] [PubMed] [Google Scholar]
- MetLife. (2011). The MetLife study of elder financial abuse: Crimes of occasion, desperation, and predation against America’s elders.
- Moran JM (2013). Lifespan development: The effects of typical aging on theory of mind. Behavioural Brain Research, 237, 32–40. [DOI] [PubMed] [Google Scholar]
- Moran JM, Jolly E, & Mitchell JP (2012). Social-cognitive deficits in Normal aging. Journal of Neuroscience, 32(16), 5553–5561. 10.1523/JNEUROSCI.5511-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AM, Mérillat S, & Jäncke L (2016). Small changes, but huge impact? The right anterior insula’s loss of connection strength during the transition of old to very old age. Frontiers in Aging Neuroscience, 8, 86. 10.3389/fnagi.2016.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L, Knutson B, & Carstensen LL (2008). Affect dynamics, affective forecasting, and aging. Emotion, 8(3), 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L, & Mather M (2011). Emerging perspectives in social neuroscience and neuroeconomics of aging. Social Cognitive and Affective Neuroscience, 6(2), 149–164. 10.1093/scan/nsr019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira D, Rocha H, Yang H, Ellis D, Dommaraju S, Muradoglu M, … Ebner NC (2017). Dissecting spear phishing emails for older vs young adults: On the interplay of weapons of influence and life domains in predicting susceptibility to phishing. CHI’17: CHI Conference Proceedings on Human Factors in Computing Systems (pp. 6412–6424). 10.1145/10.1145/3025453.3025831 [DOI] [Google Scholar]
- Opitz PC, Rauch LC, Terry DP, & Urry HL (2012). Prefrontal mediation of age differences in cognitive reappraisal. Neurobiology of Aging, 33(4), 645–655. [DOI] [PubMed] [Google Scholar]
- Palminteri S, Justo D, Jauffret C, Pavlicek B, Dauta A, Delmaire C, & Pessiglione M (2012). Critical roles for anterior insula and dorsal striatum in punishment-based Avoidance learning. Neuron, 76(5), 998–1009. 10.1016/j.neuron.2012.10.017 [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, & Frith CD (2006). Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature, 442(7106), 1042–1045. 10.1038/nature05051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E, Hess TM, Västfjäll D, Auman C, & Vastfjall D (2007). Adult age differences information in dual information processes in older adults decision making. Perspectives on Psychological Science, 2(1), 1–23. 10.1111/j.1745-6916.2007.00025.x [DOI] [PubMed] [Google Scholar]
- Peterson JC, Burnes DP, Caccamise PL, Mason A, Henderson CR, Wells MT, … Powell M (2014). Financial exploitation of older adults: A population-based prevalence study. Journal of General Internal Medicine, 29(12), 1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrican R, English T, Gross JJ, Grady C, Hai T, & Moscovitch M (2013). Friend or foe? Age moderates time-course specific responsiveness to trustworthiness cues. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 68(2), 215–223. [DOI] [PubMed] [Google Scholar]
- Pew Research Center. (2014). Global Population Estimates by Age, 1950–2050. Retrieved from http://www.pewglobal.org/2014/01/30/global-population/
- Premack D, & Woodruff G (1978). Premack and Woodruff: Chimpanzee theory of mind. Behavioral and Brain Sciences, 4, 515–526. [Google Scholar]
- Queen TL, & Hess TM (2010). Age differences in the effects of conscious and unconscious thought in decision making. Psychology and Aging, 25(2), 251–261. 10.1037/a0018856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna VF, & Brainerd CJ (2011). Dual processes in decision making and developmental neuroscience: A fuzzy-trace model. Developmental Review, 31(2–3), 180–206. 10.1016/j.dr.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger M, & Mata R (2013). On the generality of age differences in social and nonsocial decision making. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 70(2), 200–212. [DOI] [PubMed] [Google Scholar]
- Rilling JK, & Sanfey AG (2011). The neuroscience of social decision-making. Annual Review of Psychology, 62, 23–48. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Mitchell SH, Harbaugh WT, & Janowsky JS (2012). Risk, reward, and economic decision making in aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 67(3), 289–298. 10.1093/geronb/gbr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnlund M, Karlsson E, Laggnäs E, Larsson L, & Lindström T (2005). Risky decision making across three areas of choice: Are younger and older adults differently susceptible to framing effects? Journal of General Psychology, 132, 81–92. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, & Knutson B (2007). Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience, 10(6), 787–791. 10.1038/nn1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, & Knutson B (2015). Decision making in the ageing brain: Changes in affective and motivational circuits. Nature Reviews Neuroscience, 16(5), 278–289. 10.1038/nrn3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey A (2007). Social decision-making: Insights from game theory and neuroscience. Science, 318(5850), 598–602. 10.1126/science.1142996 [DOI] [PubMed] [Google Scholar]
- Scheibe S, & Carstensen LL (2010). Emotional aging: Recent findings and future trends. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 65(2), 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman KL, Brooks N, Karrer TM, Castrellon JJ, Perkins SF, Dang LC, … Samanez-Larkin GR (2018). Subjective value representations during effort, probability and time discounting across adulthood. Social Cognitive and Affective Neuroscience, 13(5), 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafir R, Schwartz N, Blechert J, & Sheppes G (2015). Emotional intensity influences pre-implementation and implementation of distraction and reappraisal. Social Cognitive and Affective Neuroscience, 10(10), 1329–1337. 10.1093/scan/nsv022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG (2011). The neural bases for empathy. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 17(1), 18–24. 10.1177/1073858410379268 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Cassidy BN, Darboh BS, DuPre E, Lockrow AW, Setton R, & Turner GR (2017). Financial exploitation is associated with structural and functional brain differences in healthy older adults. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 72(10), 1365–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter M, & Kocher MG (2007). Trust and trustworthiness across different age groups. Games and Economic Behavior, 59(2), 364–382. [Google Scholar]
- Suzuki A (2016). Persistent reliance on facial appearance among older adults when judging someone’s trustworthiness. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences, 73(4), 573–583. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Satpute AB, & Lieberman MD (2008). The sunny side of fairness. Psychological Science, 19(4), 339–347. 10.1111/j.1467-9280.2008.02091.x [DOI] [PubMed] [Google Scholar]
- Todorov A, Pakrashi M, & Oosterhof NN (2009). Evaluating faces on trustworthiness after minimal time exposure. Social Cognition, 27(6), 813–833. 10.1521/soco.2009.27.6.813 [DOI] [Google Scholar]
- True Link Financial. (2015). The true link report on elder financial abuse 2015. San Francisco, CA: True Link Financial. [Google Scholar]
- Urry HL, & Gross JJ (2010). Emotion regulation in older age. Current Directions in Psychological Science, 19(6), 352–357. [Google Scholar]
- van ‘t Wout M, & Sanfey AG (2008). Friend or foe: The effect of implicit trustworthiness judgments in social decision-making. Cognition, 108, 796–803. [DOI] [PubMed] [Google Scholar]
- von Neumann J, & Morgenstern O (1947). Theory of games and economic behavior (p. 648). Princeton, NJ: Princeton Univ. Press. [Google Scholar]
- Webb B, Hine AC, & Bailey PE (2016). Difficulty in differentiating trustworthiness from untrustworthiness in older age. Developmental Psychology, 52(6), 985–995. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Dolan KA, & Hollingworth A (2018). A blunted phasic autonomic response to errors indexes age-related deficits in error awareness. Neurobiology of Aging, 71, 13–20. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, & Dolan RJ (2002). Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience, 5(3), 277–283. 10.1038/nn816 [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Ward N, Boshyan J, Gutchess A, & Hadjikhani N (2017). Older adults’ neural activation in the reward circuit is sensitive to face trustworthiness. Cognitive, Affective, & Behavioral Neuroscience, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
FURTHER READING
- Hess TM, Strough J, & Löckenhoff C (Eds.). (2015). Aging and decision making: Empirical and applied perspectives. Academic Press. [Google Scholar]


