Abstract
Background:
Transcatheter aortic valve replacement (TAVR) causes coronary artery obstruction in 0.7% of cases, with 40–50% mortality. Bioprosthetic or native aortic scallop intentional laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA) is a procedure to prevent coronary obstruction. Safety and feasibility in a large patient cohort is lacking.
Objectives:
This study sought to determine the safety of the BASILICA procedure.
Methods:
The International BASILICA Registry was a retrospective, multicenter, real-world registry of patients at risk of coronary artery obstruction undergoing BASILICA and TAVR. VARC-2 definitions were used to adjudicate events.
Results:
Between June 2017 and December 2020, 214 patients were included from 25 centers in North America and Europe; 72.8% had bioprosthetic aortic valves and 78.5% underwent solo BASILICA. Leaflet traversal was successful in 94.9% and leaflet laceration in 94.4%. Partial or complete coronary artery obstruction was seen in 4.7%. Procedure success, defined as successful BASILICA traversal and laceration without mortality, coronary obstruction or emergency intervention, was achieved in 86.9%. Thirty-day mortality was 2.8% and stroke was 2.8%, with 0.5% disabling stroke. Thirty-day death and disabling stroke were seen in 3.4%. VARC-2 composite safety was achieved in 82.8%. One-year survival was 83.9%. Outcomes were similar between solo and doppio BASILICA, native and bioprosthetic valves, and with the use of cerebral embolic protection.
Conclusions:
BASILICA is safe, with low reported rates of stroke and death. BASILICA is feasible in the real-world setting, with a high procedure success rate and low rates of coronary artery obstruction.
Keywords: Transcatheter aortic valve replacement, coronary artery obstruction, cerebral embolic protection
CONDENSED ABSTRACT
Bioprosthetic or native aortic scallop intentional laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA) is a procedure to prevent coronary artery obstruction from transcatheter aortic valve replacement. There are no large published studies of BASILICA, which are needed to determine excess risk associated with the procedure. In this 214-patient retrospective analysis, the BASILICA procedure was completed in 94.4% of patients. Both 30-day death and stroke were seen in 2.8%, with disabling stroke in 0.5%. One-year survival was 83.9%. In conclusion, BASILICA is feasible in the real world and is safe, with low rates of death, stroke, and coronary obstruction.
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) therapy displaces the diseased native or bioprosthetic aortic valve leaflets during transcatheter heart valve implantation. In 0.7% of all cases, the displaced diseased aortic leaflets obstruct the coronary arteries, requiring rescue percutaneous coronary intervention or emergency coronary artery bypass grafting(1). The risk is higher for valve-in-valve (ViV) TAVR (2.3%)(2). This is probably an underrepresentation, as many patients are excluded from TAVR because of coronary obstruction risk. If coronary obstruction occurs, then mortality is 40% to 50%(1,2). Bioprosthetic or native aortic scallop intentional laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA) is a transcatheter electrosurgical procedure performed immediately before TAVR in which catheters and guidewires are used to first traverse, then lacerate, the aortic leaflet in front of the threatened coronary artery to preserve coronary perfusion after TAVR and, thereby, prevent coronary obstruction(3,4).
The BASILICA Investigational Device Exemption (IDE) trial demonstrated successful leaflet traversal and laceration in 93% and freedom from coronary obstruction in all 30 subjects, despite the high predicted risk in all(5). At 30 days, there was one death and one major stroke in the same patient (3%), and two non-disabling strokes (10% all stroke). A larger number of patients are required to assess whether there is excess risk associated with BASILICA. Furthermore, the BASILICA IDE trial was performed early in the development of the procedure at four centers. Real-world data using contemporary practices are lacking.
The primary objective of this study was to determine real-world safety of the BASILICA procedure in a multicenter international registry.
METHODS
Study design and oversight
Site-reported data were collected retrospectively. Subjects enrolled in the BASILICA IDE trial were excluded. The outcomes were adjudicated according to the Valve Academic Research Consortium (VARC)-2 definitions by a committee of two interventional cardiologists and one cardiothoracic surgeon (JMK, TR, and JEC). Success was defined per patient, not per leaflet.
The Medstar Washington Hospital Center Institutional Review Board (IRB) approved this registry and communication. Participating sites either obtained local IRB approval or accepted the MedStar IRB exemption. The sample size was not statistically derived. All subjects who underwent BASILICA at the participating institutions between June 2017 and December 2020 were included.
Study endpoints
The primary safety endpoint was death and disabling stroke at 30 days. The primary effectiveness endpoint was procedure success measured at exit from the catheterization laboratory. This was a composite of successful BASILICA traversal and laceration of the intended aortic leaflet(s), absence of procedural mortality, absence of acute life-threatening ostial coronary artery obstruction, and freedom from emergency cardiac surgery or reintervention related to the BASILICA procedure, including freedom from attempted implantation of coronary artery stents to treat TAVR-induced coronary artery obstruction.
Secondary endpoints included VARC-2 30-day safety endpoints (all death, all stroke, life-threatening bleeding, stage 2 or 3 acute kidney injury, coronary obstruction, major vascular complication, and valve dysfunction requiring re-intervention), 1-year survival, periprocedural and spontaneous acute myocardial infarction, pericardial effusion or tamponade, BASILICA device- or procedure-related technical failure (acute embolism, mitral valve injury, traversal into left atrium, coronary artery injury induced by BASILICA, etc.), hemodynamic instability caused by BASILICA before TAVR, TAVR thrombosis on computed tomography (CT) or echocardiography during follow-up, and endocarditis during follow-up.
Statistical analysis
All analyses were based on the intention-to-treat principle, with data from all enrolled patients. Baseline subject and procedural characteristics were summarized as means and standard deviations for continuous variables and counts and percentages for categorical variables. Means were compared using two-sample t-tests. Categorical data were compared using chi-square tests.
RESULTS
Between June 1, 2017, and December 31, 2020, 214 patients from 25 centers in North America and Europe underwent BASILICA and TAVR and were included in the analysis (Central Illustration). The baseline and procedure characteristics are detailed in Table 1; 68.7% were female, and the patients’ mean age was 74.9 years. The setting for BASILICA TAVR was in native aortic valves in 27.2% and failed bioprosthetic valves in 72.8%. The bioprosthetic valve types are listed in Table 2.
Central Illustration:
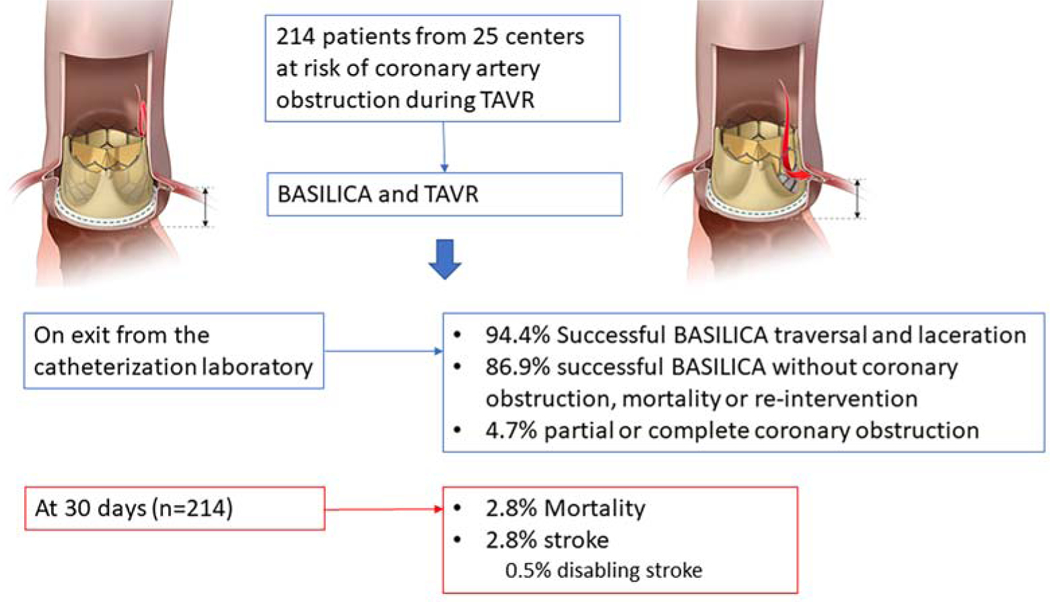
Outcomes from the International BASILICA Registry
Table 1.
Baseline and procedure characteristics
| DEMOGRAPHICS | mean ± SD or % |
|---|---|
| Age, years | 74.9 ± 10.6 |
| Female | 68.7% |
| COMORBIDITIES | |
| STS PROM % | 6.3 ± 5.3 |
| Surgical risk | |
| Low | 4.7% |
| Intermediate | 28.2% |
| High | 54.0% |
| Extreme | 13.1% |
| Aortic lesion | |
| Aortic stenosis | 85.9% |
| Aortic regurgitation | 14.1% |
| Aortic valve | |
| Native | 27.2% |
| Bioprosthetic | 72.8% |
| Prior stroke | 14.6% |
| Prior CABG | 31.6% |
| PROCEDURE | Mean ± SD or % |
| Valve type | |
| Sapien 3 | 60.1% |
| Evolut R/Pro | 39.9% |
| Nominal valve size, mm | 23.5 ± 2.3 |
| Access for TAVR | |
| Transfemoral | 91.1% |
| Transcaval | 7.0% |
| Subclavian/Axillary | 0.9% |
| Carotid | 0.9% |
| Target cusp | |
| Left solo | 68.7% |
| Right solo | 9.8% |
| Doppio | 21.5% |
| Sentinel cerebral protection | 47.7% |
Table 2.
Bioprosthetic valve types
| Bioprosthetic valve | n | Valve-related risk of obstruction (independent of aortic root anatomy) |
|---|---|---|
| CE Magna/Perimount | 64 | Moderate |
| Trifecta | 34 | High |
| Mitroflow | 32 | High |
| Freestyle | 9 | High |
| Mosaic | 7 | Low |
| Epic | 3 | Low |
| CoreValve | 2 | High |
| Homograft | 2 | High |
| Lotus | 1 | Moderate |
| Sorin Freedom Solo | 1 | High |
| Hancock | 1 | Low |
TAVR was performed via the transfemoral access in 91.1%. Of the transcatheter heart valves deployed, 60.1% were balloon-expandable valves (Sapien XT, Sapien 3, Sapien 3 Ultra, Edwards Lifesciences, Irvine, CA) and 39.9% were self-expanding valves (Evolut R, Evolut Pro, Evolut Pro Plus, Medtronic, Minneapolis, MN), with smaller valve sizes predominating. Solo BASILICA was attempted in 78.5% and doppio BASILICA in 21.5%. Sentinel cerebral protection was used in 47.7%.
Procedure outcomes are detailed in Table 3. Leaflet traversal was successful in 94.9% of all patients and leaflet laceration in 94.4% (Supplemental Table 1). Overall composite of procedure success was achieved in 86.9%. Figure 1 illustrates a representative doppio BASILICA procedure in a patient at high risk of coronary artery obstruction.
Table 3.
Procedure outcomes
| Primary Efficacy Endpoint (exit from catheter laboratory) | % |
|---|---|
| Successful BASILICA traversal | 94.9% |
| Successful BASILICA laceration | 94.4% |
| Freedom from culprit coronary obstruction | 95.3% |
| Survival | 100% |
| Freedom from emergency surgery or reintervention | 93.0% |
| Procedure success (all of above) | 86.9% |
| Primary Safety Endpoint (30 days) | |
| Death or Disabling stroke | 3.4% |
| VARC-2 Safety Endpoints (30 days) | |
| Death | 2.8% |
| All stroke Disabling Non-disabling |
2.8% 0.5% 2.4% |
| Life threatening bleeding | 3.3% |
| Major vascular complication | 3.8% |
| AKI stage 2/3 | 4.3% |
| Coronary artery obstruction (including nonculprit) | 5.7% |
| Valve-related dysfunction requiring repeat procedure | 1.4% |
| VARC-2 Early Safety (all of above) | 82.8% |
| SECONDARY ENPOINTS | |
| Periprocedural myocardial infarction | 3.3% |
| Pericardial effusion or cardiac tamponade | 0.5% |
| Hypotension requiring pressors during index procedure | 8.5% |
| Endocarditis | 1.4% |
| 1-year survival | 83.9% |
Figure 1:
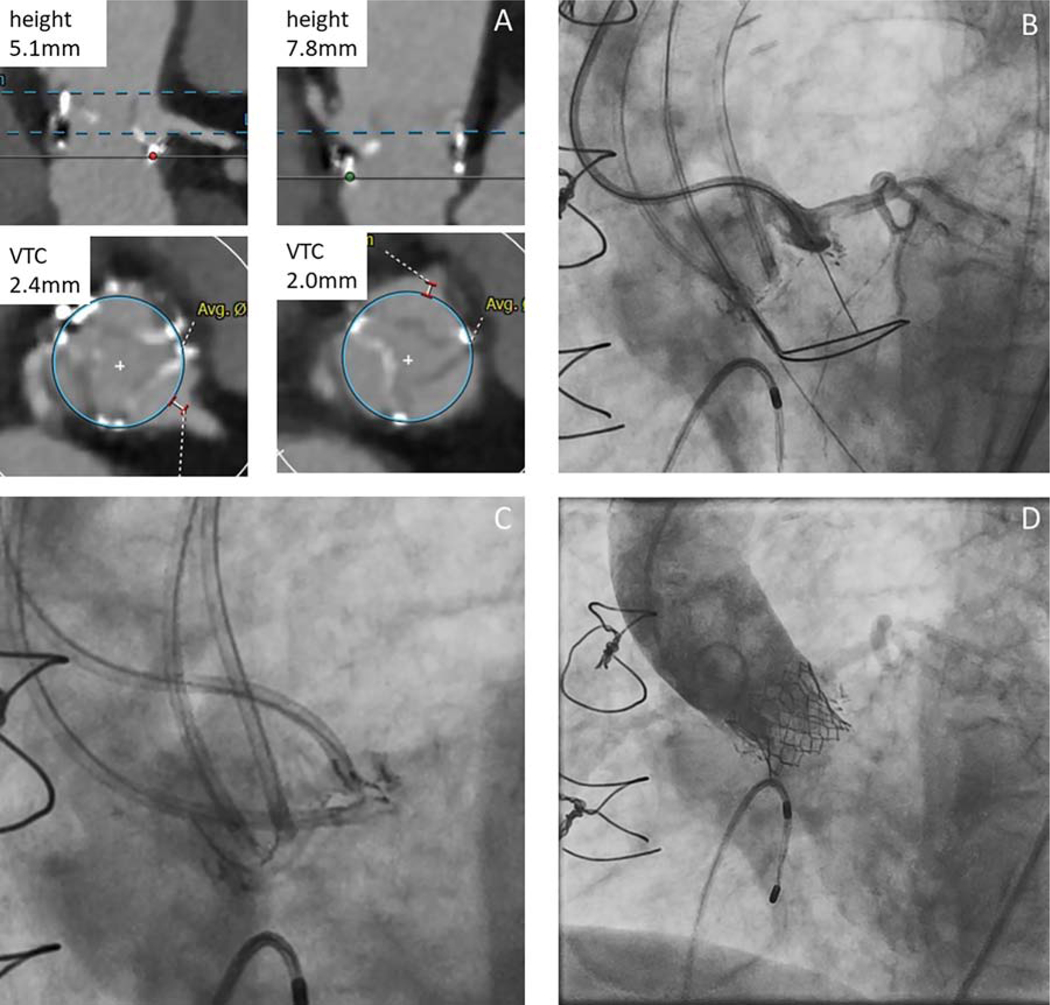
Representative BASILICA procedure. A) Pre-procedure CT demonstrates high risk of both left and right coronary artery obstruction in a patient with a degenerated Trifecta valve. B) Fluoroscopy showing left leaflet traversal with a guidewire. C) Left and right BASILICA system in place ready for laceration. D) Aortic root angiography after BASILICA and TAVR demonstrates patent coronary arteries. BASILICA: Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction; CT: computed tomography; TAVR: transcatheter aortic valve replacement; VTC: virtual transcatheter to coronary distance.
Ten patients (4.7%; 95% CI, 1.8% to 7.5%) had some degree of coronary obstruction despite BASILICA (Supplemental Table 2). Six patients had partial leaflet obstruction (five left main coronary artery; one right coronary artery), evident as haziness on angiography. All were successfully treated with orthotopic stents, with one periprocedural myocardial infarction in a patient who also received mechanical circulatory support. One patient had obstruction of the right coronary artery after likely leaflet or thrombus embolism, which was successfully treated with balloon angioplasty. One patient had significant left main coronary artery obstruction with cardiogenic shock, went on cardiopulmonary bypass and had snorkel stenting to the left main coronary artery, with subsequent unremarkable recovery. In two patients, the skirt of the Evolut valve obstructed the left main coronary artery. The valves were snared and pulled up into the ascending aorta and a second valve deployed without coronary obstruction. One of these patients developed cardiogenic shock despite these maneuvers and subsequently died during the inpatient stay.
Thirty-day survival was 97.2% and stroke rate was 2.8%, with 0.5% disabling stroke. The composite primary safety endpoint of death or disabling stroke at 30 days was 3.4%. The individual components of these endpoints, as well as of VARC-2 safety outcomes, are listed in Table 3. The overall VARC-2 composite safety endpoint was achieved in 82.8%.
The reported BASILICA procedural complications included one mitral chord laceration requiring mitral valve replacement two months later. There were three inadvertent traversals into the interventricular septum and one through the aortic root; the guidewires were re-directed without consequence. Hypotension requiring pressors during any stage of the BASILICA TAVR procedure was reported in 8.5%.
In patients who had reached 1-year follow-up post-procedure (n=124), 1-year survival was 83.9%. One patient was lost to follow-up between 30 days and one year.
Procedure success, VARC-2 safety, and death and disabling stroke were similar between solo and doppio BASILICA procedures, and between patients with native and bioprosthetic aortic valves (Table 4).
Table 4.
Comparative outcomes
| Outcomes | Single BASILICA (n=168) | Double BASILICA (n=46) | P-value |
|---|---|---|---|
| Procedure success | 88.7% | 80.4% | 0.14 |
| VARC-2 Safety | 83.6% | 79.5% | 0.52 |
| Death | 2.4% | 4.3% | 0.48 |
| All Stroke | 2.4% | 4.3% | 0.49 |
| Disabling stroke | 0.6% | 0% | 0.51 |
| Outcomes | Bioprosthetic (n=155) | Native (n=58) | P-value |
| Procedure success | 84.5% | 93.1% | 0.10 |
| VARC-2 Safety | 82.9% | 82.1% | 0.90 |
| Death | 3.3% | 1.7% | 0.55 |
| All Stroke | 3.3% | 1.7% | 0.54 |
| Disabling stroke | 0.7% | 0% | 0.54 |
| Outcomes | Cerebral protection (n=102) | No cerebral protection (n=112) | P-value |
| Procedure success | 83.3% | 90.2% | 0.14 |
| VARC-2 Safety | 85.1% | 80.7% | 0.41 |
| Death | 2.0% | 4.6% | 0.49 |
| All stroke | 1% | 4.5% | 0.12 |
| Disabling stroke | 0% | 0.9% | 0.34 |
In patients who received cerebral embolic protection using the Sentinel device (Boston Scientific, Marlborough, MA), there was no disabling stroke (versus 0.9% without protection, p=0.34), and 1% total stroke (versus 4.5% without protection, p=0.13).
DISCUSSION
The main finding of this international registry was that BASILICA is safe, with low rates of mortality and stroke in 214 patients. The study shows BASILICA is feasible in the real-world setting, with successful traversal and laceration of all intended leaflets in 94.4% of patients.
The BASILICA IDE trial of 30 patients demonstrated a 3.3% disabling and 10% overall stroke rate(5). However, with a small sample size (n=30) and few events (n=3) in a high-risk population undergoing TAVR, a larger study was needed to confirm or refute excess risk of stroke from BASILICA. In this registry, there was a 2.8% mortality and 2.8% overall stroke rate at 30 days, with 0.5% disabling stroke. This is comparable to outcomes in patients undergoing TAVR who are not specifically at risk of coronary artery obstruction. Data from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry reported a 2.5% mortality and 2.3% stroke rate at 30 days across all risk groups in 2019(6).
There is interest in whether cerebral protection should be recommended with BASILICA, as there remains mechanistic plausibility of increased stroke and embolization from excess leaflet manipulation and laceration. Cerebral protection was used in 47.7% of patients, with a stroke rate of 1% compared with 4.5% in patients who did not receive cerebral protection. However, with such a low event rate in this study, compounded by possible selection bias in this unmatched group, particularly from anatomic suitability and device availability at participating sites, we cannot comment on whether cerebral protection reduced stroke further.
These results differ from unpublished data presented on a cohort of 129 patients(7) where the investigators reported 6% 30-day mortality and 7.5% disabling stroke, with significantly worse outcomes with doppio BASILICA compared with solo BASILICA. The lower mortality and considerably lower disabling stroke rate in our study, and equivalent outcomes between solo and doppio BASILICA, could be due to chance. However, another potential mechanistic explanation is the consistent use of 5% dextrose flush during laceration by the investigators in this study and not in the Dvir cohort. Five percent dextrose is non-ionic, and therefore, concentrates charge on the leaflet to promote efficient laceration. Perhaps more important, the dextrose infusion displaces blood that would coagulate if heated with electrosurgery current through the guidewire, which could lead to thrombosis and debris embolization. Moreover, dextrose infusion improves electrosurgical laceration efficiency and may, thereby, reduce the incidence of mechanical avulsion causing cerebral and coronary embolization. The rates of cerebral embolic protection were not reported in the prior cohort but may also have contributed to differing rates of stroke.
Patients underwent BASILICA because of high predicted risk for coronary artery obstruction. Partial or complete coronary artery obstruction was noted in 10 (4.7%) patients despite BASILICA. Of these, one patient (10%) died, and four (40%) had biomarker evidence of periprocedural myocardial infarction. This compares favorably to the 40% to 50% mortality rate reported in registries when BASILICA is not performed(1,2). This is likely because the majority of the coronary artery obstruction cases reported in this registry were not flow-limiting. Furthermore, many were orthotopically stented through the struts of the transcatheter heart valve into the coronary ostium, made possible by BASILICA, rather than “snorkeled” alongside the transcatheter heart valve. Orthotopic stents may increase the chance of successful coronary arteries access in the future as compared to snorkel stents. Orthotopic stents are also more likely to be fully expanded and avoid stent thrombosis.
One important avoidable mechanism of coronary obstruction in this registry was from the skirt of the transcatheter heart valve. The skirt height of the valve must be taken into consideration when there is coronary obstruction risk and the depth of implant planned accordingly. The commissural posts may also land in front of the coronaries, causing obstruction. Another mode of failure related to BASILICA appears to be incomplete displacement of the lacerated leaflets. One possible reason may be mechanical avulsion from excessive pull, rather than controlled electrosurgical laceration, causing leaflet prolapse into the coronary arteries. It is also possible that leaflet traversal or laceration was eccentric, or the coronary arteries were not aligned with the center of the leaflets, causing partial obstruction after BASILICA TAVR.
Patients with high coronary obstruction risk anatomies remain high risk for TAVR, irrespective of their surgical risk. It is important to remember that surgery is still a safe and good option for those who are at intermediate or low risk for surgery. For high- and extreme-surgical-risk patients, protection with a guidewire with or without snorkel stenting is another option. A recent study demonstrated increased cardiac death and coronary obstruction when the guidewire was pulled and no stent deployed(8). One explanation is that the guidewire may pin the offending leaflet open and, therefore, falsely reassure the operator that the risk of coronary obstruction is low, only to discover the leaflet moves after the wire is pulled, causing obstruction. If deployed, snorkel stenting is associated with several complications. The 60-patient CHIMNEY Registry reported 5% procedural death, 1.7% stroke, 21.6% myocardial infarction, and 23.3% cardiogenic shock (9). There remains a risk of delayed coronary artery obstruction (10), especially as these stents tend to be underexpanded, if not completely crushed by the transcatheter heart valve(11). To avoid these complications, as well as problems with lifelong dual antiplatelet therapy and increased difficulty re-engaging the coronary artery for downstream transcatheter intervention, BASILICA appears to be the favorable solution.
Limitations
These are retrospective, site-reported data without independent monitoring and data verification. The participating sites determined risk of coronary obstruction and identified candidates for BASILICA, rather than an independent core laboratory and central eligibility committee. It is likely that BASILICA was performed in patients presenting across a spectrum of coronary obstruction risk, from borderline to prohibitive. The comparisons made between native and bioprosthetic valves, between solo and doppio BASILICA, and in the use of cerebral embolic protection are not between matched groups and, so, should be interpreted with caution. Data were collected for three discrete time points, on exit from the catheterization laboratory, at 30 days, and at 1 year, so Kaplan-Meier mortality estimates and duration of follow-up are not available. Data on the use of adjunctive coronary wire protection or bioprosthetic valve fracture during the procedure were not systematically collected. This study did not investigate patients who were denied TAVR with BASILICA. Therefore, it does not address the applicability of the procedure in all-comers. From the BASILICA IDE trial, 1/60 (1.7%) were excluded during screening because BASILICA was thought not to be feasible due to heavy leaflet calcification.
Despite these shortcomings, the current study represents the largest multicenter, international, real-world registry with adjudicated outcomes that includes a large number of patients for a relatively rare procedure.
CONCLUSIONS
BASILICA is safe, with low reported rates of stroke and death. BASILICA is feasible in the real-world setting, in centers with experience and appropriate training, with a high procedural success rate and low rates of coronary artery obstruction.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN?
BASILICA is a novel procedure that is effective at preventing coronary artery obstruction at expert centers. There are insufficient data on procedure safety, especially risk of stroke.
WHAT IS NEW?
BASILICA is safe, with low reported rates of stroke and death in this real-world registry.
WHAT IS NEXT?
These data should be reassuring and should facilitate wider dissemination of the BASILICA procedure at high-volume centers.
ACKNOWLEDGEMENTS
Jason P. Wermers, medical writer associate at MedStar Washington Hospital Center; Joseph A. (Jake) Sutton, III, clinical project lead at MedStar Washington Hospital Center; and Sam Feinberg, coordinator at Brigham and Women’s Hospital.
Funding:
NHLBI grant Z01-HL006061
ABBREVIATIONS AND ACRONYMS
- BASILICA
Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction
- CT
Computed tomography
- IDE
Investigational Device Exemption
- STS PROM
Society of Thoracic Surgeons’ Predicted Risk of Operative Mortality
- TAVR
Transcatheter Aortic Valve Replacement
- VARC
Valve Academic Research Consortium
- ViV
Valve in valve
Footnotes
Disclosures:
JMK, TR, and RJL are co-inventors on patents, assigned to NIH, on catheter devices to lacerate valve leaflets.
JMK has proctored for Edwards Lifesciences and Medtronic.
ABG is a proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular. He has equity in Transmural Systems.
VCB is a consultant for Edwards Lifesciences, Abbott Vascular and Transmural Systems, and his employer has research contracts for clinical investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, St Jude Medical, and Boston Scientific.
TR is a consultant/proctor for Edwards Lifesciences and Medtronic. He has equity in Transmural Systems.
RW is a consultant for Medtronic and is a consultant and receives grant support from Abbott Vascular.
RJL is the principal investigator on a Cooperative Research and Development Agreement between NIH and Edwards Lifesciences on transcatheter modification of the mitral valve.
HCH has Institutional research funding from Abbott Vascular, Boston Scientific, Edwards Lifesciences, Medtronic; consultant / speaking honoraria from Edwards Lifesciences and Medtronic.
KIM is proctor for Edwards Lifesciences, Medtronic and Abbott.
RAL is proctor for Edwards Lifesciences.
AC is on the speaker bureau for Abbott Vascular, consultant/ research grant from Boston Scientific, proctor/speakers bureau for Edwards Lifesciences, proctor/speakers bureau for Medtronic; consultant for Silk Road Medical
JPD is consultant and a member of the advisory board for Edwards Lifesciences, Boston Scientific, and WL Gore & Associates.
GHLT is a physician proctor for Medtronic and a consultant for Medtronic, Abbott Structural Heart and W. L. Gore & Associates.
PSF is proctor for Medtronic.
RK is a proctor for Medtronic and Edwards.
PBS is proctor Edwards, educational grants from Edwards, Abbott, and Medtronic.
JMM declares honoraria / consulting with Medtronic and Edwards.
AP is consultant for Edwards.
PM is consultant and proctor for Edwards and Medtronic
No other author has a financial conflict of interest related to this research.
Tweet/handle: BASILICA is safe, with low stroke and death rates, and feasible, with high procedure success and low coronary artery obstruction rate. @ron_waksman
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013;62:1552–1562. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro HB, Rodes-Cabau J, Blanke P, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J 2018;39:687–695. [DOI] [PubMed] [Google Scholar]
- 3.Khan JM, Dvir D, Greenbaum AB, et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement: Concept to First-in-Human. JACC Cardiovasc Interv 2018;11:677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lederman RJ, Babaliaros VC, Rogers T, et al. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: From Computed Tomography to BASILICA. JACC Cardiovasc Interv 2019;12:1197–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan JM, Greenbaum AB, Babaliaros VC, et al. The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc Interv 2019;12:1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2020;76:2492–2516. [DOI] [PubMed] [Google Scholar]
- 7.Dvir D BASILICA vs. control in TAVI procedures at risk for coronary obstruction: comprehensive corelab adjudicated matched comparison. Available at https://media.pcronline.com/diapos/PCReCourse2020/205-20200625_1656_Hotline_and_Innovation_Channel_Dvir_Danny_0000_(468)/Dvir_Danny_20200625_1645_Hotline_and_Innovation_-_Channel_3.pdf. Accessed February 5, 2021.
- 8.Palmerini T, Chakravarty T, Saia F, et al. Coronary Protection to Prevent Coronary Obstruction During TAVR: A Multicenter International Registry. JACC Cardiovasc Interv 2020;13:739–747. [DOI] [PubMed] [Google Scholar]
- 9.Mercanti F, Rosseel L, Neylon A, et al. Chimney Stenting for Coronary Occlusion During TAVR: Insights From the Chimney Registry. JACC Cardiovasc Interv 2020;13:751–761. [DOI] [PubMed] [Google Scholar]
- 10.Jabbour RJ, Tanaka A, Finkelstein A, et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2018;71:1513–1524. [DOI] [PubMed] [Google Scholar]
- 11.Pighi M, Lunardi M, Pesarini G, et al. Intravascular ultrasound assessment of coronary ostia following valve in valve transcatheter aortic valve implantation. EuroIntervention 2020. September 8 10.4244/EIJ-D-20-00611. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


