Abstract
A 43-year-old man was brought to our hospital with fever. The initial diagnosis was bacterial pneumonia, and ampicillin/sulbactam was administered. However, defervescence was not achieved, and relative bradycardia was observed. Detailed history-taking revealed that the patient had been involved in caring for a wild pigeon before hospitalization. We changed the antimicrobial therapy to minocycline and the patient’ s condition improved. Chlamydophila psittaci antibody was subsequently found to be increased four-fold, and psittacosis was diagnosed. This case acts a reminder to clinicians of the importance of both the history of exposure to any birds and vital signs, including relative bradycardia.
Keywords: Chlamydophila psittaci, Psittacosis, Atypical pneumonia, Relative bradycardia
Introduction
Chlamydophila psittaci is an obligate intracellular gram-negative bacterium that causes psittacosis, an important human zoonosis, and is a member of the Chlamydiaceae family [1,2]. While psittacosis is a well-known disease, appropriately diagnosed cases are rare both in Japan and other countries [3]. Chlamydophila psittaci is thought to account for approximately 1% of community-acquired pneumonias [4]. The clinical course of psittacosis can be severe if appropriate medical treatment is delayed [[5], [6], [7]]. We report here a case of Chlamydophila psittaci infection where the keys to diagnosis were careful elicitation of the history and scrutiny of vital signs, including relative bradycardia.
Case report
Clinical presentation and management
A 43-year-old man was brought to our hospital by ambulance with a 3-day history of fever >40 °C and headache at night. The headache worsened with the fever, was accompanied by photophobia and nausea, and was associated with a pain as if both eyes were being pushed into the skull. Furthermore, he coughed without the sputum. His symptoms did not improve over time, and he was brought to our hospital because he could not move by himself. He had no apparent past medical history or family history. He denied a history of keeping pets at home. He said he had been living in an approximately 20-year-old reinforced concrete apartment and had not recently visited any hot springs.
On arrival at the emergency department, he was noted to be 171.0 cm tall and weighed 78.0 kg, with a Glasgow Coma Scale score of 15 (E4V5M6), body temperature of 39.8 °C, blood pressure of 118/72 mmHg, pulse rate of 86 beats/min (regular), respiratory rate of 16 breaths/min, and percutaneous oxygen saturation of 98 % in room air. On physical examination, the conjunctivae were not anemic, and no icteric changes were observed. Signs of meningeal irritation, including cervical rigidity, were absent. Slight crepitations were audible on auscultation of the right back. No palpable surface lymph nodes, rash, or neck stiffness were evident.
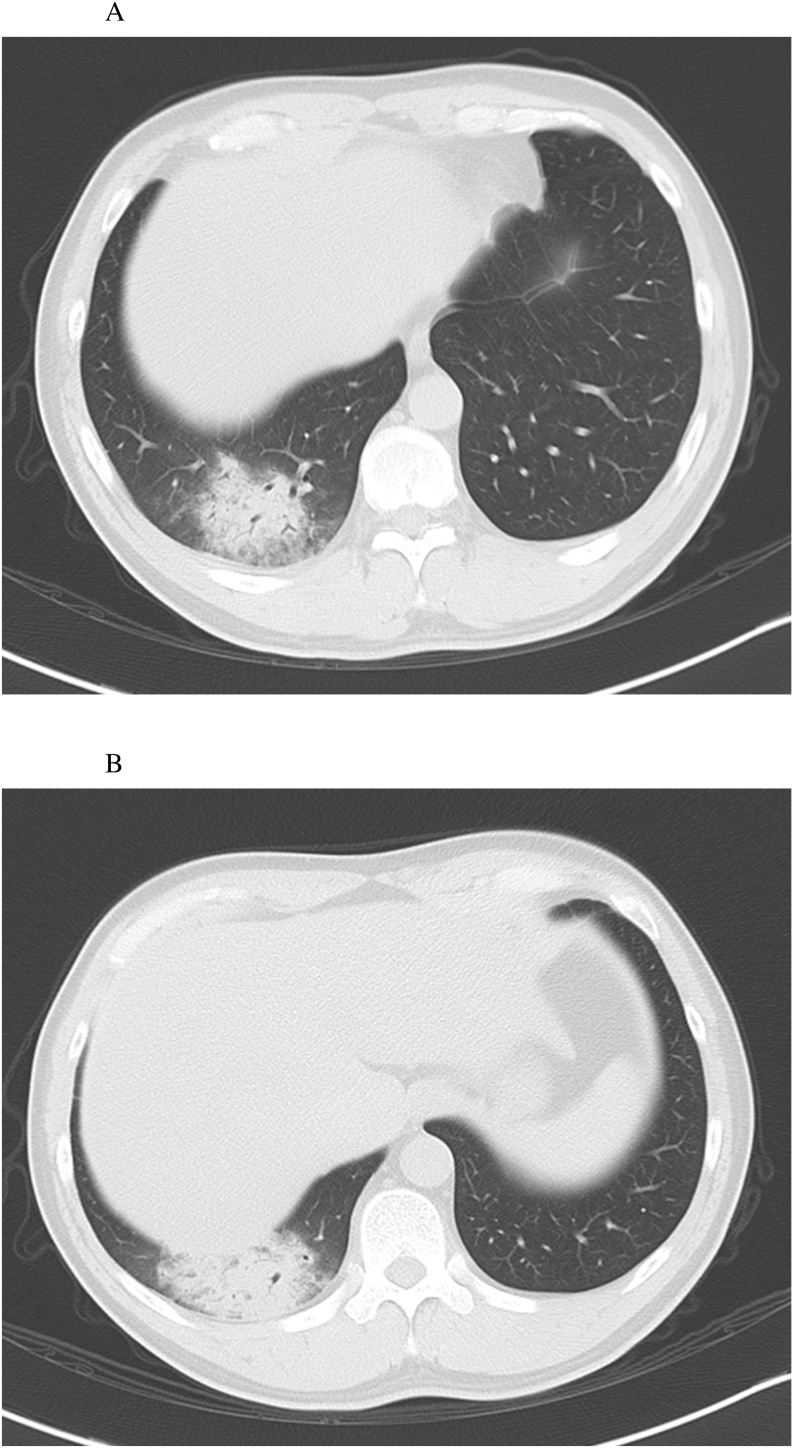
Blood tests revealed an elevated white blood cell count with dominant neutrophils and C-reactive protein (CRP) level (Table 1). Chest radiography showed infiltrative shadows in the back on a lateral projection (Fig. 1-A, -B). Chest computed tomography (CT) showed consolidation in the right lower lobe (Fig. 2-A, -B). The initial diagnosis was bacterial pneumonia, and he was hospitalized for treatment.
Table 1.
Results of blood tests on admission. White blood cell count (high neutrophil count) is slightly elevated and C-reactive protein level is high.
| Blood test | Result | (Normal range) |
|---|---|---|
| White blood cells (/μL) | 8400 | (4000–8000) |
| Neutrophils (%) | 82.4 | (40.0–75.0) |
| Lymphocytes (%) | 10.3 | (30.0–50.0) |
| Red blood cells (/μL) | 470 × 106 | (380 × 106–480 × 106) |
| Hemoglobin (g/dL) | 15.3 | (12.0–16.0) |
| Hematocrit (%) | 43.9 | (37.0–47.0) |
| Platelets (/μL) | 17.8 × 104 | (13.0 × 104–35.0 × 104) |
| Total protein (g/dL) | 6.5 | (6.7–8.3) |
| Total bilirubin (mg/dL) | 8.9 | (0.2–1.0) |
| Aspartate aminotransferase (IU/L) | 54 | (12.0–32.0) |
| Alanine aminotransferase (IU/L) | 33 | (8.0–36.0) |
| Lactate dehydrogenase (IU/L) | 242 | (127.0–221.0) |
| Creatine kinase (IU/L) | 392 | (50.0–206.0) |
| Blood urea nitrogen (mg/dL) | 9.7 | (8.0–20.0) |
| Creatinine (mg/dL) | 0.97 | (0.3–1.2) |
| Sodium (mEq/L) | 141 | (134.0–147.0) |
| Potassium (mEq/L) | 3.5 | (3.2–4.8) |
| Chloride (mEq/L) | 100 | (98–108) |
| C-reactive protein (mg/dL) | 17.86 | (0.0–0.3) |
| Prothrombin time (s) | 13.1 | (9.5–13.5) |
| Activated partial thromboplastin time (s) | 34.0 | (26.0–38.0) |
IU: International Unit, Eq: Equivalent, s: second.
Fig. 1.
(A) Chest radiograph (posterior/anterior view). An infiltrative shadow is seen in the right diaphragm. (B) Chest radiograph (lateral, left/right view). Infiltrative shadow is seen dorsally.
Fig. 2.
(A) and (B) Computed tomography (axial sections). The lower right lobe shows localized infiltrative shadows.
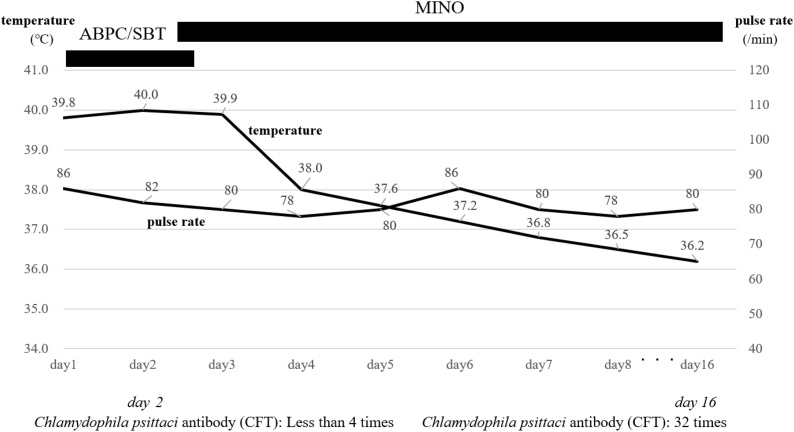
Blood and sputum cultures were obtained before administration of antibiotics, and ampicillin/sulbactam (ABPC/SBT) was intravenous administered (every six hours) during hospitalization. However, no defervescence was observed in the 2 days after hospitalization. His body temperature was 40.0 °C, and the pulse rate of 82 beats/min on hospital day 2 reflected relative bradycardia. Given his relative bradycardia, mildly elevated white blood cell count and young age, we considered the possibility of atypical pneumonia and carefully elicited the patient’s history. This second interview revealed that he had been involved in caring for an injured pigeon at his home 2 weeks before hospitalization. In the interview on admission, the patient had answered “no” to the question about keeping pets. Based on this new information, we immediately changed ampicillin/sulbactam to minocycline (intravenous administration: every 12 h) on hospital day 2. Defervescence was observed in the next several days, and minocycline was administered for 14 days in total. His overall status improved, and he was discharged on hospital day 16. More than four-fold rise of anti-Chlamydophila psittaci antibody titer from <1:4 on day 2 to 1:32 on day 16 by complement fixation method, psittacosis was diagnosed after discharge (Fig. 3). The results of the blood and sputum cultures were negative. Furthermore, other atypical pneumonias were negative. (Legionella urinary antigen, Mycoplasma blood antibody, Mycoplasma throat swab and Chlamydia pneumoniae blood antibody)
Fig. 3.
Clinical course and events of relative bradycardia are shown.
Discussion
Chlamydophila psittaci is an obligate intracellular Gram-negative bacteria that causes psittacosis, an important human zoonosis, and is a member of the Chlamydiaceae family [1,2]. The number of reported cases of Chlamydophila psittaci infection in Japan is small, with 12 cases reported in 2011, six in 2012, six in 2013, eight in 2014, five in 2015, six in 2016, and two in 2017 according to the National Institute of Infectious Diseases in Japan. Similarly, approximately 10 cases per year are reported in the United States [3].
Humans can contract psittacosis after inhaling Chlamydophila psittaci aerosolized from secretions from birds, such as bird excrement or respiratory secretions on feathers [1]. Birds are the primary reservoir for Chlamydophila psittaci, and pet birds (particularly psittacines such as parrots and parakeets) are considered the most common source of infection. Chlamydophila psittaci infection affects at least 465 bird species, spanning 30 different orders, most prominently Psittacidae (cockatoos, parrots, parakeets, and lories) [8]. This case was caused by a pigeon. While the Columbiformes order (including pigeons) has been reported to be a reservoir for Chlamydophila psittaci [9], the name of the disease, “psittacosis,” prevented us from considering less common origin species, such as pigeon, and led to a diagnostic pitfall. Furthermore, we had mistakenly considered the likelihood of psittacosis low after simply asking about having pets in the initial interview on admission. History of contact with animals within a month, including not only breeding pets, but also any contact with animals, birds, and insects is needed when obtaining a patient’s history. Relative bradycardia is a common sign in patients with psittacosis and other atypical pneumoniae, such as Legionella and chlamydial pneumonias [[10], [11], [12]]. We took a detailed history again and revealed that the patient had cared for an injured pigeon at his home 2 weeks before hospitalization. The complications such as endocarditis/myocarditis were negative from an electrocardiogram and an echocardiography. It was more inconclusive than the interview, psittacosis was the primary suspect in the differential diagnosis.
The incubation period is usually 5–14 days, although incubation periods of up to 1 month have been reported [13,14]. This case had cared for the pigeon 2 weeks before hospitalization. In addition, symptoms of Chlamydophila psittaci infection frequently include high fever accompanied by a relatively low pulse rate (as described above), chills, headache, myalgia, non-productive cough, and dyspnea [1,2]. The patient in this case presented to the hospital with a strong headache with photophobia. Patients with psittacosis often present with intense headache with photophobia [1].
In terms of hematological findings, white blood cell count is usually normal to slightly low during the acute phase of psittacosis, followed by leukopenia in approximately 25 % of cases [15]. In addition, elevated levels of CRP, aspartate aminotransferase, and alanine aminotransferase in patients with psittacosis seem to be related to the severity of the infection [9]. In this case, the CRP level was elevated, but no increase in white blood cell count was seen on admission, representing relatively typical results. In terms of radiographic findings, up to 90 % of hospitalized patients with psittacosis show abnormalities on chest radiography. Most commonly, dense consolidation in a unilateral lower lobe is observed [16]. This was indeed the case in our patient, and the lower right lobe showed local infiltrative shadows, even though it is difficult to distinguish psittacosis from other community-acquired pneumonia by chest X-ray and CT findings.
The lack of rapid diagnostic methods represents an obvious problem. To diagnose infection with Chlamydophila psittaci, the case is confirmed from laboratory data using at least one of the following methods: 1) isolation of the causative agent from respiratory secretions; 2) an increase of ≥4-fold in antibody titers between paired sera as determined by complement fixation test or the more sensitive micro-immunofluorescence (MIF) test; or 3) immunoglobulin M antibodies against Chlamydophila psittaci detected by MIF to a reciprocal titer of ≥16 (10, 27). Definitive diagnosis was reached in the present case using the second of the above methods. Definitive diagnosis was confirmed in the present case using paired serum samples after a 2-week intervals [9]. Numerous cases in which medical treatment proves successful without a definitive diagnosis are presumably attributable to the lack of rapid diagnostic methods for psittacosis and the good response to medical treatment for atypical pneumonia started on admission. Because of the risk of a severe clinical course with delayed treatment [[5], [6], [7]], starting pharmacotherapy without waiting for definitive diagnosis is important if psittacosis is suspected. Furthermore, in public health, the examination that is quicker other than the serodiagnosis, and is simple and easy is necessary.
Treatment of psittacosis preferably involves tetracyclines or macrolides for patients for whom tetracycline is contraindicated for at least 10–14 days [9]. The present case showed no improvement following the initial beta-lactam-based treatment (ABPC/SBT). The beta-lactam medicine which is ectoblast synthesis inhibitor is invalid. Additional detailed history-taking is necessary for cases of pneumonia showing no improvement with beta-lactam-based treatment, and the presence of findings associated with psittacosis (headache, relative bradycardia, and increased CRP with normal white blood cell count as in the present case). When any situation is important for a detailed interview, we emphasize last.
Certain flaws in our approach must be noted. We should have strongly suspected atypical pneumonia on admission because the present case met four criteria to suspect atypical pneumonia according to the 2017 adult pneumonia practice guidelines published by the Japanese Respiratory Society: he was <60 years old, had a white blood cell count <10,000/μL, had no or minimal underlying disease, and no sputum production.
Conclusion
Psittacosis is a rare disease, but shows characteristic findings on clinical examination, including relative bradycardia. Detailed history-taking may be needed again if the pneumonia remains unimproved following beta-lactam-based treatment, and clinical findings suggest the possibility of psittacosis. Because some patients can be severely affected, empirical treatment should be initiated as soon as possible without waiting for a definitive diagnosis if psittacosis is suspected.
Conflicts of interest
None declared.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Written informed consent was obtained.
Ethical approval
No ethical approval was required for this publication.
Author contribution
SF and WK treated patient, and SF, YU and TN drafted the manuscript. All authors critically reviewed the manuscript and approved the final version.
Acknowledgements
None.
References
- 1.Wheelhouse N., Longbottom D. Endemic and emerging chlamydial infections of animals and their zoonotic implications. Transbound Emerg Dis. 2012;59(4):283–291. doi: 10.1111/j.1865-1682.2011.01274.x. [DOI] [PubMed] [Google Scholar]
- 2.Wannaratana S., Thontiravong A., Amonsin A. Persistence of Chlamydia psittaci in various temperatures and times. Avian Dis. 2017;61(1):40–45. doi: 10.1637/11475-072216-Reg. [DOI] [PubMed] [Google Scholar]
- 3.Adams D.A., Thomas K.R., Jajosky R.A. Summary of notifiable infectious diseases and condition – United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;63:1–122. doi: 10.15585/mmwr.mm6253a1. [DOI] [PubMed] [Google Scholar]
- 4.Hogerwerf L., De Gier B., Baan B. Chlamydia psittaci as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–3105. doi: 10.1017/S0950268817002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verweij P.E., Meis J.F., Eijk R. Severe human psittacosis requiring artificial ventilation: case report and review. Clin Infect Dis. 1995;20(2):440–442. doi: 10.1093/clinids/20.2.440. [DOI] [PubMed] [Google Scholar]
- 6.Soni R., Seale J.P., Young I.H. Fulminant psittacosis requiring mechanical ventilation and demonstrating serological cross-reactivity between Legionella longbeachae and Chlamydia psittaci. Respirology. 1999;4(2):203–205. doi: 10.1046/j.1440-1843.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi H., Mukae H., Ihiboshi H. A case of fulminant psittacosis necessitating mechanical ventilation diagnosed by chlamydial isolation form BALF. Kansenshogaku Zasshi. 1995;69(12):1396–1401. doi: 10.11150/kansenshogakuzasshi1970.69.1396. [DOI] [PubMed] [Google Scholar]
- 8.Wallensten A., Fredlund H., Runehagen A. Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January/February 2013. Euro Surveill. 2014;19(42):20937. doi: 10.2807/1560-7917.es2014.19.42.20937. [DOI] [PubMed] [Google Scholar]
- 9.Beeckman D.S.A., Vanrompay D.C.G. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect. 2009;15(1):11–17. doi: 10.1111/j.1469-0691.2008.02669.x. [DOI] [PubMed] [Google Scholar]
- 10.Ye F., Hatahet M., Youniss M.A. The clinical significance of relative bradycardia. WMJ. 2018;117(2):73–78. [PubMed] [Google Scholar]
- 11.Ye F., Winchester D., Stalvey C. Proposed mechanisms of relative bradycardia. Med Hypo. 2018:11963–11967. doi: 10.1016/j.mehy.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Ostergaard L., Huniche B., Andersen P.L. Relative bradycardia in infectious diseases. J Infectol. 1996;33(3):185–191. doi: 10.1016/s0163-4453(96)92225-2. [DOI] [PubMed] [Google Scholar]
- 13.Moroney J.F., Guevara R., Iverson C. Detection of chlamydiosis in a shipment of pet birds, leading to recognition of an outbreak of clinically mild psittacosis in humans. Clin Infect Dis. 1998;26(6):1425–1429. doi: 10.1086/516368. [DOI] [PubMed] [Google Scholar]
- 14.Grayston J.T., Thom D.H. The chlamydia pneumonias. Curr Clin Top Infect Dis. 1991;11:1–18. [PubMed] [Google Scholar]
- 15.Longbottom D., Coulter L.J. Animal chlamydioses and zoonotic implications. J Comp Pathol. 2003;128(4):217–244. doi: 10.1053/jcpa.2002.0629. [DOI] [PubMed] [Google Scholar]
- 16.Gosbell I.B., Ross A.D., Turner I.B. Chlamydia psittaci infection and reinfection in a veterinarian. Aust Vet J. 1999;77(8):511–513. doi: 10.1111/j.1751-0813.1999.tb12121.x. [DOI] [PubMed] [Google Scholar]