Abstract
In vitro genotoxicity dose–response data have been investigated for their utility in modeling and assessing potential differences in mutagenic responses between machine-generated whole smoke solutions (WSSs) from combusted cigarette tobacco products. Our previous study observed that potency ranking by benchmark dose (BMD) analysis was a useful modeling approach for quantitative assessment of differences between the mutagenicity of several structurally diverse chemical constituents of cigarette smoke. To follow-up on these observations, we used the mouse lymphoma assay (MLA) to evaluate the mutagenicity of WSSs prepared from two commercial cigarettes smoked under two different smoking machine regimens. L5178Y cells were exposed to ≥5 concentrations of each WSS for 4 hr ± S9 activation. S9 reduced the cytotoxicity and enhanced the mutagenicity of the WSSs. The resulting S9-mediated mutagenicity dose–responses were compared between test articles using BMD analysis, the lowest dose exceeding the Global Evaluation Factor, the no observed or lowest observed genotoxic effect level, and the mutagenic potency. The BMD10, BMD50, BMD100, and BMD200, indicating a 10%, 50%, 100%, or 200% increase in the background mutant frequency, respectively, were calculated using the PROAST software package. Overall, the quantitative approaches resulted in a similar rank order of mutagenic potency for the MLA tested WSSs, with potency increasing with the level of tar. The BMD approach using covariate analysis produced the most informative comparisons. Differences in potency were associated with the number of cigarettes smoked, the cigarette product smoked, and the smoking machine protocol used to prepare the sample. Under the conditions of this study, these results suggest that our hypothesis of modeling MLA data using the BMD approach to quantitatively discriminate between the mutagenic potential of WSSs from combustible cigarettes might be an useful method.
Keywords: whole smoke solution, mouse lymphoma assay, quantitative analysis, benchmark dose
INTRODUCTION
Cigarette smoke is a complex and dynamic aerosol mixture of gaseous and particulate compounds consisting of more than 7,000 individual chemicals (CDC, 2014, 2016), of which hundreds have toxicological properties including carcinogenicity as reported by the International Agency for Research on Cancer (IARC) (IARC, 2012). As a clear causal relationship has been established between tobacco smoke and diseases in nearly every organ (CDC, 2014), manufacturers have invested heavily in introducing purportedly lower-risk products into the market (Warner, 2002). As early as the 1950s, filtered cigarettes became a marketing tool to support the assumption that they would reduce risk. In the late 1960s and 1970s, cigarettes labeled as “low tar and nicotine” were promoted as innovations to address smoker health concerns (Warner, 2002). The Family Smoking Prevention and Tobacco Control Act of 2009 gave the U.S. Food and Drug Administration (FDA) authority to regulate tobacco products. It is in the public health interest to reduce the morbidity and mortality associated with tobacco use, and the tobacco control provisions in the Federal Food, Drug, and Cosmetic Act (FD&C Act) have valuable tools to regulate and help protect the public from the health risks of tobacco products. Scientific evidence is required to assess the impacts of these products on public health.
Genotoxicity testing is considered an important part of hazard identification for carcinogenic potential as well as establishing a carcinogenic mode of action for risk assessment (EPA, 2005). Because tobacco smoke is classified as a Group 1 human carcinogen by IARC (2012), we previouly modelled the genotoxicity outcomes from different cigarette smoke condensates in order to measure the varying toxicity of individual tobacco products (Guo et al., 2011). A subsequent study applied point-of-departure (PoD) and other quantitative metrics to mouse lymphoma assay (MLA) data with the aim of ranking and quantitatively evaluating select chemicals representing different chemical classes found in tobacco smoke on the basis of their genotoxicity (Guo et al., 2016). The quantitative metrics we used were the benchmark dose (BMD), the no observed genotoxic effect level (NOGEL), the lowest observed genotoxic effect level (LOGEL), and mutagenic potency expressed as mutant frequency (MF) per μM chemical. The results demonstrated that the MLA is capable of discriminating the relative mutagenic potency of a set of structurally diverse chemicals in a scientifically rigorous manner. These preliminary findings confirmed the usefulness of BMD analysis in potency ranking among different chemical agents, as described in the reports by the Working Group on Quantitative Approaches to Genetic Toxicology Risk Assessment of the International Workshops on Genotoxicity Testing (IWGT) (MacGregor et al., 2015a,b). These recommendations, and previous studies employing them, however, were usually based on testing individual chemicals, mainly model mutagens (Recio et al., 2012; Gollapudi et al., 2013; Hernandez et al., 2013; Cao et al., 2014; Johnson et al., 2014). For our application, it is of interest to know whether these quantitative metrics also are useful for distinguishing between the mutagenic responses induced by complex chemical mixtures, specifically cigarette smoke generated from various tobacco products.
In the present study, we used quantitative dose–response metrics to evaluate the mutagenicity of cigarette whole smoke solutions (WSSs) in the MLA. The in vitro PoD metrics were used here for comparing mutagenic potencies, rather than for deriving human exposure limits or conducting a health risk assessment that requires a population-based in vitro–to–in vivo extrapolation model to estimate the human equivalent dose (Wetmore et al., 2012). The WSSs from two combustible tobacco products were prepared by bubbling machine-generated whole smoke through dimethyl sulfoxide (DMSO), thus resulting in a test article containing the chemicals in cigarette smoke that can be dissolved and/or trapped in DMSO. These WSSs were generated from two types of commercial cigarettes smoked under two different smoking regimens and were expected to differ in their chemical and possibly toxic properties.
MATERIALS AND METHODS
Materials
Samples of two combustible cigarette products were purchased on or before the fall of 2013 at retail outlets in Atlanta, GA. All cigarettes were stored in their original packaging at −70°C until analysis. The two cigarette products were selected because they were expected to differ in their smoke chemistry when machine smoked by the International Organization for Standardization (ISO) and Health Canada Intense (HCI) methods on the basis of the fact that smoke from Cigarette #2 contained higher amounts of the tar, nicotine, and carbon monoxide (TNCO) than Cigarette #1 as described by the manufacturer (FTC, 2000). Benzo[a]-pyrene (BaP), 4-nitroquinoline-1-oxide (4-NQO), trifluorothymidine (TFT), and DMSO were obtained from Sigma-Aldrich (St. Louis, MO). Aroclor-1254-induced male Sprague-Dawley rat liver postmitochondrial fraction (S9) was purchased from Moltox (Boone, NC). Fischer’s medium was obtained from Quality Biological (Gaithersburg, MD), and all other cell culture supplies were acquired from Invitrogen Life Technologies (Carlsbad, CA).
Preparation of the WSSs
Table I lists basic information for the six WSSs used in this study. Cigarette WSSs were prepared by the Centers for Disease Control and Prevention (CDC), Division of Laboratory Sciences (Atlanta, GA). The WSSs were generated by smoking 20 or 60 of either Cigarette #1 or Cigarette #2 using a Borgwaldt RM20H rotary smoking machine (Richmond, VA) with acid cleaned perfluoroalkoxy impingers and using the ISO smoking protocol (35 ml puff volume with a puff interval of 60 sec, 2.0 sec puff duration, 0% blocking of the filter ventilation) or the HCI smoking regimen (55 ml puff volume, 2 sec puff duration, 30 sec puff interval, with ventilation holes blocked 100%). Twenty cigarettes were smoked for preparing the HCI WSSs because a preliminary study indicated that a WSS sample generated by smoking 20 cigarettes using the ISO regimen was not mutagenic (data not shown). WSSs also were prepared by smoking 60 cigarettes using both the HCl and ISO protocols in order to observe differences in mutagenicity on the basis of dose (i.e., samples prepared with 20 vs. 60 cigarettes) and between the two machine smoking regimens (i.e., samples prepared using the ISO vs. HCI smoking machine protocol). Cigarette whole mainstream smoke was bubbled through a series of three impingers each containing 30 ml analytical grade DMSO. After completion of smoking, the solutions in the impingers were pooled and the remnants in the impingers were rinsed with 10 ml DMSO, which was combined with the original solution to make 100 ml of WSS solution. TNCO measurements were made as part of the HCI WSS preparation. The solutions were apportioned in 1-ml volumes into gas-tight vials, frozen, and shipped overnight on dry ice to the National Center for Toxicological Research (Jefferson, AR). The WSSs were stored at −70°C and each experiment used one vial of the WSSs.
TABLE I.
Six Whole Smoke Solutions (WSSs) from Two Commercial Cigarettes Generated Using Two Smoke Conditions with Different Numbers of Cigarettes
| Cigarett |
Number of cigarettes |
Smoke regimen |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| WSS ID | #1 | #2 | 20 | 60 | ISO | HCI | Tar (mg/ml) | CO (mg/ml) | Nicotine (mg/ml) |
| C1-ISO-60 | √ | √ | √ | ND | ND | ND | |||
| C2-ISO-60 | √ | √ | √ | ND | ND | ND | |||
| C1-HCI-20 | √ | √ | √ | 1.8 | 3.9 | 0.2 | |||
| C1-HCI-60 | √ | √ | √ | 6.4 | 14.1 | 0.9 | |||
| C2-HCI-20 | √ | √ | √ | 3.1 | 7.5 | 0.4 | |||
| C2-HCI-60 | √ | √ | √ | 9.3 | 22.5 | 1.3 | |||
ISO, International Organization for Standardization smoking regimen.
HCI, Health Canada Intense smoking regimen.
CO, Carbon monoxide.
ND, not determined.
Cells and Exposure Conditions
The L5178Y/Tk+/− 3.7.2C mouse lymphoma cell line was used for conducting the MLA forward mutation assay. Cells were maintained and cleansed periodically to purge spontaneous Tk−/− and Tk−/0 mutants as described previously (Mei et al., 2014). All WSSs were tested in the absence or presence of S9 metabolic activation. An S9 cofactor mixture was prepared by mixing the S9 liver fraction with a reduced nicotinamide adenine dinucleotide phosphate (NADPH)-generating system at a ratio of 1:4 (Guo et al., 2016). The standard S9 mix contained 50 mM sodium phosphate buffer (pH 8.0), 30 mM KCl, 10 mM MgCl2, 4 mM NADP, 5 mM glucose-6-phosphate, 10 mM CaCl2, and 2 mg/ml of S9 protein. The final S9 concentration in the treatment medium was 0.4 mg protein/ml (about 1% v/v).
Dose range-finding assays were performed to establish an appropriate concentration range for testing each WSS. For the definitive mutation assays, single or duplicate cultures of 6 × 106 cells were treated with 5 or 6 concentrations (based on their cytotoxicity) of each WSS in 10 ml treatment medium. The treatments were conducted in 9-ml S-Monovette neutral tubes (Sarstedt, Nümbrecht, Germany), which are gas-tight and have a small headspace to minimize the loss of the volatile constituents dissolved in the WSS. The working solutions (100×) for each WSS concentration were prepared fresh in DMSO by diluting the stocks in 1-ml amber vials. One hundred microliters of the working solutions were transferred into the cell cultures using gas-tight syringes. The cells then were placed on a roller drum (15 rpm) in a 37¼C incubator for 4 hr. In all cases, the final concentration of DMSO in the treatment medium was 1%, including the solvent controls and the positive controls (0.3 μg/ml BaP with S9 and 0.1 μg/ml 4-NQO without S9). Following treatment, the cells were washed twice with basic (serum-free) medium by centrifugation and then resuspended in 20 ml of growth medium containing 10% heat-inactivated horse serum for a 2-day phenotypic expression of Tk-deficient mutants.
The Tk Microwell Mutant Assay
Tk mutants were selected using the microwell version of the MLA as described previously (Mei et al., 2012). Briefly, following the 2-day expression period, the cultures were adjusted to a density of 1 × 104 cells/ml in cloning medium that contained 20% heat-inactivated horse serum and 3 μg/ml of TFT selective agent. Two hundred microliter portions of the cell culture then were seeded into each well of four 96-well flat-bottom plates at a final density of 2,000 cells/well. For the determination of plating efficiency, the cultures were diluted to 8 cells/ml in cloning medium without TFT and 200 μl aliquots were seeded into each well of two 96-well flat-bottom plates. All plates were incubated for 11 days at 37°C in a humidified incubator with 5% CO2 in air. Mutant colonies were counted visually and categorized as small or large (Mei et al., 2014). WSS-induced cytotoxicity was measured using relative total growth (RTG), which is an estimate of relative cell growth (compared to the solvent control) for the treated cultures during the 4-h treatment, 2-day expression, and 11-day cloning phases. Mutant frequencies (MFs) were calculated using the Poisson distribution by counting the number of TFT-resistant clones, corrected for plating efficiency (Mei et al., 2014). Each WSS sample was tested in at least three (+S9 assays) or two (−S9 assays) independent experiments conducted between 29 October 2013 and 20 August 2014.
Data Analysis
The independent experiment was used as the experimental unit for statistical evaluation of the S9-mediated mutation responses (n = 3). As described in our previous study (Guo et al., 2016), the data evaluation criteria developed by the MLA Expert Workgroup of the IWGT were used for determining whether a specific treatment was positive (Moore et al., 2006). A positive response had an induced MF in one or more treated cultures that exceeded the Global Evaluation Factor (GEF, 126 mutants per 106 cells) and where a positive dose-response was obtained. Mutagenic potency was calculated from the slope of a linear regression line fit to the dose–response data (DeMarini et al., 2008) and expressed as MF per 1% (v/v) WSS in the treatment medium using GraphPad (GraphPad Software, La Jolla, CA). The NOGEL (defined as the highest tested concentration that did not induce a significant increase in MF) and LOGEL (defined as the lowest tested concentration that induced a significant increase in MF) were calculated by one way analysis of variance (ANOVA) followed by Dunnett’s test using SigmaPlot 13.0 (Systat Software, San Jose, CA). Unpaired t tests were used to compare the cytotoxic and mutagenic effects induced by C1-HCI-20 and C2-HCI-20 (GraphPad Software).
BMD analysis was conducted using PROAST software (version 38.9, developed by the Dutch National Institute for Public Health and the Environment, RIVM) and following the technical guidance provided by RIVM (RIVM, 2013). Briefly, the concentration-dependent data were modeled using the exponential and Hill models. Next, the BMD10, BMD50, BMD100, and BMD200 values (a 10%, 50%, 100%, and 200%, respectively, increase over the background MF) were calculated for each data set without covariate analysis. Then the BMDU and BMDL, which refer to the two-sided (upper and lower bounds) 95% confidence intervals (CIs), were calculated for each BMD. The BMD, BMDU, and BMDL values for the exponential and Hill models were quite comparable except for a few data sets that produced extremely high BMDUs with the Hill model (Supporting Information Table 2). Thus, the exponential model was chosen in the present study for conducting WSS potency ranking. In addition, the BMD covariate approach, a recently developed computational method which permits combined analysis of multiple dose–response data sets (Wills et al., 2016), was performed to improve BMD precision.
RESULTS
Cytotoxicity and Mutagenicity of Six WSSs in the Absence of S9 Activation
Based on the dose-range finding tests and their cytotoxicity, concentrations ranging from 0.1 to 1.0% (v/v) were used for the genotoxicity testing of the six WSSs in the absence of S9 metabolic activation. Concentrations higher than 1.0% were not included in the present study because of the 1.0% concentration limit for DMSO solvent in the treatment medium (OECD, 2015). According to the criteria recommended by the IWGT MLA workgroup (Moore et al., 2006), only three out of the six WSSs (C2-ISO-60, C1-HCI-60, and C2-HCI-60) produced weak positive responses in the MLA at the highest one or two concentrations (Table II). All six WSSs, however, induced concentration-dependent cytotoxicity in mouse lymphoma cells. Three WSSs reached the 10–20% RTG limit for cytotoxicity at test article solvent concentrations that were lower than the maximum allowed for the assay (1.0% v/v), i.e., 0.25%, 0.4%, and 0.8% for C2-HCI-60, C1-HCI-60, and C2-HCI-20, respectively. High levels of cytotoxicity were not necessarily accompanied by mutagenicity; at an RTG of 13%, C2-HCI-20 resulted in a MF of 177 per 106 cells, a MF that did not exceed the sum of the GEF (126 per 106 cells) and background MF of 111 per 106 cells, and which, according to IWGT and OECD guidelines, is a negative mutagenicity response in the MLA. Two WSSs made from Cigarette #1 (C1-ISO-60 and C1-HCI-20) had RTGs (49–56%) that did not reach the recommended cytotoxicity level of 10–20% RTG at the recommended limit for solvent concentration (Moore et al., 2006). These WSSs were also negative for mutagenicity in the absence of S9. Because of the generally weak mutagenic responses of the WSSs in the absence of S9, these responses were not evaluated further using dose–response metrics.
TABLE II.
Cytotoxicity and Mutagenicity of Whole Smoke Solutions (WSSs) in the Mouse Lymphoma Assay Without Metabolic Activationa
| WSS IDb | Concentration (%, v/v) | RTG (%) | MF (×10−6) | SC (%) |
|---|---|---|---|---|
| C1-ISO-60 | 0 | 100 | 92 | 45 |
| 0.2 | 95 | 86 | 44 | |
| 0.4 | 76 | 127 | 28 | |
| 0.6 | 88 | 161 | 35 | |
| 0.8 | 79 | 123 | 46 | |
| 1.0 | 56 | 165 | 41 | |
| C2-ISO-60 | 0 | 100 | 72 | 39 |
| 0.2 | 83 | 92 | 42 | |
| 0.4 | 90 | 115 | 52 | |
| 0.6 | 48 | 125 | 53 | |
| 0.8 | 30 | 204c | 50 | |
| 1.0 | 24 | 207c | 46 | |
| C1-HCI-20 | 0 | 100 | 111 | 48 |
| 0.2 | 85 | 105 | 49 | |
| 0.4 | 89 | 87 | 57 | |
| 0.6 | 68 | 83 | 72 | |
| 0.8 | 61 | 109 | 57 | |
| 1.0 | 49 | 157 | 57 | |
| C1-HCI-60 | 0 | 100 | 82 | 34 |
| 0.1 | 89 | 74 | 36 | |
| 0.2 | 59 | 119 | 43 | |
| 0.25 | 44 | 140 | 41 | |
| 0.3 | 30 | 196 | 46 | |
| 0.35 | 30 | 233c | 55 | |
| 0.4 | 13 | 372c | 54 | |
| C2-HCI-20 | 0 | 100 | 111 | 48 |
| 0.2 | 63 | 74 | 60 | |
| 0.4 | 65 | 104 | 46 | |
| 0.6 | 35 | 128 | 58 | |
| 0.8 | 13 | 177 | 61 | |
| C2-HCI-60 | 0 | 100 | 82 | 34 |
| 0.1 | 83 | 102 | 46 | |
| 0.15 | 65 | 131 | 43 | |
| 0.2 | 44 | 170 | 48 | |
| 0.25 | 23 | 222c | 45 |
A positive control of 0.1 μg/ml 4-nitroquinoline 1-oxide included in each experiment.
Sixty (or 20) of Cigarette #1 (or #2) were smoked under ISO (or HCI) regimen (more information in Table I).
Positive response exceeded the Global Evaluation Factor.
RTG, relative total growth that includes a measure of growth during treatment, expression, and cloning phases.
MF, mutant frequency per 106 cells.
SC, the percentage of small colonies.
Cytotoxicity and Mutagenicity of Six WSSs in the Presence of S9 Activation
Preliminary testing showed that the addition of S9 in the treatment medium generally reduced WSS-induced cytotoxicity. WSS MFs, however, were significantly increased in the presence of metabolic activation. Table III shows WSS-induced RTG, MF, and percentage of small colony mutants expressed as the mean ± 1 standard deviation (SD) from ≥3 independent experiments with S9. Based on the GEF, C1-HCI-20 was negative in the MLA, while the five other WSSs were positive and produced concentration-related cytotoxicity and mutagenicity following the 4-hr treatment. The lowest concentrations giving a significant positive response were 0.2%, 0.3%, 0.6%, 0.6%, and 1.0% for C2-HCI-60, C1-HCI-60, C2-ISO-60, C2-HCI-20, and C1-ISO-60, respectively, indicating that a positive response was observed only at the highest concentration tested for C1-ISO-60. In the presence of S9, only two WSSs were found to have a highest testable concentration lower than 1.0%, i.e., 0.5% and 0.6% for C2-HCI-60 and C1-HCI-60, respectively (Table III).
TABLE III.
Cytotoxicity and Mutagenicity of Whole Smoke Solutions (WSSs) in the Mouse Lymphoma Assay with Metabolic Activationa
| WSS IDb | Concentration (%, v/v) | RTG (%) | MF (×10−6) | SC (%) |
|---|---|---|---|---|
| C1-ISO-60 | 0 | 100±7 | 114±14 | 43±8 |
| 0.2 | 98±15 | 162±18 | 32±7 | |
| 0.4 | 94±12 | 194±66 | 28±5 | |
| 0.6 | 77±9 | 213±58 | 39±8 | |
| 0.8 | 72±9 | 229±30 | 32±5 | |
| 1.0 | 55±12 | 273±46c | 36±7 | |
| C2-ISO-60 | 0 | 100±8 | 115±20 | 39±3 |
| 0.2 | 96±5 | 162±23 | 37±5 | |
| 0.4 | 84±6 | 170±7 | 45±6 | |
| 0.6 | 72±8 | 259±35c | 40±4 | |
| 0.8 | 68±9 | 295±53c | 43±8 | |
| 1.0 | 55±10 | 415±105c | 45±6 | |
| C1-HCI-20 | 0 | 99±5 | 116±18 | 38±7 |
| 0.2 | 96±28 | 114±48 | 38±4 | |
| 0.4 | 93±16 | 121±34 | 38±5 | |
| 0.6 | 70±36 | 157±42 | 53±10 | |
| 0.8 | 81±11 | 176±45 | 44±3 | |
| 1.0 | 63±5 | 213±30 | 41±3 | |
| C1-HCI-60 | 0 | 100±7 | 87±26 | 37±9 |
| 0.1 | 79±11 | 152±29 | 44±6 | |
| 0.2 | 76±13 | 186±28 | 48±7 | |
| 0.3 | 64±9 | 256±38c | 50±2 | |
| 0.4 | 42±5 | 449±46c | 52±5 | |
| 0.5 | 22±3 | 894±3c | 56±3 | |
| 0.6 | 11±1 | 1170±140c | 55±5 | |
| C2-HCI-20 | 0 | 99±12 | 109±15 | 40±9 |
| 0.2 | 77±20 | 152±32 | 47±6 | |
| 0.4 | 76±20 | 197±35 | 49±9 | |
| 0.6 | 57±17 | 270±45c | 48±9 | |
| 0.8 | 42±11 | 449±114c | 60±6 | |
| 1.0 | 22±4 | 709±169c | 53±7 | |
| C2-HCI-60 | 0 | 100±5 | 92±32 | 36±7 |
| 0.1 | 82±7 | 114±19 | 55±12 | |
| 0.2 | 67±7 | 222±26c | 56±6 | |
| 0.3 | 45±3 | 383±53c | 55±3 | |
| 0.4 | 22±5 | 656±104c | 59±4 | |
| 0.5 | 9±2 | 822±187c | 62±3 |
Data are expressed as the mean ± 1 standard deviation (SD) from 3 or 4 independent experiments that included a positive control of 0.3 μg/ml benzo[a]pyrene in each experiment.
Sixty (or 20) of Cigarette #1 (or #2) were smoked under ISO (or HCI) regimen (more information in Table I).
Positive response exceeded the Global Evaluation Factor.
RTG, relative total growth that includes a measure of growth during treatment, expression, and cloning phases.
MF, mutant frequency per 106 cells.
SC, the percentage of small colonies.
Quantitative Analysis of MLA Mutagenicity Data from Six WSSs
We used the same metrics as in our previous study (Guo et al., 2016) to quantify the S9-mediated mutagenic responses of the six WSSs in the MLA. Initially, the BMD10 (i.e., 10% increase above the background response) was calculated using the PROAST software package. The exponential model provided the best fit for the BMD10 calculation for all sample data. The dose–response BMD10 modeling plots using the Tk gene MF data are shown in Supporting Information Figure 1. Then, we calculated the BMDs for 50%, 100%, and 200% increases above the background response in order to better discriminate between the dose-responses (Table IV).
TABLE IV.
Comparison and Rankings of the Benchmark Doses (BMDs) Producing a 10%, 50%, 100%, and 200% Increase in the Background Frequency (BMD10–200) for the Six WSSs by PROAST
| WSS IDa | BMD10 (%, v/v) |
BMD50 (%, v/v) |
BMD100 (%, v/v) |
BMD200 (%, v/v) |
||||
|---|---|---|---|---|---|---|---|---|
| PROAST1c | PROAST2d | PROAST1c | PROAST2d | PROAST1c | PROAST2d | PROAST1c | PROAST2d | |
| C1-ISO-60 | 0.051 [1]b | 0.309 [5] | 0.361 [4] | 0.690 [5] | 0.836 [5] | 0.975 [5] | 1.940 [5] | 1.380 [5] |
| C2-ISO-60 | 0.110 [4] | 0.211 [4] | 0.343 [3] | 0.471 [4] | 0.559 [4] | 0.666 [4] | 0.912 [4] | 0.941 [4] |
| C1-HCI-20 | 0.404 [6] | 0.326 [6] | 0.724 [6] | 0.728 [6] | 1.130 [6] | 1.030 [6] | N/A [6] | 1.450 [6] |
| C1-HCI-60 | 0.069 [3] | 0.059 [2] | 0.146 [2] | 0.131 [2] | 0.201 [2] | 0.185 [2] | 0.277 [2] | 0.262 [2] |
| C2-HCI-20 | 0.178 [5] | 0.149 [3] | 0.369 [5] | 0.332 [3] | 0.504 [3] | 0.469 [3] | 0.690 [3] | 0.663 [3] |
| C2-HCI-60 | 0.064 [2] | 0.052 [1] | 0.125 [1] | 0.115 [1] | 0.168 [1] | 0.162 [1] | 0.227 [1] | 0.230 [1] |
Sixty (or 20) of Cigarette #1 (or #2) were smoked under ISO (or HCI) regimen (more information in Table I).
Number in square bracket indicates the potency ranking of the WSSs.
PROAST1, BMDs calculated from exponential model of the PROAST Software for the MLA MF data.
PROAST2, BMDs calculated from exponential model of the PROAST Software for the MLA MF data using whole smoke solution as a covariate.
One way ANOVA followed by Dunnett’s test was employed to determine the NOGEL and LOGEL (Fig. 1A). In agreement with the result that C1-HCI-20 was negative based on the GEF criteria, no statistically significant differences in the mean values among the treatment groups were observed; thus neither NOGEL nor LOGEL values could be calculated for the C1-HCI-20 gene mutation data. The mutagenic potencies of the six WSSs were calculated using the slope of the linear regression of the dose–response curves (Fig. 1B). The trend-lines had correlation coefficients (R2) of between 0.82 and 0.98. The mutagenic potencies of the six WSSs displayed an 18-fold difference between the most (C1-HCI-60) and the least (C1-HCI-20) mutagenic WSS.
Fig. 1.
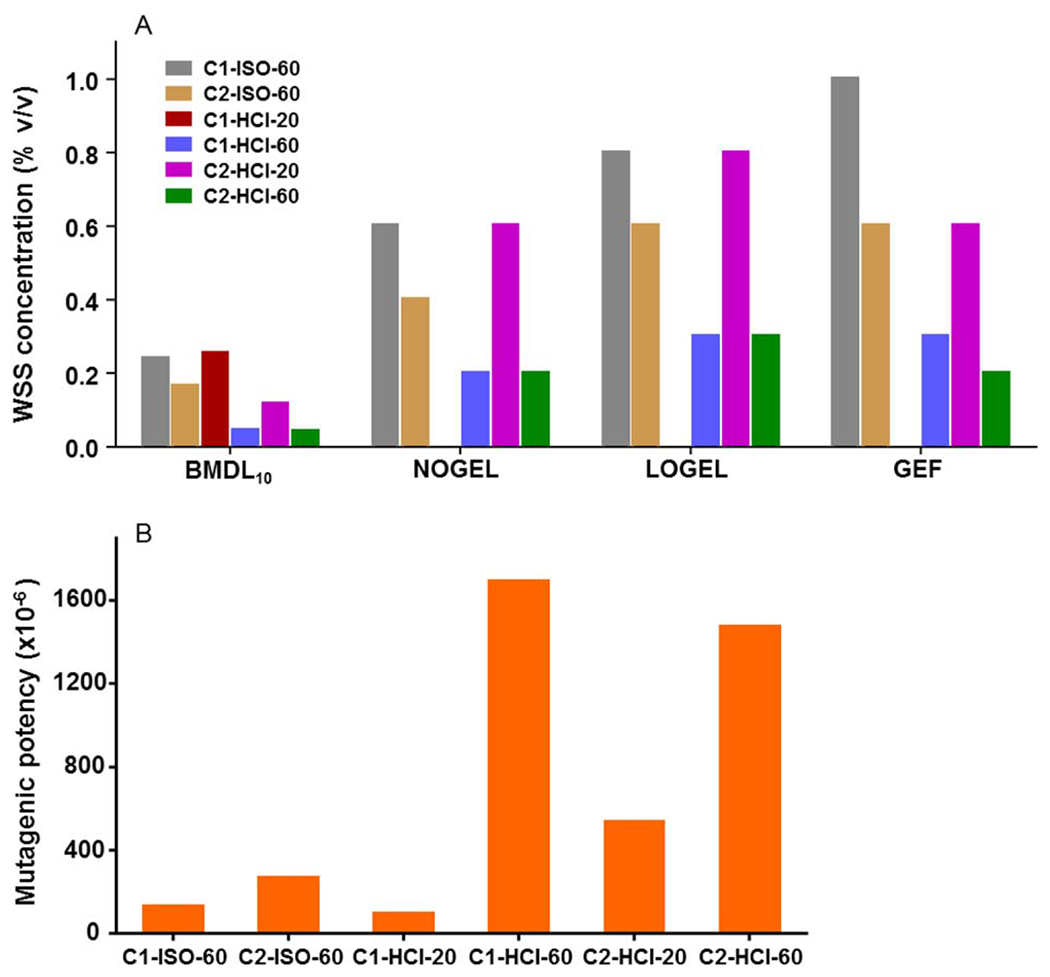
Relative mutagenicity of six whole smoke solutions (WSSs) in the mouse lymphoma assay. (A) BMDL10, calculated from the exponential model of the PROAST Software using whole smoke solution as covariate approach, is the lower limit of the BMD10 that is a dose that produces a predetermined change in the response of an adverse effect over control [in this case, 10% increase in mutant frequency (MF)]; NOGEL and LOGEL, determined by one way ANOVA followed by Dunnett’s test; GEF, Lowest positive response based on the global evaluation factor (GEF). A lower value indicates a higher mutagenic potential. No values were generated for NOGEL, LOGEL, and GEF in C1-HCI-20, as no positive response was observed over the tested concentrations. (B) Mutagenic potency, calculated from the slope of the linear regression of the dose–response curve.
Comparison of Six WSSs’ Mutagenicity Using Different Quantification Metrics
Differences in how various metrics describe dose-response data are anticipated based on the different approaches that they use and their focus on different parts of the dose-response relationships. For all the metrics except mutagenic potency, the lower the numerical values, the higher the potential mutagenicity. As shown in Figure 1A, the BMDL10s generated the lowest numerical values of all the quantitative metrics, as it evaluated a small (10%) increase in MF over the control. Even so, the rankings of the six WSSs that were produced by the different metrics all showed that C1-HCI-60, C2-HCI-60, and C2-HCI-20 were the three most potent mutagens, whereas the other three WSSs were less potent, with C1-HCI-20 ranking consistently as the weakest mutagen.
The values and the ordering using GEF and LOGEL were quite comparable (differences of no more than 1 ranking position) as both criteria defined the lowest part of the mutagenicity dose–responses. Consistent with our previous study that ranked the mutagenicity of five chemicals found in tobacco smoke (Guo et al., 2016), the mutagenic potency metric produced the highest numerical values among all the metrics evaluated (Fig. 1 and Supporting Information Table 1), as well as the widest range of values from the greatest to the least potent agent (18-fold vs. 3–5-fold for the other metrics).
Comparison of BMD Values Generated by PROAST with or Without Covariate Analysis
When the six dose–responses were analyzed individually by PROAST, a wide range of CIs was observed, which included relatively large BMDU/BMDL ratios for some of the WSSs (e.g., C1-ISO-60, Supporting Information Table 2). The large ratios resulted mainly from the extremely high upper bound of the CIs. As shown in Figures 2A–2D, when the individual dose–responses were evaluated, the BMD10 and BMD50 values for the six dose–responses overlapped each other, while the BMD100 and BMD200 values formed two groups whose upper and lower 95% CIs did not overlap. The PROAST analysis without WSS as a covariate provided discrete groupings only for BMD100s (Fig. 2C) and BMD200s (Fig. 2D).
Fig. 2.
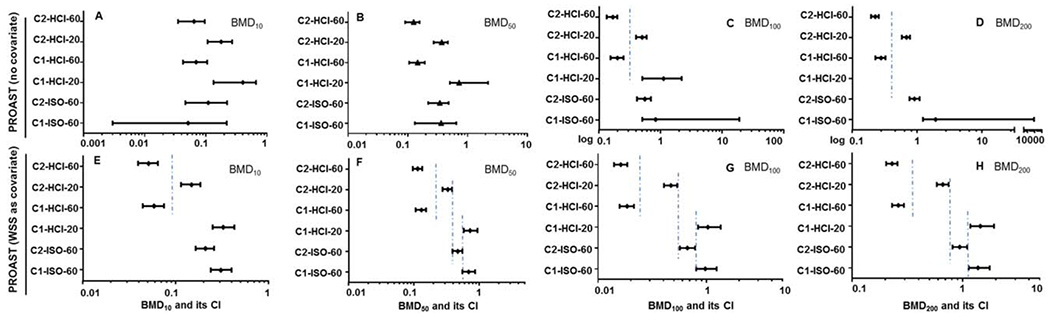
Comparison of BMD values for WSSs in the mouse lymphoma assay. The BMD (BMD10, BMD50, BMD100, and BMD200) estimates producing a 10%, 50%, 100%, or 200% increase in the background frequency were calculated using exponential model of PROAST both without (A,B,C,D) and with covariate analysis approach (E,F,G,H). The bars represent the calculated 95% confidence interval of each value. The lower and upper limits derived from the BMD estimates were used to differentiate between responses based on non-overlapping confidence intervals.
PROAST also gives the option of combining data by considering a variable (e.g., compound or tissue) as a covariate to provide additional precision to the BMD estimate (Wills et al., 2016). The BMDU/BMDL ratios were reduced when a combined analysis of the six dose–responses was performed using the BMD covariate approach (Supporting Information Table 3). The BMD50, BMD100, and BMD200 values for the six dose–responses formed four groups without overlapping 95% CIs (Figs. 2F–2H), indicating a greater degree of precision in the BMD estimates.
Mutagenicity Potency Ranking of WSSs Generated by Smoking Different Cigarettes under Different Regimens
Two types of commercial cigarettes that were previously reported to differ in levels of TNCO smoke constituents (FTC, 2000) were smoked by automated smoking machines under two different regimens (ISO and HCI) (Table I), the samples prepared by the HCI protocol were generated by smoking 20 and 60 cigarettes while the samples prepared with the ISO protocol only were generated by smoking 60 cigarettes. This allowed us to investigate the effect of varying levels of TNCO from the two types of cigarettes, number of cigarettes smoked, and smoking intensity on WSS-induced cytotoxicity and mutagenicity. There were no significant differences between the cytotoxicity or mutagenicity responses produced by the WSSs containing the highest tar levels generated from 60 of Cigarette #1 or 60 of Cigarette #2 (Figs. 3A and 3B). The data show that both test articles, Cigarettes #1 and #2 using 60 cigarettes, were ranked in the top two by all metrics of mutagenic potency (Table IV). The responses produced by C2-HCI-20, prepared by machine smoking 20 of Cigarette #2, however, were significantly greater than those of C1-HCI-20, prepared by smoking 20 of Cigarette #1 (Figs. 3C and 3D). For the HCl WSSs, the WSSs prepared with 60 cigarettes resulted in a higher mutagenic responses in the MLA than did the WSSs with 20 cigarettes, i.e., a dose–response was seen for mutagenic response.
Fig. 3.
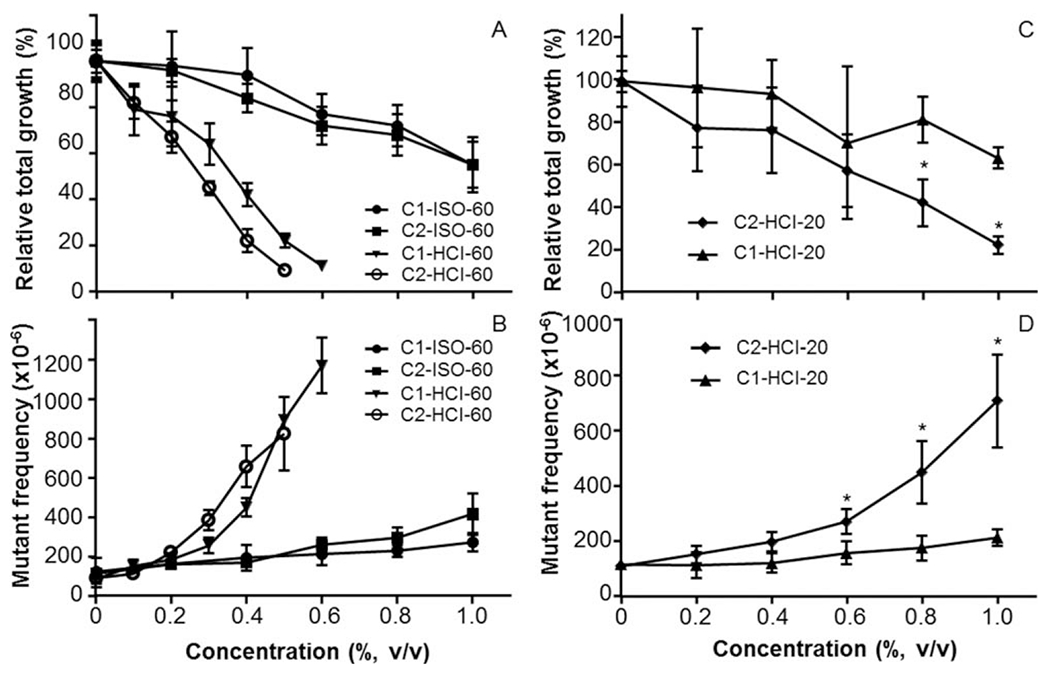
Comparison of cytotoxic and mutagenic effects in mouse lymphoma cells treated with WSSs from cigarettes machine-smoked under different regimens. (A,C) Cytotoxicity is presented as relative total growth (RTG, %); (B,D) mutagenicity is presented as mutant frequencies (MF) per 106 cells. All data are expressed as the mean ± 1 SD from 3 or 4 independent experiments.
Increased machine smoking intensity (larger puff volume and 100% blocking of filter vent holes) also resulted in the changes in mutagenic response; for both types of cigarettes, the WSS prepared using the HCI protocol resulted in higher mutagenic responses than the WSS prepared with the ISO protocol (Fig. 1 and Table IV). BMD potency ranking analysis also showed that when 60 of Cigarette #1 or #2 were smoked under the HCI smoking regimen, the CIs of the BMD50s, BMD100s, and BMD200s overlapped each other. The data shown in Figures 2F–2H indicate that the mutagenic responses (i.e., BMDs) produced by C2-HCI-60 and C1-HCI-60 were not significantly different. This could suggest the sensitivity of the modeling technique could not distinguish between the tar levels emitted from these products (6.4 and 9.3 mg tar/ml). In contrast, it can be seen in these same three panels in Figure 2 that BMD potency ranking at BMD50, BMD100, and BMD200 was able to separate the mutagenicity of samples produced by smoking 60 of Cigarette #1 or #2 under the ISO protocol (C1-ISO-60 vs. C2-ISO-60), or by smoking 20 of Cigarette #1 or #2 under the HCI protocol (C1-HCI-20 vs. C2-HCI-20), or by smoking 60 or 20 Cigarette #2 (C2-HCI-60 vs. C2-HCI-20) or by smoking 60 or 20 Cigarette #1 (C1-HCI-60 vs. C1-HCI-20) under the HCI protocol. In addition, BMD potency rankings at BMD50, BMD100, and BMD200 were able to separate the mutagenicity responses of samples prepared by smoking either 60 of Cigarette #1 or Cigarette #2 smoked under the HCI or ISO protocol (C2-HCI-60 vs. C2-ISO-60 and C1-HCI-60 vs. C1-ISO-60).
DISCUSSION
We applied the BMD approach to analyzing in vitro MLA data in order to evaluate if it is possible to discriminate among the mutagenic responses of WSSs. The WSSs were generated from the two different types of combustible cigarette products. These cigarettes were smoked using two different and widely recognized smoking machine regimens. In a previously reported pilot study, we evaluated MLA dose–response data for five representative carcinogenic compounds contained in cigarette smoke using several quantitative methods (Guo et al., 2016). In the present study, we extended our work to quantitatively evaluate the ability of these methods to differentiate between the mutagenicity produced by a series of WSSs generated under different smoking machine conditions. Six WSSs were prepared using two different combustible cigarette tobacco products (Cigarette #1 or Cigarette #2), smoked under the ISO or HCI smoking-machine regimens, and using different numbers of cigarettes (20 or 60). Cigarettes #1 and #2 were analyzed and found to produce different tar levels (Table I).
We first evaluated the genotoxicity of the six WSSs in the MLA both with and without metabolic activation. The results showed that WSSs were more cytotoxic in cells treated in the absence of S9 compared to treatments conducted with S9. In contrast, S9 activation increased the mutagenicity of the WSSs. This later finding is consistent with previous reports indicating that many human carcinogens, including four well recognized chemical constituents of cigarette smoke [4-aminobiphenyl (4-ABP), BaP, 2-amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline (MeIQ), and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)] that were tested in our previous study (Guo et al., 2016), require metabolic activation to exert their mutagenic and carcinogenic activities (Luch, 2005). This observation was also consistent with the findings of a study that evaluated the genotoxicity of machine-generated mainstream smoke using the Ames bacterial reverse-mutation assay (Kilford et al., 2014). Mainstream smoke from Kentucky Research Reference Cigarettes (3R4F), which are cigarettes not intended for use by humans but are used in research for benchmarking in chemical analysis, produced concentration-related increases in the number of revertants in four strains of Salmonella typhimurium (TA98, TA100, YG1024, and YG1042) in the presence of S9, but produced no clear concentration-related increases in revertant numbers without S9 activation. Another study tested mainstream smoke total particulate matter (TPM) from 1R4F reference cigarettes and three experimental single tobacco type cigarettes (Bright, Burley, or Oriental) in the MLA. Although higher concentrations of TPM were required for producing positive responses in the treatments with S9 as compared to treatments without S9, both treatments induced the same fold-increases in MF (Schramke et al., 2006). It should be noted that TPM, unlike WSSs and smoke generated from a smoking machine, contains only the particulate phase of cigarette smoke, and testing TPM does not reflect the contribution of gas phase smoke constituents to the toxicity of cigarette smoke (Li et al., 2014). Overall, the results of our studies are consistent with previous observations that exogenous metabolic activation increases the genotoxicity of cigarette smoke (Kilford et al., 2014), and suggest that, because WSSs would be anticipated to contain compounds derived from both the aerosol and particulate phases of cigarette smoke, they may have a unique value for conducting toxicological assessments of tobacco products.
One strength of this study is the utilization of a mammalian cell-based test for assessing mutagenicity of different chemicals. An advantage of this test system is the ability to characterize the diverse range of possible mutations induced through sequencing the mutated Tk gene, thereby increasing our understanding of the mutational origin. By knowing the mechanism of action, additive effects of structurally similar chemicals from a mixture of chemicals might potentially be evaluated effectively. In addition compared to use of a nonmammalian cell-based assay, the use of a mammalian cell-based assay system might be better suited for comparative in vitro genotoxicity potency ranking.
As we found in our previous study (Guo et al., 2016), all the quantitative metrics, i.e., BMDL10 by PROAST, NOGEL, LOGEL, the lowest positive response by the GEF, and the mutagenic potency, ordered the relative mutagenicity of the six WSSs in a manner consistent with their anticipated mutagenic potency. NOGEL, LOGEL, and the lowest positive response by GEF are highly dependent on study design features such as dose selection and the scatter in the data (Johnson et al., 2014; Slob and Setzer, 2014). When a weak mutagenic response is observed, NOGEL, LOGEL, and GEF may not generate any values, and that was the case for C1-HCI-20 in the current study. Also, these approaches do not allow calculation of CIs (Soeteman-Hernandez et al., 2015; Wills et al., 2016). Both BMD analysis and mutagenic potency estimates take the entire data set into account for estimating mutagenic potential. However, the mutagenic potency calculation uses a linear regression fit to the increasing portion of the dose-response curve (DeMarini et al., 2008), while the BMD estimates are calculated from a dose–response model that best fits the shape of the dose–response relationship (Slob and Setzer, 2014). A mutagenic potency expressed as a linear slope is assumed to be proportional to a BMD that is based on fitting a linear model. However, dose-response data are not always linear. Also, mutagenic potency is greatly influenced by the upper values of the mutagenicity dose–response, while BMDs can be estimated at multiple points along the dose–response curve. This present study calculated BMD10s, BMD50s, BMD100s, and BMD200s, which represent a 10%, 50%, 2-fold, and 3-fold increase in the background MF, respectively. The most important practical feature of BMD analysis is its ability to generate CIs for the BMDs (i.e., BMDL-BMDU metrics), which can be used to separate dose–response mutagenic potencies in a scientifically rigorous manner (Soeteman-Hernandez et al., 2015; Wills et al., 2016). Thus, the BMDL and BMDU values for the BMD50s, BMD100s, and BMD200s that were calculated by PROAST software using covariate analysis divided the six dose–responses into four groups whose upper and lower 95% CIs did not overlap (Fig. 2). In contrast, mutagenic potency divided the six dose–responses into only two groups without overlapping two-sided CIs. (It should be noted that when the CIs of two BMDs overlap, this is evidence that the BMDs are not significantly different, not that they are identical) (Supporting Information Figure 2). In addition, the BMD potency ranking was able to use the MLA mutagenicity dose–responses to identify differences in the mutagenic potencies of the WSSs that were expected based on their chemical compositions. As shown in Figure 2 and Table IV, we observed different potency levels for the test articles in a manner reflecting their expected toxicities based on the level of tar.
PROAST employs exponential and Hill models for describing dose–responses using continuous data endpoints. Both models produced similar BMD values; suggesting that model selection was not a crucial issue for this data set. For the present analysis, we used the exponential model for estimating BMDs, because the Hill model produced extremely high BMDU values for two WSSs (Supporting Information Table 2). Our findings suggest that, when the covariate approach was used, the BMD50s, BMD100s, and BMD200s were capable of dividing the six WSS dose–responses into four groups that differed significantly from one another (Fig. 2 and Supporting Information Table 3). However, we also found that when the dose–responses were analyzed individually, i.e., no covariate approach was employed, PROAST generated high or infinite upper CIs for weak positive or negative (by the GEF rule) mutagenicity data (Table III and Supporting Information Table 2; see results for C1-ISO-60 and C1-HCI-20). These observations indicate that the weak mutagenic effects and the imprecision in measuring dose–responses in independent studies may contribute to the variability of CI values; and that it is useful to conduct covariate analysis for improving the BMD precision.
A second consideration was whether or not the ordering of the four groups defined by the potency estimates was consistent with the toxicity expected for the WSSs. It is expected that WSSs generated from a greater number of cigarettes (i.e., 60) contain a higher concentration of chemical components as compared to that generated from a smaller number of the same cigarettes (i.e., 20). As evaluated by their BMD50s, BMD100s, and BMD200s (Table IV and Fig. 2), the HCI WSSs produced by smoking 60 cigarettes consistently produced greater mutagenic potencies (smaller BMDs) than the WSSs prepared under the same conditions using 20 cigarettes (C2-HCI-60 > C2-HCI-20 and C1-HCI-60 > C1-HCI-20). In agreement with the fact that Cigarette #2 produced higher TNCO yields when smoked with an automated smoking machine compared to Cigarette #1, WSSs prepared from Cigarette #2 displayed higher mutagenic potency than WSSs from Cigarette #1 when 60 cigarettes were smoked under the ISO regimen (C2-ISO-60 > C1-ISO-60) or when 20 cigarettes were smoked under the HCI protocol (C2-HCI-20 > C1-HCI-20) (Figs. 2E–2H and Table IV). It should be noted that these results are valid under the experimental conditions used in this study; when smoked by humans, the mutagenic potencies of Cigarettes #1 and # 2 may vary because of the different smoking topography of consumers (i.e., how a person smokes a cigarette including the puff frequency, puff volume, and puff duration). In addition, the WSSs generated by smoking 60 cigarettes under the HCI regimen were more mutagenic than WSSs prepared by smoking 60 cigarettes using the ISO regimen, i.e., C1-HCI-60 > C1-ISO-60 and C2-HCI-60 > C2-ISO-60 (Fig. 2). However, when 60 cigarettes were smoked under the intensive HCI smoking regimen, C1-HCI-60 and C2-HCI-60 displayed a similar rank order of mutagenic potency. We speculate that this may have occurred because the metabolic capacity of the S9 used in our assays was saturated by the relatively high levels of chemical constituents contained in these samples or because the chemical constituents contained in tobacco smoke became saturated in the solvent during WSS preparation.
As mentioned previously, there are limitations with this study. For example, we did not perform chemical analysis on the smoke generated by the ISO protocol that was used to prepare the C1-ISO-60 and C2-ISO-60 samples. However, it is helpful to know that studies reported in the literature have found higher levels of TPM and tar in smoke generated by the HCI as compared with the ISO smoking protocol (Roemer et al., 2012). In addition, this study tested only two different commercial combustible cigarette products; therefore, more studies are warranted to assert that this technique and the specific models might be helpful to understand the in vitro genotoxicity profile of different tobacco products. Another factor to consider with the modeling methodology is that varying BMDs can be generated under different model fits (e.g., “best” - fitting model, range of “sufficiently” fitting models, or “average model”), depending upon the selection and analysis of the data. Fortunately, the BMD approach accounts for statistical variability of the dose–response data as calculated by the two-sided confidence interval.
In summary, this follow-up study performed quantitative dose–response analysis of MLA data produced by testing six WSSs. The WSSs were generated from the whole mainstream smoke of two commercial cigarettes (#1 and #2), using two different smoking regimens (ISO and HCI), and using different numbers of cigarettes (20 and 60). Further research is needed, such as increasing the sampling size to better understand the variability, and transferability of this method, as well as its precision, to establish this approach. Although testing additional cigarette types and more investigation is needed to establish this approach, the results of this integrative modeling and in vitro study are encouraging in that they suggest that these methods (MLA outcomes on WSS and BMD modeling) may provide useful information to help assess differing mutagenic responses to WSSs generated from combustible cigarettes by machine protocols. This is the first study that uses quantitative tools for assessing the mutagenic potency of cigarette WSSs. As mentioned above, additional studies can determine which quantitative approach (i.e., metric) or combination of quantitative metrics might be helpful for understanding the mutagenic potency of WSSs generated from different combustible tobacco products. The objective was to evaluate the approaches described in this study to learn if they could distinguish between in vitro mutagenic potencies of different combustible cigarettes using different machine smoking regimes and if the metrics showed any similar rank order in potency. Overall, the CIs generated by the BMD approach resulted in the most informative comparisons between the dose–responses; and covariate analysis improved the precision of individual BMD estimates. The results from this study using machine generated WSSs and a small sample size of combustible cigarettes support further investigation towards understanding the utility of quantitative dose-response modeling of in vitro MLA data using BMD techniques.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by an inter-center agreement within the U. S. Food and Drug Administration (FDA) between the Center for Tobacco Products (CTP) and the National Center for Toxicological Research (NCTR). The information in this manuscript is not a formal dissemination of information by the FDA. This publication represents the views of the authors and does not represent FDA position or policy.
Footnotes
This publication represents the views of the author(s) and does not represent the United States Food and Drug Administration/Center for Tobacco Products position or policy.
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Cao X, Mittelstaedt RA, Pearce MG, Allen BC, Soeteman-Hernandez LG, Johnson GE, Bigger CA, Heflich RH. 2014. Quantitative dose–response analysis of ethyl methanesulfonate genotoxicity in adult gpt-delta transgenic mice. Environ Mol Mutagen 55:385–399. [DOI] [PubMed] [Google Scholar]
- CDC. 2014. Mechanisms of cancer induction by tobacco smoke. 2014 Surgeon General’s Report: The health consequences of smoking—50 years of progress. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf (accessed 10/2/2017).
- CDC. 2016. Tobacco. National Biomonitoring Program. https://www.cdc.gov/biomonitoring/tobacco.html (accessed 10/2/2017). [Google Scholar]
- DeMarini DM, Gudi R, Szkudlinska A, Rao M, Recio L, Kehl M, Kirby PE, Polzin G, Richter PA. 2008. Genotoxicity of 10 cigarette smoke condensates in four test systems: Comparisons between assays and condensates. Mutat Res 650:15–29. [DOI] [PubMed] [Google Scholar]
- EPA. 2005. Guildelines for carcinogen risk assessment. http://www.epa.gov/sites/production/files/2013-09/documents/cancer_guidelines_final_3-25-05.pdf (accessed 10/2/2017).
- FTC. 2000. “Tar”, nicotine, and carbon monoxide of the smoke of 1294 varieties of domestic cigarettes for the year of 1998. https://wwwftcgov/sites/default/files/documents/reports/2000-report-tar-nicotine-and-carbon-monoxide-covering-1998/1998tarnicotinereport_0pdf (accessed 10/2/2017).
- Gollapudi BB, Johnson GE, Hernandez LG, Pottenger LH, Dearfield KL, Jeffrey AM, Julien E, Kim JH, Lovell DP, Macgregor JT, Moore MM, van Benthem J, White PA, Zeiger E, Thybaud V. 2013. Quantitative approaches for assessing dose–response relationships in genetic toxicology studies. Environ Mol Mutagen 54:8–18. [DOI] [PubMed] [Google Scholar]
- Guo X, Heflich RH, Dial SL, Richter PA, Moore MM, Mei N. 2016. Quantitative analysis of the relative mutagenicity of five chemical constituents of tobacco smoke in the mouse lymphoma assay. Mutagenesis 31:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Verkler TL, Chen Y, Richter PA, Polzin GM, Moore MM, Mei N. 2011. Mutagenicity of 11 cigarette smoke condensates in two versions of the mouse lymphoma assay. Mutagenesis 26:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LG, van Benthem J, Johnson GE. 2013. A mode-of-action approach for the identification of genotoxic carcinogens. PLoS One 8:e64532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. 2012. Tobacco smoking, a review of human carcinogens: Personal habits and indoor combustions. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 100E:43–212. [PMC free article] [PubMed] [Google Scholar]
- Johnson GE, Soeteman-Hernandez LG, Gollapudi BB, Bodger OG, Dearfield KL, Heflich RH, Hixon JG, Lovell DP, MacGregor JT, Pottenger LH, et al. 2014. Derivation of point of departure (PoD) estimates in genetic toxicology studies and their potential applications in risk assessment. Environ Mol Mutagen 55:609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilford J, Thorne D, Payne R, Dalrymple A, Clements J, Meredith C, Dillon D. 2014. A method for assessment of the genotoxicity of mainstream cigarette-smoke by use of the bacterial reverse-mutation assay and an aerosol-based exposure system. Mutat Res Genet Toxicol Environ Mutagen 769:20–28. [DOI] [PubMed] [Google Scholar]
- Li X, Nie C, Shang P, Xie F, Liu H, Xie J. 2014. Evaluation method for the cytotoxicity of cigarette smoke by in vitro whole smoke exposure. Exp Toxicol Pathol 66:27–33. [DOI] [PubMed] [Google Scholar]
- Luch A. 2005. Nature and nurture—Lessons from chemical carcinogenesis. Nat Rev Cancer 5:113–125. [DOI] [PubMed] [Google Scholar]
- MacGregor JT, Frotschl R, White PA, Crump KS, Eastmond DA, Fukushima S, Guerard M, Hayashi M, Soeteman-Hernandez LG, Johnson GE, et al. 2015a. IWGT report on quantitative approaches to genotoxicity risk assessment II. Use of point-of-departure (PoD) metrics in defining acceptable exposure limits and assessing human risk. Mutat Res Genet Toxicol Environ Mutagen 783:66–78. [DOI] [PubMed] [Google Scholar]
- MacGregor JT, Frotschl R, White PA, Crump KS, Eastmond DA, Fukushima S, Guerard M, Hayashi M, Soeteman-Hernandez LG, Kasamatsu T, Levy DD, Morita T, Muller L, Schoeny R, Schuler MJ, Thybaud V, Johnson GE. 2015b. IWGT report on quantitative approaches to genotoxicity risk assessment I. Methods and metrics for defining exposure-response relationships and points of departure (PoDs). Mutat Res Genet Toxicol Environ Mutagen 783:55–65. [DOI] [PubMed] [Google Scholar]
- Mei N, Guo X, Moore MM. 2014. Methods for using the mouse lymphoma assay to screen for chemical mutagenicity and photomutagenicity. In: Caldwell GW, Yan Z, editors. Optimization in Drug Discovery: In-vitro Methods. Totowa, NJ:Humana Press. pp 561–592. [Google Scholar]
- Mei N, Zhang Y, Chen Y, Guo X, Ding W, Ali SF, Biris AS, Rice P, Moore MM, Chen T. 2012. Silver nanoparticle-induced mutations and oxidative stress in mouse lymphoma cells. Environ Mol Mutagen 53:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MM, Honma M, Clements J, Bolcsfoldi G, Burlinson B, Cifone M, Clarke J, Delongchamp R, Durward R, Fellows M, et al. 2006. Mouse lymphoma thymidine kinase gene mutation assay: Follow-up meeting of the International Workshop on Genotoxicity Testing–Aberdeen, Scotland, 2003–Assay acceptance criteria, positive controls, and data evaluation. Environ Mol Mutagen 47: 1–5. [DOI] [PubMed] [Google Scholar]
- OECD. 2015. In vitro mammalian cell gene mutation assays using the thymidine kinase gene. OECD Guideline For The Testing Of Chemicals No:490. http://www.oecd-ilibrary.org/environment/test-no-490-in-vitro-mammalian-cell-gene-mutation-tests-using-the-thymidine-kinase-gene_9789264242241-en (accessed 10/2/2017). [Google Scholar]
- Recio L, Shepard KG, Hernandez LG, Kedderis GL. 2012. Dose–response assessment of naphthalene-induced genotoxicity and glutathione detoxication in human TK6 lymphoblasts. Toxicol Sci 126:405–412. [DOI] [PubMed] [Google Scholar]
- RIVM. 2013. PROAST. http://www.rivm.nl/en/Documents_and_publications/Scientific/Models/PROAST (accessed 10/2/2017).
- Roemer E, Schramke H, Weiler H, Buettner A, Kausche S, Weber S, Berges A, Stueber M, Muench M, Trelles-Sticken E, Pype J, et al. 2012. Mainstream smoke chemistry and in vitro and in vivo toxicity of the reference cigarettes 3R4F and 2R4F. Beiträge zur Tabakforschung International/Contributions to Tobacco Research 25:316–335. [Google Scholar]
- Schramke H, Meisgen TJ, Tewes FJ, Gomm W, Roemer E. 2006. The mouse lymphoma thymidine kinase assay for the assessment and comparison of the mutagenic activity of cigarette mainstream smoke particulate phase. Toxicology 227:193–210. [DOI] [PubMed] [Google Scholar]
- Slob W, Setzer RW. 2014. Shape and steepness of toxicological dose–response relationships of continuous endpoints. Crit Rev Toxicol 44:270–297. [DOI] [PubMed] [Google Scholar]
- Soeteman-Hernandez LG, Fellows MD, Johnson GE, Slob W. 2015. Correlation of in vivo versus in vitro benchmark doses (BMDs) derived from micronucleus test data: A proof of concept study. Toxicol Sci 148:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KE. 2002. Tobacco harm reduction: Promise and perils. Nicotine Tob Res 4: S61–S71. [DOI] [PubMed] [Google Scholar]
- Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ 3rd, Dix DJ, Andersen ME, Houck KA, et al. 2012. Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol Sci 125:157–174. [DOI] [PubMed] [Google Scholar]
- Wills JW, Johnson GE, Doak SH, Soeteman-Hernandez LG, Slob W, White PA. 2016. Empirical analysis of BMD metrics in genetic toxicology Part I: In vitro analyses to provide robust potency rankings and support MOA determinations. Mutagenesis 31:255–263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


