Abstract
Primary squamous cell carcinoma of the stomach is a rare type of gastric malignancies. Diagnosis criteria are well defined but diagnosis is generally late being made at an advanced stage with metastases explaining its poor diagnosis.
Keywords: fistula, ovarian metastases, Primary squamous cell carcinoma, stomach
Primary squamous cell carcinoma of the stomach is a rare type of gastric malignancies. Diagnosis criteria are well defined but diagnosis is generally late being made at an advanced stage with metastases explaining its poor diagnosis.
![]()
1. INTRODUCTION
Stomach cancer is the fifth most common cancer worldwide. There are different histological types of gastric carcinoma and adenocarcinoma is the most frequent one accounting for more than 90% of gastric malignancies, while primary gastric squamous cell carcinoma (PSCC) is rare, accounting for only 0.04% to 0.09% of all gastric carcinomas. 1 It is more prevalent in the sixth decade of life with a male to female ratio of 5 to 1. 2
The pathogenesis of this tumor is controversial, and many theories have been proposed in this respect. The treatment is also controversial, and its prognosis is generally poor. 3
We report a case of gastric PSCC with a bilio‐gastric fistula and Krukenberg syndrome in a women.
2. CASE PRESENTATION
A 66‐year‐old woman, with no medical history, consulted the emergency department for dyspnea with a three‐month history of intermittent epigastric pain associated with weight loss. She also reported a prior episode of melena.
Physical examination revealed stable vital signs, pale conjunctiva, and an epigastric 3‐cm hard painful mass. The digital rectal examination did not show any signs of bleeding. Cardiopulmonary examination was normal.
Laboratory findings revealed a biological inflammatory syndrome (WBC=13 570 /mm3, CRP =133 mg/ml) and a normochromic normocytic anemia at 4,5g/dl. Carcinoembryonic antigen as well as CA19‐9 levels were normal.
An upper gastrointestinal endoscopy was performed, showing a normal esophagus and fundus. In the prepyloric region, we found a tumor reducing the lumen and there was a pertuis which seems to correspond to a fistula. By crossing this pertuis, a large cavity with a necrotic bottom and a black brown stasis fluid was noted. The duodenum was normal (Figure 1). Biopsies of the tumor and the gastric mucosa were performed, and pathological examination showed well‐differentiated keratinizing squamous cell carcinoma (Figure 2).
FIGURE 1.
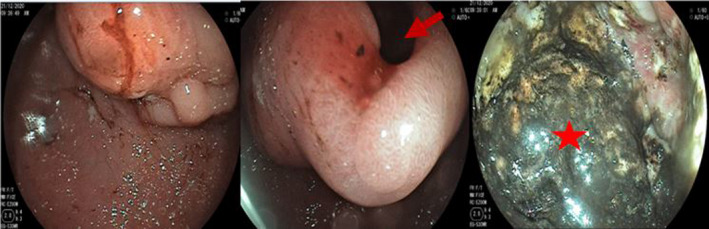
Upper gastrointestinal endoscopy : A tumor reducing the lumen in the prepyloric region with a pertuis (arrow) corresponding to a fistula conducting to a large cavity with a necrotic bottom and a black brown stasis fluid, bleeding easily (star)
FIGURE 2.
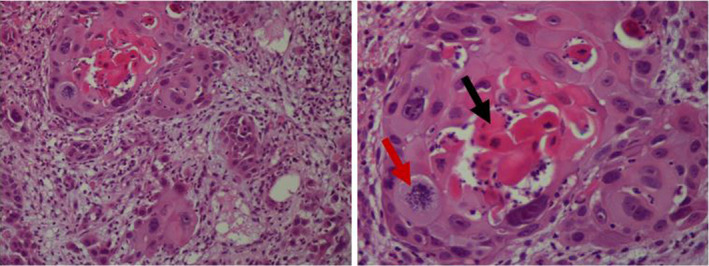
Infiltrating carcinomatous proliferation made up of lobules and clusters of atypical squamous cells (Gx200) centered by keratin (black arrow) with mitoses (red arrow) (G×400)
Computed tomography (CT) revealed a nonstenosing irregular circumferential thickening of the antropyloric region invading the segments IV and III of the liver by contiguity resulting in an heterogeneous mass measuring 71x60 mm with visibility of the left intrahepatic bile ducts. Suspicious ganglia of the hepatic hilum, the gastrohepatic ligament and the greater omentum were identified. The CT also showed a right well‐limited ovarian mass measuring 37x29mm, strongly enhanced in the portal phase, suggesting an ovarian metastasis: Krukenberg syndrome, as well as a thrombosis of the left renal vein and a subsegmental pulmonary embolism. No other metastasis was individualized (Figure 3).
FIGURE 3.
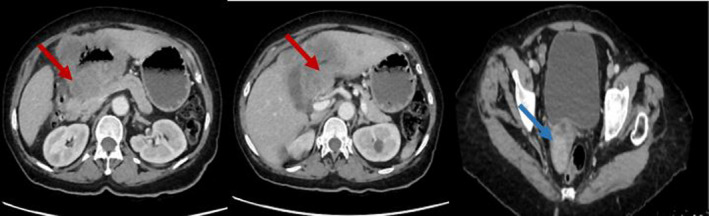
CT images in longitudinal section showing a nonstenosing irregular circumferential thickening of the antropyloric region invading the liver (red arrows) and ovarian metastases (blue arrow)
A bowel opacification was also performed showing a passage of the contrast agent in the biliary ducts, as well as a pneumobilia revealing a bilio‐gastric fistula (Figure 4).
FIGURE 4.
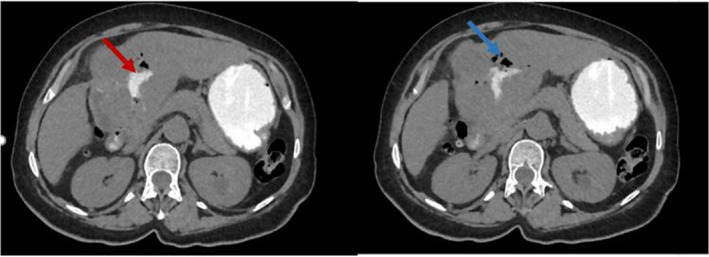
CT images in longitudinal section with opacification showing a passage of the contrast agent in the biliary ducts (red arrow), as well as an aerobilia (blue arrow) revealing a bilio‐gastric fistula
Extensive evaluation, including that of the ear, nose and throat, as well as gynecologic examination, revealed no other possible primary sites of involvement, confirming the primary squamous cell gastric carcinoma.
A palliative symptomatic treatment with transfusion and analgesics was indicated since the operative risk of the patient was high (performance status=3, recurrence of anemia after transfusion and pulmonary embolism) and the late stage of the tumor. Chemotherapy was not indicated because performance status of the patient was stage 3 and the infectious risk was high due to the biliodigestive fistula, as well as recurrence of the anemia and pulmonary embolism which worsen the prognosis of the patient.
She unfortunately died after few months.
3. DISCUSSION
Primary gastric squamous cell carcinoma amounts to less than one percent of all gastric carcinomas. 1 It is more prevalent in the sixth decade of life and in males.
The first case of PSCC was described in 1905. 4 Since then, less than 100 cases have been reported in the related literature.
The diagnostic criteria of PSCC of the stomach were defined by Parks: (a) tumor must not be occurring in the cardia; (b) tumor must not extend into the esophagus; and (c) there should be no evidence of squamous cell carcinoma in any other organ, such as lung or cervix. 5 These criteria were met in our case report. Indeed, the tumor was antropyloric, there was no evidence of extending into the esophagus and no other possible primary sites of involvement were found.
Boswell and Helwig described 4 histopathologic criteria for diagnosis of PSCC, of which at least 1 must be present: (a) keratinized cell masses forming keratin pearls, (b) mosaic cell arrangement, (c) intercellular bridges and (d) high concentration of sulfhydryl and/or disulfide groups, indicating the presence of keratin or prekeratin. 2
The Japanese had proposed an other classification for the diagnosis of PSCC of the stomach (a) all the tumor cells are squamous cell carcinoma cells and any part does not contain gland cancer cells, and (b) there is sufficient evidence to show that squamous cell carcinoma originates in the gastric mucosa. 2
Strong staining for p63 and high molecular weight cytokeratin (CK5/6) were associated with PSCC with a specificity of 99% and a sensitivity of 98%. Thus, immunohistochemistry can help the diagnosis. 3
The pathogenesis of this tumor is controversial and many theories have been proposed : totipotent stem cells, metaplastic squamous focus, heterotopic squamous epithelium in the stomach, the overgrowth of a squamous epithelium element in a primary adenocarcinoma, Epstein‐Barr virus or human papilloma virus. 6 , 7
The diagnosis is always late, at an advanced stage. It was the case in our patient in whom the CT scan revealed a liver infiltration. Different organs could be involved. Some cases of PSCC with other organs involvement reported in the literature are summarized in Table 1. 2 , 8 , 9 , 10 , 11 , 12 , 13
TABLE 1.
Some cases of PSCC with other organs involvement
| Authors | involved Organs |
|---|---|
| Our case, Juan Antonio González‐Sánchez et al 2 and Gil R Faria et al 8 | Liver infiltration |
| Kehua Zhou et al 9 | the pancreas, the spleen and the left kidney |
| Shengqiang Gao et al 10 | the head of the pancreas and the transverse mesocolon |
| Kchaou et al, 11 Michael Beattie et al 12 and Kimura et al 13 | Liver metastases |
However, no cases of PSCC of the stomach with ovarian metastases have been reported in the literature.
In fact, secondary tumors of the ovary account for 10%‐25% of all ovarian malignancies. The most common primary sites are the gastrointestinal tract especially the stomach followed by the colon and the appendix, the breast and less frequently the gallbladder, the biliary tract, the pancreas, the small intestine, the ampulla of Vater, the cervix and the urinary bladder. 14 , 15
Krukenberg tumor (KT) is rare, most commonly in the stomach‐ovarian axis, and it is often defined as a metastatic signet ring cell adenocarcinoma of the ovary. 15 So, signet ring cells that produce mucin and the sarcomatoid proliferation of the stroma are the distinguishing features for the diagnosis of KT. 14 Thus, ovarian metastases are always associated with adenocarcinoma, however, some cases of squamous cell carcinoma have been reported. 16
In our case, the diagnosis of KT has been suggested on the findings of the CT scan but no histopathological evidence is available to confirm the presence of signet ring cells producing mucin.
As far as treatment is concerned, there are no clear recommendations for the management of Gastric PSCC. It is still controversial. Certainly, surgery remains the best option, since it represents the only radical treatment. Some cases of gastric PSCC have responded well to the combination of radiotherapy and chemotherapy with 5FU and cisplatin. 11 , 17
Gastric PSCC has a poor prognosis as it is usually diagnosed at an advanced stage with local infiltration and metastases to the liver, the lymph nodes and other organs. 3 The overall survival rates range from 7 months to 8 years. 17
In our patient, the prognosis was very poor as the tumor was associated with liver infiltration, a bilio‐gastric fistula, ovarian metastases and thromboembolic complications.
4. CONCLUSION
Primary squamous cell carcinoma of the stomach is a rare entity. Although diagnosis criteria are well defined, the pathogenesis and the treatment remain controversial. Further researches are needed to standardize the management of this tumor, whose prognosis remains very pejorative.
CONFLICT OF INTEREST
Authors report no conflicts of interest for this submission.
AUTHOR CONTRIBUTIONS
MS and GG: wrote the paper. GG: reviewed the literature. NB : Referring Doctor. IH: contributed by the pathology pictures as well as the interpretation of figures. CC : contributed by the CT scan pictures. DG : The head of the gastroenterology department in the Habib Thameur Hospital and contributed in the therapeutic decisions.
ETHICAL APPROVAL
Patient's personal data have been respected.
DATA AVAILABILITY STATEMENT
No data is available for this submission.
ACKNOWLEDGMENTS
Published with written consent of the patient.
Sabbah M, Gharbi G, Bellil N, Helal I, Chamakhi C, Gargouri D. Primary gastric squamous cell carcinoma with a bilio‐gastric fistula and Krukenberg syndrome. Clin Case Rep. 2021;9:e04325. 10.1002/ccr3.4325
REFERENCES
- 1. Dong C, Jiang M, Tan Y, et al. The clinicopathological features and prognostic factors of gastric squamous cell carcinoma. Medicine. 2016;95(34):e4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. González‐Sánchez JA, Vitón R, Collantes E, Rodríguez‐Montes JA. Primary squamous cell carcinoma of the stomach. Clinical Medicine Insights: Oncology. 2017;11:1‐4. 10.1177/1179554916686076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Y, Zhu H, Xu F, et al. Clinicopathological Characteristics, Treatment, and Prognosis of 21 Patients with Primary Gastric Squamous Cell Carcinoma. Gastroenterology Research and Practice. 2016;2016:1‐6. 10.1155/2016/3062547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer. 1965;18:181‐192. https://doi.org/10.1002/1097‐0142(196502)18:2<181::AID‐CNCR2820180209>3.0.CO;2‐3 [DOI] [PubMed] [Google Scholar]
- 5. Parks RE. Squamous neoplasms of the stomach. Am J Roentgenol Radium Ther Nucl Med. 1967;101:447‐449. [DOI] [PubMed] [Google Scholar]
- 6. Chang YS, Kim MS, Kim DH, et al. Primary squamous cell carcinoma of the remnant stomach after subtotal gastrectomy. J Gastric Cancer. 2016;16:120‐124. 10.5230/jgc.2016.16.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu X‐D, Zhou Y, Fan R‐G, Zhou B, Shi Q, Jia J. Primary squamous cell carcinoma of the stomach presenting as a huge retroperitoneal tumor: a case report. Rev Española Enferm Dig. 2016;108:283‐284. 10.17235/reed.2015.3795/2015 [DOI] [PubMed] [Google Scholar]
- 8. Faria GR, Eloy C, Preto JR, et al. Primary gastric adenosquamous carcinoma in a Caucasian woman: a case report. Journal of Medical Case Reports. 2010;4:351. 10.1186/1752-1947-4-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou K, Faraz A, Magotra M, Tahir M. Exophytic primary gastric squamous cell carcinoma and H. pylori gastritis. BMJ Case Rep. 2019;12(7). 10.1136/bcr-2019-230310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao S, Chen D, Huang L, Dai R, Shan Y. Primary squamous cell carcinoma of the stomach: a case report and literature review. Int J Clin Exp Pathol. 2015;8:9667‐9671. PMID: 26464735; PMCID: PMC4583967. [PMC free article] [PubMed] [Google Scholar]
- 11. Kchaou A, Ben Ameur H, Rejab H, et al. Carcinome épidermoïde de l’estomac : une entité rare. J Afr Hepato Gastroenterol. 2014;8:223‐225. 10.1007/s12157-014-0569-1 [DOI] [Google Scholar]
- 12. Beattie M, Mansour R, Thigpin D, Haus C. Metastatic primary gastric squamous cell carcinoma: an uncommon presentation of a rare malignancy. Case Reports in Gastrointestinal Medicine. 2019;2019:1‐4. 10.1155/2019/5305023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kimura Y, Matsuda H, Saeki H, et al. Case of early adenosquamous carcinoma of the stomach. Fukuoka Igaku Zasshi. 2013;104(9):315‐320. PMID: 24364267. [PubMed] [Google Scholar]
- 14. Lionetti R, De Luca M, Travaglino A, et al. Treatments and overall survival in patients with Krukenberg tumor. Arch Gynecol Obstet. 2019;300:15‐23. [DOI] [PubMed] [Google Scholar]
- 15. Al‐Agha OM, Nicastri AD. An in‐depth look at Krukenberg tumor: an overview. Arch Pathol Lab Med. 2006;130:1725‐1730. [DOI] [PubMed] [Google Scholar]
- 16. Ge HJ, Bi R, Cheng YF, et al. Clinicopathologic analysis of primary carcinoid of the ovary. Zhonghua Bing Li Xue Za Zhi = Chinese Journal of Pathology. 2018;47:517‐521. 10.3760/cma.j.issn.0529-5807.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 17. Bonnheim DC, Sarac OK, Fett W. Primary squamous cell carcinoma of the stomach. Am J Gastroenterol. 1985;80:91‐94. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data is available for this submission.


