Abstract
Background
The route of transmission of severe acute respiratory syndrome coronavirus 2 has challenged dentistry to improve the safety for patients and the dental team during various treatment procedures. The purpose of this study was to evaluate and compare the effectiveness of dental evacuation systems in reducing aerosols during oral prophylactic procedures in a large clinical setting.
Methods
This was a single-center, controlled clinical trial using a split-mouth design. A total of 93 student participants were recruited according to the inclusion and exclusion criteria. Aerosol samples were collected on blood agar plates that were placed around the clinic at 4 treatment periods: baseline, high-volume evacuation (HVE), combination (HVE and intraoral suction device), and posttreatment. Student operators were randomized to perform oral prophylaxis using ultrasonic scalers on 1 side of the mouth, using only HVE suction for the HVE treatment period and then with the addition of an intraoral suction device for the combination treatment period. Agar plates were collected after each period and incubated at 37 °C for 48 hours. Colony-forming unit (CFU) counts were determined using an automatic colony counter.
Results
The use of a combination of devices resulted in significant reductions in CFUs compared with the use of the intraoral suction device alone (P < .001). The highest amounts of CFUs were found in the operating zone and on patients during both HVE and combination treatment periods.
Conclusions
Within limitations of this study, the authors found significant reductions in the amount of microbial aerosols when both HVE and an intraoral suction device were used.
Practical Implications
The combination of HVE and intraoral suction devices significantly decreases microbial aerosols during oral prophylaxis procedures.
Key Words: Dental aerosols, oral prophylaxis, dental evacuation systems, dental suctions
Abbreviation Key: ACH, Air changes per hour; CDC, Centers for Disease Control and Prevention; CFU, Colony-forming unit; HVE, High-volume evacuation
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19 has caused worldwide concerns.1 The main modes of transmission include contact, droplets, airborne respiratory droplets, and fomite transmissions.2 , 3 It is contagious, with a reported estimated basic reproductive number between 1.40 and 6.47.4 , 5 The transmission of this virus has become a new challenge to clinical dentistry, in which many routine procedures, such as dental prophylaxis and cavity preparation, produce aerosols.
According to the Centers for Disease Control and Prevention (CDC), aerosols generated during the use of an ultrasonic scaler or high-speed dental drill may impose risks to oral health care personnel and patients.6 Such aerosols consist of droplets and debris that are greater than 50 micrometers in diameter and can remain suspended in the air.7, 8, 9 Aerosols generated from such procedures may contain bacterial cells or spores, fungal spores, or viruses.10 The size of some of these specific pathogens generally range from 0.30 through 10 μm for bacteria and approximately 0.02 through 0.30 μm for viruses.10 For severe acute respiratory syndrome coronavirus 2, its size has been reported to be in the range of 0.25 through 4 μm.11 , 12 The authors of a 2020 study found that the virus can remain viable in aerosols for up to 3 hours,13 and other studies report up to days3 , 11 on certain surfaces.
Although no cases of COVID-19 infection from dental procedures have been reported, it is imperative that dentistry rise to the challenge that this virus has imposed by means of reducing the risk of spreading COVID-19 and protecting patients and dental professionals. Dentistry is an essential part of health care that requires the use of aerosol-generating instruments and equipment so that appropriate care can be provided. On the basis of the recommendations of the CDC,6 , 14 the American Dental Association Council on Scientific Affairs has recommended that control measures be implemented in reducing aerosols during dental procedures.15 In studies conducted in vitro on manikins and typodonts, researchers have evaluated various techniques and devices to control, reduce, and eliminate aerosols during dental procedures and have reported positive outcomes.16, 17, 18, 19, 20, 21 Several clinical studies have also been conducted, but the methodologies have been limited to an enclosed operatory with microbial collection plates or litmus paper strips being positioned within the operating field.7 , 22, 23, 24, 25, 26, 27 To date, to our knowledge, no study has evaluated microbial aerosol generation in a large clinic and in areas further from the operating field. The purpose of our study was to evaluate and compare the effectiveness of 2 dental evacuation systems in reducing aerosols during ultrasonic scaling procedures in a large clinical setting.
Methods
Study design and sample size calculation
This clinical study was a single-center, controlled trial using a split-mouth design. Before conducting the study, we submitted protocols and pertinent documents, which were approved by the Loma Linda University Health Institutional Review Board (5200303). We performed a preliminary investigation to collect preliminary data, which showed a mean (standard deviation) colony-forming unit (CFU) difference of 1.00 (1.70) between pre- and posttreatment periods. Using an effect size of 0.588, a minimum of 27 patients would be required to achieve 80% power with a significance level of α at 0.05. To allow for 10% attrition, a minimum of 30 patients would need to be recruited.
Study participants
We recruited incoming third- and fourth-year predoctoral dental, second-year international dental, and second-year dental hygiene students. The students had the choice of signing up as an operator, an assistant, or a patient. A total of 93 student participants were recruited via university email. All participants had to pass the COVID-19 screening examination, which included taking their temperatures. Students who signed up as patients were enrolled according to the inclusion and exclusion criteria, which included a COVID-19 test. All patients who qualified for this study signed the informed consent form, were in good general and oral health, had negative COVID-19 test results, and had at least 20 natural teeth present. Patients were excluded according to the following criteria: pregnant or nursing, allergy to materials found in the intraoral suction device, tumors or significant pathology of the soft or hard tissues of the oral cavity, presence of orthodontic bands, advanced periodontal disease, presence of a removable prosthesis, history of infectious disease or bloodborne diseases, and having had dental prophylaxis within 2 weeks before commencement of the study. All patients were informed about their right to withdraw from the study at any time for any reason. In the event a patient elected to withdraw from the study, a closing examination and follow-up would be requested.
Description of the clinic setting
This study was conducted in a clinic area that had multiple open bay cubicles and was surrounded by panels that were approximately 5 feet high. The clinic was approximately 3,118 square feet, and each cubicle was 78 ft2. Each dental chair was equipped with the normal hoses for saliva ejector, high-volume evacuation (HVE) suction, and air and water syringe. An additional HVE hose was connected to the air pipes within the walls and was used to connect the intraoral suction device (Mr. Thirsty, Zirc). The amount of airflow measured from 1 source of hoses was 12.4 meters per second for the HVE and greater than 30.0 m/s for the additional HVE. When all cubicle hoses were turned on, the amount of airflow decreased to 5.8 m/s for the HVE and 28.0 m/s for the additional HVE.
Study procedures
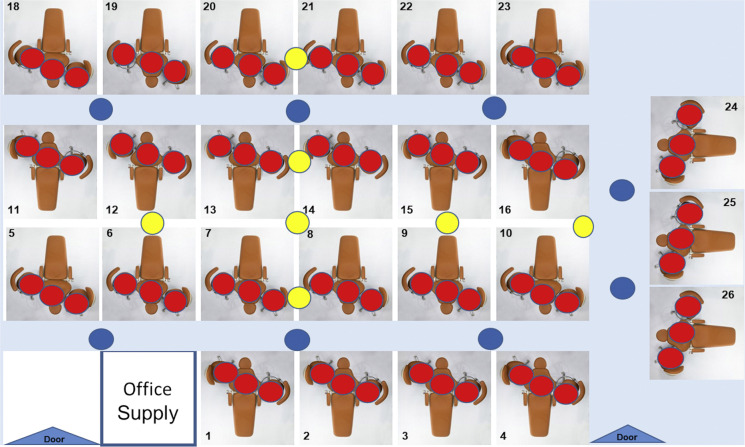
Patients were asked to refrain from performing oral hygiene care for at least 10 hours before and from eating or drinking (except for water) 4 hours before the appointment. Trypticase soy agar with 5% sheep blood plates were placed in various locations around the room in the operating zone (zone 1: operator, assistant, and patient), on mobile trays (zone 2: 2-4 feet horizontal distance), and on shelves or countertops (zone 3: > 4 feet horizontal distance) (Figure 1 ). The plates were attached to disposable bibs and were placed at chest level and on the chest for zone 1.
Figure 1.
Clinical test area with trypticase soy agar with 5% sheep blood plates placed in zone 1 (red; horizontal distance, < 2.0 feet; vertical distance, ≈ 3.0 feet); zone 2 (blue; horizontal distance, 2.0-4.0 feet; vertical distance, 3.0 feet); and zone 3 (yellow, horizontal distance, > 4.0 feet; vertical distance, 5.0 feet).
Agar plates were placed at 4 treatment periods:
-
▪
baseline;
-
▪
using the HVE alone;
-
▪
using the combination (HVE and intraoral suction device);
-
▪
posttreatment.
Each plate was exposed for 50 minutes. To obtain baseline microbial levels, agar plates were exposed to the air for 50 minutes. No participants were allowed in the room during baseline and posttreatment times. Student operators were instructed to connect the plastic suction tip to the regular HVE hose and the intraoral suction device to the additional HVE hose. Before the treatment periods, student operators were instructed to set the ultrasonic scaler (Cavitron 124 SPS, Dentsply) to moderate level and to use the highest output of water through the ultrasonic scaler tip. They were also instructed not to change the ultrasonic scaler settings and to continue using the instrument for the full duration (20 minutes) of the treatment periods. Student assistants were instructed to provide assistance in reducing aerosols generated from the ultrasonic scaler and removing accumulated saliva and debris using the HVE only, following normal clinical procedures. For treatment period 2, operators and assistants were instructed to use only the HVE and perform oral prophylaxis using ultrasonic scalers only for 20 minutes on 1 randomized side of the mouth. The clinical procedure was stopped, and all participants exited the room. An additional 30 minutes was allowed for aerosols to settle, and the agar plates were collected. For treatment period 3, operators and assistants were instructed to use both HVE and intraoral suction device (combination) and to perform the same treatment procedure for 20 minutes on the other side of the mouth (Figure 2 ). After 20 minutes, all participants exited the room, and the same aerosol collection procedure was completed. Posttreatment agar plates were placed for an additional 50 minutes. All agar plates were incubated at 37 oC for 48 hours. The CFU counts were determined using an automatic colony counter (ProtoCOL Systems, Synbiosis). The general procedure is illustrated in a flowchart (Figure 3 ).
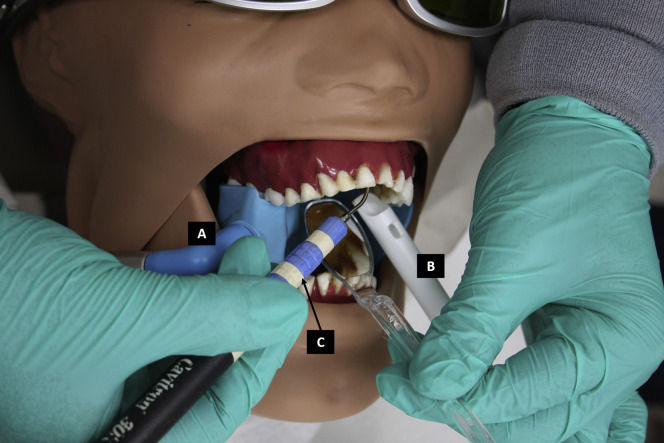
Figure 2.
Clinical illustration of the intraoral suction device (Mr. Thirsty, Zirc) connected to the additional high-volume evacuation hose (A), plastic suction tip connected to the high-volume evacuation hose (B), and ultrasonic scaler (Cavitron 124 SPS, Dentslpy) (C) using a manikin.
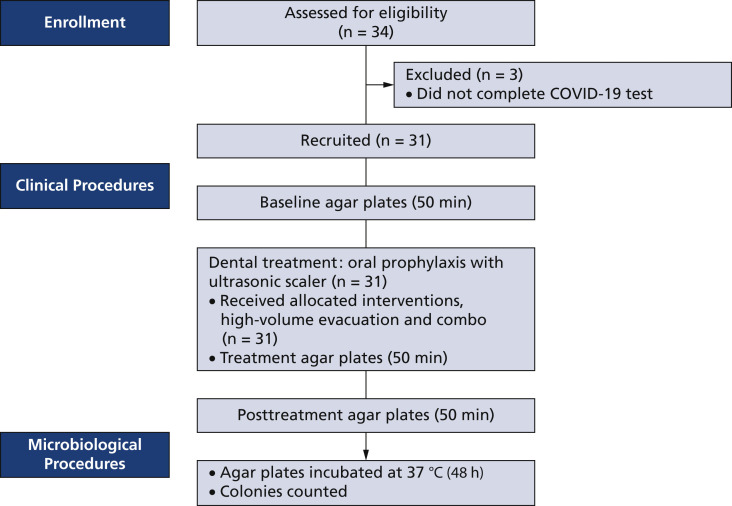
Figure 3.
General flowchart of clinical and microbiological procedures. Thirty-four patients were assessed for eligibility.
Statistical analysis
We performed Kruskal-Wallis and Wilcoxon signed rank tests to compare the mean CFUs between devices and with the baseline and posttreatment results. We used a generalized estimating equation mixed-effects analysis of variance model to estimate and test the treatment effect, time effect, and their interactions and adjusted for the correlations among observations of the split-mouth data. We conducted all tests using statistical software (R Version 3.6.2, R Core Team) with a significance level of α at 0.05.
Results
The descriptive statistics showing the means (standard deviations) of CFUs per treatment period are presented in Table 1 . CFU levels for baseline and posttreatment periods were lower than the treatment periods (HVE and combination), with HVE alone having the highest amount. The pairwise comparisons are presented in Table 2 . When comparing the treatment periods with the baseline, we found highly significant differences for HVE and combination treatment periods (P < .001), whereas we found no significant difference with the posttreatment period (P = .274). Comparing the treatment periods revealed statistically significant differences (P < .001).
Table 1.
Descriptive statistics showing means (standard deviations) of colony-forming units by treatment periods.
| TREATMENT PERIOD | AGAR PLATES, NO.∗ | MEAN (STANDARD DEVIATION) |
|---|---|---|
| Baseline | 124 | 2.26 (1.69) |
| High-Volume Evacuation | 124 | 132.00 (353.00) |
| Combination (High-Volume Evacuation + Intraoral Suction Device) | 124 | 46.10 (178.00) |
| Posttreatment | 124 | 2.62 (2.23) |
Total number of 5% sheep blood agar plates.
Table 2.
Pairwise comparisons of treatment periods.
| TREATMENT PERIOD | AGAR PLATES, NO.∗ | MEAN (STANDARD ERROR) | 95% CI | P VALUE |
|---|---|---|---|---|
| Δ†HVE‡ | 124 | 117.00 (30.36) | 56.90 to 177.10 | < .001 |
| Δ Combination (HVE + Intraoral Suction Device) | 124 | 43.80 (15.94) | 12.24 to 75.36 | < .001 |
| Δ Posttreatment | 124 | 0.36 (0.22) | −0.08 to 0.80 | .274 |
| HVE Versus Combination | 124 | 81.59 (35.27) | 11.91 to 151.27 | < .001 |
Total number of 5% sheep blood agar plates.
Δ: Mean difference compared with baseline.
HVE: High-volume evacuation.
For the within effects, the generalized estimating equation model revealed statistically significant differences for the treatment periods (F2,228 = 7.33; P < .001) and for the treatment periods by zones interaction (F8,228 = 8.39, P < .001) compared with the baseline treatment period. Scheffé post hoc test for HVE revealed significant differences within zone 1 for patient and operator (P < .001), patient and assistant (P < .001), and patient and zones 2 and 3 (P < .001). We found no statistical differences in CFUs among the zones within the combination and posttreatment periods.
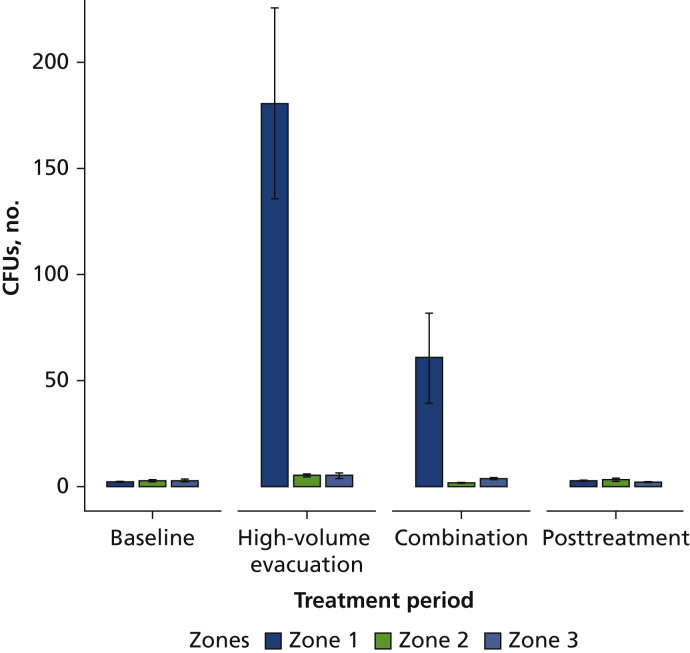
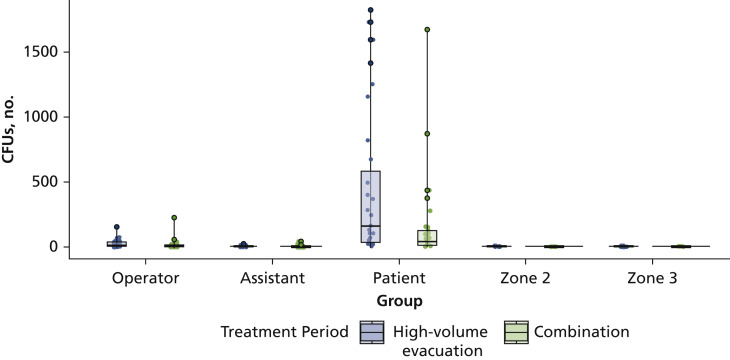
For the between effects, we found statistically significant differences for the zones (F4,114 = 17.87; P < .001) only. The highest amount of CFUs were found on the patient (zone 1) compared with all other zones, including operators and assistants (Figure 4 ). Scheffé post hoc test revealed significant differences between patient and operator (P < .001), between patient and assistant (P < .001), and between zones 2 and 3 (P < .001) when compared with baseline.
Figure 4.
Mean colony-forming units (CFUs) and treatment periods among zone 1 (horizontal distance, < 2.0 feet; vertical distance, ≈ 3.0 feet), zone 2 (horizontal distance, 2.0-4.0 feet; vertical distance, 3.0 feet), and zone 3 (horizontal distance, > 4.0 feet; vertical distance, 5.0 feet).
We found statistically significant differences (P < .05) within each zone for the HVE and combination treatment periods only (Table 3 ). We found highly significant differences (P < .001) when patient (zone 1) was compared with all other zones in the treatment periods. We further separated zone 1 to evaluate CFUs by group (operator, assistant, patient) for the HVE and combination treatment periods (Figure 5 ). We found the highest amount of CFUs on the patients and during the HVE treatment period, whereas we found the lowest amount of CFUs for the assistants (Table 3). We observed low levels of CFUs for zones 2 and 3, with modest changes in mean CFU from baseline to the treatment and posttreatment periods.
Table 3.
Mean colony-forming units in zones within treatment periods.∗
| TREATMENT PERIODS |
||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
High-Volume Evacuation |
Combination |
Posttreatment |
|||||
| ZONE | Mean (SD†) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI |
| Zone 1 | ||||||||
| Operator | 2.68 (1.68) | 1.95 to 3.41 | 26.16 (33.00) | 19.45 to 32.87‡,§ | 18.84 (40.65) | 13.24 to 24.44‡,§,¶ | 3.10 (2.49) | 2.29 to 3.91 |
| Assistant | 1.61 (1.02) | 1.32 to 1.90 | 6.68 (5.06) | 5.19 to 8.17# | 4.23 (7.95) | 0.22 to 8.24¶,# | 2.61 (1.99) | 1.89 to 3.33 |
| Patient | 1.84 (1.68) | 1.25 to 2.43 | 441.61 (569.17) | 248.25 to 634.97‡,#,∗∗ | 158.71 (331.55) | 61.14 to 256.28‡,#,∗∗ | 2.00 (1.63) | 1.48 to 2.52 |
| Zone 2 | 2.94 (1.65) | 2.12 to 3.76 | 4.88 (2.96) | 3.77 to 5.99§,∗∗ | 1.94 (1.61) | 1.47 to 2.41§,∗∗ | 3.31 (3.28) | 2.21 to 4.41 |
| Zone 3 | 2.87 (2.26) | 2.02 to 3.72 | 5.47 (3.58) | 3.98 to 6.96∗∗ | 3.00 (1.77) | 2.68 to 3.32§,∗∗ | 2.20 (1.61) | 1.06 to 3.34 |
Dwass-Steel-Critchlow-Fligner pairwise comparison was used to determine P values within treatment periods.
SD: Standard deviation.
Statistically significant differences (P < .001) between operator and patient.
Statistically significant differences (P < .05) compared with zone 1, operator.
Statistically significant differences (P < .001) between operator and assistant.
Statistically significant differences (P < .001) between assistant and patient.
Statistically significant differences (P < .001) compared with zone 1, patient.
Figure 5.
Comparison of colony-forming units (CFUs) by treatment periods for zone 1 (horizontal distance, < 2.0 feet; vertical distance, ≈ 3.0 feet), zone 2 (horizontal distance, 2.0-4.0 feet; vertical distance, 3.0 feet), and zone 3 (horizontal distance, > 4.0 feet; vertical distance, 5.0 feet).
Discussion
Aerosols generated during clinical dental procedures have raised concerns and awareness. Control of aerosols designed to protect dental providers and patients are critical, and certain guidelines have been proposed for dental settings.5 , 15 To date, there is no direct evidence that dental procedures are a major cause of airborne infections. However, given the pandemic situation, the possibility of potential risks cannot be ignored. Therefore, it is important to identify effective measures to control aerosols associated with performing dental procedures.
HVE is a standard device for saliva control during dental procedures, and it alone has been shown to reduce aerosol and spatter to some degree.9 Most oral health care providers do not use an additional means of suction for many dental procedures owing to decreased visualization and accessibility. Our results show the limitations of using HVE alone in reducing microbial aerosols, especially in the operating field (zone 1, < 2 feet distance). Nevertheless, it was encouraging to observe that during both treatment periods (HVE and combination) microbial aerosols were confined to zone 1. It was interesting to find that within zone 1, the highest amounts of CFUs were found on patients. Similar results were found by Bentley and colleagues,7 who reported higher levels of CFUs on patients after various dental procedures, and by King and colleagues,26 who reported high levels of CFUs at a distance of 6 inches from the patient’s mouth. However, the limitations of their studies included low sample size (n = 5 and n = 12, respectively) and placement of plates that were positioned mainly around the patient’s head.7 , 26 Using a slightly different methodology and positioning of agar plates (angled instead of flat on the patient’s chest), Devker and colleagues22 also reported increased CFU levels during ultrasonic scaling procedures on patients before using an antibacterial mouthrinse. They also reported higher levels of mean CFUs for the operator and assistant, which we did not observe in our study. The difference in the observations could be attributed to the position of the plates on the operator and assistants. In our study, the position of the plates were on the chest for all participants, whereas in the previous studies, the plates were positioned on the mask at the level of the nose and in front of the mouth of the operator and assistant.7 , 22 For the student operators and assistants in our study, the position of the plates were at or slightly above the patients' mouths.
To our knowledge, this is the first clinical study to evaluate 2 methods for controlling aerosol in a large clinic area with multiple operators and assistants who have limited clinical experiences. The clinic area in our study was an open bay design that consisted of several operatories that were divided by walls that were 5 feet high (Figure 1). Positions of the plates provided a general idea of the microbial aerosols that are generated during an exaggerated clinical setting. The plate positions also allowed us to evaluate specific zones. We were surprised that low levels of CFUs were found in zones 2 and 3. Plausible explanations include positioning of the HVE during treatment procedures, addition of the intraoral suction device, the positions of the agar plates (height range, 3-5 feet), and the airflow through the ventilation systems. Still, the positioning of the HVE during the dental procedure may not be as strong of an explanation because the levels of expertise were low. In addition, even though the airflow through the additional hose was high (additional HVE, > 30.0 m/s) and provided enhanced control of the aerosol, the intraoral suction device was placed posteriorly and provided limited suction when working in the anterior areas. Therefore, the height of the plates could better explain the low levels of CFUs. However, the level of the plates in zone 2 was slightly higher than the typical level of a patient’s mouth (Figure 1). Given the ballistic nature of aerosols and spatter that has been described in the literature,8 , 9 we assumed that those plates would have higher amounts of CFUs. We did not observe this, which warrants further investigation in understanding the pattern or flow of dental aerosols during various clinical procedures and assessments of airflow from ventilation systems.
The CDC recommends allowing at least 15 minutes for aerosols to settle after a treatment procedure before disinfection of the operatory.14 The CDC recommendation is based on the time required for removal of 99% efficiency and having air changes per hour (ACH) of 15 through 20. On the basis of our evaluation of the clinic area and estimates of the ACH, we determined that 30 minutes would allow adequate time for aerosols to settle and for sufficient ACH. Normal time for dentists and dental hygienists to complete full-mouth oral prophylaxis is less than 1 hour. We considered the participants’ level of expertise and determined that 20 minutes per side would be appropriate. We kept the baseline and posttreatment times the same, with the treatment periods of oral prophylaxis for 20 minutes and aerosol settling for 30 minutes (total of 50 minutes per treatment period). The posttreatment period revealed that CFU levels returned to levels that were observed for the baseline periods. Furthermore, modification of the cubicles via the addition of the additional HVE hose proved to be beneficial because it reduced microbial aerosols and was a desirable improvement for clinical dental settings.
In our study, we did not evaluate the use of pre- and postantibacterial mouthrinses, although other studies have reported significant decrease in CFUs after various dental procedures.7 , 22 Our study was also limited to microbial evaluation and did not include viruses; however, a potential relationship could be generated, because the size of some oral bacteria is within the range of that of some viruses.10 Further studies are needed to validate our results. Additional research into the data collected in this study is needed to better understand the dynamics of aerosols produced during dental procedures. Furthermore, the design of our study should be expanded to other aerosol-producing procedures like restorations, crowns, and placement of dental implants.
Conclusions
Within the limitations of our study in a large clinical setting, the combination of HVE and an intraoral suction device significantly reduced the amount of microbial aerosol during treatment periods. The interval time of 30 minutes between treatments for aerosol settling and air change appeared to be adequate according to the findings in our study.
Biographies
Dr. Suprono is an associate professor, Center for Dental Research, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Won is an assistant professor, Division of General Dentistry, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Savignano is an assistant professor, Center for Dental Research, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Zhong is an assistant professor, Center for Dental Research, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Ahmed is a research associate, Center for Dental Research, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Roque-Torres is an assistant professor, Center for Dental Research, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Zhang is a professor, Center for Dental Research, Loma Linda University School of Dentistry, Loma Linda, CA.
Mr. Udochukwu Oyoyo is an assistant professor, Dental Education Services, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Richardson is an associate professor, Division of General Dentistry, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Caruso is a distinguished professor, Orthodontics, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Handysides is an associate professor, Endodontics, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Li is a distinguished professor, Center for Dental Research, Loma Linda University School of Dentistry, Loma Linda, CA.
This article has an accompanying online continuing education activity available at: http://jada.ada.org/ce/home.
Footnotes
Disclosures. None of the authors resported any disclosures.
The authors would like to thank all students for their participation.
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12(1):9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions Accessed January 4, 2021.
- 4.Rahman B., Sadraddin E., Porreca A. The basic reproduction number of SARS-CoV-2 in Wuhan is about to die out, how about the rest of the world? Rev Med Virol. 2020;30(4):e2111. doi: 10.1002/rmv.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome Coronavirus 2. Emerg Infect Dis. 2020;26(7):1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidance for dental settings Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html Accessed September 12, 2021.
- 7.Bentley C.D., Burkhart N.W., Crawford J.J. Evaluating spatter and aerosol contamination during dental procedures. JADA. 1994;125(5):579–584. doi: 10.14219/jada.archive.1994.0093. [DOI] [PubMed] [Google Scholar]
- 8.Harrel S.K., Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. JADA. 2004;135(4):429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micik R.E., Miller R.L., Mazzarella M.A., Ryge G. Studies on dental aerobiology, I: bacterial aerosols generated during dental procedures. J Dent Res. 1969;48(1):49–56. doi: 10.1177/00220345690480012401. [DOI] [PubMed] [Google Scholar]
- 10.Cole E.C., Cook C.E. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am J Infect Control. 1998;26(4):453–464. doi: 10.1016/S0196-6553(98)70046-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chia P.Y., Coleman K.K., Tan Y.K. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun. 2020;11(1):2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y., Ning Z., Chen Y. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 13.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appendix B: air—guidelines for environmental infection control in health-care facilities (2003). Centers for Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html Accessed November 5, 2021.
- 15.Infection control recommendations for the dental office and the dental laboratory ADA Council on Scientific Affairs and ADA Council on Dental Practice. JADA. 1996;127(5):672–680. doi: 10.14219/jada.archive.1996.0280. [DOI] [PubMed] [Google Scholar]
- 16.Dahlke W.O., Cottam M.R., Herring M.C., Leavitt J.M., Ditmyer M.M., Walker R.S. Evaluation of the spatter-reduction effectiveness of two dry-field isolation techniques. JADA. 2012;143(11):1199–1204. doi: 10.14219/jada.archive.2012.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravenel T.D., Kessler R., Comisi J.C., Kelly A., Renne W.G., Teich S.T. Evaluation of the spatter-reduction effectiveness and aerosol containment of eight dry-field isolation techniques. Quintessence Int. 2020;51(8):660–670. doi: 10.3290/j.qi.a44919. [DOI] [PubMed] [Google Scholar]
- 18.Harrel S.K., Barnes J.B., Rivera-Hidalgo F. Reduction of aerosols produced by ultrasonic scalers. J Periodontol. 1996;67(1):28–32. doi: 10.1902/jop.1996.67.1.28. [DOI] [PubMed] [Google Scholar]
- 19.Rivera-Hidalgo F., Barnes J.B., Harrel S.K. Aerosol and splatter production by focused spray and standard ultrasonic inserts. J Periodontol. 1999;70(5):473–477. doi: 10.1902/jop.1999.70.5.473. [DOI] [PubMed] [Google Scholar]
- 20.Harrel S.K., Barnes J.B., Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. JADA. 1998;129(9):1241–1249. doi: 10.14219/jada.archive.1998.0421. [DOI] [PubMed] [Google Scholar]
- 21.Veena H.R., Mahantesha S., Joseph P.A., Patil S.R., Patil S.H. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. J Infect Public Health. 2015;8(3):260–265. doi: 10.1016/j.jiph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Devker N.R., Mohitey J., Vibhute A. A study to evaluate and compare the efficacy of preprocedural mouthrinsing and high volume evacuator attachment alone and in combination in reducing the amount of viable aerosols produced during ultrasonic scaling procedure. J Contemp Dent Pract. 2012;13(5):681–689. doi: 10.5005/jp-journals-10024-1209. [DOI] [PubMed] [Google Scholar]
- 23.Miller R.L., Micik R.E., Abel C., Ryge G. Studies on dental aerobiology, II: microbial splatter discharged from the oral cavity of dental patients. J Dent Res. 1971;50(3):621–625. doi: 10.1177/00220345710500031701. [DOI] [PubMed] [Google Scholar]
- 24.Toroglu M.S., Haytac M.C., Koksal F. Evaluation of aerosol contamination during debonding procedures. Angle Orthod. 2001;71(4):299–306. doi: 10.1043/0003-3219(2001)071<0299:EOACDD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Hallier C., Williams D.W., Potts A.J., Lewis M.A.O. A pilot study of bioaerosol reduction using an air cleaning system during dental procedures. Br Dent J. 2010;209(8):E14. doi: 10.1038/sj.bdj.2010.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King T.B., Muzzin K.B., Berry C.W., Anders L.M. The effectiveness of an aerosol reduction device for ultrasonic scalers. J Periodontol. 1997;68(1):45–49. doi: 10.1902/jop.1997.68.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Chuang C.-Y., Cheng H.-C., Yang S., Fang W., Hung P.C., Chuang S.Y. Investigation of the spreading characteristics of bacterial aerosol contamination during dental scaling treatment. J Dent Sci. 2014;9(3):294–296. [Google Scholar]