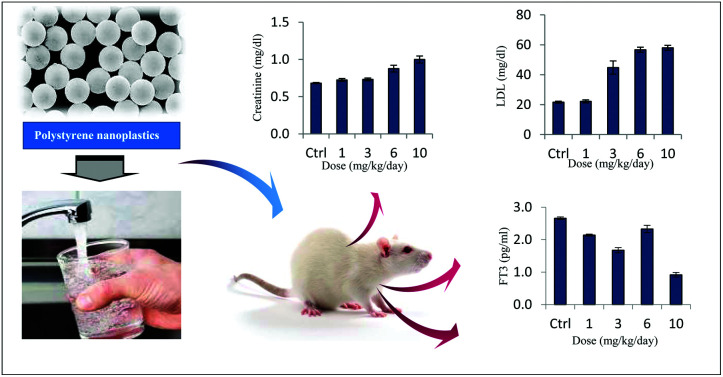
 At this time, little is known about the long-term effects of micro(nano)plastics on public health. In the present study, early warning signs of thyroid endocrine disruption were noticed in adult Wistar rats from the ingestion of virgin polystyrene nanoparticles.
At this time, little is known about the long-term effects of micro(nano)plastics on public health. In the present study, early warning signs of thyroid endocrine disruption were noticed in adult Wistar rats from the ingestion of virgin polystyrene nanoparticles.
Abstract
Toxicity evaluations of micro- or nano-sized plastics in rodent species commonly employed for toxicity analyses based on which risk assessment for humans could be performed are still largely lacking. Given this knowledge gap, the present work was aimed at determining the potential impact of chronic exposure to polystyrene nanoplastics (PS NPs) on the thyroid endocrine status and biochemical stress in a rat model. Young adult male rats were orally administered with PS NPs (1, 3, 6 and 10 mg kg–1 day–1) for five weeks. Thyroid hormones (THs) l-thyroxine (T4), l-triiodothyronine (T3), l-free triiodothyronine (FT3), and l-free thyroxine (FT4) as well as thyroid stimulating hormone (TSH) serum levels of normal rats and those exposed to PS NPs were compared. Serum levels of high-density lipoprotein (HDL), low-density lipoprotein (LDL), cholesterol, and creatinine, as well as glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) enzymes were also measured. Exposure to PS NPs suppressed the serum levels of T3 and circulating levels of THs, whereas TSH increased significantly. Though exposure to PS NPs did not affect the molar ratios of T3/T4, it induced a slight, but significant, increase in FT3/FT4. In addition, exposure to plastic nanoparticles showed signs of nephrotoxicity induction and kidney injury in exposed organisms as can be inferred from the significantly higher serum levels of creatinine in exposed groups. Our results provide clear evidence of an association between exposure to plastic NPs and thyroid endocrine disruption as well as metabolic deficit, and generate new leads for future research efforts.
1. Introduction
In 2015, the worldwide plastic production exceeded 322 million tons. With continuing current trends, approximately another 33 billion tons will have built up in the earth by 2050.1 The rapidly growing consumption of plastics inevitably produces large quantities of waste, and generous portions of them will eventually end up in different environmental compartments.2,3 Once in the environment, plastic debris undergoes size reduction as a result of biotic and abiotic degradation processes resulting in its progressive fragmentation into micro- (MPs; fragments < 5 mm) and nano-sized plastic particles (NPs; fragments < 100 nm).4–6 Additionally, plastic microbeads, used in many consumer facial cleansers and personal care products, are able to reach aquatic and terrestrial environments mainly via the effluents of wastewater treatment plants.7,8 Though plastics may be considered biochemically inert, the possibility that plastic particles and more importantly nano-sized plastics may penetrate biological membranes, become involved in biochemical reactions and affect the functioning of cells, including the endocrine system, is extremely worrying.6,9 Translocation (i.e., across the lung and gut epithelium) would imply that internal organs and tissues are exposed to these particles, resulting in systemic exposure.10 Therefore, it seems plausible to speculate about higher health and environmental risks of nano-sized plastics, as opposed to other common plastic debris.
Health risks stem from ingestion/inhalation of NPs, dispersion throughout the body and plausible exposure of vulnerable organs and tissues with associated toxicity. The uptake of fluorescent carboxylated polystyrene microspheres (50 nm) by rats is followed by migration from the gastrointestinal tract to the kidney, heart, stomach wall and small intestinal wall.11 Translocation of polystyrene NPs from the gut and subsequent body distribution have indeed been previously reported.12,13 Evidence from limited animal studies and experimental data suggests that plastic nanoparticles may affect the endocrine system.5,9,14,15 Estrogen and androgen antagonists and agonists, aromatase inhibitors, and thyroid disruptors are the common modes of action of endocrine disrupting compounds (EDCs).16
The thyroid gland is suspected to be targeted by various biotic and abiotic factors.17,18 Its function is primarily kept under the control of specific receptors that bind to THs. The production of pro-hormone thyroxine (tetra-iodothyronine or T4) in the thyroid gland is regulated by the hypothalamus and pituitary axis. Triiodothyronine (T3, the active form of THs), however, is primarily synthesized in peripheral tissues through the action of deiodinases,19 and helps regulate the basal metabolic rate in cells throughout the body, as well as the polypeptide hormone calcitonin. Thyroid function is involved in various critical physiological processes in the body such as metabolism, development, energy balance, synthesis and catabolism of proteins, plasma and liver fats, sexual function, the function of other endocrine glands, respiration, sleep, growth and development of the body. Cardiovascular activity also demands a certain level of thyroid function involvement.20–24 Accordingly, the interactions and mechanisms of the toxicity of nano-sized plastic particulates in relation to general human health problems, especially those broadening the term of endocrine disruption to ‘metabolic disruption’, should be deeply investigated. The present study was therefore conducted to investigate the serum concentrations of high-density lipoprotein (HDL), low-density lipoprotein (LDL), and cholesterol and evaluate their associations with thyroid hormones and several hepatotoxicity markers (GOT and GPT) as well as the kidney injury index (creatinine) in exposed animals.20,25
To the best of our knowledge, so far information on in vivo or in vitro toxicity studies of plastic nanoparticles in rodent species commonly exploited for toxicity studies has not been available based on which the risk assessment for humans could be carried out. Our previous study strongly suggests that chronic exposure to plastic nanoparticles may negatively affect the neurobehavioral status of exposed rats.26 The aim of this work was therefore to investigate if and how nano-sized plastic particles affect thyroid function in a model animal of Wistar rats. In addition to the suspicious endocrine disruption effects, less is known about the toxicity of plastic nanoparticles at the biochemical level. An attempt was thence made to analyse the alterations in the activity of some biochemical biomarkers and association between thyroid hormones and these metabolic parameters to assist in delving into the toxic effects and mode of action of these particles on living organisms. To address this concern, we developed a chronic dietary exposure bioassay protocol to investigate the toxicity of polystyrene NPs (PS NPs). Polystyrene is not only one of the primary components of plastic debris observed in the environment,27–29 but also has been the focus for most of the similar studies30,31 and was used as a model NP in our study. This polymer persists for several hundred years in the environment and undergoes remarkably slow de-polymerization, resulting in the formation of micro- and nano-sized plastic debris.32–34
2. Materials and methods
2.1. Preparation and characterization of nanoplastics
Pristine spherical polystyrene NPs (PS NPs) were purchased from Kisker Biotech (G. Kisker GbR, Steinfurt, Germany). We used two different sizes of PS NPs [PPS-0.025 (1% w/v solid suspension, 10 mL) and PPS-0.05 (2.5% w/v solid suspension, 15 mL)] with nominal diameters of 25 and 50 nm. The particle suspensions were stored at 4 °C in the dark and vortexed before each use.
Binary mixtures of nanoplastics were freshly prepared by the addition of predetermined volumes of 25 and 50 nm PS NP stocks to distilled water (pH 6.9 ± 0.1), considering equal weights of each chemical. The particle size distribution and ζ-potential of PS NPs (in prepared suspensions) were determined in triplicate by laser diffraction using a Malvern Zetasizer (Malvern Instruments Ltd, Malvern, UK). The aggregation and deposition kinetics of PS NPs were quantified through time-resolved dynamic light scattering (DLS) measurements.
2.2. Experimental design
2.2.1. Animals
Eight week old male Wistar rats were obtained from the breeding colony at the Neuroscience Research Center. The animals were housed under controlled temperature (20–22 °C) and humidity (about 60%) conditions and presented with a 12 : 12 h light/dark cycle. Animals were fed ad libitum on commercial pellets for 3 weeks until they were used in the experiment. All attempts were made to minimize animal suffering.
2.2.2. Exposure experiment
A total of thirty adult rats were randomly and equally distributed among five fiberglass cages (six rats per cage) a week prior to the beginning of the experiment. The study was performed in strict accordance with the treatment and care of laboratory animals published by the US National Institute of Health guidelines for the care and use of laboratory animals and was approved by the Institutional ethics Committee of Shahid Beheshti University of Medical Science, Iran (reference no: IR.SBMU.PHNS.REC.1395.111). The exposure experiment comprised the following treatments: normal control and PS NP treatments (1, 3, 6, and 10 mg PS NPs in distilled water per kg of body weight per day). It is worth noting here that the exact mechanisms by which these fragments mediate toxicity and lead to physiological malfunctions still remain largely unknown. Studies using animal and cell culture models conducted with higher exposure dosages to better understand the cellular and molecular mechanisms linking pollution and the mentioned possible disorders are highly recommended. Hence, in the current study, we used four different concentrations of polystyrene particles (1, 3, 6, and 10 mg kg–1 day–1). The environmentally relevant exposure dosages of nano-sized polystyrene particles were chosen to be 1–3 mg kg–1 day–1, according to the literature,11,34 and the relatively higher dosages (6 and 10 mg kg–1 day–1) of PS NPs were also chosen to be investigated due to the wider large-scale production of plastics and growing accumulation of plastic debris.
All groups were administered PS NPs through oral gavage using a 5 ml gavage needle to the pharyngeal region to minimize the loss of particles, which was however minimal. This strategy ensured the uptake of NPs. The administered dosages of PS NPs were adjusted every week, according to the increase in the body weight of the rats. The control groups received the same volume of vehicle solution (distilled water) only. Rats had ad libitum access to tap water and a standard low-calorie diet. The diets were in pellet form and their composition was as follows: glucose, 710 g; ovalbumin, 155 g; cellulose, 30 g; corn oil, 28 g; coconut fat, 17.75 g; NaH2PO4-2H2O, 14.5 g; CaCO3, 12.4 g; KHCO3, 7.7 g; MgCO3, 1.4 g; KCl, 1.0 g; KIO3, 0.25 mg; vitamin premix, 12 g; and mineral premix, 10 g, according to the information provided by the supplier. Drinking water was exclusively supplied through plastic bottles capped with steel stoppers. Water bottles were assigned to each experimental group in order to avoid cross contamination. Administration was ceased 24 h before the end of the exposure period. After a 35-day exposure period, the animals were anaesthetized with a liquid solution of chloroform. Blood samples were immediately taken by intra-cardiac puncture. Serum samples were obtained by centrifugation at 6000 rpm for 5 min and preserved at –70 °C until analysis.
2.2.3. Hormonal assays
Triiodothyronine (T3), thyroxine (T4), and their free active circulating forms (FT3 and FT4, respectively), as well as thyroid-stimulating hormone (TSH) concentrations in serum samples were obtained using enzyme-linked immunosorbent assay (ELISA) with commercially available kits following the manufacturer's instructions.
T3 and T4 were determined using Padtangostarisar human ELISA test kits, and their levels were expressed as ng ml–1. Accordingly, FT3 was determined using an FT3 DiaMetra human ELISA test kit, and its level was expressed as pg ml–1. Likewise, FT4 was determined using an FT4 Padtangostarisar human ELISA test kit, and its level was expressed as pg ml–1. Free active hormones were separated through ultrafiltration steps. TSH was determined using a rat TSH ZellBio ELISA test, and its level was expressed as m IU ml–1. Some researchers have also argued that the ratios of T3 to T4 (T3/T4) and FT3 to FT4 (FT3/FT4) may be better indicators of adverse outcomes than the individual hormones alone.17 The ratios of the two hormones were therefore worked out for the purposes of these analyses.
The linearity of the assay for all ELISA assays was examined by serial dilutions of the samples which were subsequently fitted to the dilutions of the respective standards supplied with the kits. Study samples revealed decent linearity demonstrating an average correlation coefficient (R2) of 0.92 ± 0.04 with lines lain on standard curves.
2.2.4. Biochemical evaluation of serum
Cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), creatinine, glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) were determined using an automatic dry-chemistry analyzer system (TS/technology Alpha – Classic; Tajhizat Sanjesh, Iran). The analyses were tripled and the arithmetic mean of the three measurement results is presented here.
2.3. Statistical analysis
All statistical analyses were conducted using SPSS software (IBM Corporation, USA; version 21.0) and Excel for Windows-2013. Results are expressed as the means ± standard deviation. Before the analyses, the data obtained were tested for normality (Shapiro-Wilks test) and homogeneity of variance (Levene's test). Multiple comparisons were carried out using one-way analysis of variance (ANOVA). If ANOVA showed a statistically significant difference (p < 0.05), the analysis was followed by post hoc procedures (Tukey's test). The strength of relationships between each parameter was assessed using Pearson's correlations. To examine whether non-significant outcomes in the present study originated from a plausible lack of statistical power, a post hoc power analysis was performed using the Power and Sample Size Calculation program (PS) considering the power (1 – β) set at 0.80 at a significance level of 5%, two-tailed.
3. Results
3.1. PS NP characterization
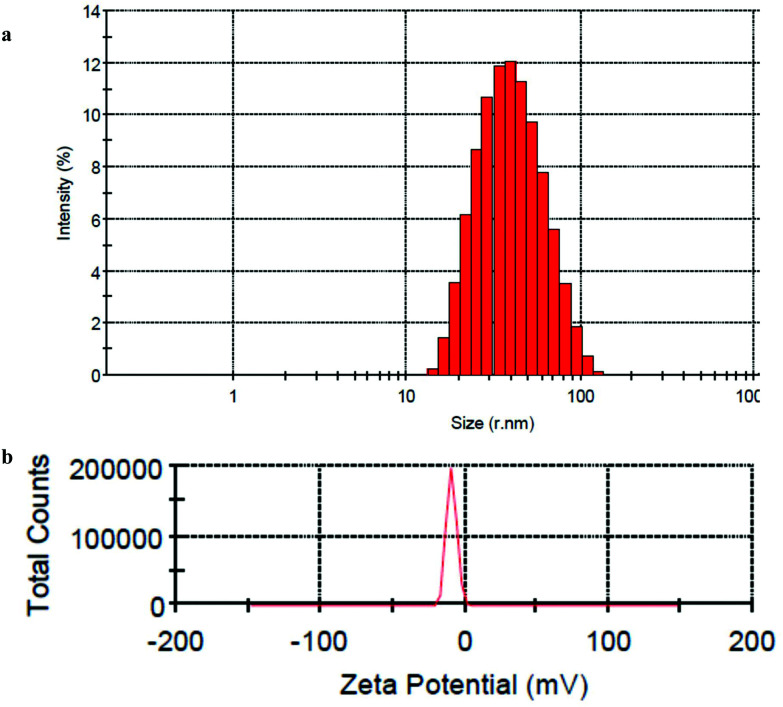
DLS analysis of the prepared PS NP suspension demonstrates isolated particles with an average hydrodynamic diameter (dh) of 38.92 nm in deionized water (Fig. 1a). The NP size distribution is also presented in this figure. ζ-Potential measurements revealed that PS NPs have a negative surface charge (–9.27 ± 3.57 mV) at pH 6.9 ± 0.1 (Fig. 1b), demonstrating an incipient stability of particles. Although this finding cannot be extrapolated to the gastrointestinal mucus, the ζ-potential of PS NPs in our study, though relatively small, has been sufficient to prevent NP adherence and thus formation of larger aggregates.
Fig. 1. Particle size histogram (a) and zeta-potential of PS-NPs (b) obtained from DLS measurements.
3.2. Thyroid hormone levels
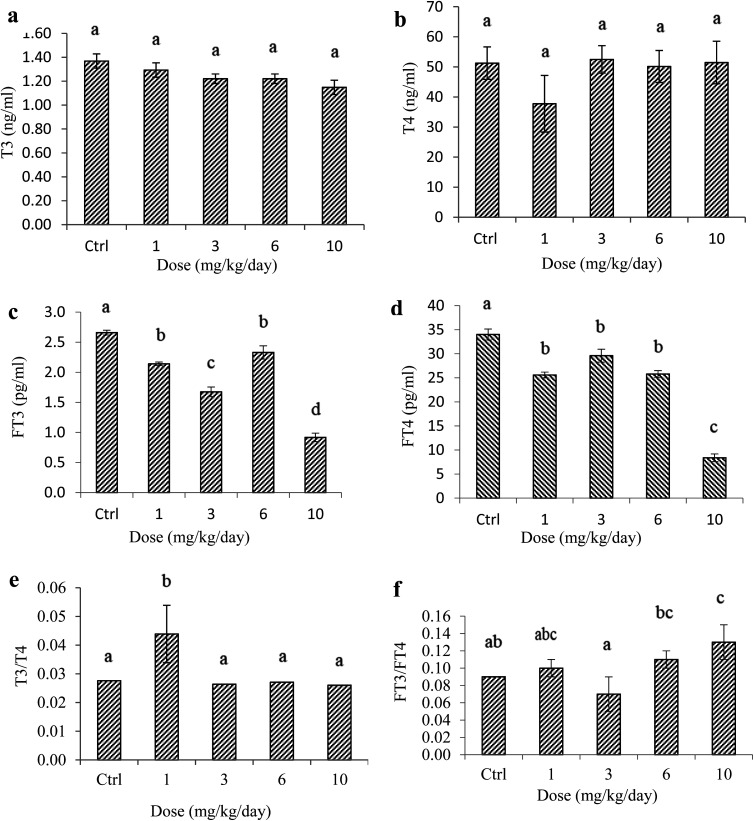
Thyroid hormone levels were analyzed in the blood serum of normal and exposed animals. Although the serum concentrations of T3 were affected by PS NP exposure (Fig. 2a), no statistically significant differences were observed among groups for the T3 level which ranged from 1.29 ± 0.06 to 1.37 ± 0.06 m IU ml–1 (ANOVA, Fig. 2; also see ESI†). The graphs indeed present error bars obtained from the standard error analysis of exposure tests and hormone analysis. The maximum error from error analysis was ±6%, suggesting a high homogeneity of the values in each group. It is clear that the T3 values decreased with an increase in the exposure dosage. Following exposure to polystyrene nanoparticles, on the other hand, the T4 levels of administered rats appeared unchanged as compared to those of control samples (p > 0.05) (Fig. 2b; also see the ESI†), demonstrating that exposure to PS nanoplastics has no considerable effects on the synthesis of this hormone, with one notable exception – a mild suppression of T4 activity by exposure to the lowest PS dosage (1 mg kg–1 day–1).
Fig. 2. Serum concentrations of (a) T3, (b) T4, (c) FT3, and (d) FT4, and the ratios of (e) T3/T4 and (f) FT3/FT4 in rats orally administered with PS NPs and control. Error bars are means ± SEM (n = 6). Different letters for the significance demonstrate that there are significant differences among groups (p < 0.05).
The toxicity of PS NPs towards thyroid gland function was also studied through the analyses of the circulating active levels of thyroid hormones (FT3 and FT4) (Fig. 2c and d). Error analysis revealed that the standard error ranged from 0.07 to ±0.03 for FT3 and from 0.1 to ±0.05 for FT4. As it is difficult to present such small error bars for some experimental groups, only arithmetic average values of hormone concentrations are visible in the graphs. Exposure to PS NPs had a mild disrupting effect in male rates, resulting in a decrease of FT3 levels, though these effects were strengthened with the exposure dosage especially in exposure to 10 mg PS per kg per day (by 55.33%), without much effect in the case of a mid-dose of 6 mg kg–1 day–1. In comparison, although the measured levels of FT4 were suppressed in all exposure levels compared to those of the normal rats (Fig. 2d), the activity of freely circulating thyroxin was rather stable except for a substantial and statistically significant suppression (by 75.29%) in the animals exposed to the highest dosage (10 mg kg–1 day–1). It was further confirmed by ANOVA analysis where the serum FT4 levels of the control group were slightly different from 1 to 6 – and different from the 10 mg PS NPs per kg per day administered unit (Fig. 2d; also see the ESI†). The analysis of free active T3 against T3 bound to serum thyroid-hormone binding proteins revealed that ≤0.08 to 0.19% of triiodothyronine freely circulated in the blood, whereas about 0.02 to 0.07% of thyroxine circulated freely in the blood plasma.
It has been reported that the adverse consequences of exposure to environmental contaminants on thyroid function are associated with changes in the peripheral deiodination and/or metabolism of T4, illustrated by the FT3/FT4 ratio.17,35 In the case of this work, the molar ratios of FT3/FT4 ranged between 0.07 and 0.13 (Fig. 2f). Analysis of this ratio suggests that higher polystyrene exposure affected the synthesis of free active thyroxine (FT4). The highest nanoparticle dosage in the present study (10 mg PS NPs per kg per day) revealed a significantly higher FT3 concentration as opposed to FT4 measurements, resulting in sizable FT3/FT4 ratios for this group (p < 0.05).
3.3. TSH levels
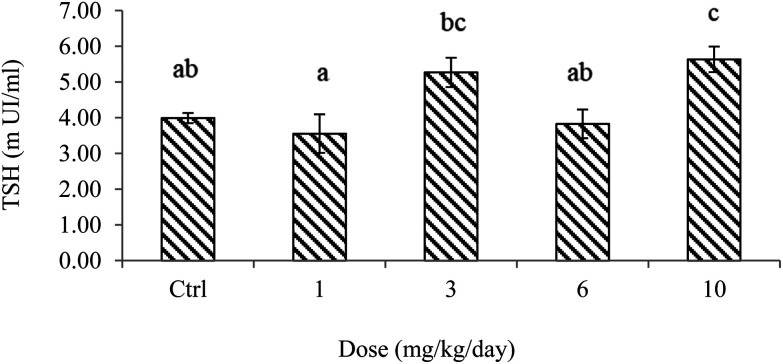
The effect of PS NPs on the serum levels of thyroid-stimulating hormones is presented in Fig. 3. This plot suggests strong block correlation among exposure levels. It is seen from the comparison of the control and the lowest exposure dosage (1 mg PS NPs per kg per day) that the exposure to polystyrene nanoparticles did not cause much change in the serum hormone level. However, the blood serum levels of TSH were strongly elevated by exposure to PS NPs and plummeted in the 6 mg kg–1 day–1 exposed group. Although ANOVA of the results showed that different dosages of PS NPs had different effects on TSH levels, Tukey's multiple comparison analysis revealed that there were significant differences between PS NP dosages of 1 and 10 as well as 6 and 10 mg kg–1 day–1 at a significance level of 5%. Recalling the post hoc multiple comparison analysis showed that 10 mg PS NPs per kg per day slightly stimulated TSH activity, but an insignificant difference was observed between the control and all other groups (p > 0.05) (Fig. 3; also see the ESI†).
Fig. 3. Serum concentrations of TSH in rats orally administered with PS NPs and control. Error bars are means ± SEM (n = 6). Different letters for the significance demonstrate that there are significant differences among groups (p < 0.05).
3.4. Blood biochemistry
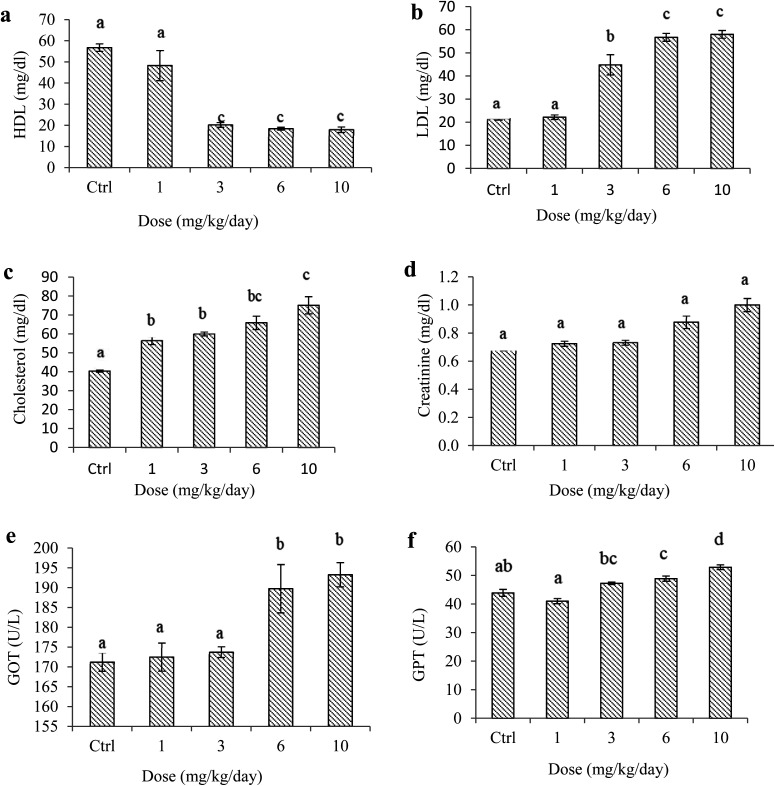
Exposure to PS NPs significantly lowered the plasma HDL levels (Fig. 4a). Variance analysis of the results also demonstrated that different concentrations of PS NPs affected the serum levels of HDL at a significance level of 5%. Tukey's multiple comparison analysis revealed that there was no significant difference in the serum levels of HDL between the animals in the control group and samples treated with a dosage of 1 mg kg–1 day–1, and also samples treated with dosages from 3 to 10 mg kg–1 day–1 (Fig. 4a; also see the ESI†). A notably high variability in HDL levels was indeed seen within the group administered with a dosage of 1 mg kg–1 day–1. In contrast, PS treatments significantly increased serum LDL, cholesterol, GOT, and GPT levels notably at dosages ≥3 mg kg–1 day–1 (Fig. 4b–f). Variance analyses of the results again revealed that exposing the rats to different dosages of PS NPs had significantly different effects on the blood levels of LDL and cholesterol (p < 0.05). Additionally, the serum creatinine levels of normal and exposed rats were measured. Our results implied that the mean serum creatinine levels of the exposed animals were considerably higher than the level in the control (p < 0.05) (Fig. 4d). Post hoc multiple comparison analyses further illustrated that there were significant disparities among exposed groups in view of GOT and, remarkably, GPT enzymes at a significance level of 5% (Fig. 4e and f; also see the ESI†).
Fig. 4. Biochemical parameters of rats orally administered with PS NPs and control. Error bars are means ± SEM (n = 6). Serum levels of (a) HDL, (b) LDL, (c) cholesterol, (d) creatinine, (e) GOT and (f) GPT.
3.5. Correlations between TH levels and serum biochemical parameters
The correlations between TH levels and some serum biochemical parameters were also taken into account in our study. As depicted in Table 1, the mean serum levels of LDL, cholesterol, and GPT were negatively correlated with T3 (r = –0.532, –0.604, and –0.632, p < 0.05; respectively). Also, a nearly significant negative correlation between T3 and GOT was observed (r = –0.404, p = 0.078). In contrast, T3 strongly positively correlated with HDL (r = 0.533, p = 0.015).
Table 1. Correlation coefficients (r) and p-values of the relationships between thyroid function (serum T3, T4, FT3, FT4, TSH concentrations, and T3/T4 and FT3/FT4 ratios) and biochemical variables (LDL, HDL, cholesterol, creatinine, GOT and GPT). Asterisks indicate significant Pearson correlation coefficients.
| LDL | HDL | Cholesterol | GOT | GPT | Creatinine | ||
| T3 | r | –0.532* | 0.533* | –0.604** | –0.404 | –0.632** | –0.46* |
| p | 0.016 | 0.015 | 0.005 | 0.078 | 0.003 | 0.041 | |
| T4 | r | 0.254 | –0.32 | –0.119 | –0.055 | 0.218 | 0.06 |
| p | 0.28 | 0.169 | 0.617 | 0.817 | 0.355 | 0.802 | |
| FT3 | r | –0.454* | 0.407 | –0.533* | –0.41 | –0.64** | –0.614** |
| p | 0.045 | 0.075 | 0.015 | 0.072 | 0.002 | 0.004 | |
| FT4 | r | –0.578** | 0.504* | –0.756** | –0.628** | –0.623** | –0.791** |
| p | 0.008 | 0.023 | 0.000 | 0.003 | 0.003 | 0.000 | |
| FT3/FT4 | r | 0.425 | –0.328 | –0.51* | 0.566* | 0.395 | –0.565* |
| p | 0.062 | 0.158 | 0.022 | 0.009 | 0.085 | 0.009 | |
| T3/T4 | r | –0.418 | 0.379 | –0.199 | –0.31 | –0.46* | –0.235 |
| p | 0.67 | 0.1 | 0.4 | 0.183 | 0.041 | 0.319 | |
| TSH | r | 0.374 | –0.49* | 0.394 | 0.308 | 0.58** | 0.349 |
| p | 0.104 | 0.028 | 0.086 | 0.186 | 0.007 | 0.131 |
A roughly similar pattern was observed between FT3 and biochemical variables. Two parameters revealed strong negative correlations with circulating levels of triiodothyronine: GPT (r = –0.64, p = 0.002) and creatinine (r = –0.614, p = 0.004). Significant negative associations were indeed evidenced between FT3 levels and LDL (r = –0.454, p = 0.045) as well as cholesterol (r = –0.533, p = 0.015), which was not observed with GOT and HDL.
Unlike lots of significant correlations pointed out for serum levels of both freely circulating and protein bound triiodothyronine (FT3 and T3, respectively) and biochemical variables, no significant correlations were noticed between T4 and these variables. Similar results were observed in the case of the T3/T4 ratio, except for GPT which exhibited a significant positive correlation. In contrast, the mean serum levels of biochemical variables demonstrated strong inverse associations with the levels of FT4. A notable exception from this pattern was a robust positive correlation with HDL (r = 0.504, p = 0.023). Interestingly, the circulating levels of THs were positively correlated with the biochemical variables in our study, as corroborated by the close positive association between the ratio of FT3/FT4 and cholesterol (r = 0.51, p = 0.022), GOT (r = 0.566, p = 0.009) as well as creatinine (r = 0.565, p = 0.009). LDL and GPT levels were both nearly correlated with the FT3/FT4 ratio (r = 0.425, p = 0.062; r = 0.395, p = 0.085, respectively).
The mean blood levels of HDL were negatively correlated with the levels of TSH (r = 0.49, p = 0.028). Robust positive correlation was also noticed between TSH and GPT levels (r = 0.58, p = 0.007). In addition, our data demonstrated a nearly significant association between cholesterol and serum levels of TSH (r = 0.394, p = 0.086). No association was observed with other biochemical variables.
4. Discussion
The literature already includes several reports of exposure routes and eco-toxicity of micro- and nanoplastics (e.g., ref. 13 and 36). Though the biochemical activity of plastics in the body may be determined,2 exposure to PS NPs in our study affected the serum levels of thyroid hormones and their ratios as well as TSH in a dose-dependent manner. Thyroid disruption may take place through several pathways, including alterations in the hypothalamus and/or pituitary status, hormone synthesis and secretion, regulation, transport and metabolism, and/or interference with a receptor.37 Lower levels of T3 were found in the exposed groups which demonstrate a common stress signature, but this change was not statistically significant. Despite the limited literature on the subject, this finding was consistent with that of Miao et al.38 who reported that zebrafish exposure to lead significantly suppressed TH (T4 and T3) levels, whereas titanium dioxide (TiO2) nanoparticles did not produce detectable changes. A study on short-term exposure of Sprague-Dawley rats to TiO2 nanoparticles, however, found significantly lower T3 levels in the exposed group than in the controls (0.1 vs. 0.07 ng mL–1, p < 0.05).39 Another study suggested a significantly higher T3 level in zebrafish larvae exposed to ZnO nanoparticles.40 Similarly, elevated amounts of T3 were observed in zebrafish larvae on cobalt ferrite (CoFe2O4) nanoparticle treatment.41 It is worth noting here that extrapolations from such studies on engineered nanoparticles such as metal and metal oxides have to be made cautiously, since toxicity relies heavily on the physicochemical aspects of nanomaterials.42 Despite remarkable disagreements among these studies, it is important to bear in mind that a minor decrease in mean T3 is clinically considerable,43 even though it was not significant statistically. It has been widely accepted that the importance of clinical outcomes takes precedence over statistical significance.44 Furthermore, T3 levels in our study appeared highly correlated with the decrease of cholesterol, LDL and GPT as well as the increase of HDL, suggesting a dominant effect of T3 in fat metabolism.
As shown in Fig. 2b, none of the experimental exposure to PS NPs resulted in disturbance of TH synthesis, as indicated by the maintained stable values of T4 secretion. Meanwhile, it is seen from the comparison of the nanoparticle dosage and T4 levels that low exposure to PS NPs was associated with decreased T4 levels in animals. Likewise, a difference of 13.5 ng mL–1 in mean T4 could be clinically significant, even though it was just suggestively significant (p = 0.085). Observed changes in T4 levels could be assigned to alterations in the thyroid follicles and regulation of TSH, as well as changes in iodothyronine deiodinase activity.35,45
Reports in contrast to our findings demonstrating increased levels of THs because of exposure to engineered nanoparticles, however, exist.37–40 The T3/T4 ratio is an index which reflects the thyroid function and action of hormones on tissues.17 During the PS NP administration, the highest T3/T4 ratio was observed in rats administered the lowest dosage. Molar ratios of T3/T4 remained, however, unchanged by further administration of higher dosages in our study. The high T3/T4 ratio exhibited by the lowest exposed group was related to lower T4 levels in the serum. It is encouraging to compare this result with that reported by Gong et al.,46 who found higher T3 to T4 ratios in goldfish exposed to diluted leachate, a matrix probably enriched with plastic nanoparticles.
Exposure to PS NPs led to suppressed circulating levels of thyroid hormones (FT3 and FT4) coupled with an evident stimulation in TSH levels. This finding contrasts with earlier studies demonstrating an activation of thyroid function during Cyprinus carpio exposure to copper sulfate and copper nanoparticles.37 The decrease in serum levels of both free and protein-bound T3 in our study could be explained by alterations in the peripheral TH metabolism.47,48 In addition, a decline of FT4 paves the way for the decrease in the FT3 level.
Exposure to virgin polyethylene pre-production pellets has been reported to promote endocrine disrupting effects in Japanese medaka.15 Results of two recent studies further highlight the ability of pristine low-density polyethylene fragments to cause toxicity in zebrafish larvae49 and African catfish.50 Even insignificant variations in circulating levels of thyroid hormones have been reported to have permanent effects on metabolism and development.51 It implies that long-term exposure to polystyrene nanoplastics could exhaust the thyroid endocrine function, weakening its driving force in the regulation of growth, development, metabolism, and reproduction.52,53 In fact, the carcinogenic and endocrine disrupting effects of a styrene monomer have been highlighted before.28 Also more complex exposure scenarios, including e.g. chemical burden and individual susceptibility, need to be taken into account to be able to estimate the health risks of plastic debris. Given that other adverse events accumulate with respect to the thyroid axis life history, effects in older rats might be much more pronounced. An analysis of the data available in the literature already confirms that effects of different goitrogenic substances differ in young and old experimental animals.54,55 Additionally, in spite of subtle and insignificant changes in T3/T4, the molar ratios of FT3/FT4 underwent a considerable increase as a function of exposure dosage. It implies that PS NPs in our study changed the consumption of these hormones, due to alterations in metabolism.
These responses are in good agreement with thyroid stimulating hormone levels (Fig. 3). The highest dosage exposure group is distinguished from the non-exposed control primarily by the excellently enhanced TSH levels. This result is further supported by an increase in the corresponding FT3 values, as observed in Fig. 2c. It has been reported that exposure to cobalt, zinc and their organic nano-complexes led to TSH disruption in frogs.14 The stimulating effects of this pituitary hormone encourage the thyroid gland to synthesize thyroxine, which is thence transformed to its active form (T3) through peripheral deiodination of its aromatic ring mostly in the liver.56 It has previously been reported that prolonged stimulation of the pituitary gland by suppressed levels of THs leads to the release of highly elevated levels of TSH by the thyrotrophs. The latter, in turn, may result in thyroid gland neoplasia.57,58 The dominant effect of TSH on specific receptors on the membrane of follicular cells is sufficient to invigorate the activity of a sodium–iodine symporter and also of intracellular enzymes entailed in thyroid hormone homeostasis. Accordingly, the feedback inhibition of TSH is expected to attenuate in response to decreased serum levels of active THs and more TSH is secreted. Such a process favors cell hyperplasia and hypertrophy and provokes thyroid function to lie in a dynamic state in order to sustain the required levels of thyroid hormones in the body.45 Although our results provided no evidence of possible endocrine disrupting mechanisms, it can be deduced that the downstream transformation of T4 toT3 by deiodination and upstream mechanisms controlling T4 synthesis (i.e., TSH plasma levels) are by far the most important modes of action, and the latter seems to be more probable. Nevertheless, the mechanism behind the dysregulation of thyroid functions at a mild nano-sized PS dosage (6 mg kg–1 day–1) in this work, compared to those with other exposed groups, remains unidentified. This was in line, however, with a generally assumed pattern, where endocrine disruptors show signs of a nonlinear or nonmonotonic dose–response. It denotes that tiny dosages could result in significant consequences than mid-level dosages.59,60
In addition, our data revealed significant correlations between THs and lipoproteins (Table 1). Exposed animals were considered to be accompanied by increased levels of cholesterol and LDL, as well as decreased HDL levels (Fig. 3), suggesting dominant effects of polystyrene NP exposure on lipid metabolism. Decreased thyroid secretion considerably increases the plasma concentrations of cholesterol, phospholipids, and triglycerides and almost always causes excessive deposition of fat in the liver as well.20 Ingestion of PS particles has been reported to disturb the fat metabolism in freshwater fishes.61 Our results are also in accord with earlier studies on rodents in which thyroid hormones enhanced the synthesis of HDL.62
One of the mechanisms by which thyroid hormones decrease the plasma cholesterol concentration is increasing significantly the rate of cholesterol secretion in the bile and consequent loss in the feces. A possible mechanism for the increased cholesterol secretion is that thyroid hormones induce increased numbers of low-density lipoprotein receptors on the liver cells, causing fast removal of low-density lipoproteins from the plasma by the liver and subsequent secretion of cholesterol in these lipoproteins by the liver cells.20 Thyroxine (T4), on the other hand, may promote the reverse cholesterol transport expression, making improvements in lipid metabolism.63 Furthermore, the correlations between these two lipoproteins and circulating levels of thyroid hormones were investigated. The serum levels of FT4 were negatively correlated with LDL and positively associated with HDL. It was reported that alterations of LDL in hypo- or hyperthyroidism are associated with free active T4 levels.64 In another study, a concomitant increase in TSH and suppression of HDL were observed among patients with metabolic syndrome.65
Interestingly, FT4 values were inversely correlated with serum GOT and GPT production. The mechanisms behind these relationships are not quite clear; however, there is an underlying assumption that such an association may reflect hepatotoxicity.61 These enzymes are predominantly found in the cytoplasm of hepatic cells, and thence their excretion to blood might result from cell damage.66 Additionally, serum concentrations of creatinine were positively associated with a dose-dependent increase in PS NP exposure in rats. Serum creatinine is a well-known clinical indicator of kidney function. A recent study reported that serum creatinine levels of rats with kidney damage were significantly higher than those of control subjects.67 Interestingly, it was shown that fluorescent polystyrene nanoparticles were taken up by rats and localized in the kidney.11
5. Conclusion
The present study reports new information on the thyroid endocrine status of experimental male rats orally exposed to polystyrene plastic nanoparticles. To summarize, PS NPs induced a general state of thyroid endocrine disruption and metabolic deficit. A concomitant decrease in the levels of T3, free active THs (FT3 and FT4), and biochemical variables except for HDL was found. This metabolic deficit may account for possible associations between exposure to polystyrene nanoparticles and thyroid function dysregulation. Given the current concerns over the eco-toxicity of engineered nano-materials, the outcomes of this study reflect early warning symptoms of the endocrine disrupting potential of plastic nanoparticles in experimental animals, indicating that plastic fragments may induce changes in the functioning of the endocrine system in rodents. Also more complex exposure scenarios which could highly represent actual environmental nanoplastics, including e.g. chemical burden, individual susceptibility and NP bioavailability, need to be taken into account to be able to estimate the health risks of plastic debris. Nevertheless, the exact dose to which the population is exposed has to be clarified, since there are different sources of exposure with different nano-sized plastic types and concentrations. The time of exposure indeed varies with lifestyle; so it becomes important to study a broad range of sizes and concentrations. However, taking the species differences into account and that exposure levels in humans are probably tens of thousands of times lower than the lowest effect levels in the present study, we cannot argue that the outcomes obtained with rat samples will be the same in humans. These and many other implications, however, remain far from clear but will warrant scrutiny over the next months and years. Finally, we attempted to show how the public health status could be affected by the ever increasing accumulation of plastic debris.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the Shahid Beheshti University of Medical Sciences, Tehran, Iran (grant no: 10790). The authors want to express their special thanks to the experts of Neuroscience Research Center, Shahid Beheshti University of Medical Sciences for the animal feeding work and for their collaboration in this study.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tx00147f
References
- Ecoclimax, Average daily plastic waste production per capita, 2016. [Google Scholar]
- Hammer J., Kraak M. H. and Parsons J. R., Plastics in the marine environment: the dark side of a modern gift, in Reviews of Environmental Contamination and Toxicology, Springer, 2012, pp. 1–44. [DOI] [PubMed] [Google Scholar]
- Peters C. A., Bratton S. P. Environ. Pollut. 2016;210:380–387. doi: 10.1016/j.envpol.2016.01.018. [DOI] [PubMed] [Google Scholar]
- Andrady A. L. Mar. Pollut. Bull. 2011;62(8):1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Lambert S., Sinclair C. and Boxall A., Occurrence, degradation, and effect of polymer-based materials in the environment, in Reviews of Environmental Contamination and Toxicology, Springer, 2014, vol. 227, pp. 1–53. [DOI] [PubMed] [Google Scholar]
- Mattsson K., Hansson L.-A., Cedervall T. Environ. Sci.: Processes Impacts. 2015;17(10):1712–1721. doi: 10.1039/c5em00227c. [DOI] [PubMed] [Google Scholar]
- Fendall L. S., Sewell M. A. Mar. Pollut. Bull. 2009;58(8):1225–1228. doi: 10.1016/j.marpolbul.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Ziajahromi S., Neale P. A., Leusch F. D. Water Sci. Technol. 2016;74(10):2253–2269. doi: 10.2166/wst.2016.414. [DOI] [PubMed] [Google Scholar]
- da Costa J. P., Santos P. S., Duarte A. C., Rocha-Santos T. Sci. Total Environ. 2016;566:15–26. doi: 10.1016/j.scitotenv.2016.05.041. [DOI] [PubMed] [Google Scholar]
- EFSA, EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) EFSA J. 2016;14(6):4501. [Google Scholar]
- Walczak A. P., Hendriksen P. J., Woutersen R. A., van der Zande M., Undas A. K., Helsdingen R., van den Berg H. H., Rietjens I. M., Bouwmeester H. J. Nanopart. Res. 2015;17(5):231. doi: 10.1007/s11051-015-3029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baun A., Hartmann N. B., Grieger K., Kusk K. O. Ecotoxicology. 2008;17(5):387–395. doi: 10.1007/s10646-008-0208-y. [DOI] [PubMed] [Google Scholar]
- Pitt J. A., Kozal J. S., Jayasundara N., Massarsky A., Trevisan R., Geitner N., Wiesner M., Levin E. D., Di Giulio R. T. Aquat. Toxicol. 2018;194:185–194. doi: 10.1016/j.aquatox.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falfushynska H., Gnatyshyna L., Fedoruk O., Sokolova I. M., Stoliar O. Aquat. Toxicol. 2016;170:62–71. doi: 10.1016/j.aquatox.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Rochman C. M., Kurobe T., Flores I., Teh S. J. Sci. Total Environ. 2014;493:656–661. doi: 10.1016/j.scitotenv.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Quintaneiro C., Patrício D., Novais S., Soares A., Monteiro M. Sci. Total Environ. 2017;586:390–400. doi: 10.1016/j.scitotenv.2016.11.153. [DOI] [PubMed] [Google Scholar]
- Couderc M., Marchand J., Zalouk-Vergnoux A., Kamari A., Moreau B., Blanchet-Letrouvé I., Le Bizec B., Mouneyrac C., Poirier L. Sci. Total Environ. 2016;550:391–405. doi: 10.1016/j.scitotenv.2015.12.136. [DOI] [PubMed] [Google Scholar]
- Jarque S., Piña B. Environ. Res. 2014;135:361–375. doi: 10.1016/j.envres.2014.09.022. [DOI] [PubMed] [Google Scholar]
- Köhrle J. Cell. Mol. Life Sci. 2000;57(13):1853–1863. doi: 10.1007/PL00000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. E., Guyton and Hall Textbook of Medical Physiology e-Book, Elsevier Health Sciences, 2015, pp. 910–914. [Google Scholar]
- Brouwer A., Morse D. C., Lans M. C., Schuur A. G., Murk A. J., Klasson-Wehler E., Visser T. J. Toxicol. Ind. Health. 1998;14(1–2):59–84. doi: 10.1177/074823379801400107. [DOI] [PubMed] [Google Scholar]
- Chan W. K., Chan K. M. Aquat. Toxicol. 2012;108:106–111. doi: 10.1016/j.aquatox.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Lema S. C., Dickey J. T., Schultz I. R., Swanson P. Environ. Health Perspect. 2008;116(12):1694–1699. doi: 10.1289/ehp.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E. N., Braverman L. E. Best Pract. Res., Clin. Endocrinol. Metab. 2009;23(6):801–813. doi: 10.1016/j.beem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Xu P., Lou X., Ding G., Shen H., Wu L., Chen Z., Wang X. Sci. Total Environ. 2015;536:215–222. doi: 10.1016/j.scitotenv.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Rafiee M., Dargahi L., Eslami A., Beirami E., Jahangiri-rad M., Sabour S., Amereh F. Chemosphere. 2018;193:745–753. doi: 10.1016/j.chemosphere.2017.11.076. [DOI] [PubMed] [Google Scholar]
- Baldwin A. K., Corsi S. R., Mason S. A. Environ. Sci. Technol. 2016;50(19):10377–10385. doi: 10.1021/acs.est.6b02917. [DOI] [PubMed] [Google Scholar]
- Lithner D., Larsson Å., Dave G. Sci. Total Environ. 2011;409(18):3309–3324. doi: 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Sadri S. S., Thompson R. C. Mar. Pollut. Bull. 2014;81(1):55–60. doi: 10.1016/j.marpolbul.2014.02.020. [DOI] [PubMed] [Google Scholar]
- Bergami E., Bocci E., Vannuccini M. L., Monopoli M., Salvati A., Dawson K. A., Corsi I. Ecotoxicol. Environ. Saf. 2016;123:18–25. doi: 10.1016/j.ecoenv.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Lu Y., Zhang Y., Deng Y., Jiang W., Zhao Y., Geng J., Ding L., Ren H. Environ. Sci. Technol. 2016;50(7):4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A., Basak G. C. Mater. Sci. Technol. 2007;23(3):307–314. [Google Scholar]
- Degli Innocenti F., Biodegradability and Compostability. Biodegradable polymers and plastics, Springer, 2003, pp. 33–45. [Google Scholar]
- Lambert S., Wagner M. Chemosphere. 2016;145:265–268. doi: 10.1016/j.chemosphere.2015.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J. A., Patiño R. Gen. Comp. Endocrinol. 2011;170(2):299–312. doi: 10.1016/j.ygcen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Cole M., Galloway T. S. Environ. Sci. Technol. 2015;49(24):14625–14632. doi: 10.1021/acs.est.5b04099. [DOI] [PubMed] [Google Scholar]
- Hoseini S. M., Hedayati A., Mirghaed A. T., Ghelichpour M. Exp. Toxicol. Pathol. 2016;68(9):493–503. doi: 10.1016/j.etp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Miao W., Zhu B., Xiao X., Li Y., Dirbaba N. B., Zhou B., Wu H. Aquat. Toxicol. 2015;161:117–126. doi: 10.1016/j.aquatox.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Tassinari R., Cubadda F., Moracci G., Aureli F., D'Amato M., Valeri M., De Berardis B., Raggi A., Mantovani A., Passeri D. Nanotoxicology. 2014;8(6):654–662. doi: 10.3109/17435390.2013.822114. [DOI] [PubMed] [Google Scholar]
- Du J., Wang S., You H., Liu Z. J. Environ. Sci. 2016;47:153–164. doi: 10.1016/j.jes.2016.01.018. [DOI] [PubMed] [Google Scholar]
- Ahmad F., Liu X., Zhou Y., Yao H., Zhao F., Ling Z., Xu C. Environ. Toxicol. 2016;31(12):2068–2080. doi: 10.1002/tox.22206. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Dekkers S., Noordam M. Y., Hagens W. I., Bulder A. S., De Heer C., Ten Voorde S. E., Wijnhoven S. W., Marvin H. J., Sips A. J. Regul. Toxicol. Pharmacol. 2009;53(1):52–62. doi: 10.1016/j.yrtph.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Mescher A. L., in JUNQUEIR'S Basic Histology: Text and Atlas, McGraw-Hill Education, USA, 15th edn, 2018. [Google Scholar]
- Rothman K. J., Greenland S. and Lash T. L., Modern epidemiology, Lippincott Williams & Wilkins, 2008. [Google Scholar]
- Chiamolera M. I., Wondisford F. E. Endocrinology. 2009;150(3):1091–1096. doi: 10.1210/en.2008-1795. [DOI] [PubMed] [Google Scholar]
- Gong Y., Tian H., Dong Y., Zhang X., Wang J., Wang W., Ru S. Sci. Total Environ. 2016;554:64–72. doi: 10.1016/j.scitotenv.2016.02.188. [DOI] [PubMed] [Google Scholar]
- Li D., Xie P., Zhang X. Chemosphere. 2008;74(1):13–18. doi: 10.1016/j.chemosphere.2008.09.065. [DOI] [PubMed] [Google Scholar]
- Liu Z., Li D., Wang Y., Guo W., Gao Y., Tang R. Environ. Toxicol. Chem. 2015;34(9):2033–2040. doi: 10.1002/etc.3024. [DOI] [PubMed] [Google Scholar]
- Karami A., Groman D. B., Wilson S. P., Ismail P., Neela V. K. Environ. Pollut. 2017;223:466–475. doi: 10.1016/j.envpol.2017.01.047. [DOI] [PubMed] [Google Scholar]
- Karami A., Romano N., Galloway T., Hamzah H. Environ. Res. 2016;151:58–70. doi: 10.1016/j.envres.2016.07.024. [DOI] [PubMed] [Google Scholar]
- Zoeller T. R., Dowling A. L., Herzig C. T., Iannacone E. A., Gauger K. J., Bansal R. Environ. Health Perspect. 2002;110(Suppl 3):355. doi: 10.1289/ehp.02110s3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloas W., Urbatzka R., Opitz R., Würtz S., Behrends T., Hermelink B., Hofmann F., Jagnytsch O., Kroupova H., Lorenz C. Ann. N. Y. Acad. Sci. 2009;1163(1):187–200. doi: 10.1111/j.1749-6632.2009.04453.x. [DOI] [PubMed] [Google Scholar]
- Opitz R., Hartmann S., Blank T., Braunbeck T., Lutz I., Kloas W. Toxicol. Sci. 2006;90(2):337–348. doi: 10.1093/toxsci/kfj083. [DOI] [PubMed] [Google Scholar]
- Bajaj J. K., Salwan P., Salwan S. J. Clin. Diagn. Res. 2016;10(1):FE01. doi: 10.7860/JCDR/2016/15195.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad B., Hoffman M. M. Am. J. Physiol. 1955;182(3):497–502. doi: 10.1152/ajplegacy.1955.182.3.497. [DOI] [PubMed] [Google Scholar]
- Brown D. D. Thyroid. 2005;15(8):815–821. doi: 10.1089/thy.2005.15.815. [DOI] [PubMed] [Google Scholar]
- Boelaert K. Endocr.-Relat. Cancer. 2009;16(4):1065–1072. doi: 10.1677/ERC-09-0150. [DOI] [PubMed] [Google Scholar]
- Hood A., Liu Y. P., Gattone 2nd V., Klaassen C. D. Toxicol. Sci. 1999;49(2):263–271. doi: 10.1093/toxsci/49.2.263. [DOI] [PubMed] [Google Scholar]
- Seltenrich N. Environ. Health Perspect. 2015;123(2):A34. doi: 10.1289/ehp.123-A34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons W. V., Thayer K. A., Judy B. M., Taylor J. A., Curran E. M., Vom Saal F. S. Environ. Health Perspect. 2003;111(8):994. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedervall T., Hansson L.-A., Lard M., Frohm B., Linse S. PLoS One. 2012;7(2):e32254. doi: 10.1371/journal.pone.0032254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A., Xia T., Mädler L., Li N. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Canton I., Battaglia G. Chem. Soc. Rev. 2012;41(7):2718–2739. doi: 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]
- Gutch M., Rungta S., Kumar S., Agarwal A., Bhattacharya A., Razi S. M. Biomed. J. 2017;40(3):147–153. doi: 10.1016/j.bj.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulivo M., de Alda M. L., Capri E., Barceló D. Environ. Res. 2016;151:251–264. doi: 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Amacher D. E. Regul. Toxicol. Pharmacol. 1998;27(2):119–130. doi: 10.1006/rtph.1998.1201. [DOI] [PubMed] [Google Scholar]
- Rana K., Verma Y., Rani V., Rana S. V. S. Chemosphere. 2018;193:142–150. doi: 10.1016/j.chemosphere.2017.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.