Abstract
Hyponatremia is a very common electrolyte abnormality, associated with poor short- and long-term outcomes in patients with heart failure (HF). Two opposite processes can result in hyponatremia in this setting: Volume overload with dilutional hypervolemic hyponatremia from congestion, and hypovolemic hyponatremia from excessive use of natriuretics. These two conditions require different therapeutic approaches. While sodium in the form of normal saline can be lifesaving in the second case, the same treatment would exacerbate hyponatremia in the first case. Hypervolemic hyponatremia in HF patients is multifactorial and occurs mainly due to the persistent release of arginine vasopressin (AVP) in the setting of ineffective renal perfusion secondary to low cardiac output. Fluid restriction and loop diuretics remain mainstay treatments for hypervolemic/dilutional hyponatremia in patients with HF. In recent years, a few strategies, such as AVP antagonists (Tolvaptan, Conivaptan, and Lixivaptan), and hypertonic saline in addition to loop diuretics, have been proposed as potentially promising treatment options for this condition. This review aimed to summarize the current literature on pathogenesis and management of hyponatremia in patients with HF.
Keywords: Hyponatremia, sodium, heart failure, congestive heart failure, vaptans, pathogenesis
1. Introduction
Despite advances in medicine, heart failure (HF) remains one of the most common principal diagnoses for hospital admission worldwide [1-5]. In the United States (U.S.), total costs, including indirect costs for HF, are estimated to increase from $31 billion in 2012 to $70 billion in 2030 [6]. Patients with chronic HF are well known to have neuro-hormonal abnormalities including activations of the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS), which commonly lead to derangements in electrolytes and acid-base balance [7, 8]. In addition to patient’s functional impairment, these metabolic abnormalities such as hyponatremia, hypochloremia and hypokalemia have been considered as poor prognostic indicators of all-cause mortality in HF patients [9-11].
Hyponatremia, defined as a serum sodium concentration <135 mEq/L, is the most common electrolyte abnormality in the hospitalized patients associated with a prolonged hospital length of stay, and higher risks of readmission and mortality [12-14]. We analyzed the ESCAPE Trial analysis and found a benign nature of mild hyponatremia on discharge in HF patients with normal admission Na [15]. In fact, depending on the patient population, the prevalence of hyponatremia in HF patients ranges between 11% and 27% [10, 16-22]. In addition to being well demonstrated in general patient population [23], the presence of hyponatremia on or after admission in patients with HF is also associated with increased risk of morbidities (i.e., falls and bone fractures), discharge to acute care facilities, longer hospital stays, and mortality [23-25]. Furthermore, studies have shown that HF patients with hyponatremia were more likely to be admitted to the ICU reflecting the severity of HF and/or the direct effect of hyponatremia [11, 26]. Among patients with HF, persistent hyponatremia after hospitalization has been shown to be associated with a 1.5 to 1.7-fold increase risk of 30-day readmission or mortality [24, 27].
In the U.S., the direct medical costs of hyponatremia have been estimated between $1.6 billion and $3.6 billion [14, 28]. This review aimed to present the current literature and future directions regarding the pathogenesis and management of hyponatremia in patients with HF.
2. Hypervolemic hyponatremia
Hyponatremia in patients with HF can be multifactorial as summarized in Table 1 [8, 11, 12, 18, 25, 26, 29-32]. The main mechanisms of hyponatremia in HF include 1) adaptive neurohormonal activation with a predominance and leading role of arginine vasopressin (AVP) (dilution state); 2) potent thirst stimulation from both low cardiac output and angiotensin II, and 3) free water retention in the setting of severely reduced glomerular filtration rate (GFR).
Table 1.
| Causes of Hyponatremia in Patients with Heart Failure |
|---|
| 1. Continued release AVP despite a reduction in osmolality due to the following reasons: • Low cardiac output • Decreased renal blood flow • Reduced baroreceptor stimulation mediated by low blood pressure 2. Low cardiac output leads to activation of RAAS and increase in angiotensin II levels, potent stimuli to thirst, resulting in enhanced water intake 3. Medications used to treat heart failure, hypertension, or cardiac-related conditions • Diuretics (especially thiazides, less commonly aldosterone antagonists, amiloride, loop diuretics) 4. Hyponatremia in the setting of advanced CKD/ESRD due to Cardiorenal syndromes • Hypotonic hyponatremia due to increased free water intake in the setting of low GFR |
Abbreviations: ACEIs, Angiotensin-Converting Enzyme Inhibitors; AVP, Arginine Vasopressin; CKD, Chronic Kidney Disease; ESRD, End-Stage Renal Disease; GFR, Glomerular Filtration Rate; RAAS, Renin-Angiotensin-Aldosterone System.
2.1. Neurohormonal Activation in Dilutional Hyponatremia
AVP is synthesized in the supraoptic and paraventricular nuclei of the anterior hypothalamus [33]. Through axonal transport, AVP is transported into nerve terminals within the posterior lobe of the pituitary gland and then released into the bloodstream in response to hypertonicity [34]. AVP secretion is increased by two different stimuli: baroreceptor activation and decreased effective circulating volume [33]. Arterial underfilling from the reduced cardiac output along with activation of the sympathetic nervous system and renin-angiotensin-aldosterone system (RAAS) lead to increased release of AVP. AVP binds to the vasopressin 2 (V2) receptor in the collecting ducts of the nephron and via a cyclic guanosine monophosphate (GMP) mediated mechanism increases aquaporin-2 water channel expression on the luminal side. This, in turn, leads to increased permeability of water in the collecting ducts and enhanced free water retention [34].
In the usual state of health, AVP is degraded and excreted by the liver and kidneys rapidly. At low levels, AVP also stimulates V1a receptors in the liver, vasa recta, and collecting ducts increasing the hypertonicity in the renal medulla through urea production in the liver, urea reabsorption in the collecting duct and by decreasing flow through the vasa recta. When vasa recta receive high flow, interstitial tonicity reduces by washout of osmoles in the medulla [35].
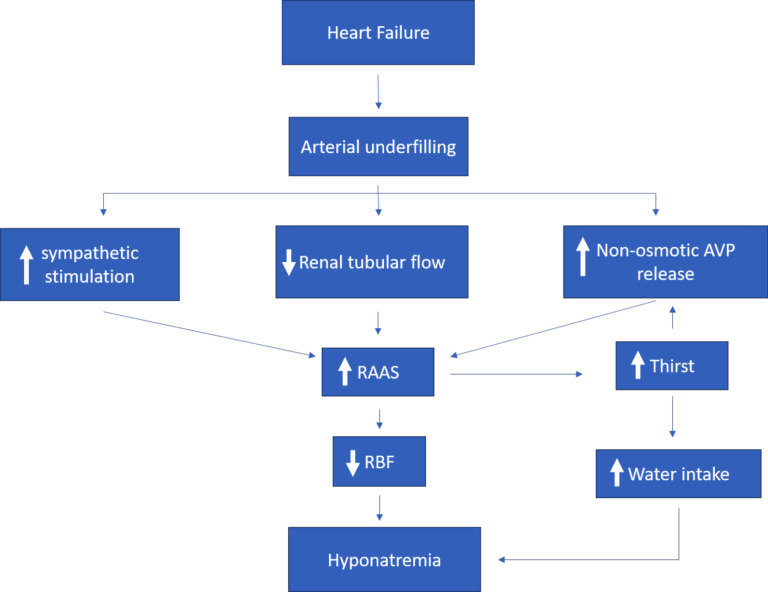
In patients with HF, the reduction in cardiac output induces activation of the sympathetic nervous system [8]. The peripheral vasoconstriction instigated by this phenomenon coupled with the reduced cardiac output leads to sodium and water retention as well as RAAS activation [5, 9]. RAAS activation can increase angiotensin II levels, leading to thirst stimulation and worsening hyponatremia in patients with HF. Compared with patients without HF, AVP levels are higher in patients with severe HF [33], which does not appropriately decrease even with acute water loading [36, 37], thus results in hyponatremia [29, 34, 36] (Fig. 1). AVP role in acute HF and hyponatremia is crucial since it has been shown that HF patients exhibit not only increased AVP production but also a dysregulation of AVP characterized by an elevation of its levels despite the presence of volume overload, atrial distension, and low plasma osmolality [36, 38-40].
Fig. (1).
Pathophysiology of dilutional hyponatremia in heart failure.
2.2. Increase Free Water Consumption in the Setting of Low GFR
Cardiorenal syndrome (CRS) encompasses a disorder of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ [41]. The Acute Dialysis Quality Initiative outlined a consensus approach in 2008 which phenotyped CRS into two major groups: cardio-renal and reno-cardiac syndromes, based on the primum movens of the disease process [42]. This was further grouped into five subtypes of the cardio-renal syndromes based on disease acuity and sequential organ involvement, which are outlined in Table 2. Over 40% of HF patients had some degree of renal impairment (cardio-renal syndrome) [3]. Conversely, HF is highly prevalent approximately up to 60% in patients with advanced CKD and end-stage renal disease (reno-cardiac syndrome) [43]. In patients with CRS, hyponatremia can be dilutional, due to excess water or hypotonic fluid intake or impaired free water clearance in the setting of elevated AVP levels and reduced GFR [44].
Table 2.
Summary of cardio-renal syndromes (CRS) phenotypes [117].
| CRS Type | Denomination | Description | Model |
|---|---|---|---|
| I | Acute Cardio-renal | Acute cardiac decompensation leads to acute kidney injury which can be reversible | Acute coronary syndrome/acute HF triggering acute kidney failure |
| II | Chronic Cardio-renal | Chronic cardiac dysfunction leads to progressive chronic kidney dysfunction which is mostly irreversible | Chronic heart failure causing chronic kidney disease |
| III | Acute Reno-cardiac | Abrupt primary kidney dysfunction leads to acute cardiac dysfunction | Acute glomerulonephritis instigating acute HF |
| IV | Chronic Reno-cardiac | Primary kidney dysfunction leads to cardiac impairment | Chronic kidney disease causing coronary artery disease/diastolic heart dysfunction |
| V | Secondary Cardio-renal | Acute/chronic systemic disease leading to combined cardiac and kidney dysfunction | Sepsis, vasculitis, diabetes mellitus, amyloidosis |
2.3. Depletion Induced Hyponatremia
Diuretics are one of the most common reasons for drug-induced hyponatremia (Table 1). This is particularly characteristic of thiazide diuretics because they work entirely in the distal tubules and do not interfere with urinary concentrating capacity and the action of AVP to promote water retention [45, 46]. Diuretic-induced hyponatremia is usually mild, but idiosyncratic reactions have been reported [31, 32]. Thiazides more commonly cause hyponatremia compared to loop diuretics, with a thiazide-induced hyponatremia incidence that ranging 4.1-14% [46]. Female gender, advanced age, low body mass, multiple comorbidities and increasing dosage of thiazides are risk factors for hyponatremia from this drug class. Thiazides have more propensity to cause hyponatremia because of inhibition of urinary dilution through decreased sodium-chloride re-absorption in the distal tubule, a mechanism that loop diuretics do not have. In general, the pathophysiological factors implicated in thiazide-induced hyponatremia are increased water intake from AVP stimulation through thirst from water loss, reduced free water clearance from antidiuretic hormone (ADH) stimulus and as mentioned above, renal sodium /potassium loss.
Although less commonly, loop diuretics can also contribute to hypovolemic hyponatremia. According to the Acute Decompensated Heart Failure National Registry (ADHERE) registry, loop diuretics are utilized in up to 88% of HF patients as the mainstay of management [47]. Following management of acute HF decompensation, up to 70% of patients continue loop diuretics chronically [47, 48]. Loop diuretics block the sodium-potassium-chloride cotransporter in the thick ascending limb of the loop of Henle [48]. This part of the nephron makes a substantial contribution to the tonicity of the renal interstitium. Loop diuretics interfere with the concentrating capacity of kidneys which leads to reduced free water reabsorption that in turn leads to the production of hypotonic urine [47]. Nevertheless, if profound volume depletion with robust neurohormonal activation ensues, water diuresis will be less pronounced leading to decreased glomerular filtration rate and distal nephron flow in the setting of increased AVP levels, which constitutes an ideal scenario for hyponatremia development [49].
Addition of thiazides (e.g., metolazone or chlorothiazide) to loop diuretics, so-called sequential nephron blockade has been shown to be an effective approach to overcome diuretic resistance in patients with HF [50, 51]. However, this combination of diuretic therapy can result in significant electrolyte abnormalities including hypokalemia, hyponatremia, and hypochloremic metabolic alkalosis [51, 52]. Hyponatremia can occur in this setting because the increase in urine sodium excretion is much higher than the increase in urine water excretion, resulting in more hypertonic urine after sequential nephron blockade than after treatment with loop diuretics alone [51, 53].
Aldosterone antagonist such as spironolactone, work by blocking reabsorption of sodium in the distal convoluted tubules and collecting ducts, generating less hypotonic urine. Their use in heart failure with reduced ejection fraction is usually after betablocker and ACEI have been started. In a retrospective study by Goland et al. [54] they analyzed 157 patients of whom 100 were given spironolactone (were already on ACEI and betablocker). At 1 year follow up, 6 patients developed hyperkalemia and 2 patients developed hyponatremia [54].
2.4. Management of Hyponatremia
Management of hyponatremia, in general, represents a challenge, especially for the patient with expanded extracellular volume. In patients that are admitted with acute HF exacerbation, the concomitant presence of hyponatremia on admission has shown to be associated with higher all-cause and cardiovascular mortality, especially when there is a further decrease in the sodium level during the hospitalization [55]. Currently, there are no specific guidelines for the management of hyponatremia in the setting of HF; data from a U.S. registry shows that the most commonly used treatment modalities for hyponatremia in HF are fluid restriction, and non-specific management strategies including diuretic therapy for congestion along with isotonic or hypertonic saline, or V2 receptor antagonists [55].
2.5. Hyponatremia with Severe Symptoms
The initial management of the patient with hyponatremia should be guided by the severity of symptoms and the acuity of the hyponatremia [56, 57]. The most urgent complication of hyponatremia is cerebral edema [57]. Acute hyponatremia, defined as the one that develops in <48 hours, and can often present with neurological symptoms, given that there has been no time for counter-regulatory mechanisms to help abate its clinical impacts [56, 57]. These neurologic symptoms are due to brain edema resulting from fluids shifts from a hypotonic extracellular media into relatively hypertonic brain tissue. The patient with hyponatremia and severe symptoms (coma, seizures, abnormal deep somnolence, vomiting) should be managed in the intensive care unit, where frequent clinical and laboratory monitoring can be provided [57]. Hyponatremia with severe symptoms requires emergent treatment regardless of the patient’s volume status, whether the onset is acute or chronic. The European guidelines for the management of hyponatremia with severe symptoms recommend an infusion of 150 ml of 3% hypertonic saline over 20 minutes, to a goal of 5 mEq/L increase in serum sodium in the first hour, with further management guided by clinical and laboratory response [58]. Correction should not exceed 8 mEq/L in the first 24 hours of treatment [56-58]. However, the additional sodium load provided by the use of hypertonic saline in HF patients could worsen their symptoms [59, 60]. In patients admitted with HF, the use of intravenous fluids during the first 48 hours of hospitalization has been associated with a higher risk of intubation, renal replacement therapy, and hospital mortality [61]; therefore, hypertonic saline treatment should be reserved only for patients with severe neurologic symptoms in which benefits of immediate treatment to decrease neurological dysfunction, outweigh the risk of congestion. In addition, concurrent infusion of loop diuretics such as furosemide is also suggested when HF patients are treated with hypertonic saline solution Fortunately, acute presentation of hyponatremia is infrequent in HF. Typically, a decrease in serum sodium occurs gradually and causes mild symptoms or is asymptomatic.
2.6. Hyponatremia without Severe Symptoms
In patients without symptoms of acute neurological dysfunction due to hyponatremia, it is often difficult to establish whether the hyponatremia has developed acutely (<48 hours) or chronically [56, 57]. Despite limited data on the chronicity of hyponatremia in the setting of HF, it is reasonable to presume the condition is chronic especially in the patient with progressively worsening HF [34, 36]. Importantly, even mild hyponatremia increases the risk of falls and cognitive deficits [62]. In the setting of chronic hyponatremia, plasma sodium level correction should not exceed the 8mEq/L in a 24-hour period in order to avoid the risk of osmotic demyelination syndrome (ODS) [59]. Currently, mainstay treatments of chronic hyponatremia in HF patients (hypervolemic state) with acceptable/stable kidney functions remain fluid restriction and loop diuretics [29, 34, 36]. Other modalities that have been studied include loop diuretics with hypertonic saline [60, 61, 63, 64], angiotensin-converting enzyme inhibitors (ACEIs) [63], and AVP receptor antagonists [64, 65], depending on whether depletion or dilutional hyponatremia is thought to be the problem. In challenging cases, right heart catheterization should be performed to differentiate between volume overload and volume depletion.
3. Treatment of hypovolemic hyponatremia
In uncommon instances, HF patients can present with hypovolemic hyponatremia due to volume depletion related to prominent gastrointestinal, or third-space losses [11]. These HF patients with volume depletion and concurrent hyponatremia can have a severely reduced effective circulating volume, especially with inadequate dietary intake of osmoles (protein and electrolytes) and use of thiazide diuretics [11]. In this setting, optimization of mean arterial pressure (MAP), in order to maintain adequate renal perfusion is suggested by withholding blood pressure lowering medications, and treatments to improve heart function by the use of inotropes [21, 26, 66, 67]. It is also suggested that hyponatremia in this setting (depletion induced hyponatremia) can potentially be improving with normal saline administration [11]. However, clinicians have to do a careful evaluation of the patient’s volume status, since intravenous fluids in patients with HF has never been shown to provide benefits and is associated with worse outcomes [57, 60, 68]. Independent of the pathophysiological mechanism, magnesium, and potassium deficiencies have to be corrected, and thiazide diuretics should be discontinued [11].
4. Treatment of hypervolemic hyponatremia
For hypervolemic hyponatremia (dilution), the mainstay of management is focused on fluid restriction, promoting free water excretion by increasing the distal nephron flow and lowering of AVP levels or its function (i.e., use of AVP receptor antagonists) [64, 65]. Tables 3 and 4 summarized the proposed treatments for hypervolemic hyponatremia in HF patients.
4.1. Fluid Restriction
Fluid restriction is helpful in the treatment of dilutional hyponatremia [69]. This approach has been studied in a randomized study allocating patients to a group with usual care vs. fluid restriction of <1L per day [70]. After 60 days, patients in the fluid restricted group had better scores of symptoms burden and overall quality of life [70]. In a small study, comparing fluid restriction vs. tolvaptan (a V2 receptor antagonist) in hyponatremic patients, the sodium level increased by 1 mEq/L in the fluid restriction group at outpatient follow up (although the increase was greater in the tolvaptan arm) [71]. A clinically significant increase in serum sodium with water restriction alone might be difficult, and free water restriction is difficult to maintain following hospital discharge as patients with HF often have increased thirst [70, 72]. In most cases, however, more liberal fluid intake of 2L per day is appropriate.
4.2. Diuretics
An increase in distal nephron flow can be achieved with loop diuretics [49]. Loop diuretics will also decrease tonicity in the renal medulla and facilitate increased free water excretion. Loop diuretics represent the first line of management in dilutional hyponatremia of acute HF [11, 29, 36, 73, 74]. In the ESCAPE trial, we found that patients with ADHF and hyponatremia on admission had a higher degree of congestion and required higher doses of furosemide, compared with normonatremic subjects [75]. Interestingly, our group has shown that the association between the percentage increase in loop diuretic utilization in-hospital relative to baseline and the development of discharge hyponatremia [76]. Whether diuretics should be accompanied by hypertonic saline has been debated [77], since it is suggested that osmotic action of hypertonic saline admiration can result in instantaneous mobilization of extravascular fluid into the intravascular space [77, 78]. Small studies in patients with severe refractory HF have shown that the addition of small-volume hypertonic saline to high dose furosemide can be well tolerated, can cause an increase in serum sodium, improve signs and symptoms of congestion, and has a reduction in readmission rate and mortality [77, 79]. However, the use of hypertonic saline in addition to diuretics for the sole management of hyponatremia in HF still requires validations in future studies. Currently, there is no evidence to support the use of hypertonic saline in HF patients with hyponatremia without neurological symptoms [55, 56]. When renal replacement therapy is indicated in subjects with diuretic refractoriness, advanced CKD or severe AKI, careful serial sodium monitoring during dialysis must be ensured to avoid rapid correction of serum sodium [80].
4.3. ACEI
Angiotensin-converting enzyme inhibitors (ACEI) help improve the hemodynamic perturbations intrinsic to CRS [81]. In addition, they have been shown to increase serum sodium level in patients with HF in small studies [82, 83]. This effect is likely due to enhanced cardiac output, and via their AVP antagonist activity in the collecting ducts, they increase the ability of the kidney to excrete diluted urine [33]. Thus, it is suggested that ACEI should be utilized in the admitted patients with HF who are also hyponatremic unless there are contraindications to their use [11]. However, in the long term, an analysis of the Italian HF registry showed that the use of ACEI did not modify the relationship between hyponatremia and 1-year mortality in HF patients [84].
4.4. AVP-receptor Antagonists
AVP antagonists (i.e., vaptans) were thought to be pathophysiologically promising in the management of HF and CRS, but evidence has shown otherwise [21, 60, 71]. AVP antagonists act on the collecting ducts of the distal nephrons by preventing aquaporin 2-channel availability in production [85]. Conivaptan is an intravenous aquaretic agent that inhibits both V1 and V2 receptors, whereas tolvaptan and lixivaptan are oral selective V2 receptor antagonist agents [64]. Vaptans increase plasma sodium levels without compromising the hemodynamics or impairing renal function [74, 81]. Regarding AVP receptors, cardiovascular, renal and other effects are mediated through 3 main receptors: V1A, V1B, V2. These receptors are G protein-coupled and they differ mainly by their second messenger mechanism. V1A receptor is coupled to a phosphoinositol (IP3) second messenger mechanism and is found mainly on vascular smooth muscle, myocardium and platelets, meaning activation mediates vasoconstriction in peripheral and coronary circulation as well as platelet aggregation [86]. Contrasting, V1B (sometimes referred to as V3) receptors are calcium and IP3 second messenger receptors that are mainly located in the anterior pituitary and mediate adrenocorticotropic hormone release. V2 receptors use cyclic adenosine monophosphate as a second messenger and are found on the collecting tubules of the kidney and mediate free water absorption [86].
Vaptans should be used carefully as they can cause xerostomia, polydipsia and sodium overcorrection. It is recommended that the therapy is initiated in the hospital at a lower dose with slow up-titration [64]. Fluids should not be restricted, and serum sodium should be monitored every 6-8 hours when vaptans are being used. Vaptans should not be used in patients with hypovolemic hyponatremia, and their effectiveness might be reduced in patients with renal failure [87]. Due to the risk of liver injury, FDA issued a warning to not administer vaptans for more than 30 days or in patients with the underlying chronic liver disease [88]. In general, the role of vaptans in hyponatremic patients with HF is limited mainly to patients who failed adequate correction with other medical management strategies.
4.5. Tolvaptan
In an investigation called “Study of Ascending Levels of Tolvaptan in Hyponatremia” (SALT-1 and -2), an improvement in serum sodium level and cognitive ability in the tolvaptan group in comparison with the control arm was reported, but only 30% of subjects had HF [87]. Several other studies have also shown improvement in serum sodium with the use of vaptans, but outcomes in the long term remained unknown [64, 74]. The EVEREST trial (efficacy of Vasopressin antagonism in HF outcome study with tolvaptan) was an event-driven, randomized, double-blind, placebo-controlled trial designed to examine the acute and chronic effects of a fixed dose (30 m/day) of tolvaptan [88]. Tolvaptan induced normalization of serum sodium. However, its use did not show a significant reduction in all-cause mortality, readmission rates in patients with Na <135 while indicated an improved survival free from cardiovascular death or readmission in patients with Na <130 [89, 90]. A post hoc analysis of the EVEREST database showed that in the hyponatremia subgroup tolvaptan was associated with greater weight loss, greater likelihood of normalization of serum sodium, less likelihood of having a decrease to a more severe level of hyponatremia; and greater relief of dyspnea, with no effects on long-term outcomes [90]. The Targeting Acute Congestion with Tolvaptan in Congestive Heart Failure (TACTICS-HF) trial showed no improvement in dyspnea relief or long-term outcomes despite greater weight loss and fluid loss [91]. At higher doses of 60 to 90 mg/day, Tolvaptan has shown a non-statistically significant increase in adverse effects, and renal failure [92]. Tolvaptan has been approved in the US for patients with clinically significant hypervolemic and euvolemic hyponatremia with a serum sodium of <125, who did not respond to fluid restriction and diuretics [56, 57].
4.6. Conivaptan
Conivaptan is a V1A and V2-receptor blocker that is administered intravenously [64]. The hemodynamic effects of conivaptan have been evaluated in small studies with chronic stable HF patients on standard medical treatment [64]. The addition of conivaptan to furosemide showed an increase in urine volumes, as well as urine sodium excretion [93]; pulmonary capillary wedge pressure and right atrial pressure showed a decrease [94]. Despite potentially favorable tolerability profile of conivaptan in these small studies [64, 95, 96], it is still currently suggested that conivaptan should not be used in patients with HF; given there is no strong evidence suggesting benefits as treatment for HF [96]. In addition, significant hypotension can occur in patients receiving conivaptan therapy [97, 98].
4.7. Lixivaptan
Lixivaptan is an oral, V2-receptor antagonist [64]. Lixivaptan’s safety and effects in hospitalized patients with worsening HF and hyponatremia are currently being evaluated in a randomized, double-blind, placebo-controlled trial [99]. The BALANCE (Treatment of Hyponatremia Based on Lixivaptan in NYHA Class III/IV Cardiac Patient Evaluation) study is enrolling only patients with HF hyponatremia and utilizes tailored therapeutic dosing [99]. In addition to assessing whether Lixivaptan can correct hyponatremia, The Balance study will assess improvement in cognitive function and clinical outcomes in HF patients [99]. Recruitment status has been completed. However, the publication of results is currently awaited [100]. In general, there is no strong evidence that vaptans improve outcomes in HF, and current guidelines recommend considering them (IIb) only in patients hospitalized with volume overload, with persistent severe and who are at risk for or having active cognitive symptoms despite water restriction [101].
5. Future directions
In recent years, many risk prediction models combining clinical models with biomarkers have emerged to facilitate prognosis assessment of HF patients in either reduced or preserved ejection fraction in different settings [102-113]. Since hyponatremia is independently associated with adverse clinical outcomes in patients with HF [103-105], a number of predictor models incorporating serum sodium have been developed [106-109] such as the Heart Failure Survival Score (HFSS) [107], the Seattle Heart Failure Model (SHFM) [108], and the metabolic exercise test data combined with cardiac and kidney indexes [106]. While these models require further external validation on their performance [102], these predictor models suggest the usefulness of serum sodium assessment in clinical care for patients with HF. In the era of big data, implementing sophisticate analytical techniques such as artificial intelligence to analyze serum sodium from multiple EHRs may be useful [114-116].
Hyponatremia in patients with HF remains a challenge for clinicians, and available treatment options are limited. Ongoing studies are still underway to determine the utility of vasopressin receptor antagonists whether they have an impact on long-term outcomes [109]. Currently, there is ongoing randomized controlled trial NCT02183792, The Aquaresis Utility for Hyponatremic Acute Heart Failure Study (AQUA-HF), comparing the effects of tolvaptan-based aquaretic regimen versus furosemide infusion in acute HF with hyponatremia. The findings from this study will potentially provide more information on the efficacy and safety outcomes of a tolvaptan-based diuretic regimen, compared to a furosemide-based regimen in hyponatremic acute HF patients.
Conclusion
Pathophysiology of hyponatremia in HF patients is often multifactorial. Clinical and biochemical evaluation often helps in differentiating dilutional from depletional hyponatremia to guide treatment strategies. The presence of hyponatremia in patients with HF is associated with increased risk of morbidity and mortality reflecting the severity of HF, or a direct effect of hyponatremia. Hyponatremia with severe symptoms is a medical emergency, and due to the high risk of neurologic sequelae, should be treated regardless of the patient’s volume status. Currently, fluid restriction and loop diuretics remain cornerstones of dilutional hyponatremia management in patients with HF. Although V2 receptor antagonists are considered as potentially promising treatments for refractory hypervolemic hyponatremia in patients with HF, resulting in improvement in serum sodium, improved dyspnea, negative fluid balance, and weight loss, these potential short-term effects have not translated in improvements in long-term clinical outcomes and require future studies.
Table 3.
Summary of proposed treatment options for HF patients with hyponatremia [11, 21, 29, 38, 69, 70, 72, 73, 78, 80, 108].
| Proposed Treatment Options for HF Patients with Hyponatremia (Hypervolemic Hyponatremia) |
|---|
| Current Standard/ Conventional Approaches • Fluid restriction less than 800-1000 mL/day • Loop diuretics • Renal replacement therapy for cases diuretic resistance, advanced CKD/ESRD, severe AKI |
| Proposed/Possible Optional Approaches • ACEIs • AVP antagonists: Tolvaptan and Lixivaptan • Hypertonic saline in addition to diuretics |
Abbreviations: ACEIs, Angiotensin-Converting Enzyme Inhibitors; AKI, Acute Kidney Injury; AVP, Arginine Vasopressin; CKD, Chronic Kidney Disease; ESRD, End-Stage Renal Disease.
Table 4.
Summary of clinical trials for treatment of HF patients with hyponatremia.
| First Author | Treatment | Outcomes |
|---|---|---|
| Albert et al. [70] | Fluid restriction (<800 ml/day) in hyponatremic patients with heart failure | Better quality of life at 60 days |
| Aliti et al. [118] | Fluid (<800 ml/day) and sodium (<800 mg /day) restriction in patients with acute heart failure | Nonstatistically significant decrease in rehospitalizations. Aggressive fluid and sodium restriction has no effect on weight loss or clinical stability. |
| Gheorghiade et al. [119] | Tolvaptan 30,45 or 60 mg vs. placebo | Normalization of serum sodium after 24 h, greater decrease in body weight and edema, increase urine output with tolvaptan. |
| Gheorghiade et al. [120] | Tolvaptan 30,60 or 90 mg vs. placebo | Normalization of serum sodium with tolvaptan |
| Ghali et al. [121] | Conivaptan 40 or 80 mg vs. placebo | Normalization of serum sodium wit conivaptan |
| Zeltser et al. [122] | Conivaptan 40 or 80 mg vs. placebo | Increase in serum sodium concentration with conivaptan |
| Konstam et al. [123] | Tolvaptan 30 mg vs. placebo | No effect on mortality or hospitalization, significant increase in serum sodium with tolvaptan |
| Felker et al. [91] | Tolvaptan 30 mg vs. placebo added to furosemide | Greater weight loss and fluid loss, but no improvement in number of responders at 24 hrs |
Abbreviations: BID, twice (two times) a day; IV, Intravenous; vs., Versus.
Acknowledgements
Declared none.
Authors' contributions
All authors had access to the data and a role in writing the manuscript.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Akintoye E., Briasoulis A., Egbe A., et al. National trends in admission and in-hospital mortality of patients with heart failure in the united states (2001-2014). J. Am. Heart Assoc. 2017;6(12):e006955. doi: 10.1161/JAHA.117.006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal S.K., Wruck L., Quibrera M., et al. Temporal trends in hospitalization for acute decompensated heart failure in the united states, 1998-2011. Am. J. Epidemiol. 2016;183:462–470. doi: 10.1093/aje/kwv455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghali J.K., Cooper R., Ford E. Trends in hospitalization rates for heart failure in the united states, 1973-1986. Evidence for increasing population prevalence. Arch. Intern. Med. 1990;150:769–773. [PubMed] [Google Scholar]
- 4.Mozaffarian D., Benjamin Emelia J., et al. Executive summary: Heart disease and stroke statistics-2016 update. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosy A.P., Fonarow G.C., Butler J., et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich P.A., Albert N.M., Allen L.A., et al. Forecasting the impact of heart failure in the united states: A policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braith R.W., Edwards D.G. Neurohormonal abnormalities in heart failure: Impact of exercise training. Congest. Heart Fail. 2003;9:70–76. doi: 10.1111/j.1527-5299.2003.00277.x. [DOI] [PubMed] [Google Scholar]
- 8.Jackson G., Gibbs C.R., Davies M.K., Lip G.Y. Abc of heart failure. Pathophysiology. BMJ. 2000;320:167–170. doi: 10.1136/bmj.320.7228.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oster J.R., Preston R.A., Materson B.J. Fluid and electrolyte disorders in congestive heart failure. Semin. Nephrol. 1994;14:485–505. [PubMed] [Google Scholar]
- 10.Gheorghiade M., Rossi J.S., Cotts W., et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the escape trial. Arch. Intern. Med. 2007;167:1998–2005. doi: 10.1001/archinte.167.18.1998. [DOI] [PubMed] [Google Scholar]
- 11.Verbrugge F.H., Steels P., Grieten L., Nijst P., Tang W.H., Mullens W. Hyponatremia in acute decompensated heart failure: Depletion versus dilution. J. Am. Coll. Cardiol. 2015;65:480–492. doi: 10.1016/j.jacc.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Holland-Bill L., Christiansen C.F., Heide-Jorgensen U., et al. Hyponatremia and mortality risk: A danish cohort study of 279 508 acutely hospitalized patients. Eur. J. Endocrinol. 2015;173:71–81. doi: 10.1530/EJE-15-0111. [DOI] [PubMed] [Google Scholar]
- 13.Waikar S.S., Mount D.B., Curhan G.C. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am. J. Med. 2009;122:857–865. doi: 10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corona G., Giuliani C., Parenti G., et al. The economic burden of hyponatremia: Systematic review and meta-analysis. Am. J. Med. 2016;129:823–35.e824. doi: 10.1016/j.amjmed.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Omar H.R., Charnigo R., Guglin M. Prognostic significance of discharge hyponatremia in heart failure patients with normal admission sodium (from the escape trial). Am. J. Cardiol. 2017;120:607–615. doi: 10.1016/j.amjcard.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Adrogue H.J., Madias N.E. Hyponatremia. N. Engl. J. Med. 2000;342:1581–1589. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 17.Klein L., O’Connor C.M., Leimberger J.D., et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: Results from the outcomes of a prospective trial of intravenous milrinone for exacerbations of chronic heart failure (optime-chf) study. Circulation. 2005;111:2454–2460. doi: 10.1161/01.CIR.0000165065.82609.3D. [DOI] [PubMed] [Google Scholar]
- 18.Sato N., Gheorghiade M., Kajimoto K., et al. Hyponatremia and in-hospital mortality in patients admitted for heart failure (from the attend registry). Am. J. Cardiol. 2013;111:1019–1025. doi: 10.1016/j.amjcard.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Binanay C., Califf R.M., Hasselblad V., et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The escape trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.E., Lee H.Y., Cho H.J., et al. Clinical characteristics and outcome of acute heart failure in korea: Results from the korean acute heart failure registry (korahf). Korean Circ. J. 2017;47:341–353. doi: 10.4070/kcj.2016.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunlap M.E., Hauptman P.J., Amin A.N., et al. Current management of hyponatremia in acute heart failure: A report from the hyponatremia registry for patients with euvolemic and hypervolemic hyponatremia (hn registry). J. Am. Heart Assoc. 2017;6(8):e005261. doi: 10.1161/JAHA.116.005261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upadhyay A., Jaber B.L., Madias N.E. Incidence and prevalence of hyponatremia. Am. J. Med. 2006;119:S30–S35. doi: 10.1016/j.amjmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Callahan M.A., Do H.T., Caplan D.W., Yoon-Flannery K. Economic impact of hyponatremia in hospitalized patients: A retrospective cohort study. Postgrad. Med. 2009;121:186–191. doi: 10.3810/pgm.2009.03.1991. [DOI] [PubMed] [Google Scholar]
- 24.Donze J.D., Beeler P.E., Bates D.W. Impact of hyponatremia correction on the risk for 30-day readmission and death in patients with congestive heart failure. Am. J. Med. 2016;129:836–842. doi: 10.1016/j.amjmed.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 25.Albabtain M., Brenner M.J., Nicklas J.M., et al. Hyponatremia, cognitive function, and mobility in an outpatient heart failure population. Med. Sci. Monit. 2016;22:4978–4985. doi: 10.12659/MSM.898538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romanovsky A., Bagshaw S., Rosner M.H. Hyponatremia and congestive heart failure: A marker of increased mortality and a target for therapy. Int. J. Nephrol. 2011;2011:732746. doi: 10.4061/2011/732746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Vecchis R., Di Maio M., Di Biase G., Ariano C. Effects of hyponatremia normalization on the short-term mortality and rehospitalizations in patients with recent acute decompensated heart failure: A retrospective study. J. Clin. Med. 2016;5:92. doi: 10.3390/jcm5100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boscoe A., Paramore C., Verbalis J.G. Cost of illness of hyponatremia in the United States. Cost Eff. Resour. Alloc. 2006;4:10. doi: 10.1186/1478-7547-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kazory A. Hyponatremia in heart failure: Revisiting pathophysiology and therapeutic strategies. Clin. Cardiol. 2010;33:322–329. doi: 10.1002/clc.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepshelovich D., Schechter A., Calvarysky B., Diker-Cohen T., Rozen-Zvi B., Gafter-Gvili A. Medication-induced siadh: Distribution and characterization according to medication class. Br. J. Clin. Pharmacol. 2017;83:1801–1807. doi: 10.1111/bcp.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos-Levi A.M., Duran Rodriguez-Hervada A., Mendez-Bailon M., Marco-Martinez J. Drug-induced hyponatremia: An updated review. Minerva Endocrinol. 2014;39:1–12. [PubMed] [Google Scholar]
- 32.Liamis G., Milionis H., Elisaf M. A review of drug-induced hyponatremia. Am. J. Kidney Dis. 2008;52:144–153. doi: 10.1053/j.ajkd.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Uretsky B.F., Verbalis J.G., Generalovich T., Valdes A., Reddy P.S. Plasma vasopressin response to osmotic and hemodynamic stimuli in heart failure. Am. J. Physiol. 1985;248:H396–H402. doi: 10.1152/ajpheart.1985.248.3.H396. [DOI] [PubMed] [Google Scholar]
- 34.Oren R.M. Hyponatremia in congestive heart failure. Am. J. Cardiol. 2005;95:2–7. doi: 10.1016/j.amjcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Mavani G.P., DeVita M.V., Michelis M.F. A review of the nonpressor and nonantidiuretic actions of the hormone vasopressin. Front. Med. 2015;2:19. doi: 10.3389/fmed.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filippatos T.D., Elisaf M.S. Hyponatremia in patients with heart failure. World J. Cardiol. 2013;5:317–328. doi: 10.4330/wjc.v5.i9.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldsmith S.R., Francis G.S., Cowley A.W., Jr Arginine vasopressin and the renal response to water loading in congestive heart failure. Am. J. Cardiol. 1986;58:295–299. doi: 10.1016/0002-9149(86)90065-2. [DOI] [PubMed] [Google Scholar]
- 38.Lee C.R., Watkins M.L., Patterson J.H., et al. Vasopressin: A new target for the treatment of heart failure. Am. Heart J. 2003;146:9–18. doi: 10.1016/S0002-8703(02)94708-3. [DOI] [PubMed] [Google Scholar]
- 39.Kalra P.R., Anker S.D., Coats A.J. Water and sodium regulation in chronic heart failure: The role of natriuretic peptides and vasopressin. Cardiovasc. Res. 2001;51:495–509. doi: 10.1016/s0008-6363(01)00297-8. [DOI] [PubMed] [Google Scholar]
- 40.Goldsmith S.R. Congestive heart failure: Potential role of arginine vasopressin antagonists in the therapy of heart failure. Congest. Heart Fail. 2002;8:251–256. doi: 10.1111/j.1527-5299.2002.01158.x. [DOI] [PubMed] [Google Scholar]
- 41.Ronco C. Cardiorenal syndromes: Definition and classification. Contrib. Nephrol. 2010;164:33–38. doi: 10.1159/000313718. [DOI] [PubMed] [Google Scholar]
- 42.Ronco C., McCullough P., Anker S.D., et al. Cardio-renal syndromes: Report from the consensus conference of the acute dialysis quality initiative. Eur. Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segall L., Nistor I., Covic A. Heart failure in patients with chronic kidney disease: A systematic integrative review. BioMed Res. Int. 2014;2014:937398. doi: 10.1155/2014/937398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovesdy C.P., Lott E.H., Lu J.L., et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125:677–684. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukushima A., Kinugawa S. Hyponatremia as a surrogate marker for optimal diuretic selection in acute heart failure. J. Cardiol. 2018;71:547–549. doi: 10.1016/j.jjcc.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Liamis G., Filippatos T.D., Elisaf M.S. Thiazide-associated hyponatremia in the elderly: What the clinician needs to know. J. Geriatr. Cardiol. 2016;13:175–182. doi: 10.11909/j.issn.1671-5411.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peacock W.F., Costanzo M.R., De Marco T., et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: Insights from the adhere registry. Cardiology. 2009;113:12–19. doi: 10.1159/000164149. [DOI] [PubMed] [Google Scholar]
- 48.Felker G.M., O’Connor C.M., Braunwald E. Loop diuretics in acute decompensated heart failure: Necessary? Evil? A necessary evil? Circ Heart Fail. 2009;2:56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spital A. Diuretic-induced hyponatremia. Am. J. Nephrol. 1999;19:447–452. doi: 10.1159/000013496. [DOI] [PubMed] [Google Scholar]
- 50.Moranville M.P., Choi S., Hogg J., Anderson A.S., Rich J.D. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc. Ther. 2015;33:42–49. doi: 10.1111/1755-5922.12109. [DOI] [PubMed] [Google Scholar]
- 51.Jentzer J.C., DeWald T.A., Hernandez A.F. Combination of loop diuretics with thiazide-type diuretics in heart failure. J. Am. Coll. Cardiol. 2010;56:1527. doi: 10.1016/j.jacc.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 52.Kroger N., Szuba J., Frenzel H. Metolazone in the treatment of advanced therapy-resistant dilated cardiomyopathy. Med. Klin. 1991;86:305–308. [PubMed] [Google Scholar]
- 53.De Vecchis R., Ariano C., Esposito C., Giasi A., Cioppa C., Cantatrione S. In right or biventricular chronic heart failure addition of thiazides to loop diuretics to achieve a sequential blockade of the nephron is associated with increased risk of dilutional hyponatremia: Results of a case-control study. Minerva Cardioangiol. 2012;60:517–529. [PubMed] [Google Scholar]
- 54.Goland S., Naugolny V., Korbut Z., Rozen I., Caspi A., Malnick S. Appropriateness and complications of the use of spironolactone in patients treated in a heart failure clinic. Eur. J. Intern. Med. 2011;22:424–427. doi: 10.1016/j.ejim.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Spasovski G., Vanholder R., Allolio B., et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur. J. Endocrinol. 2014;170:G1–G47. doi: 10.1530/EJE-13-1020. [DOI] [PubMed] [Google Scholar]
- 56.Spasovski G., Vanholder R., Allolio B., et al. Hyponatraemia diagnosis and treatment clinical practice guidelines. Nefrologia. 2017;37:370–380. doi: 10.1016/j.nefro.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 57.Hoorn E.J., Zietse R. Diagnosis and treatment of hyponatremia: Compilation of the guidelines. J. Am. Soc. Nephrol. 2017;28:1340–1349. doi: 10.1681/ASN.2016101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spasovski G., Vanholder R., Allolio B., et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol. Dial. Transplant. 2014;29(Suppl. 2):i1–i39. doi: 10.1093/ndt/gfu040. [DOI] [PubMed] [Google Scholar]
- 59.Wan Y., Li L., Niu H., et al. Impact of compound hypertonic saline solution on decompensated heart failure. Int. Heart J. 2017;58:601–607. doi: 10.1536/ihj.16-313. [DOI] [PubMed] [Google Scholar]
- 60.Lafrenière G., Béliveau P., Bégin J.Y., et al. Effects of hypertonic saline solution on body weight and serum creatinine in patients with acute decompensated heart failure. World J. Cardiol. 2017;9:685–692. doi: 10.4330/wjc.v9.i8.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bikdeli B., Strait K.M., Dharmarajan K., et al. Intravenous fluids in acute decompensated heart failure. JACC Heart Fail. 2015;3:127–133. doi: 10.1016/j.jchf.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Renneboog B., Musch W., Vandemergel X., Manto M.U., Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am. J. Med. 2006;119:71.e71–71.e78. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 63.Licata G., Di Pasquale P., Parrinello G., et al. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: Long-term effects. Am. Heart J. 2003;145:459–466. doi: 10.1067/mhj.2003.166. [DOI] [PubMed] [Google Scholar]
- 64.Vinod P., Krishnappa V., Chauvin A.M., Khare A., Raina R. Cardiorenal syndrome: Role of arginine vasopressin and vaptans in heart failure. Cardiol. Res. 2017;8:87–95. doi: 10.14740/cr553w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin T.E., Adams K.F., Jr, Patterson J.H. Potential roles of vaptans in heart failure: Experience from clinical trials and considerations for optimizing therapy in target patients. Heart Fail. Clin. 2014;10:607–620. doi: 10.1016/j.hfc.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Hashim T., Sanam K., Revilla-Martinez M., et al. Clinical characteristics and outcomes of intravenous inotropic therapy in advanced heart failure. Circ Heart Fail. 2015;8:880–886. doi: 10.1161/CIRCHEARTFAILURE.114.001778. [DOI] [PubMed] [Google Scholar]
- 67.Mebazaa A., Motiejunaite J., Gayat E., et al. Long-term safety of intravenous cardiovascular agents in acute heart failure: Results from the European society of cardiology heart failure long-term registry. Eur. J. Heart Fail. 2018;20:332–341. doi: 10.1002/ejhf.991. [DOI] [PubMed] [Google Scholar]
- 68.Vaduganathan M., Pallais J.C., Fenves A.Z., Butler J., Gheorghiade M. Serum chloride in heart failure: A salty prognosis. Eur. J. Heart Fail. 2016;18:669–671. doi: 10.1002/ejhf.546. [DOI] [PubMed] [Google Scholar]
- 69.Ghali J.K., Tam S.W. The critical link of hypervolemia and hyponatremia in heart failure and the potential role of arginine vasopressin antagonists. J. Card. Fail. 2010;16:419–431. doi: 10.1016/j.cardfail.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 70.Albert N.M., Nutter B., Forney J., Slifcak E., Tang W.H. A randomized controlled pilot study of outcomes of strict allowance of fluid therapy in hyponatremic heart failure (salt-hf). J. Card. Fail. 2013;19:1–9. doi: 10.1016/j.cardfail.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Gheorghiade M., Gottlieb S.S., Udelson J.E., et al. Vasopressin v(2) receptor blockade with tolvaptan versus fluid restriction in the treatment of hyponatremia. Am. J. Cardiol. 2006;97:1064–1067. doi: 10.1016/j.amjcard.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 72.Allida S.M., Hayward C.S., Newton P.J. Thirst in heart failure: What do we know so far? Curr. Opin. Support. Palliat. Care. 2018;12:4–9. doi: 10.1097/SPC.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 73.Sica D.A. Hyponatremia and heart failure--treatment considerations. Congest. Heart Fail. 2006;12:55–60. doi: 10.1111/j.1527-5299.2006.04844.x. [DOI] [PubMed] [Google Scholar]
- 74.Goldsmith S.R. Current treatments and novel pharmacologic treatments for hyponatremia in congestive heart failure. Am. J. Cardiol. 2005;95:14b–23b. doi: 10.1016/j.amjcard.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Omar H.R., Guglin M. Higher diuretic requirements in acute heart failure with admission hyponatraemia versus normonatraemia. Heart Lung Circ. 2019 doi: 10.1016/j.hlc.2018.12.014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 76.Omar H.R., Guglin M. Etiology of discharge hyponatremia in decompensated heart failure and normal admission na(+): Effect of diuretics. Eur. J. Intern. Med. 2018;48:e15–e17. doi: 10.1016/j.ejim.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 77.Gandhi S., Mosleh W., Myers R.B. Hypertonic saline with furosemide for the treatment of acute congestive heart failure: A systematic review and meta-analysis. Int. J. Cardiol. 2014;173:139–145. doi: 10.1016/j.ijcard.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 78.Paterna S., Parrinello G., Amato P., et al. Tolerability and efficacy of high-dose furosemide and small-volume hypertonic saline solution in refractory congestive heart failure. Adv. Ther. 1999;16:219–228. [PubMed] [Google Scholar]
- 79.Okuhara Y., Hirotani S., Ando T., et al. Comparison of salt with low-dose furosemide and carperitide for treating acute decompensated heart failure: A single-center retrospective cohort study. Heart Vessels. 2017;32:419–427. doi: 10.1007/s00380-016-0883-1. [DOI] [PubMed] [Google Scholar]
- 80.Kazory A. Haemodialysis, not ultrafiltration, can correct hyponatraemia in heart failure. Eur. J. Heart Fail. 2010;12:208. doi: 10.1093/eurjhf/hfp188. [DOI] [PubMed] [Google Scholar]
- 81.Elisaf M., Theodorou J., Pappas C., Siamopoulos K. Successful treatment of hyponatremia with angiotensin-converting enzyme inhibitors in patients with congestive heart failure. Cardiology. 1995;86:477–480. doi: 10.1159/000176926. [DOI] [PubMed] [Google Scholar]
- 82.Balling L., Kober L., Schou M., Torp-Pedersen C., Gustafsson F. Efficacy and safety of angiotensin-converting enzyme inhibitors in patients with left ventricular systolic dysfunction and hyponatremia. J. Card. Fail. 2013;19:725–730. doi: 10.1016/j.cardfail.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Oster J.R., Materson B.J. Renal and electrolyte complications of congestive heart failure and effects of therapy with angiotensin-converting enzyme inhibitors. Arch. Intern. Med. 1992;152:704–710. [PubMed] [Google Scholar]
- 84.Baldasseroni S., Urso R., Orso F., et al. Relation between serum sodium levels and prognosis in outpatients with chronic heart failure: Neutral effect of treatment with beta-blockers and angiotensin-converting enzyme inhibitors: Data from the Italian network on congestive heart failure (in-chf database). J. Cardiovasc. Med. (Hagerstown) 2011;12:723–731. doi: 10.2459/JCM.0b013e32834ae87e. [DOI] [PubMed] [Google Scholar]
- 85.Cheungpasitporn W., Erickson S.B., Rule A.D., Enders F., Lieske J.C. Short-term tolvaptan increases water intake and effectively decreases urinary calcium oxalate, calcium phosphate and uric acid supersaturations. J. Urol. 2016;195:1476–1481. doi: 10.1016/j.juro.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ali F., Guglin M., Vaitkevicius P., Ghali J.K. Therapeutic potential of vasopressin receptor antagonists. Drugs. 2007;67:847–858. doi: 10.2165/00003495-200767060-00002. [DOI] [PubMed] [Google Scholar]
- 87.Schrier R.W., Gross P., Gheorghiade M., et al. Tolvaptan, a selective oral vasopressin v2-receptor antagonist, for hyponatremia. N. Engl. J. Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 88.Konstam M.A., Gheorghiade M., Burnett J.C., Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The everest outcome trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 89.McGreal K., Budhiraja P., Jain N., Yu A.S. Current challenges in the evaluation and management of hyponatremia. Kidney Dis. 2016;2:56–63. doi: 10.1159/000446267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hauptman P.J., Burnett J., Gheorghiade M., et al. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J. Card. Fail. 2013;19:390–397. doi: 10.1016/j.cardfail.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 91.Felker G.M., Mentz R.J., Cole R.T., et al. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J. Am. Coll. Cardiol. 2017;69:1399–1406. doi: 10.1016/j.jacc.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 92.Wu M.Y., Chen T.T., Chen Y.C., et al. Effects and safety of oral tolvaptan in patients with congestive heart failure: A systematic review and network meta-analysis. PLoS One. 2017;12:e0184380. doi: 10.1371/journal.pone.0184380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldsmith S.R., Gilbertson D.T., Mackedanz S.A., Swan S.K. Renal effects of conivaptan, furosemide, and the combination in patients with chronic heart failure. J. Card. Fail. 2011;17:982–989. doi: 10.1016/j.cardfail.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 94.Udelson J.E., Smith W.B., Hendrix G.H., et al. Acute hemodynamic effects of conivaptan, a dual v(1a) and v(2) vasopressin receptor antagonist, in patients with advanced heart failure. Circulation. 2001;104:2417–2423. doi: 10.1161/hc4501.099313. [DOI] [PubMed] [Google Scholar]
- 95.Annane D., Decaux G., Smith N. Efficacy and safety of oral conivaptan, a vasopressin-receptor antagonist, evaluated in a randomized, controlled trial in patients with euvolemic or hypervolemic hyponatremia. Am. J. Med. Sci. 2009;337:28–36. doi: 10.1097/MAJ.0b013e31817b8148. [DOI] [PubMed] [Google Scholar]
- 96.Cajaiba M.M., Parks W.T., Fuhrer K., Randhawa P.S. Evaluation of human polyomavirus bk as a potential cause of villitis of unknown etiology and spontaneous abortion. J. Med. Virol. 2011;83:1031–1033. doi: 10.1002/jmv.22082. [DOI] [PubMed] [Google Scholar]
- 97.Der-Nigoghossian C., Lesch C., Berger K. Effectiveness and tolerability of conivaptan and tolvaptan for the treatment of hyponatremia in neurocritically ill patients. Pharmacotherapy. 2017;37:528–534. doi: 10.1002/phar.1926. [DOI] [PubMed] [Google Scholar]
- 98.Izumi Y., Miura K., Iwao H. Therapeutic potential of vasopressin-receptor antagonists in heart failure. J. Pharmacol. Sci. 2014;124:1–6. doi: 10.1254/jphs.13r13cp. [DOI] [PubMed] [Google Scholar]
- 99.Abraham W.T., Aranda J.M., Boehmer J.P., et al. Rationale and design of the treatment of hyponatremia based on lixivaptan in nyha class III/IV cardiac patient evaluation (the balance) study. Clin. Transl. Sci. 2010;3:249–253. doi: 10.1111/j.1752-8062.2010.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flaegstad T., Traavik T., Kristiansen B.E. Age-dependent prevalence of bk virus igg and igm antibodies measured by enzyme-linked immunosorbent assays (ELISA). J. Hyg. (Lond.) 1986;96:523–528. doi: 10.1017/s0022172400066328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology foundation/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 102.Doumouras B.S., Lee D.S., Levy W.C., Alba A.C. An appraisal of biomarker-based risk-scoring models in chronic heart failure: Which one is best? Curr. Heart Fail. Rep. 2018;15:24–36. doi: 10.1007/s11897-018-0375-y. [DOI] [PubMed] [Google Scholar]
- 103.Hamaguchi S., Kinugawa S., Tsuchihashi-Makaya M., et al. Hyponatremia is an independent predictor of adverse clinical outcomes in hospitalized patients due to worsening heart failure. J. Cardiol. 2014;63:182–188. doi: 10.1016/j.jjcc.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 104.Yoo B.S., Park J.J., Choi D.J., et al. Prognostic value of hyponatremia in heart failure patients: An analysis of the clinical characteristics and outcomes in the relation with serum sodium level in asian patients hospitalized for heart failure (coast) study. Korean J. Intern. Med. (Korean. Assoc. Intern. Med.) 2015;30:460–470. doi: 10.3904/kjim.2015.30.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu D.Y., Cheng H.M., Cheng Y.L., et al. Hyponatremia and worsening sodium levels are associated with long-term outcome in patients hospitalized for acute heart failure. J. Am. Heart Assoc. 2016;5:e002668. doi: 10.1161/JAHA.115.002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Agostoni P., Corra U., Cattadori G., et al. Metabolic exercise test data combined with cardiac and kidney indexes, the mecki score: A multiparametric approach to heart failure prognosis. Int. J. Cardiol. 2013;167:2710–2718. doi: 10.1016/j.ijcard.2012.06.113. [DOI] [PubMed] [Google Scholar]
- 107.Aaronson K.D., Schwartz J.S., Chen T.M., Wong K.L., Goin J.E., Mancini D.M. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 108.Levy W.C., Mozaffarian D., Linker D.T., et al. The seattle heart failure model: Prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 109.Oh C., Chang H.J., Sung J.M., et al. Prognostic estimation of advanced heart failure with low left ventricular ejection fraction and wide qrs interval. Korean Circ. J. 2012;42:659–667. doi: 10.4070/kcj.2012.42.10.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alba A.C., Walter S.D., Guyatt G.H., et al. Predicting survival in patients with heart failure with an implantable cardioverter defibrillator: The heart failure meta-score. J. Card. Fail. 2018;24:735–745. doi: 10.1016/j.cardfail.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 111.Krittanawong C., Kukin M.L. Current management and future directions of heart failure with preserved ejection fraction: A contemporary review. Curr. Treat. Options Cardiovasc. Med. 2018;20:28. doi: 10.1007/s11936-018-0623-1. [DOI] [PubMed] [Google Scholar]
- 112.Omar H.R., Guglin M. Rise of first follow-up sodium in patients hospitalized with acute heart failure is associated with better outcomes. Int. J. Cardiol. 2018;269:201–206. doi: 10.1016/j.ijcard.2018.06.071. [DOI] [PubMed] [Google Scholar]
- 113.Omar H.R., Guglin M. Community acquired versus hospital acquired hyponatremia in acute heart failure: Association with clinical characteristics and outcomes. Int. J. Cardiol. 2016;225:247–249. doi: 10.1016/j.ijcard.2016.09.135. [DOI] [PubMed] [Google Scholar]
- 114.Krittanawong C., Zhang H., Wang Z., Aydar M., Kitai T. Artificial intelligence in precision cardiovascular medicine. J. Am. Coll. Cardiol. 2017;69:2657–2664. doi: 10.1016/j.jacc.2017.03.571. [DOI] [PubMed] [Google Scholar]
- 115.Krittanawong C., Johnson K.W., Hershman S.G., Tang W.H.W. Big data, artificial intelligence, and cardiovascular precision medicine. Expert Rev. Precis. Med. Drug Dev. 2018;3:305–317. [Google Scholar]
- 116.Krittanawong C., Johnson K.W., Rosenson R.S., et al. Deep learning for cardiovascular medicine: A practical primer. Eur. Heart J. 2019;••• doi: 10.1093/eurheartj/ehz056. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ronco C., Haapio M., House A.A., Anavekar N., Bellomo R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 118.Aliti G.B., Rabelo E.R., Clausell N., Rohde L.E., Biolo A., Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: A randomized clinical trial. JAMA Intern. Med. 2013;173:1058–1064. doi: 10.1001/jamainternmed.2013.552. [DOI] [PubMed] [Google Scholar]
- 119.Gheorghiade M., Konstam M.A., Burnett J.C., Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The everest clinical status trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 120.Gheorghiade M., Gattis W.A., O’Connor C.M., et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA. 2004;291:1963–1971. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- 121.Ghali J.K., Koren M.J., Taylor J.R., et al. Efficacy and safety of oral conivaptan: A v1a/v2 vasopressin receptor antagonist, assessed in a randomized, placebo-controlled trial in patients with euvolemic or hypervolemic hyponatremia. J. Clin. Endocrinol. Metab. 2006;91:2145–2152. doi: 10.1210/jc.2005-2287. [DOI] [PubMed] [Google Scholar]
- 122.Zeltser D., Rosansky S., van Rensburg H., Verbalis J.G., Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am. J. Nephrol. 2007;27:447–457. doi: 10.1159/000106456. [DOI] [PubMed] [Google Scholar]
- 123.Konstam M.A., Kiernan M., Chandler A., et al. Short-term effects of tolvaptan in patients with acute heart failure and volume overload. J. Am. Coll. Cardiol. 2017;69:1409–1419. doi: 10.1016/j.jacc.2016.12.035. [DOI] [PubMed] [Google Scholar]