Abstract
Background
Coronary computed tomography angiography (CCTA) is now widely used in the diagnosis of coronary artery disease since it is a rapid, minimally invasive test with a diagnostic accuracy comparable to coronary angiography. However, to meet demands for increasing spatial and temporal resolution, higher x-ray radiation doses are required to circumvent the resulting increase in image noise. Exposure to high doses of ionizing radiation with CT imaging is a major health concern due to the potential risk of radiation-associated malignancy. Given its increasing use, a number of dose saving algorithms have been implemented to CCTA to minimize radiation exposure to “as low as reasonably achievable (ALARA)” without compromising diagnostic image quality.
Objective
The purpose of this review is to outline the most recent advances and current status of dose saving techniques in CCTA.
Method
PubMed, Medline, EMBASE and Scholar databases were searched to identify feasibility studies, clinical trials, and technology guidelines on the technical advances in CT scanner hardware and reconstruction software.
Results
Sub-millisievert (mSv) radiation doses have been reported for CCTA due to a combination of strategies such as prospective electrocardiogram-gating, high-pitch helical acquisition, tube current modulation, tube voltage reduction, heart rate reduction, and the most recent novel adaptive iterative reconstruction algorithms.
Conclusion
Advances in radiation dose reduction without loss of image quality justify the use of CCTA as a non-invasive alternative to coronary catheterization in the diagnosis of coronary artery disease.
Keywords: Coronary computed tomography angiography, effective radiation dose, image quality, prospective electrocardiogram-gating, tube voltage reduction, tube current modulation, iterative reconstruction
1. INTRODUCTION
Coronary computed tomography angiography (CCTA) is increasingly being used in the diagnosis of coronary artery disease (CAD) since it is rapid and minimally invasive [1, 2]. Owing to its high negative predictive value, recently updated guidelines from the National Institute for Health and Clinical Excellence (NICE) on Chest Pain of Recent Onset: Assessment and Diagnosis propose using CCTA as a primary screening tool in patients with stable chest pain being assessed for possible coronary artery disease [2, 3]. However, imaging coronary arteries presents increased challenges in CT, as it requires both high temporal resolution to reduce motion artifacts caused by the cardiac motion and a high spatial resolution to differentiate small coronary structures [4]. These requirements indicate that the previous high radiation doses, ranging from 18-31.4 mSv [5] required for optimizing the image signal-to-noise ratio (SNR) in CCTA, are a major healthcare concern due to an associated increase in lifetime risk of radiation-induced malignancy [6, 7].
Conventionally, invasive coronary angiography is considered the gold standard for diagnosing and treating CAD [3]. Radiation doses from coronary angiography are estimated to range from 4.2 to 21.8 mSv depending on the study [8-11] and vascular access site [12, 13]. While the image quality produced by CCTA scanners is approaching that of the standard of reference [14], the mean effective radiation dose is reportedly higher for CCTA than conventional angiography in studies with directly comparable patients [15]. However, not only is coronary angiography invasive, it requires longer examination times compared with CCTA, including patient preparation and recovery time [16].
Given its increasing use, CCTA has thus been the key driver in developing state-of-the-art multi-slice CT over noncardiac CT imaging [17]. A number of strategies have been developed over the last decade to optimize the trade-off between the scan parameters that affect image quality—temporal resolution, spatial resolution, and pitch—while minimizing the radiation exposure to “As Low As Reasonably Achievable” (ALARA) [4, 18]. Temporal resolution is modified by acquisition mode, reconstruction method and gantry rotation time, while spatial resolution is modified by the detector size and configuration, focal spot size, and the reconstruction interval [19]. Image contrast is influenced by noise, tube current and beam voltage. We will review the most current dose reduction methods used in routine clinical CCTA including prospective electrocardiogram (ECG)-gated tube current modulation, anatomy-based tube current modulation, tube voltage reduction, iterative reconstruction (IR) and heart rate reduction [20-22]. Table 1 summarizes the main dose reduction methods applied to various CT parameters to achieve a low effective radiation dose in cardiac CT.
2. Scan Mode
2.1. Prospective Electrocardiogram-gating
Prospective ECG-gated tube current modulation is reported to be one of the most effective strategies at reducing the radiation dose. Contrary to retrospective gating, where data are acquired over the whole heart phase, prospective gating uses the step-and-shoot (SAS) mode [20, 23-29]. In this mode, the x-ray tube is switched on only at predefined time-points of the cardiac cycle, usually in mid-diastole, while keeping the table stationary. The x-ray exposure time of this technique is short, and thus low radiation doses have been reported while maintaining accuracy [30].
A systematic review by Menke et al. reported a pooled effective dose of 3.5 mSv with prospective triggering, a factor of 3.5 lower than the pooled effective dose of 12.3 mSv with retrospective gating with comparable CCTA image quality and diagnostic accuracy [31]. Furthermore, prospective ECG-gating allows extra low-dose cardiac imaging with high sensitivity, specificity, negative predictive value, positive predictive value, and accuracy for detecting CAD [32]. This method, however, reaches its limits with patients with severe arrhythmia since it relies on the prediction of the patient’s next cardiac cycle.
2.2. Helical CT
An alternative to the SAS mode is the helical- or spiral-scan mode, where data are acquired while the scanner is constantly spinning and the table moves continuously during image acquisition. Thus, no two CT projections are acquired at the same slice (z-position). Cardiac axial images are reconstructed from “half-scan” data, i.e. a data segment covering 180° plus the fan beam angle (about 50-60° depending on system geometry) rather than 360° of data [33]. The pitch is given by the ratio of the table increment per rotation to the total nominal beam width [19].
Along with the helical mode, the development of multislice CT (MSCT) and a widened z-axis x-ray enabled greater coverage per gantry rotation [19, 34]. In MSCT, each detector in the z-direction is divided into multiple, parallel rows of smaller detector elements, forming a two-dimensional array [35, 36].
Typically, single-source MSCT scanners need to use multi-segment reconstruction to increase temporal resolution at high heart rates, e.g. a two-segment reconstruction doubles the temporal resolution [37]. In multi-segment reconstruction, images are reconstructed from portions of projection data from multiple sequential cardiac cycles, which yields sufficient data to perform partial scan reconstructions. This requires a smaller pitch to avoid discontinuities in anatomic coverage from consecutive cycles [38]. Furthermore, in cardiac MSCT, the number of photons, and hence noise, is proportional to tube current–time product but is independent of pitch. To maintain the same noise, the same tube current–time product value is used which, in conjunction with a smaller pitch, results in a higher radiation dose. Hence for most single-source MSCT systems, better temporal resolution in cardiac spiral CT requires a higher dose [38].
2.3. Dual-source CT and Ultra-high Pitch
The advent of second-generation, dual-source CT (DSCT) allows data acquisition with prospectively-gated CCTA in the helical high-pitch mode, or “Flash Spiral” mode [39].
Early phantom and animal studies demonstrated the feasibility of high-pitch spiral DSCT for cardiac CT without a noticeable difference in image quality [40]. Spatial resolution in the z-direction was unaffected by pitch factors up to a value of 3 [40].
In the high-pitch mode, data acquisition is also prospectively triggered with the ECG of the patient, but the entire heart can be scanned within one single cardiac cycle, again usually during diastole. While both the high-pitch and the SAS mode for low-dose CCTA provide high accuracy for the assessment of significant coronary stenoses, the high-pitch mode significantly lowers the radiation dose even further [30].
A key advantage of DSCT is improved temporal resolution in cardiac scanning without the need for multi-segment reconstruction. As cardiac axial images are reconstructed from half-scan data in single-source MSCT, the fastest rotations times are limited to ⅓s due to mechanical stresses from gantry rotation [41]. Since the DSCT scanner simultaneously uses two x-ray tubes and two detectors arranged at an angle of 90° in the same relative phase of the patient’s cardiac cycle and with the same centered region of the scan field of view, only one-quarter of a rotation of the gantry is necessary to acquire the x-ray data for one cross-sectional image [35]. This enables a table feed of up to four times the detector width per entire rotation, resulting in a pitch that is twice as high as that of single-source CT [42]. This gives greater z-axis coverage in the same amount of time and is thus able to cover the entire heart in a single rotation.
For a gantry rotation time, trot = 0.33 s, the temporal resolution is thus ΔT = trot/4 = 83 ms, independent of the patient’s heart rate. Since data from only one cardiac cycle are used to reconstruct an image, the basic mode of operation corresponds to single-segment reconstruction [41]. Consequently, the table feed can be adapted efficiently to the patient’s heart rate and significantly increased at elevated heart rates.
For a single-segment ECG-gated spiral reconstruction, the maximum pitch, p, has been shown to be related to the patient’s heart cycle time TRR via the relation [43]
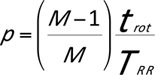
for gapless volume coverage in any phase of the cardiac cycle. M is the number of collimated detector rows [41]. The increased pitch at higher heart rates reduces the examination time and thus the radiation dose.
In summary, with a single-source CT, the pitch cannot be increased at higher heart rates because multi-segment reconstruction must be used to improve temporal resolution. DSCT however, allows pitch values to be increased as heart rate increases using single-segment reconstruction. This offsets the increased dose that accompanies improved temporal resolution in single-source cardiac MSCT [41].
In practice, a maximum pitch of 3.4 is feasible for image reconstruction in a sufficiently centered scanning field of view. The high pitch and fast table speed permit the entire volumetric data set of the heart within 250-300 ms, usually during diastole of a single cardiac cycle [7, 11, 17-19].
Since radiation exposure is inversely proportional to pitch [38], extra low dose cardiac imaging with mean effective radiation doses ranging from 0.9 ± 0.1 mSv to 2.04 ± 0.94 mSv have been recorded with high-pitch (pitch 3.4) prospective ECG-gated helical CCTA, Table 2. These values are significantly lower than the standard SAS mode [30, 42, 44-47]. In all these studies, image quality showed consistently high objective and subjective image quality [45-47], and diagnostic image quality was found in at least 97% of evaluated coronary segments without significant differences to other modes [42, 47].
However, some studies report that the image quality of high-pitch ECG-gated CCTA is more vulnerable to heart rate and motion artifacts compared with previous conventional scan protocols [42, 46]. Other feasibility studies with high pitch spiral mode-acquired images are thus limited to patients in sinus rhythm with heart rates of ≤ 65 bpm because motion-free images must be obtained during a period of approximately 270 ms [48]. In the PROTECTION IV study, CCTA was repeated in cases of insufficient image quality or nondiagnostic data sets. In the high-pitch helical group, repeat scanning was necessary for 21 patients compared with 14 patients in the conventional scan group (p = 0.25) [46].
The main limitation of only acquiring one data set in high-pitch protocols means that no additional reconstructions are possible in case of timing errors, and no functional information can be obtained from the acquisitions [49].
2.4. Detector Width and Scan Range
Another approach to cover the entire heart in a single cycle is to increase the detector width. Recently, expansion from a 256-detector row to a 320-detector row system has enabled whole heart coverage and reduced patient irradiation by eliminating helical oversampling [50, 51]. Initial 320-detector row coronary CT images have excellent quality and iodinated demonstrated contrast opacification [29]. Lower radiation doses with improved image quality with 320-MDCT scanners compared with 64-MDCT scanners are also confirmed by Zimmerman et al. [51] and Ropers et al. [52].
However, in CT, a sufficient contrast-to-noise ratio is required to resolve small and low-contrast structures such as plaques. With the increasing number of CT detectors in the z-direction, the contrast-to-noise ratio has been reported to degrade due to increased scattered radiation that can reach detectors in the z direction [19].
Patient irradiation can be further limited by tailoring the field of view since radiation dose is directly related to the craniocaudal scan range, a factor that the physician and technologist must control [53]. The wide area detector row CT scanner can be used with less than the maximum 16 cm (320-detector) craniocaudal coverage. For example, imaging over a 14 cm (280 detectors) craniocaudal field of view will decrease patient dose by 12.5% [29, 54].
3. Heart rate and premedication
In a recent phantom study evaluating the dose performance of DSCT, the most severe dose reductions were shown at increased heart rates and raised pitch [54]. Stolzmann et al. demonstrated that the radiation dose, associated with their DSCT protocol to reduce the tube current to 20% outside the pulsing window, significantly decreases with increasing heart rates, despite using wider pulsing windows at higher heart rates to maintain diagnostic image quality [55].
Oda et al. [56] compared the image quality of a 256-slice CT scanner at a gantry rotation speed of 270 ms with a 64-slice CT at a rotation speed of 420 ms in patients undergoing CCTA. While there was no significant difference in the image quality scores between 64- and 256-slice scans in patients whose heart rates were < 60 bpm, the 256-slice CT scanner yielded significantly better image quality in patients with an HR exceeding 60 bpm.
Sun et al. also reported a similar image quality at a much lower radiation dose compared with retrospectively ECG-gated low-pitch spiral acquisition mode. Their study included patients with heart rates > 65 bpm without cardiac arrhythmia with the image-acquired timing set at 20-30% of the R-R interval [57].
Premedication with beta-blockers to lower the resting heart rate has previously shown to be a safe practice [58] to reduce radiation exposure and improve image quality [5, 59]. Premedication may be restricted however, due to contraindications in 5-11% of patients (e.g. reactive airway disease) and inadequate heart rate reduction despite attempted beta blockade in 25-30% of patients [50]. Achieving beta-blockade is also time consuming and delays time-to-scan acquisition [51].
In their study of 100 patients without beta-blocker pre-medication, Ropers et al. [52] demonstrated that DSCT preserved high diagnostic accuracy in patients with high heart rates, thus circumventing the issues that arise with the use of beta-blocker medication. However, the patient group in this study was small and the prevalence of stenoses (40% on a per-patient basis) and multi-vessel disease was low. Therefore, results cannot be transferred to other clinical settings, such as patients with known coronary artery disease and a higher prevalence of stenoses.
Zimmerman et al. [51] evaluated the diagnostic quality of second-generation dual-source coronary CT examinations performed in a cohort of mostly overweight and obese subjects with no beta-blocker premedication. Overall, on a per-vessel basis, the number of coronary arteries scored as excellent quality was similar between the first three heart rate categories (88.1% for HR < 70 bpm, 91.7% for HR 70-79 bpm, 92.3% for HR 80-89). There was a significant decrease in the number of excellent quality coronary arteries in the highest heart rate category (HR ≥ 90 bpm, 73.7%, p = 0.001).
4. Tube voltage
Since radiation dose increases with the square of the tube voltage at a constant tube current, another effective method to lower radiation exposure is the reduction of tube voltage [20]. A low tube potential can also enhance the iodine-induced contrast since the attenuation coefficient of iodine-based contrast increases at lower x-ray photon energies i.e. contrast agent absorbs lower energy x-rays more efficiently, and thus improves the CCTA image quality [60].
Traditionally, CCTA has been performed with a tube voltage setting of 120 kVp. The PROTECTION II Trial demonstrated that data acquisition at a reduced tube voltage of 100 kVp is possible and has been suggested as an effective means to lower radiation dose in non-obese patients without compromising diagnostic CCTA image quality [61].
Earlier work by Pflederer et al. on dual-source CCTA on patients with a body-weight ≤ 85 kg, demonstrated a reduction of mean radiation exposure from 12.7 ± 1.7 mSv at 120 kV to 7.8 ± 2.0 mSv at 100 kV with no significant difference between image quality and vessel-based score. Contrast enhancement and image noise were significantly higher for 100 kV, whereas SNR and contrast-to-noise-ratios were not different between the two scanning protocols [62].
In a study by Lei et al. patients with very low body mass index (BMI) < 18.5 kg/m2 were investigated with retrospective ECG-gated dual-source CCTA at 120-, 100-, and 80-kV tube voltage imaging giving mean estimated dose values of 9.27 ± 1.63, 4.56 ± 2.29, and 2.29 ± 1.69 mSv, respectively [63]. They suggested that for the patients with low BMI, the dual-source CCTA with low tube voltage can obtain satisfactory image quality, and simultaneously, significantly reduce the radiation dose [63].
Using prospective ECG-gating CCTA on patients with a body mass index (BMI) ≤ 35 kg/m2, Leipsic et al. reported a further reduction in effective radiation dose of 2.6 ± 0.4 mSv versus 1.3 ± 0.5 mSv in standard (100-120 kVp) versus reduced tube voltage (80-100 kVp) with no difference in image quality score [64].
In previously available CT scanners, tube voltages below 80 kVp (kilo-voltage peak) were initially limited by x-ray tubes that were unable to provide sufficiently high tube current at low peak voltages. New third-generation, dual-source CT systems are equipped with x-ray tubes with substantially increased power (120 kW each) that enable tube currents to reach up to 1300 mAs for tube voltage as low as 70 kVp [65].
Feasibility, image quality, and radiation exposure were evaluated by Mangold et al in a selected patient population who underwent CCTA using prospectively ECG-triggered spiral acquisition with automated tube voltage selection (ATVS) in the range 70-120 kV [66]. ATVS uses an algorithm to custom-tailor the tube potential to an individual patient’s attenuation profile determined by the planning “scout” scan. The selection of low tube voltages significantly reduced the radiation dose from 10.7 ± 4.1 mSv at 120 kV to 1.5 ± 1.2 mSv at 70 kV while maintaining image quality [66]. Similarly, Wang et al. demonstrated a significant reduction in estimated dose of 1.25 ± 1.24 mSv in an ATVS group compared with 2.19 ± 1.77 mSv in a control group of patients undergoing dual-source CCTA [67].
A sub-analysis by Oliveira et al. [68], demonstrated that patients classed as obese received a similar radiation dose as the normal patients, confirming a need to optimize the protocols used in routine CCTA examinations.
In another study, Wang et al. [69] used prospective ECG-gated high-pitch spiral (pitch 3.4) CCTA to achieve average effective doses of 0.86 ± 0.08 mSv and 1.77 ± 0.18 mSv in tube voltages of 100 kV and 120 kV respectively. Use of a tube voltage of 80 kV for patients with BMI ≤ 22.5 kg/m2 resulted in a further dose reduction of 58 and 80% compared with 100 and 120 kV protocols with an effective dose of 0.36 ± 0.03 mSv, with excellent image quality, demonstrating the feasibility of BMI optimized patient-specified voltage protocol.
5. Iterative reconstruction
Earlier works combining high-pitch spiral acquisition with 100 kV tube voltage report high diagnostic accuracy at a radiation dose below 1 mSv, are limited to selected, non-consecutive, non-obese patients [30, 70]. However, dose reduction by lowering tube voltage and current is invariably accompanied by a substantial increase in noise, especially in obese patients [18]. Traditional CT image reconstruction techniques have used filtered back projection (FBP) due to faster reconstruction times. FBP, however, is limited by the process of filtering the back-projection, usually with high-pass filter, which has the effect of accentuating noise and streak artefacts and is thus less favorable at low currents for generating consistent diagnostic-quality images. To overcome these limitations and allow further dose reduction, new commercially available IR algorithms developed for routine clinical use represent another milestone in CCTA technology [71]. IR algorithms adaptively apply noise correction at a reduced x-ray exposure without compromising spatial resolution [72]. Formerly too computationally expensive, improved computer-processing power means IR can now produce images of higher quality with very low SNR within clinically acceptable reconstruction times [71, 73].
CCTA using adaptive iterative dose reduction (AIDR, Toshiba Medical Systems, Japan) and more recently three-dimensional AIDR (AIDR3D, Toshiba Medical Systems, Japan), has been reported to decrease the image noise thus allowing for reductions in tube current while preserving overall image quality [74]. Comparing AIDR3D at a lower tube current compared with the standard FBP at a higher tube current with a 320-row CT scanner, Tomizawa et al. measured a 22% reduction in the median effective radiation dose in CCTA (4.2 vs. 5.4; p = 0.0001) while no significant difference was found between their respective image noise, SNR, and contrast-to-noise ratio (CNR) [75]. In a similar study by Tatsugami et al. image noise using AIDR was reduced by 42% when compared with FBP [75-77]. Yoo et al. assessed the image quality of 640-slice CCTA using AIDR3D (AIDR3D, Toshiba Medical Systems, Japan) and automatic exposure control to optimise the tube current and voltage. The AIDR3D images had a significant noise reduction of 39% and higher SNR and CNR of the proximal coronary arteries compared with FBP while maintaining CT density. The mean subjective image quality score was also significantly higher with AIDR3D than FBP with a mean effective radiation dose of 2.0 ± 1.0 mSv [78, 79]. An increase in BMI confers a higher image noise in CCTA. BMI-adapted tube voltage and current work synergistically with AIDR3D to reduce image noise while achieving a 75% radiation dose reduction relative to a scan reconstructed with FBP [80].
Siemens (Siemens Healthcare, Forchheim, Germany) introduced the Sinogram Affirmed Iterative Reconstruction (SAFIRE) IR algorithm in 2010, and built upon this with their latest release, Advanced Model Iterative Reconstruction (ADMIRE) [93]. A significant decrease of image noise with each ADMIRE strength level (strength levels 1-5) increase and in comparison to FBP was demonstrated by Gordic et al. [86] using high-pitch 192-slice dual-source CCTA with standard settings (ref. 100 kVp, ref. 270 mAs/rot) in 25 patients. They recorded a stepwise improvement in vessel sharpness and CNR with each data set reconstructed with an ADMIRE level increase and a significant increase in comparison to FBP (p < 0.05). A sub-analysis of CCTA images using ADMIRE strength level 4, as the most often selected preferred data set for making the diagnosis, demonstrated a noise reduction of 42% compared with FBP with an estimated effective radiation dose of 0.3 ± 0.1 mSv.
iDose4 and Iterative Model Reconstruction (IMR) are alternative IR algorithms released by Philips Healthcare (Philips Healthcare, Best, the Netherlands) reported to maintain image quality at 80% reduction in radiation exposure [94]. Kordolaimi et al. compared iDose4 with FBP in terms of image quality for both retrospective electrocardiographically gated and prospective electrocardiographically triggered CCTA. A dose reduction of 43% (from 15.0 ± 3.1 mSv to 8.5 ± 2.5 mSv) was recorded in the retrospective helical ECG-gated protocol and 27% (from 3.3 ± 1.1 mSv to 2.4 ± 0.8 mSv) in the prospective axial ECG-triggered protocol on a 64-slice MDCT scanner with the use of iDose4 level 4 compared with FBP [95].
In their preliminary study, Stehli et al. demonstrated a significant reduction in the estimated radiation dose exposure of 0.29 ± 0.12 mSv (range 0.16 to 0.53 mSv) with CCTA using a model-based iterative reconstruction (MBIR, GE Healthcare, Waukesha, Wisconsin) algorithm compared
with a mean radiation dose of 13.7 ± 9.7 mSv (range 1.4 to 31.0 mSv) from invasive coronary angiography [84]. CCTA images of 36 patients reconstructed with MBIR and acquired using very low tube voltage (80 to 100 kV) and current (150 to 210 mA) resulted in a sensitivity, specificity, positive, and negative predictive value and accuracy of 100%, 74%, 77%, 100%, and 86% respectively per patient.
Most of these studies however, are limited by a small population size. Moreover, although all images were anonymized, a potential bias could arise from obvious differences in image appearance between reconstruction methods. While these studies validate a reduction in radiation dose, they have not compared the diagnostic accuracy of IR with coronary catheterization.
6. Sub-millisievert CCTA
Sub-millisievert CCTA was proven feasible in 2009 using prospective ECG-triggered high-pitch spiral acquisition [48, 70, 96]. The combination of IR techniques with second-generation 128 slice dual-source CT scanners and reduced tube voltage has demonstrated mean effective radiation dose reduction down to 0.06–0.87 mSv with robust CCTA diagnostic images [53, 83, 97]. Table 2 summarises published clinical and feasibility studies with CCTA in which a mean or median effective dose ≤ 2.2 mSv are reported.
Schuhbaeck et al. [97] demonstrate the feasibility of ultra-low radiation dose CCTA in coronary artery disease screening with an average radiation dose of 0.06 ± 0.01 mSv. However, their study was carried out in a highly selected population of young patients (mean age = 52 ± 14 years) with medium to low body weight (mean body weight = 71.5 ± 12.2 kg; mean height = 173 ± 7 cm; mean BMI = 23.9 ± 3.2 kg/m2), and low heart rate ≤ 60/min. The study was also limited by the extremely low prevalence of coronary artery disease limiting the assessment of its ability to evaluate coronary artery disease [81].
Wei-Hua Yin et al. [81] explored the feasibility and diagnostic accuracy of high-pitch spiral CCTA acquisition with IR in a consecutive patient population unselected for body habitus, mean BMI 25.5 ± 3.1 kg/m2 (range 19.8-31.1). The mean effective radiation dose was 0.58 mSv ± 0.17 and all per-patient studies were performed with a radiation dose equivalent to less than 1 mSv (0.28-0.91 mSv), even in patients with higher BMI [81]. While they investigated consecutive patients regardless of body type, they did select for slow, stable heart rates ≤ 60 bpm to conform to institution protocol for this particular acquisition technique rather than resorting to pharmaceutical rate control.
The mean effective radiation dose of 0.29 mSv reported by Stehli et al. was again limited to a study population with low heart rates, average 73 bpm [84]. Similarly, Hell et al. [85] have shown that IR techniques coupled with prospectively ECG-triggered high-pitch spiral acquisition allowed for ultra-low mean effective radiation dose of 0.3 mSv with clinically acceptable diagnostic images.
While demonstrating the feasibility of ultra-low dose CCTA, these studies were limited to carefully selected patents with a low and regular heart rate (< 60 bpm) and a body weight of less than 100 kg.
At our institute, we have since demonstrated a median effective radiation dose of 0.88 mSv (IQR, 0.6–1.4 mSv) with diagnostic image quality in 99% of CCTA images from 543 unselected patients with suspected CAD [98]. This ultra-low dose exposure was achieved by a combination of prospective ECG-gated acquisition with reduced tube current and voltage and the latest AIDR3D image reconstruction algorithm. This represents a minimum 56% reduction compared with previous reports from the 320- detector row CT scanner (Aquilion One, Toshiba Medical Systems, Japan) and AIDR3D [99]. This study verifies that submillisievert radiation doses are possible in unselected, real-world patients. However, the CCTA images were limited to subjective evaluation by two experienced cardiologists and were not objectively assessed with a quantitative evaluation of signal-to-noise and contrast-to-noise.
The conversion factor to determine effective radiation dose equivalents has been a point of considerable controversy [8, 100]. Previous ICRP conversion factors for the chest have varied from 0.012–0.026 mSv mGy-1 cm-1 potentially yielding still lower radiation estimates [101]. Moreover, CCTA is usually limited to patients in sinus rhythm [102, 103]. In MSCT coronary angiography there is an inverse relationship between heart rate and image quality.
This study does not address the lowest temporal resolution for which all patients can be imaged without motion artefacts. Dual-source CT can reach a temporal resolution as low as 83 ms, significantly greater than the 30 ms temporal resolution of catheter angiography, which is considered universally sufficient [29].
CONCLUSION
The level of radiation exposure in CCTA is comparable to the radiation range reported for a chest x-ray in two views [84]. Advancements in radiation dose reduction without compromising image quality justify the use of CCTA as a non-invasive alternative to coronary catheterization for the diagnosis of CAD [104].
Table 1.
Dose reduction strategies in coronary CT angiography.
| Parameter | Dose Reduction Method |
|---|---|
| Iterative reconstruction | Improves signal-to-noise and contrast-to-noise ratio making it possible to maintain image quality, even when current is reduced. Starts with an initial estimate of the image, which is improved iteratively by comparing the synthesized image to the one acquired with projection data and improving the previous estimation. |
| Multi-row detectors Scout view acquisition |
z-axis coverage of the scan is linearly proportional to radiation dose. Multi-row detector (or multi-slice) CT uses multiple rows of CT detectors instead of one. Faster scanning times result from an increase in the number of detectors in the z-direction allowing a larger volume of the heart to be covered per gantry rotation. Limits range covered to only part of the thorax required for scan. |
| High-pitch prospective ECG-triggered helical acquisition (recommended for low and stable heart rates). Increase pitch ≥ 3 |
Projection data is acquired for only part of the complete gantry rotation (i.e., a partial scan). The minimum projection data required to construct a complete CT image is 180° plus the fan angle of the CT detectors in the axial plane. Full tube current is only applied during a single phase of the cardiac cycle. Radiation dose is inversely proportional to the pitch. |
| Heart rate reduction with beta-blockers | Minimum cardiac motion is observed during diastolic phase; however, the diastolic phase narrows with increasing heart rate. Desired temporal resolution for motion-free cardiac imaging is 250 ms for heart rates up to 70 beats per minute and up to 150 ms for heart rates greater than 100 beats per minute, at the limit of gantry rotation. |
| Automated tube current modulation | CT dose decreases linearly with tube current and tube current–time product. Angular-modulation adjusts tube current for each projection angle [antero-posterior vs lateral] according to the size and attenuation characteristics of the human body. z-axis modulation provides noise index to allow users to select x-ray noise level of reconstructed images and attempt to maintain a constant noise level for all images irrespective of patient size and anatomy. |
| Tube voltage | CT dose is approximately proportional to the square of the tube voltage. Image quality maintained in studies as low as 70 kV in non-obese adult patients (body mass index ≤ 25 kg/m2). Reducing tube voltage increases attenuation of vessel lumen and cardiac chambers with iodinated contrast media resulting in greater image contrast. |
Table 2.
Studies with ultra-low-dose coronary CT angiography with mean effective radiation dose < 2.2 mSv.
| Study | Number of Patients | Heart Rate/bpm | BMI/ kg m-2 | CT Scanner | Dose Reduction | Mean Effective Dose/ mSv | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yin et al. (2013) [81] | 21 | 50 ± 6 | 23.9 ± 3.2 | 128 detector row, second-generation dual Source CT (Definition Flash, Siemens Healthcare, Forchheim, Germany). | High-pitch spiral acquisition and raw data-based iterative reconstruction. Tube voltage was set to 80 kV and tube current was 50 mAs. | 0.06 ± 0.01 | ||||||||||
| Zhang et al. (2014) [82] | 58 | 60.4 ± 5.7 | 22.5 ± 1.9 | Dual-source CT system (Somatom Flash; Siemens Medical Solutions, Forchheim, Germany). | Prospective ECG-triggered high-pitch spiral acquisition (3.4) at 70 kVp with 30 mL of contrast agent. Automated tube current modulation (CAREDose 4D, Siemens). Image reconstruction with iterative reconstruction SAFIRE (SAFIRE, Siemens, strength-level 3). | 0.17 ± 0.02 | ||||||||||
| Zhang et al. (2016) [83] | 43 | 69.4 ± 13.6 | 23.3 ± 2.8 | 64-slice, second-generation dual-source CT system (Somatom Flash; Siemens Medical Solutions, Forchheim, Germany). | Prospective ECG-triggered high-pitch spiral acquisition (3.4) at 70 kVp. Automated tube current modulation (CAREDose 4D, Siemens) was enabled. | 0.20 ± 0.00 | ||||||||||
| Stehli et al. (2014) [84] | 36 | 57.6 ± 6.2 | 27.6 ± 4.7 | 64-slice CT scanner (Discovery HD 750, GE Healthcare). | Prospective ECG triggering. Body mass index (BMI)–adapted tube voltage and tube current. Images reconstructed using MBIR algorithms. | 0.29 ± 0.12 | ||||||||||
| Hell et al. (2014) [85] | 26 | 54 ± 5 | 27.7 ± 3.8 | 192-slice, third-generation dual-source CT system (Somatom Force; Siemens Healthcare, Forchheim, Germany). | A prospectively ECG-triggered high-pitch (3.2) spiral (flash) acquisition was performed. Tube voltage was set at 70 kVp and tube current at 450 mAs. Images were reconstructed using iterative algorithm ADMIRE (ADMIRE; Siemens Healthcare, Forchheim, strength level 2) |
0.30 ± 0.03 | ||||||||||
| Gordic et al. (2016) [86] | 25* | 61 ± 5 | 25.3 ± 3.4 | 192-slice dual-source CT (SOMATOM Force, Siemens Healthcare). | Prospective ECG-triggered, high-pitch spiral acquisition. Automated attenuation-based tube voltage selection (CAREkV, Siemens) and tube current modulation (CAREDose, Siemens) was applied. In Images reconstructed with ADMIRE (ADMIRE; Siemens Healthcare, Forchheim, strength level 4). | 0.3 ± 0.1 | ||||||||||
| Wang et al. (2012) [69] | 40* | 55.4 ± 4.8 | 20.6 ± 1.4 | Dual source CT scanner (Definition Flash, Siemens AG, Forchheim, Germany). | Prospective ECG-triggering high-pitch spiral (3.4) at 80 kV. | 0.36 ± 0.03 | ||||||||||
| Yin et al. (2013) [81] | 40 | 54 ± 4 | 25.5 ± 3.1 | 64-slice, second-generation dual-source CT system (Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany). | Prospective ECG-triggered high-pitch spiral acquisition (3.4). Attenuation-based tube current modulation (CareDose 4D, Siemens). | 0.58 ± 0.17 | ||||||||||
| Neefjes et al. (2011) [87] |
80* | 58 ± 7 | 28 ± 4 | 64-slice, dual-source CT scanner (Somatom Definition Flash; Siemens Healthcare, Forchheim, Germany). | Prospective ECG-triggered high-pitch spiral (3.4) | 0.81 ± 0.3 | ||||||||||
| Study | Number of Patients | Heart Rate/bpm | BMI/ kg m-2 | CT Scanner | Dose Reduction | Mean Effective Dose/ mSv | ||||||||||
| Achenbach et al. (2010) [70] | 50 | 68 ± 9 | 25.4 ± 4.8 | 128 slice, dual-source CT system (‘Definition Flash’, Siemens Healthcare, Forchheim, Germany) | Prospective ECG-triggered high-pitch spiral acquisition ((pitch was 3.2 in first 28 patients and 3.4 in last 22 patients. Tube voltage was 100 kV and tube current was 320 mA s/rot. | 0.87 ± 0.07 | ||||||||||
| Alkadhi et al. (2010) [30] |
50* | 56 ± 10 | 25.9 ± 2.8 | 128-slice, second-generation dual-source CT scanner (Somatom Definition Flash; Siemens Healthcare, Forchheim, Germany). | Prospectively ECG-gated high-pitch mode (3.4). | 0.9 ± 0.1 | ||||||||||
| Chen et al. (2013) [53] | 107* | 57.1 ± 11.2 | 27.3 (24.6–32.3) | 320 row, second-generation CT scanner (Aquilion One Vision Edition; Toshiba Medical Systems, Otawara, Japan). | Prospectively ECG-gated. Tube potential and tube current were determined with use of automatic exposure control (SURE Exposure3D, Toshiba Medical Systems). Image reconstruction with iterative reconstruction AIDR-3D ((Toshiba Medical Systems). | 0.93 | ||||||||||
| Stolzmann et al. (2011) [42] | 100 | 66 ± 20 | 27.7 ± 4.2 | Second-generation dual-source 128-MDCT CT scanner (Somatom Definition Flash, Siemens Healthcare). | Prospective ECG-triggered high-pitch spiral acquisition (3.4). Tube voltage 100 kVp and tube current-time product 320 mAs per rotation | 1.0 ± 0.2 | ||||||||||
| Lell et al. (2009) [48] | 25 | 69 ± 9 | 26.8 ± 5.6 | 64 detector row, dual source CT system (Definition Flash, Siemens Healthcare, Forchheim, Germany). | Prospective ECG-triggered high-pitch spiral acquisition (3.2). Tube settings were 100 kV/320 mAs and 120 kV/400 mAs for patients below and above 100-kg weight, respectively. | 1.0 ± 0.3 | ||||||||||
| Sun et al. (2012) [57] | 134* | 79 ± 9 | 23.8 ± 2.7 | Second-generation DSCT system (Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany). | Prospective ECG-gated high-pitch spiral acquisition (3.4). Tube voltage 100 kVp and tube current-time product 320 mAs per rotation | 1.04 ± 0.16 | ||||||||||
| Sommer et al. (2010) [49] |
33 | 57.3 ± 7.0 | 24.7 ± 2.0 | Second generation dual source CT scanner (Somatom Definition Flash, Siemens Healthcare). | Prospective ECG-triggered high-pitch spiral acquisition (3.4). | 1.11 ± 0.14 | ||||||||||
| Wang et al. (2014) [80] | 172* | 59.8 ± 7.6 | 24.2 ± 2.5 | Dual-source CT scanner (Somatom Definition Flash; Siemens, Munich, Germany). | Prospective ECG-triggered high-pitch helical mode (flash mode) d if the patient’s HR < 65 bpm and prospectively ECG-triggered sequential mode if the patient’s HR between 65 and 90 bpm. Automatic tube potential selection (CARE kV; Siemens) and automatic tube current selection algorithm (CARE Dose4D; Siemens). | 1.25 ± 1.24 | ||||||||||
| Wichmann et al. (2015) [47] | 25* | 53 ± 2 | … | 128-slice second-generation dual-source CT (Somatom Definition Flash, Siemens Healthcare). | Prosepctive ECG-gated high-pitch spiral. Automatic tube potential selection (CARE kV; Siemens) and automatic tube current selection algorithm (CARE Dose4D; Siemens). | 1.27 ± 0.62 | ||||||||||
| Huang et al. (2015) [34] |
70* | 58.6 ± 4.5 | 24.8 ± 2.8 | 64-slice DSCT scanner (Somatom Definition, Siemens Healthcare, Forchheim, Germany). | Single-phase, prospective ECG-triggered acquisition. | 1.27 ± 0.57 | ||||||||||
| Study | Number of Patients | Heart Rate/bpm | BMI/ kg m-2 | CT Scanner | Dose Reduction | Mean Effective Dose/ mSv | ||||||||||
| Koplay et al. (2016) [88] |
186 | 66.52 ± 11 | 27.97 (19–40) | 128 slice dual-source CT (Somatom Definition Flash, Siemens, Germany). | Prospective ECG-triggered high-pitch spiral acquisition. Automatic tube potential selection (CARE kV; Siemens) and automatic tube current selection algorithm (CARE Dose4D; Siemens). | 1.3 ± 0.4 | ||||||||||
| Leipsic et al. (2011) [64] |
24* | 54 ± 5 | 27 ± 4 | Discovery HD 750 (GE Healthcare, Waukasha, WI, USA). | Prospective ECG-triggering. Reduced tube voltage was defined as 80 or 100 kVp for individuals with BMI < 25 kg/m2 or 25–35 kg/m2, respectively; whereas standard tube voltage was defined as 100 or 120 kVp for individuals with BMI < 25 kg/m2 or 25–35 kg/m2, respectively. | 1.3 ± 0.5 | ||||||||||
| Matsubara et al. (2016) [45] | 17* | 59.1 ± 6.0 | 21.0 ± 2.0 | 128-slice dual-source CT Somatom Definition Flash scanner (Siemens Healthcare) | Prospective ECG-triggered high-pitch spiral acquisition (3.4). | 1.5 ± 0.2 | ||||||||||
| Pflederer et al. (2010) [89] |
56* | … | … | Dual-source CT (Definition, Siemens Healthcare, Forchheim, Germany). | Prospective ECG-triggering. Tube settings were 100 kV/330 mAs. | 1.5 ± 0.4 | ||||||||||
| Mangold et al. (2016) [66] |
43* | 68.1 ± 18.4 | 23.5 ± 3.3 | 3rd generation dual-source CT (Somatom Force, Siemens Healthcare, Forchheim, Germany). | Prospective ECG-triggered spiral acquisition. Automated tube current selection and advanced iterative reconstruction. Tube voltage at 70 kV group. | 1.5 ± 1.2 | ||||||||||
| Kim et al. (2011) [28] | 23* | 59 ± 7 | 24.3 ± 3.5 | 128-slice MDCT (Definition AS Plus 128; Siemens, Forchheim, Germany). | Step-and-shoot prospective ECG-gated group. | 1.75 ± 0.83 | ||||||||||
| Leipsic et al. (2013) [90] |
109* | 59 ± 4 | 27.23 ± 4.27 | Discovery HD 750 (GE Healthcare, Waukasha, WI, USA) and a Toshiba Aquilion One (Toshiba Medical Systems, Tokyo, Japan). | Prospective ECG-triggering with a narrow window acquisition window. | 1.78 | ||||||||||
| Deseive et al. (2015) [46] |
150* | 55.5 ± 5.0 | 26.1 ± 3.5 | Second-generation dual-source CT scanner (Somatom, Definition Flash scanner, Siemens Medical Solutions) | PROTECTION IV study. Prospective ECG-gated high-pitch helical acquisition. 100-kV tube potentials up to a body mass index of 30 kg/m2. | 2.0 ± 2.4 | ||||||||||
| Yoo et al. (2013) [78] | 51 | 55 (39–65) | 25.3 (18.8–32.8) | 640-multi-slice CT scanner (Aquilion ONE; Toshiba Medical Systems, Tochiki-ken, Japan). | Prospective ECG-triggering. Automatic exposure control system (SUREExposure; Toshiba Medical Systems, Tochiki-ken, Japan). Image reconstruction with AIDR-3D (standard). | 2 ± 1 | ||||||||||
| Duarte et al. (2010) [91] |
40* | 60 ± 5 | Weight/kg (70 ± 10) | 128-MDCT (Somatom Definition AS128, Siemens Medical Solutions, Germany). | Prospective ECG-gating with full tube current at 70%. Automated tube current to patient-specific parameters such as size and attenuation of body region (CAREDose system). | 2.1 ± 0.9 | ||||||||||
| Husmann et al. (2009) [92] |
100 | 57 ± 6 | 27 ± 4 | 64-slice, LightSpeed VCT XT scanner (GE Healthcare). | Prospective ECG-triggering. | 2.2 ± 0.7 | ||||||||||
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- AIDR
Adaptive Iterative Dose Reduction
- ATVS
Automated Tube Voltage Selection
- BMI
Body Mass Index
- bpm
Beats per minute
- CAD
Coronary Artery Disease
- CCTA
Coronary CT angiography
- CNR
Contrast-to-Noise Ratio
- DLP
Dose-Length Product
- DSCT
Dual Source CT
- ED
Effective Dose
- FBP
Filtered Back-projection
- HR
Heart Rate
- IR
Iterative Reconstruction
- MBIR
Model-Based Iterative Reconstruction
- MSCT
Multislice CT
- SAS
Step-And-Shoot
- SNR
Signal-to-Noise Ratio
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Salavati A., Radmanesh F., Heidari K., et al. Dual-source computed tomography angiography for diagnosis and assessment of coronary artery disease: Systematic review and meta-analysis. J. Cardiovasc. Comput. Tomogr. 2012;6(2):78–90. doi: 10.1016/j.jcct.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Haberl R., Tittus J., Böhme E., et al. Multislice spiral computed tomographic angiography of coronary arteries in patients with suspected coronary artery disease: An effective filter before catheter angiography? Am. Heart J. 2005;149(6):1112–1119. doi: 10.1016/j.ahj.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Clinical Excellence 2010 http://guidance.nice.org.uk/CG95
- 4.Yu L., Liu X., Leng S., et al. Radiation dose reduction in computed tomography: Techniques and future perspective. Imaging Med. 2009;1(1):65–84. doi: 10.2217/iim.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jr Hausleiter. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 6.Einstein A.J., Henzlova M.J., Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 7.Halliburton S.S., Abbara S., Chen M.Y., et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J. Cardiovasc. Comput. Tomogr. 2011;5:198–224. doi: 10.1016/j.jcct.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratis A.I., Anthopoulos P.L., Gavaliatsis I.P., et al. Patient dose in cardiac radiology. Hellenic J. Cardiol. 2009;50:17–25. [PubMed] [Google Scholar]
- 9.Betsou S., Efstathopoulos E.P., Katritsis D., Faulkner K., Panayiotakis G. Patient radiation doses during cardiac catheterization procedures. Br. J. Radiol. 1998;71:634–639. doi: 10.1259/bjr.71.846.9849387. [DOI] [PubMed] [Google Scholar]
- 10.Vijayalakshmi K., Kelly D., Chapple C-L., et al. Cardiac catheterisation: Radiation doses and lifetime risk of malignancy. Heart. 2007;93:370–371. doi: 10.1136/hrt.2006.098731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Coultre R., Bize J., Champendal M., et al. Exposure of the Swiss population by radiodiagnostics: 2013 review. Radiat. Prot. Dosimetry. 2016;169(1-4):221–224. doi: 10.1093/rpd/ncv462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plourde G.B., Pancholy S., Nolan J., et al. Radiation exposure in relation to the arterial access site used for diagnostic coronary angiography and percutaneous coronary intervention: A systematic review and meta-analysis. Lancet. 2015;386:2192–2203. doi: 10.1016/S0140-6736(15)00305-0. [DOI] [PubMed] [Google Scholar]
- 13.Pancholy S.B., Joshi P., Shah S., et al. Effect of vascular access site choice on radiation exposure during coronary angiography. The REVERE trial (randomized evaluation of vascular entry site and radiation exposure). JACC Cardiovasc. Interv. 2015;8(9):1189–1196. doi: 10.1016/j.jcin.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann M.H.K., Shi H., Schmid F.T., et al. Noninvasive coronary imaging with MDCT in comparison to invasive conventional coronary angiography: A fast-developing technology. AJR Am. J. Roentgenol. 2004;182:601–608. doi: 10.2214/ajr.182.3.1820601. [DOI] [PubMed] [Google Scholar]
- 15.Coles D.R., Smail M.A., Negus I.S., et al. Comparison of radiation doses from multislice computed tomography coronary angiography and conventional diagnostic angiography. J. Am. Coll. Cardiol. 2006;47(9):1840–1845. doi: 10.1016/j.jacc.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 16.Gorenoi V., Schönermark M.P., Hagen A. CT coronary angiography vs. invasive coronary angiography in CHD. GMS Health Technol. Assess. 2012;8:1–16. doi: 10.3205/hta000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budoff M.J., Achenbach S., Blumenthal R.S., et al. Assessment of coronary artery disease by cardiac computed tomography. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 18.Xu L., Zhang Z. Coronary CT angiography with low radiation dose. Int. J. Cardiovasc. Imaging. 2010;26:17–25. doi: 10.1007/s10554-009-9576-5. [DOI] [PubMed] [Google Scholar]
- 19.Mahesh M., Cody D.D. Physics of cardiac imaging with multiple-row detector CT. Radiographics. 2007;27:1495–1510. doi: 10.1148/rg.275075045. [DOI] [PubMed] [Google Scholar]
- 20.Sabarudin A., Sun Z. Coronary CT angiography: Dose reduction strategies. World J. Cardiol. 2013;5(12):465–472. doi: 10.4330/wjc.v5.i12.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dey D., Slomka P.J., Berman D.S. Achieving very-low-dose radiation exposure in cardiac computed tomography, single-photon emission computed tomography, and positron emission tomography. Circ Cardiovasc Imaging. 2014;7:723–734. doi: 10.1161/CIRCIMAGING.113.000897. [DOI] [PubMed] [Google Scholar]
- 22.Litmanovich D.E., Tack D.M., Shahrzad M., Bankier A.A. Dose reduction in cardiothoracic CT: Review of currently available methods. Radiographics. 2014;34(3):1469–1489. doi: 10.1148/rg.346140084. [DOI] [PubMed] [Google Scholar]
- 23.Klass O., Walker M., Siebach A., et al. Prospectively gated axial CT coronary angiography: comparison of image quality and effective radiation dose between 64- and 256-slice CT. Eur. Radiol. 2010;20(5):1124–1131. doi: 10.1007/s00330-009-1652-7. [DOI] [PubMed] [Google Scholar]
- 24.Hirai N., Horiguchi J., Fujioka C., et al. Prospective versus retrospective ECG-gated 64-detector coronary CT angiography: assessment of image quality, stenosis, and radiation dose. Radiology. 2008;248(2):424–430. doi: 10.1148/radiol.2482071804. [DOI] [PubMed] [Google Scholar]
- 25.Shuman W.P., Branch K.R., May J.M., et al. Prospective versus retrospective ECG gating for 64-Detector CT of the coronary arteries: Comparison of image quality and patient radiation dose. Radiology. 2008;248(2):431–437. doi: 10.1148/radiol.2482072192. [DOI] [PubMed] [Google Scholar]
- 26.Earls J.P., Berman E.L., Urban B.A., et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: Improved image quality and reduced radiation dose. Radiology. 2008;246(3):742–753. doi: 10.1148/radiol.2463070989. [DOI] [PubMed] [Google Scholar]
- 27.Goitein O., Beigel R., Matetzky S., et al. Prospectively gated coronary computed tomography angiography: Uncompromised quality with markedly reduced radiation exposure in acute chest pain evaluation. Isr. Med. Assoc. J. 2011;13:463–467. [PubMed] [Google Scholar]
- 28.Kim J.S., Choo K.S., Jeong D.W., et al. Step-and-shoot prospectively ECG-gated vs. retrospectively ECG-gated with tube current modulation coronary CT angiography using 128-slice MDCT patients with chest pain: Diagnostic performance and radiation dose. Acta Radiol. 2011;52(8):860–865. doi: 10.1258/ar.2011.110006. [DOI] [PubMed] [Google Scholar]
- 29.Rybicki F.J., Otero H.J., Steigner M.L., et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int. J. Cardiovasc. Imaging. 2008;24:535–546. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 30.Alkadhi H., Stolzmann P., Desbiolles L., et al. Low-dose, 128-slice, dual-source CT coronary angiography: Accuracy and radiation dose of the high-pitch and the step-and-shoot mode. Heart. 2010;96:933–938. doi: 10.1136/hrt.2009.189100. [DOI] [PubMed] [Google Scholar]
- 31.Menke J., Unterberg-Buchwald C., Staab W., et al. Head-to-head comparison of prospectively triggered vs retrospectively gated coronary computed tomography angiography: Meta-analysis of diagnostic accuracy, image quality, and radiation dose. Am. Heart J. 2012;165(2):154–163. doi: 10.1016/j.ahj.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Sabarudin A., Sun Z., Ng K-H. Coronary computed tomography angiography with prospective electrocardiography triggering: A systematic review of image quality and radiation dose. Singapore Med. J. 2013;54(1):15–23. doi: 10.11622/smedj.2013005. [DOI] [PubMed] [Google Scholar]
- 33.Lewis M.A., Pascoal A., Keevil S.F., Lewis C.A. Selecting a CT scanner for cardiac imaging: The heart of the matter. Br. J. Radiol. 2016;89(1065):20160376. doi: 10.1259/bjr.20160376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang W., Xu Y., Lu D., Shi Y., Lu G. Single- versus multi-phase acquisition protocol for prospective-triggered sequential dual-source CT coronary angiography: Comparison of image quality and radiation dose. Clin. Imaging. 2015;39:597–602. doi: 10.1016/j.clinimag.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Goldman L.W. Principles of CT: Multislice CT. J. Nucl. Med. Technol. 2008;36(2):57–68. doi: 10.2967/jnmt.107.044826. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann U., Ferencik M., Cury R.C., Pena A.J. Coronary CT angiography. J. Nucl. Med. 2006;47:797–806. [PubMed] [Google Scholar]
- 37.Lin E., Alessio A. What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J. Cardiovasc. Comput. Tomogr. 2009;3(6):403–408. doi: 10.1016/j.jcct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Primak A.N., McCollough C.H., Bruesewitz M.R., Zhang J., Fletcher J.G. Relationship between noise, dose, and pitch in cardiac multi–detector row CT. Radiographics. 2006;26(6):1785–1794. doi: 10.1148/rg.266065063. [DOI] [PubMed] [Google Scholar]
- 39.Achenbach S., Marwan M., Schepis T., et al. High-pitch spiral acquisition: A new scan mode for coronary CT angiography. J. Cardiovasc. Comput. Tomogr. 2009;3(2):117–121. doi: 10.1016/j.jcct.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Ertel D., Lell M.M., Harig F., et al. Cardiac spiral dual-source CT with high pitch: A feasibility study. Eur. Radiol. 2009;19:2357–2362. doi: 10.1007/s00330-009-1503-6. [DOI] [PubMed] [Google Scholar]
- 41.Flohr T.G., McCollough C.H., Bruder H., et al. First performance evaluation of a dual-source CT (DSCT) system. Eur. Radiol. 2006;16(2):256–268. doi: 10.1007/s00330-005-2919-2. [DOI] [PubMed] [Google Scholar]
- 42.Stolzmann P., Goetti R.P., Maurovich-Horvat P., et al. Predictors of image quality in high-pitch coronary CT angiography. AJR Am. J. Roentgenol. 2011;197(4):851–858. doi: 10.2214/AJR.10.6072. [DOI] [PubMed] [Google Scholar]
- 43.Flohr T., Ohnesorge B.M. Heart rate adaptive optimization of spatial and temporal resolution for electrocardiogram-gated multislice spiral CT of the heart. J. Comput. Assist. Tomogr. 2001;25(6):907–923. doi: 10.1097/00004728-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Leschka S., Stolzmann P., Desbiolles L., et al. Diagnostic accuracy of high-pitch dual-source CT for the assessment of coronary stenoses: First experience. Eur. Radiol. 2009;19:2896–2903. doi: 10.1007/s00330-009-1618-9. [DOI] [PubMed] [Google Scholar]
- 45.Matsubara K., Sakuda K., Nunome H., et al. 128-slice dual-source CT coronary angiography with prospectively electrocardiography-triggered high-pitch spiral mode: Radiation dose, image quality, and diagnostic acceptability. Acta Radiol. 2016;57(1):25–32. doi: 10.1177/0284185114562467. [DOI] [PubMed] [Google Scholar]
- 46.Deseive S., Pugliese F., Meave A., et al. Image quality and radiation dose of a prospectively electrocardiography-triggered high-pitch data acquisition strategy for coronary CT angiography: The multicenter, randomized PROTECTION IV study. J. Cardiovasc. Comput. Tomogr. 2015;9:278–285. doi: 10.1016/j.jcct.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Wichmann J.L., Hu X., Engler A., et al. Dose levels and image quality of second‐generation 128‐slice dual‐source coronary CT angiography in clinical routine. Radiol. Med. (Torino) 2015;120:1112–1121. doi: 10.1007/s11547-015-0546-9. [DOI] [PubMed] [Google Scholar]
- 48.Lell M., Marwan M., Schepis T., et al. Prospectively ECG-triggered high-pitch spiral acquisition for coronary CT angiography using dual source CT: Technique and initial experience. Eur. Radiol. 2009;19:2576–2583. doi: 10.1007/s00330-009-1558-4. [DOI] [PubMed] [Google Scholar]
- 49.Sommer W.H., Albrecht E., Bamberg F., et al. Feasibility and radiation dose of high-pitch acquisition protocols in patients undergoing dual-source cardiac CT. AJR Am. J. Roentgenol. 2010;195(6):1306–1312. doi: 10.2214/AJR.10.4416. [DOI] [PubMed] [Google Scholar]
- 50.Mahabadi A.A., Achenbach S., Burgstahler C., et al. Safety, efficacy, and indications of beta-adrenergic receptor blockade to reduce heart rate prior to coronary CT angiography. Radiology. 2010;257(3):614–623. doi: 10.1148/radiol.10100140. [DOI] [PubMed] [Google Scholar]
- 51.Zimmerman S.L., Kral B.G., Fishman E.K. Diagnostic quality of dual-source coronary CT exams performed without heart rate control: importance of obesity and heart rate on image quality. J. Comput. Assist. Tomogr. 2014;38(6):949–955. doi: 10.1097/RCT.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ropers U., Ropers D., Pflederer T., et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography coronary angiography. J. Am. Coll. Cardiol. 2007;50(225):2393–2398. doi: 10.1016/j.jacc.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 53.Chen M.Y., Shanbhag S.M., Arai A.E. Submillisievert median radiation dose for coronary angiography with a second-generation 320–detector row CT scanner in 107 consecutive patients. Radiology. 2013;267(1):76–85. doi: 10.1148/radiol.13122621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCollough C.H., Primak A.N., Saba O., et al. Dose performance of a 64-Channel dual-Source CT scanner. Radiology. 2007;243(3):775–784. doi: 10.1148/radiol.2433061165. [DOI] [PubMed] [Google Scholar]
- 55.Stolzmann P., Scheffel H., Schertler T., et al. Radiation dose estimates in dual-source computed tomography coronary angiography. Eur. Radiol. 2008;18:592–599. doi: 10.1007/s00330-007-0786-8. [DOI] [PubMed] [Google Scholar]
- 56.Oda S., Katahira K., Utsunomiya D., et al. Improved image quality at 256-slice coronary CT angiography in patients with a high heart rate and coronary artery disease: Comparison with 64-slice CT imaging. Acta Radiol. 2015;56(11):1308–1314. doi: 10.1177/0284185114555152. [DOI] [PubMed] [Google Scholar]
- 57.Sun K., Han R-J., Ma L-J., et al. Prospectively electrocardiogram-gated high-pitch spiral acquisition mode dual-source CT coronary angiography in patients with high heart rates: Comparison with retrospective electrocardiogram-gated spiral acquisition mode. Korean J. Radiol. 2012;13(6):684–693. doi: 10.3348/kjr.2012.13.6.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts W., Wright A., Timmis J., Timmis A. Safety and efficacy of a rate control protocol for cardiac CT. Br. J. Radiol. 2009;82(976):267–271. doi: 10.1259/bjr/24574758. [DOI] [PubMed] [Google Scholar]
- 59.Dewey M., Vavere A.L., Arbab-Zadeh A., et al. Patient characteristics as predictors of image quality and diagnostic accuracy of MDCT compared with conventional coronary angiography for detecting coronary artery stenoses: CORE-64 multicenter international trial. AJR Am. J. Roentgenol. 2010;194(1):93–102. doi: 10.2214/AJR.09.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prasad S.R., Wittram C., Shepard J-A., McLoud T., Rhea J. Standard-dose and 50%–reduced-dose chest CT: Comparing the effect on image quality. AJR Am. J. Roentgenol. 2002;179:461–465. doi: 10.2214/ajr.179.2.1790461. [DOI] [PubMed] [Google Scholar]
- 61.Jr Hausleiter. Image quality and radiation exposure with a low tube voltage protocol for coronary CT angiography: Results of the PROTECTION II trial. JACC Cardiovasc. Imaging. 2010;3(11):1113–1123. doi: 10.1016/j.jcmg.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 62.Pflederer T., Rudofsky L., Ropers D., et al. Image Quality in a Low Radiation exposure protocol for retrospectively ECG-gated coronary CT angiography. AJR Am. J. Roentgenol. 2009;192(4):1045–1050. doi: 10.2214/AJR.08.1025. [DOI] [PubMed] [Google Scholar]
- 63.Lei Z.Q., Han P., Xu H.B., Yu J.M., Liu H.L. Correlation between low tube voltage in dual source CT coronary artery imaging with image quality and radiation dose. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014;34(4):616–620. doi: 10.1007/s11596-014-1326-9. [DOI] [PubMed] [Google Scholar]
- 64.Leipsic J., LaBounty T.M., Mancini G.B.J., et al. A prospective randomized controlled trial to assess the diagnostic performance of reduced tube voltage for coronary CT angiography. AJR Am. J. Roentgenol. 2011;196(4):801–806. doi: 10.2214/AJR.10.5786. [DOI] [PubMed] [Google Scholar]
- 65.Meinel F.G., Canstein C., Schoepf U.J., et al. Image quality and radiation dose of low tube voltage 3rd generation dual-source coronary CT angiography in obese patients: A phantom study. Eur. Radiol. 2014;24:1643–1650. doi: 10.1007/s00330-014-3194-x. [DOI] [PubMed] [Google Scholar]
- 66.Mangold S., Wichmann J.L., Schoepf U.J., et al. Automated tube voltage selection for radiation dose and contrast medium reduction at coronary CT angiography using 3rd generation dual-source CT. Eur. Radiol. 2016;26:3608–3616. doi: 10.1007/s00330-015-4191-4. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Wang X., Zhang Y., et al. Image quality and required radiation dose for coronary computed tomography angiography using an automatic tube potential selection technique. Int. J. Cardiovasc. Imaging. 2014;30:89–94. doi: 10.1007/s10554-014-0526-5. [DOI] [PubMed] [Google Scholar]
- 68.Oliveira L.C.G., Gottlieb I., Rizzi P., Lopes R.T., Kodlulovich S. Radiation dose in cardiac CT angiography: Protocols and image quality. Radiat. Prot. Dosimetry. 2013;155(1):73–80. doi: 10.1093/rpd/ncs313. [DOI] [PubMed] [Google Scholar]
- 69.Wang D., Hu X.H., Zhang S.Z., et al. Image quality and dose performance of 80 kV low dose scan protocol in high-pitch spiral coronary CT angiography: Feasibility study. Int. J. Cardiovasc. Imaging. 2012;28:415–423. doi: 10.1007/s10554-011-9822-5. [DOI] [PubMed] [Google Scholar]
- 70.Achenbach S., Marwan M., Ropers D., et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur. Heart J. 2010;31:340–346. doi: 10.1093/eurheartj/ehp470. [DOI] [PubMed] [Google Scholar]
- 71.Fleischmann D., Boas F.E. Computed tomography—old ideas and new technology. Eur. Radiol. 2011;21(3):510–517. doi: 10.1007/s00330-011-2056-z. [DOI] [PubMed] [Google Scholar]
- 72.Padole A., Khawaja R.D.A., Kalra M.K., Singh S. CT radiation dose and iterative reconstruction techniques. AJR Am. J. Roentgenol. 2015;204:W384–W92. doi: 10.2214/AJR.14.13241. [DOI] [PubMed] [Google Scholar]
- 73.Yin W-H., Lu B., Gao J-B., et al. Effect of reduced x-ray tube voltage, low iodine concentration contrast medium, and sinogram-affirmed iterative reconstruction on image quality and radiation dose at coronary CT angiography: Results of the prospective multicenter REALISE trial. J. Cardiovasc. Comput. Tomogr. 2015;9:215–224. doi: 10.1016/j.jcct.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 74.Yamashiro T., Miyara T., Honda O., et al. Adaptive iterative dose reduction using three dimensional processing (AIDR3D) improves chest CT image quality and reduces radiation exposure. PLoS One. 2014;9(8):e105735. doi: 10.1371/journal.pone.0105735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomizawa N., Nojo T., Akahane M., et al. Adaptive iterative dose reduction in coronary CT angiography using 320-row CT: Assessment of radiation dose reduction and image quality. J. Cardiovasc. Comput. Tomogr. 2012;6:318–324. doi: 10.1016/j.jcct.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 76.Tatsugami F., Matsuki M., Nakai G., et al. The effect of adaptive iterative dose reduction on image quality in 320-detector row CT coronary angiography. Br. J. Radiol. 2012;85:e378–e82. doi: 10.1259/bjr/10084599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams M.C., Weir N.W., Mirsadraee S., et al. Iterative reconstruction and individualized automatic tube current selection reduce radiation dose while maintaining image quality in 320-multidetector computed tomography coronary angiography. Clin. Radiol. 2013;68(11):e570–e7. doi: 10.1016/j.crad.2013.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoo R-E., Park E-A., Lee W., et al. Image quality of adaptive iterative dose reduction 3D of coronary CT angiography of 640-slice CT: Comparison with filtered back-projection. Int. J. Cardiovasc. Imaging. 2013;29(3):669–676. doi: 10.1007/s10554-012-0113-6. [DOI] [PubMed] [Google Scholar]
- 79.Feger S., Rief M., Zimmermann E., et al. The impact of different levels of adaptive iterative dose reduction 3D on image quality of 320-Row coronary CT angiography: A clinical trial. PLoS One. 2015;10(5):e0125943. doi: 10.1371/journal.pone.0125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang G., Gao J., Zhao S., et al. Achieving consistent image quality and overall radiation dose reduction for coronary CT angiography with body mass index-dependent tube voltage and tube current selection. Clin. Radiol. 2014;69:945–951. doi: 10.1016/j.crad.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 81.Yin W.H., Lu B., Hou Z.H., et al. Detection of coronary artery stenosis with sub-milliSievert radiation dose by prospectively ECG-triggered high-pitch spiral CT angiography and iterative reconstruction. Eur. Radiol. 2013;23:2927–2933. doi: 10.1007/s00330-013-2920-0. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L.J., Qi L., Wang J., et al. Feasibility of prospectively ECG-triggered high-pitch coronary CTangiography with 30 mL iodinated contrast agent at 70 kVp: Initial experience. Eur. Radiol. 2014;24:1537–1546. doi: 10.1007/s00330-014-3157-2. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L.J., Wang Y., Schoepf U.J., et al. Image quality, radiation dose, and diagnostic accuracy of prospectively ECG-triggered high-pitch coronary CT angiography at 70 kVp in a clinical setting: Comparison with invasive coronary angiography. Eur. Radiol. 2016;26:797–806. doi: 10.1007/s00330-015-3868-z. [DOI] [PubMed] [Google Scholar]
- 84.Stehli J., Fuchs T.A., Bull S., et al. Accuracy of coronary CT angiography using a submillisievert fraction of radiation exposure: Comparison with invasive coronary angiography. J. Am. Coll. Cardiol. 2014;64(8):772–780. doi: 10.1016/j.jacc.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 85.Hell M.M., Bittner D., Schuhbaeck A., et al. Prospectively ECG-triggered high-pitch coronary angiography with third-generation dual-source CT at 70 kVp tube voltage: Feasibility, image quality, radiation dose, and effect of iterative reconstruction. J. Cardiovasc. Comput. Tomogr. 2014;8:418–425. doi: 10.1016/j.jcct.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 86.Gordic S., Desbiolles L., Sedlmair M., et al. Optimizing radiation dose by using advanced modelled iterative reconstruction in high-pitch coronary CT angiography. Eur. Radiol. 2016;26:459–468. doi: 10.1007/s00330-015-3862-5. [DOI] [PubMed] [Google Scholar]
- 87.Neefjes L.A., Dharampal A.S., Rossi A., et al. Image quality and radiation low-dose scan protocols in dual-source CT coronary angiography: Randomized study. Radiology. 2011;261(3):779–786. doi: 10.1148/radiol.11110606. [DOI] [PubMed] [Google Scholar]
- 88.Koplay M., Erdogan H., Avci A., et al. Radiation dose and diagnostic accuracy of high-pitch dual-source coronary angiography in the evaluation of coronary artery stenoses. Diagn. Interv. Imaging. 2016;97:461–469. doi: 10.1016/j.diii.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Pflederer T., Jakstat J., Marwan M., et al. Radiation exposure and image quality in staged low-dose protocols for coronary dual-source CT angiography: A randomized comparison. Eur. Radiol. 2010;20:1197–1206. doi: 10.1007/s00330-009-1645-6. [DOI] [PubMed] [Google Scholar]
- 90.Leipsic J., LaBounty T.M., Ajlan A.M., et al. A prospective randomized trial comparing image quality, study interpretability, and radiation dose of narrow acquisition window with widened acquisition window protocols in prospectively ECG-triggered coronary computed tomography angiography. J. Cardiovasc. Comput. Tomogr. 2013;7(1):18–24. doi: 10.1016/j.jcct.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 91.Duarte R., Fernandez G., Castellon D., Costa J.C. Prospective coronary CT angiography 128-MDCT versus retrospective 64-MDCT: Improved image quality and reduced radiation dose. Heart Lung Circ. 2011;20:119–125. doi: 10.1016/j.hlc.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 92.Husmann L., Herzog B.A., Gaemperli O., et al. Diagnostic accuracy of computed tomography coronary angiography and evaluation of stress-only single-photon emission computed tomography/ computed tomography hybrid imaging: Comparison of prospective electrocardiogram-triggering vs. retrospective gating. Eur. Heart J. 2009;30:600–607. doi: 10.1093/eurheartj/ehn536. [DOI] [PubMed] [Google Scholar]
- 93.Moscariello A., Takx R., Schoepf U., et al. Coronary CT angiography: image quality, diagnostic accuracy, and potential for radiation dose reduction using a novel iterative image reconstruction technique—comparison with traditional filtered back projection. Eur. Radiol. 2011;21(10):2130–2138. doi: 10.1007/s00330-011-2164-9. [DOI] [PubMed] [Google Scholar]
- 94.Naoum C., Blanke P., Leipsic J. Iterative reconstruction in cardiac CT. J. Cardiovasc. Comput. Tomogr. 2015;9(4):255–263. doi: 10.1016/j.jcct.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 95.Kordolaimi S., Argentos S., Mademli M., et al. Effect of iDose4 iterative reconstruction algorithm on image quality and radiation exposure in prospective and retrospective electrocardiographically gated coronary computed tomographic angiography. J. Comput. Assist. Tomogr. 2014;38(6):956–962. doi: 10.1097/RCT.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 96.Cademartiri F., Maffei E., Arcadi T., Catalano O., Midiri M. CT coronary angiography at an ultra-low radiation dose (<0.1 mSv): Feasible and viable in times of constraint on healthcare costs. Eur. Radiol. 2013;23:607–613. doi: 10.1007/s00330-012-2767-9. [DOI] [PubMed] [Google Scholar]
- 97.Schuhbaeck A., Achenbach S., Layritz C., et al. Image quality of ultra-low radiation exposure coronary CT angiography with an effective dose <0.1 mSv using high-pitch spiral acquisition and raw data-based iterative reconstruction. Eur. Radiol. 2012;23(3):597–606. doi: 10.1007/s00330-012-2656-2. [DOI] [PubMed] [Google Scholar]
- 98.Richards C., Dorman S., John P., et al. Low-radiation and high image quality coronary computed tomography angiography in “real-world” unselected patients. World J. Radiol. 2018;10(10):135–142. doi: 10.4329/wjr.v10.i10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cesare E.D., Gennarelli A., Sibio A.D., et al. Assessment of dose exposure and image quality in coronary angiography performed by 640-slice CT: A comparison between adaptive iterative and filtered back-projection algorithm by propensity analysis. Radiol. Med. (Torino) 2014;119(8):642–649. doi: 10.1007/s11547-014-0382-3. [DOI] [PubMed] [Google Scholar]
- 100.Christner J.A., Kofler J.M., McCollough C.H. Estimating effective dose for CT using dose-length product compared with using organ doses: Consequences of adopting international commission on radiological protection publication 103 or dual-energy scanning. AJR Am. J. Roentgenol. 2010;194(4):881–889. doi: 10.2214/AJR.09.3462. [DOI] [PubMed] [Google Scholar]
- 101.Gosling O., Loader R., Venables P., et al. A comparison of radiation doses between state-of-the-art multislice CT coronary angiography with iterative reconstruction, multislice CT coronary angiography with standard filtered back-projection and invasive diagnostic coronary angiography. Heart. 2010;96:922–926. doi: 10.1136/hrt.2010.195909. [DOI] [PubMed] [Google Scholar]
- 102.Cesare E.D., Gennarelli A., Sibio A.D., et al. Image quality and radiation dose of single heartbeat 640-slice coronary CT angiography: a comparison between patients with chronic atrial fibrillation and subjects in normal sinus rhythm by propensity analysis. Eur. J. Radiol. 2015;84:631–636. doi: 10.1016/j.ejrad.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 103.Yang L., Xu L., Schoepf U.J., et al. Prospectively ECG-triggered sequential dual-source coronary CT angiography in patients with atrial fibrillation: Influence of heart rate on image quality and evaluation of diagnostic accuracy. PLoS One. 2015;10:e0134194. doi: 10.1371/journal.pone.0134194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moscariello A., Takx R.A.P., Schoepf U.J., et al. Coronary CT angiography: image quality, diagnostic accuracy, and potential for radiation dose reduction using a novel iterative image reconstruction technique—comparison with traditional filtered back projection. Eur. Radiol. 2011;21:2130–2138. doi: 10.1007/s00330-011-2164-9. [DOI] [PubMed] [Google Scholar]


