Abstract
Transient receptor potential vanilloid channel 2 (TRPV2) is required for normal cardiac contractility. The stimulation of TRPV1 in isolated cardiomyocytes can aggravate the effect of hypoxia/reoxygenation (H/R) on H9C2 cells. The knockout of the TRPV1 gene promotes increased tolerance of the isolated perfused heart to the impact of ischemia/reperfusion (I/R). However, activation of TRPV1 increases the resistance of the heart to I/R due to calcitonin gene-related peptide (CGRP) release from afferent nerve endings. It has been established that TRPV1 and TRPV2 are involved in the pathogenesis of myocardial infarction and, in all likelihood, ensure the cardiac tolerance to the ischemia/reperfusion. It has also been documented that the activation of TRPV4 negatively affects the stability of cardiomyocytes to the H/R. The blockade of TRPV4 can be considered as a new approach to the prevention of I/R injury of the heart. Studies also indicate that TRPV1 is involved in the pathogenesis of cardiac hypertrophy and that TRPV2 channels participate in the pathogenesis of dilated cardiomyopathy. Excessive expression of TRPV2 leads to chronic Ca2+-overload of cardiomyocytes, which may contribute to the development of cardiomyopathy.
Keywords: Heart, TRPV1, TRPV2, TRPV4, cardiomyocytes, cardiomyopathy
1. introduction
Transient receptor potential channels (TRP) represent a group of nonselective channels that conduct cations (mainly Na+ and Ca2+) [1, 2]. These channels are localized or translocated into the plasma membrane of most of the animal cells [1]. In total, there are 28 TRP channels structurally similar to each other [1]. Many of these channels respond to pain stimuli, heat, cold, pressure, stretching, vibration; while some of these channels function as microscopic thermometers [3]. Structurally TRP channel is a tetramer, which is embedded in the cell membrane and consists of four subunits. Each subunit has 6 transmembrane domains and two sufficiently extended intracellular regions located at the beginning of the polypeptide chain (NH2-terminal) as well as at the end of the polypeptide chain (COOH-terminal) [1]. This review is specifically focused on the transient receptor potential vanilloid (TRPV) channels, formerly named vanilloid receptor (VR) [4-6]. The activation of TRPV1 leads to an increase in the level of intracellular calcium [Ca2+]i [7]. Here we analyze the three types of TRPV channels (TRPV1, TRPV2, TRPV4) and the investigations which document their role in the regulation of the functional state of the heart.
The TRPV1 has been a widely studied channel which was cloned in 1997 by the D. Julius’ group [7]. These receptors are activated by heat (t>43°C) or pH <5.5 and the physiological role of these receptors is to convey the sensations of heat and pain [8]. The TRPV1 channels are expressed on the different cells, including cardiomyocytes [9, 10]. More specifically, they are present in the nociceptive afferent neural C-fibers innervating the heart [4]. Capsaicin has been the most commonly employed agonist of TRPV1 and another selective agonist of TRPV1 i.e., resiniferatoxin has also been employed [11]. Several TRPV1 antagonists have also been identified [12]. Capsazepine is the nonselective TRPV1 antagonist [13-15]. The activation of TRPV1 leads to an increase in the level of intracellular calcium [Ca2+]I [7]. In 1999, the D. Julius’ group cloned TRPV2, which is activated at t> 52°C [16]. Their studies indicate that TRPV2 responds to the changes in osmolarity and membrane stretch [17]. Transcripts encoding the TRPV2 channel in the heart have also been detected [18]. The existence of TRPV2 channels in cardiomyocytes has also been confirmed by other researchers [19]. It has been reported that these channels are located on the sarcolemma of cardiomyocytes [20, 21]. An agonist of TRPV2 i.e., probenecid, activates TRPV2 with EC50 of 32 μM and it has minimal effect on other channels [22]. Tranilast is a non-selective TRPV2 antagonist [20, 23] and ruthenium red is non-specific and non-competitive vanilloid antagonist [16]. In 2000, TRPV4 was first identified and characterized as an osmolarity sensitive channel [24]. TRPV4 channels are expressed on H9C2 cardiomyoblasts [25].
2. The role of TRPV in the regulation of the cardiac contractility and vascular relaxation
In 2012, an in vivo study in mice showed that the TRPV2 activator probenecid, at a dose of 100 mg/kg intravenously, had a pronounced inotropic effect, which was recorded echocardiographically [18]. The inotropic effect of probenecid could not be detected in animals with the knockout gene encoding TRPV2. The TRPV2 agonist did not affect the heart rate, the QRS complex or the duration of the PR interval. In an in vitro study, the isolated heart was perfused with a solution containing probenecid (1 μM) for 5 min which increased the rate of contraction and relaxation [18]. In a study on isolated cardiomyocytes, the effect of probenecid on the contractility and transport of Ca2+ was studied. The probenecid had a positive inotropic effect, increased [Ca2+]i and elevated the amplitude of [Ca2+]i oscillations. The thapsigargin, which disrupts the accumulation of Ca2+ in the sarcoplasmic reticulum (SR), prevented a probenecid-induced rise in the level of Ca2+ in the cell. On the basis of this data, the authors have concluded that probenecid causes mobilization of Ca2+ from SR by activation of TRPV2 [18]. It has also been documented that the deletion of the gene encoding TRPV2 in mice results in a disruption of the systolic and diastolic function of the heart [26]. Furthermore, the non-selective TRPV2 blocker tranilast blunted the hypertrophic and fibrotic responses induced by transverse aortic constriction in vivo after 4 weeks and was associated with improved cardiac function, but after longer administration (8 weeks) cardiac function was similar to the control group. In vitro studies showed tranilast response was not at the cardiomyocyte level [27]. The stimulation of TRPV2 with the TRPV2 agonist probenecid (100 mg/kg intravenously) had a positive inotropic effect, which was absent in TRPV2-/- mice. The authors have concluded that the activation of TRPV2 was a prerequisite for normal pumping function of the heart [26]. In 2014, the results of a study utilizing cardiomyocytes of genetically modified mice with the deletion of a region of DNA (exon 4) encoding TRPV2 were published [28]. This deletion disturbs the cardiomyocyte contractility and Ca2+ transport in the cell. These data confirm the view that the TRPV2 is necessary for normal cardiac contractility. Utilizing knockout mice which had the gene encoding TRPV2 deleted have shown that TRPV2 channels participate in the cardiacresponse to physical activity [29]. In a study that was performed on an isolated rat papillary muscle, it was shown that 4-α-phorbol-12,13-didecanoate (4α-PDD), an agonist of TRPV4, had no effect on papillary muscle contractility under isosmotic conditions [30]. It has been shown that GSK1016790A, a novel TRPV4 activator, induced circulatory collapse in the dogs and rat [31]. However, GSK1016790A had no effect on rate or contractility in the isolated perfused rat heart [31]. This compound produced potent endothelial-dependent relaxation of isolated vascular ring segments which was abolished by pretreatment with the NO-synthase inhibitor L-NAME, the TRPV inhibitor ruthenium red, and by endothelial NO-synthase gene deletion. The circulatory collapse was not abolished by L-NAME or endothelial NO-synthase gene deletion and was associated with profound vascular leakage and hemorrhage in the intestine, lung, and kidney [31]. Thus, these data suggest that TRPV4 is not involved in the regulation of cardiac contractility. Circulatory collapse, apparently, was a consequence of the toxic effect of GSK1016790A.
The aforementioned studies suggest that TRPV2 channels are necessary for normal heart contractility. They also indicate that TRPV4 channels are not involved in the regulation of heart contractility. The role of other TRPV channels in the regulation of heart contractility in normal conditions has yet to be studied.
3. TRPV2 Channel Activation Induces Cardiac Tolerance to Ischemia and Reperfusion
The TRPV2 channels also appear to play an important role in the pathogenesis of acute myocardial infarction. The knockout of TRPV2 gene in mice decreases the cardiac tolerance to ischemia/reperfusion (I/R) [32]. Three to four days after coronary occlusion, the TRPV2 mRNA levels were increased more than ten-fold in the left ventricular tissue as compared to sham-operated controls [33]. The authors associated this increase in the TRPV2 mRNA levels in entire left ventricular tissue with the migration of macrophage (monocytes) to the infarction zone. Indeed, flow cytofluorometry indicated that on the third day after coronary occlusion, the number of cells that had a monocyte marker (CD11B/C) and TRPV2 at the same time was increased several-fold in the myocardial infarction. After ten days, the number of such cells in the infarcted myocardium was only 2-fold higher than the values characteristic for the sham-operated animals. In another study, it was shown that the NR8383 alveolar macrophages migrate towards the incubation medium of the H9C2 cardiomyoblast by exposing cardiomyoblasts to hypoxia for 4 hours. However, this migration was significantly increased following treatment of macrophages with small interfering RNA (siRNA) of TRPV2 mRNA [33]. These siRNAs disrupt the expression of TRPV2 on macrophages NR8383 and thus, the authors propose that macrophages expressing TRPV2 participate in the pathogenesis of the acute myocardial infarction by migrating to the infarction zone [33]. These studies indicate that activation of TRPV2 channels increases the tolerance of cardiomyocytes to the impact of I/R.
4. Role of TRPV1 in cardiac tolerance to ischemia/reperfusion
It has been shown that endogenous vanilloid 12(S)-hydroperoxyeicosatetraenoic acid limits the infarct size which has been attributed to the stimulation of TRPV1 channels on the afferent nerves [34]. Moreover, deletion of the genes encoding TRPV1 in experimental animals has been associated with the increased production of pro-inflammatory cytokines and pathological post-infarction cardiac remodeling [35]. In contrast, the study of Sun et al indicated the negative role of TRPV1 channels in regulating the tolerance of cardiomyoblasts in response to hypoxia-reoxygenation (H/R). The Sun et al reported that capsaicin [10], a TRPV1 agonist, during H/R enhances the apoptosis of H9C2 cells, increases [Ca2+]i, enhances the superoxide production by mitochondria and reduces the mitochondrial membrane potential. Moreover, capsazepine (a TRPV1 antagonist) or TRPV1 siRNA attenuated H/R-induced H9C2 cell apoptosis. The hearts isolated from TRPV1-/- mice were resistant to I/R in comparison to the hearts of wild mice. On the basis of these contradictory results, it may be proposed that the direct activation of TRPV1 channels on the cardiomyocytes exacerbates H/R-induced injury is mainly due to calcium overload and to an increase in mitochondrial superoxide production [10]. However, the infarct-limiting effect observed in in vivo may be due to the activation of TRPV1 located on the endings of the afferent fibers, which may lead to increase in the release of cardioprotective peptides such as calcitonin gene-related peptide (CGRP) and substance P. Using TRPV1 gene knockout (TRPV1KO) and wild-type (WT) mice, it has been shown that the activation of TRPV1 channels protect hearts from inflammation and apoptosis in I/R injury subjected mice by releasing CGRP from afferent nerve terminals [36]. Remote preconditioning-induced cardioprotection against I/R injury has been attributed to TRPV1-dependent CGRP release [37]. Moreover, it is also reported that release of activation of TRPV1 channels during remote preconditioning may inhibit the enzymatic activity of glycogen synthase kinase-3β (GSK-3β), which subsequently may enhance gap junction coupling to produce cardioprotective effects against I/R injury [38]. A very recent study has shown that the activation of TRPV1 protects the heart against I/R through up-regulation of the phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway [39]. In 2009 [40], it was shown that TRPV1 agonist capsaicin can reduce reperfusion contractile dysfunction of the isolated perfused murine heart. Capsaicin reduced the reperfusion LDH leakage from the heart. In 2016, it was shown that capsaicin (0.1, 0.3, 1.0 mg/kg) reduces the infarct size/area at risk (IS/AAR) when administered prior to ischemia or after ischemia. Capsaicin exhibited a maximum infarct-reducing effect at a dose of 0.3 mg/kg intravenously [41, 42]. The TRPV1 antagonist capsazepine eliminated the infarct-sparing effect of capsaicin. It has been shown that a cream containing capsaicin can also exhibit infarct-reducing properties [43]. Cannabidiol (CBD), a non-psychoactive Cannabis constituent, is active in the functional assays of several TRP channels including rat and human TRPV1 [44, 45], and rat TRPV2 [45, 46]. Studies have shown that it reduces the infarct size in models of I/R in rats [47] and reactive oxygen species (ROS) generation in human cardiomyocytes [48]. Among its multiform therapeutic potential in a number of disease states [49], CBD also shows beneficial effects in rodent models of myocardial infarction [50]. The cardioprotective effects observed in in vivo studies appears to be associated with the activation of TRPV1 channels located on the afferent nerve endings and appears to be unrelated to the stimulation of TRPV1 located in cardiomyocytes. Thus, the cardioprotective effects observed in in vivo studies are associated with the activation of TRPV1 and these appear to be unrelated to the stimulation of TRPV1 located on cardiomyocytes.
5. ROLE OF TRPV IN CARDIAC HYPERTROPHY AND CARDIOMYOPATHY
Vanilloid receptors are involved not only in I/R injury of the heart, but also in the pathogenesis of hypertrophy and cardiomyopathy. It has been shown that the combined effect of abdominal aortic constriction (AAC) prior to exposure to cold temperature (4°C) for 4 weeks causes cardiac hypertrophy and contractile myocardial dysfunction in mice [51]. Western blot analysis showed upregulation of TRPV1 in the hearts of these mice. The TRPV1 antagonist SB366791 reduced manifestations of contractile dysfunction of the heart of these animals. These studies indicate that TRPV1 is involved in the formation of cardiac hypertrophy. A study in the cultured cardiomyocytes has also reported that activation of TRPV1 increased cell size and intracellular calcium level effects via of p38 mitogen-activated protein (MAP) kinases [52].
There is also evidence that vanilloid receptors can participate in the pathogenesis of cardiomyopathy. In 2013, a model of dilated cardiomyopathy (DC) caused by a deficiency of δ-sarcoglycan in hamsters was used [20]. Moreover, the DC model which was used was caused by excessive expression of the sialyltransferase in 4C30 mice. In hamsters with DC, the expression of TRPV2 in the myocardium was 3 times higher than the values typical for normal hamsters. In cardiomyocytes of hamsters with DC, significant accumulation of Ca2+ was observed after addition of the TRPV2 agonist 2-aminoethoxydiphenyl borate to the medium of incubation. While no significant increase in Ca2+ was observed in the cytoplasm of cardiomyocytes of wild hamsters. In the myocardium of human subjects with DC, there was also an increase in the expression of TRPV2 [20]. HEK293 cells were transfected with a vector containing DNA that encodes the NH2-terminal (NT) fragment of TRPV2. This transfection leads to the internalization of TRPV2, that is, the transport of these receptors from the sarcolemma to the cytoplasm [20]. The authors found that the creation of transgenic 4C30 mice with NT expression leads to a decrease in the number of TRPV2 in cardiomyocytes. In these mice, unlike the initial line of 4C30 mice, DC does not develop and their longevity was greater than that of 4C30. This is the same effect that NT expression has in hamsters with congenital DC. The long-term (14 days) administration of the TRPV2 inhibitor tranilast into hamsters with DC contributed to a decrease in the number of TRPV2 on the sarcolemma of cardiomyocytes and improved cardiac contractility parameters [20]. These authors hypothesize that overexpression of TRPV2 leads to chronic Ca2+-loading of cardiomyocytes, which in turn causes DC.
The aforementioned studies indicate that TRPV2 is involved in the pathogenesis of dilated cardiomyopathy, and that excessive expression of TRPV2 leads to chronic Ca2+-overload of cardiomyocytes, which in turn causes cardiac dilatation.
Duchenne muscular dystrophy (DMD) is a disease that is accompanied by degeneration of skeletal muscles due to a mutation leading to the lack of dystrophin protein [21]. In addition to muscular dystrophy, heart failure and arrhythmias develop with DMD. The lack of dystrophin leads to Ca2+-overload in the cardiomyocytes due to over-stretching of the cell membrane [21]. Studies were performed using C57BL/10 mice and C57BL/10ScScn-mdx mice with muscular dystrophy [21]. They indicate that osmotic stress causes a marked rise in the level of Ca2+ in cardiomyocytes isolated from the heart of mice with dystrophy and does not significantly affect the level of Ca2+ in the cardiomyocytes of wild mice. It has now also been documented that TRPV2 is located on the sarcolemma of cardiomyocytes. The number of these receptors is 1.5 times higher in mice from the C57BL/10ScScn-mdx line than in wild mice. TRPV2 activators (probenecid and 2-aminoethyl diphenylborinate) increased the level of Ca2+ in the cytoplasm of cardiomyocytes in mice with dystrophy, and TRPV2 inhibitors eliminate this effect of agonists [21]. These investigations were performed on cardiomyocytes, which were exposed to compounds depleting Ca2+ stores in the SR (thapsigargin + ryanodine + caffeine). Consequently, the elevation of [Ca2+]i could only be the result of the entry of Ca2+ from the extracellular medium through the TRPV2 channels. The rise of Ca2+ in the cytoplasm of cardiomyocytes of C57BL/10ScScn-mdx mice in response to osmotic stress disappeared after pretreatment with antibodies to TRPV2 or TRPV2 siRNA [21]. The data presented indicate that TRPV2 is an osmoreceptor whose overexpression can lead to Ca2+ overload of cardiomyocytes, which in turn can cause cardiomyopathy in C57BL/10ScScn-mdx mice. In 2016, Aguettaz et al. [53] published the results of investigations with isolated cardiomyocytes of wild mice and mice with muscular dystrophy C57BL/10ScScn-mdx. The stress of stretching was reproduced using micro carbon fibres technique. In response to the stretching of [Ca2+]i in the cardiomyocytes of wild mice, it increased 20-fold, and in the cardiomyocytes of mdx mice, the increase in the Ca2+ level was even more significant. Nifedipine, the blocker of L-type Ca2+ channels did not affect the rise of [Ca2+]i in cardiomyocytes after their extension. Streptomycin, the non-selective inhibitor of ionic channels, reacting to tension, completely eliminated the rise of [Ca2+]i. The selective inhibitor of these channels, the GsMTx-4 isolated from tarantula venom reduced, but did not eliminate the increase in [Ca2+]i in response to the stretching. The antibodies to TRPV2 completely eliminated the increase in [Ca2+]i in the cardiomyocytes of mdx mice in response to stretching, and the inhibitor TRPV2 tranilast also acted [53]. The antibodies to TRPV2 and tranilast did not affect the rise of [Ca2+]i in the cardiomyocytes of wild mice. The authors found that the density of TRPV2 is significantly larger in the cardiomyocytes of mdx mice than in wild animals [53]. The data presented indicate that overexpression of TRPV2 can promote Ca2+-overload of cardiomyocytes in mice with muscular dystrophy. Such an overload of cells with calcium ions may be one of the causes of cardiomyopathy.
The foregoing studies indicate that TRPV2 channel can function as an osmoreceptor, the overexpression of which promotes Ca2+-overload of cardiomyocytes, and it, in turn, can lead to cardiomyopathy.
6. TRPV4 channel activation exacerbates ischemic injury
In 2017, research results in mice that received coronary occlusion (30 min) following reperfusion were published [54]. The reperfusion time was 24 h. The selective TRPV4 inhibitor HC-067047 was administered intraperitoneally every 8 h, the first injection was done at the time of reperfusion initiation. The HC-067047 had an infarct-limiting effect, starting at a dose of 5 mg/kg. The optimal dose was 10 mg/kg. In mice with a deletion of the gene encoding TRPV4, the infarct size was 2-fold less than in wild mice. In the blood of TRPV4-/- mice, the troponin T (a marker of myocardial necrosis) level was lower than in the blood of wild mice. The left ventricular ejection fraction, in contrast, was higher in TRPV4-/- mice than in TRPV4+/+ mice. In TRPV4-/- mice the apoptotic index was 3-fold lower in comparison with TRPV4+/+ animals. HC-067047 reduced the apoptotic index and decreased the activity of the key enzyme of apoptosis of Caspase 3. In another group, HC-067047 was administered 1 h after reperfusion, the reperfusion duration was 4 hours. This study indicated that the blockade of phosphatidylinositol-3-kinase (PI3K) and extracellular signal-regulated kinase (ERK) eliminated the infarct-limiting and anti-apoptotic effect of HC-067047 [54]. The infarct-limiting effect of HC-067047, when administered 1 h after reperfusion was a surprise. Usually, the drugs are administered intravenously 5 minutes before reperfusion. G.J. Gross’s laboratory data have shown that opioid U-50,488 reduced the infarct size if it was injected 5 minutes before reperfusion, and did not have a cardioprotective effect if it was administered 10 s after reperfusion [55]. Consequently, the data of these Chinese physiologists require independent re-examination [54]. At the same time, experiments utilizing knockout mice convincingly demonstrate that TRPV4 channels play a negative role in regulating cardiac tolerance to the impact of I/R. In another study, performed by the same author's team [25] confirmed the data regarding the ability of HC-067047 to have an infarct-limiting effect when administered 1 h after the onset of reperfusion. The TRPV4 agonist GSK1016790A, on the contrary, increased the size of the infarction following Ischemia-reperfusion and resulted in a decrease in the left ventricular ejection fraction.
The TRPV4 agonist GSK1016790A aggravated the negative inotropic effect of coronary occlusion and reperfusion while the TRPV4 antagonist HC-067047 improved the pumping function of the heart. In experiments on the H9C2 cardiomyoblasts culture, the existence of functionally active TRPV4 in cardiac cells has been demonstrated [25]. The TRPV4 activator GSK1016790A caused the rise of [Ca2+]i, starting at a concentration of 100 nM, the TRPV4 antagonist HC-067047 (1 μM) eliminated this effect.
Hypoxia-reoxygenation of H9C2 cardiomyoblasts caused an increase in [Ca2+]i, and the addition of GSK1016790A in the incubation medium aggravated Ca2+-overload. The inhibitor TRPV4 HC-067047, on the contrary, reduced the reoxygenation Ca2+-overload. This data indicates that TRPV4 is involved in reoxygenation Ca2+-loading of the cardiac cells, and the blockade of these vanilloid receptors can increase the resistance of the cardiac cells to H/R. Indeed, it was found that the H/R of H9C2 cells resulted in an increase in the concentration of lactate dehydrogenase (LDH), a marker of necrosis, in the incubation medium. The TRPV4 antagonist HC-067047 (1 μM) contributed to the decrease in LDH concentration. The TRPV4 agonist GSK1016790A (300 nM), in contrast, aggravated the necrosis of the cardiomyoblasts. Survival of the cells, which were subjected to H/R under the influence of HC-067047, was increased while cells subjected to GSK1016790A had decreased survival time. The effect of H/R led to a fivefold increase in the number of H9C2 cells in the state of apoptosis, GSK1016790A enhanced apoptosis, HC-067047 reduced the number of apoptotic cells. It has also been documented that hypoxia/reoxygenation (H/R) leads to an increase in the number of reactive oxygen species (ROS). GSK1016790A enhances the production of ROS, and HC-067047 inhibits the ROS formation. The authors obtained data that the H/R leads to the opening of the mitochondrial permeability transition pore (MPT pore).
It is well known that the opening of the MPT pore causes apoptosis of the cells and that the pore opens by the action of Са2+ and ROS [56-58]. The TRPV4 antagonist HC-067047 inhibited the MPT pore opening while the TRPV4 agonist GSK1016790A promoted pore opening MPT, which accordingly affected the apoptosis of the H9C2 cardiomyoblasts. The antioxidant N-acetyl-L-cysteine prevented the MPT pore opening under the action of H/R and GSK1016790A [25]. The presented data demonstrate that events in cardiomyocytes in response to H/R develop as follows:
H/R → TRPV4 → ↑ [Ca2+]i + ↑ ROS → MPT pore opening → apoptosis, necrosis
Moreover, a recent study has shown that the blockade of TRPV4 may reduce apoptosis and myocardial injury via the activation of reperfusion injury salvage kinase (RISK) pathway comprising of phosphorylation of Akt, ERK1/2, and GSK-3β [54]. GSK-3β is a unique enzyme whose activity is decreased on its phosphorylation. Therefore, the study of Dong et al showing phosphorylation of GSK-3β suggests that inhibition of GSK-3β and activation of Akt along with ERK 1/2 is involved in producing cardioprotection, which is consistent with earlier discussed studies [38, 39, 54]. The data presented indicate that activation of TRPV4 negatively affects the tolerance of cardiomyocytes to the H/R. The blockade of TRPV4 can be considered as a new approach to the prevention of ischemic and reperfusion injury of the heart.
7. Interaction of TRPV1 channels and CGRP
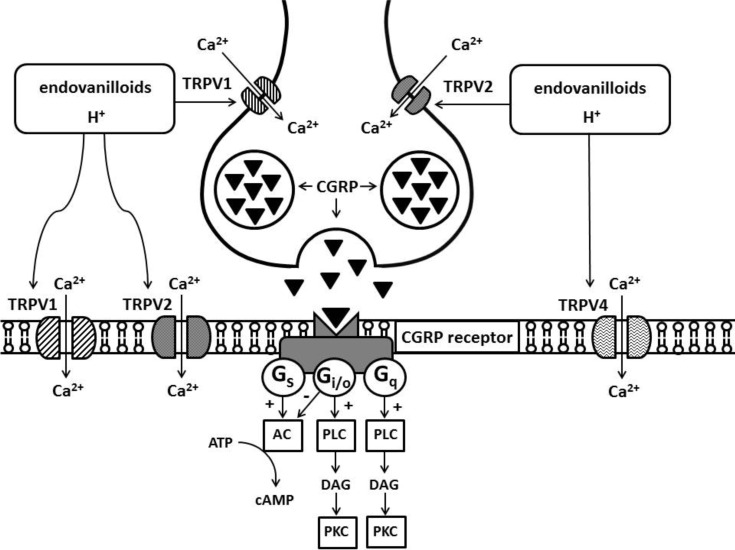
In documenting the role of the TRPV1 channels in the implementation of cardiac effects of capsaicin, one also has to focus on the role of calcitonin gene-related peptide (CGRP). Activation of TRPV1, which is located on afferent terminals, causes mobilization of these CGRP from endings (Fig. 1). In this case, CGRP acts as a neurotransmitter [59, 60]. In the study with isolated afferent nerves, it was shown that activation of TRPV2 channels also leads to the CGRP release (Fig. 1) [46]. The CGRP peptide consists of 37 amino acid residues and belongs to the family of calcitonin peptides. It is represented by two peptides that differ by three amino acid residues: α-CGRP, β-CGRP [61]. CGRP is found in the gastrointestinal tract [59, 60], heart [62]. CGRP transcripts are also found in the brain [61] and, in the liver [61]. The CGRP receptor refers to G-protein-conjugated receptors comprised of, CGRP1 and CGRP2 receptors [63, 64]. The antagonist of these receptors is the peptide CGRP (8-37) [63]. It has been reported that activation of CGRP receptors leads to enhanced cAMP synthesis [64, 65], but there is evidence that CGRP receptors are associated with pertussis toxin-sensitive Gi/o proteins [66]. Data show that there are two CGRP pools: one is coupled to Gi/o-proteins, the other is not [66] (Fig. 1). CGRP receptors are widely represented in the body, they are present in the gastrointestinal tract [59, 60], cells of the heart and blood vessels [63-65, 67]. In the study with an isolated perfused guinea pig heart, it has been shown that capsaicin (1 μM) induces a CGRP release into the coronary effluent [62]. Capsaicin-induced CGRP release was Ca2+-dependent. Capsaicin-induced the heart rate increases and decreased the force of contractions. The maximum CGRP content is found in the atria - 30 pM/g, the ventricular content is 10 pM/g [62].
Fig. (1).
Role TRPV and endovanilloid in the neurotransmission in the heart.
Abbreviations: TRPV, transient receptor potential vanilloid; CGRP, calcitonin gene-related peptide; AC, adenylyl cyclase; PLC, phospholipase C; DAG, diacylglycerol; PKC, protein kinase C.
The perfusion of the isolated heart of the rats with a solution containing CGRP (5 nM) for 5 min before total ischemia (30 min) contributed to an improvement in heart contractility in the reperfusion period, reduced the incidence of reperfusion ventricular arrhythmias, and the release of the creatine kinase (a cardiomyocyte marker of necrosis) into the coronary effluent [68]. The same effect was achieved by perfusion (5 min) of the heart with a solution containing capsaicin (3 μM). The CGRP receptor antagonist CGRP (8-37) eliminated the effect of CGRP while the non-selective TRPV1 antagonist ruthenium red eliminated the effect of capsaicin [68]. Similar data were obtained by Randhawa and Jaggi [69]. Capsaicin (5 mg/kg) was injected intraperitoneally 40 minutes prior to cardiac isolation. It turned out that capsaicin increases the resistance of the heart to the global ischemia and reperfusion. Pretreatment with capsaicin-evoked an improvement in the parameters of heart contractility during the reperfusion period, and a decrease in creatine kinase release into the coronary effluent [69].
The aforementioned studies suggest that the activation of TRPV1 increases the resistance of the heart to the pathogenic impact of I/R due to the mobilization of CGRP from the afferent nerve endings.
CONCLUSION
It is concluded that the optimal activation of TRPV2 channels is necessary for normal heart contractility. However, excessive expression of TRPV2 leads to chronic Ca2+-overload-induced dilated cardiomyopathy. The activation of TRPV1 may provide protection to hearts by increasing the CGRP release from the afferent nerve endings, inhibiting GSK-3β activity, enhancing gap junctional coupling and activating PI3K/Akt signaling pathway. The activation of TRPV4 may negatively affect the stability of cardiomyocytes to H/R and the blockade of TRPV4 may be a new approach for preventing ischemia-reperfusion-induced myocardial injury.
Acknowledgements
The authors are grateful for the technical assistance of D. Sipkova and N.S. Voronkov.
Consent for Publication
Not applicable.
Funding
The article was supported by Russian Science Foundation 19-15-00037. The chapter on cardiomyopathy was prepared within the framework of state task AAAA-A15-115120910024-0. The chapter on the role of TRPV channels in the regulation of cardiac contractility was supported by Russian Foundation of Basic Research.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Li M., Yu Y., Yang J. 2011. Structural biology of TRP channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Petrocellis L., Nabissi M., Santoni G., Ligresti A. Actions and regulation of ionotropic cannabinoid receptors. Adv. Pharmacol. 2017;80:249–289. doi: 10.1016/bs.apha.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Vriens J., Nilius B., Voets T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 2014;15(9):573–589. doi: 10.1038/nrn3784. [DOI] [PubMed] [Google Scholar]
- 4.Guo A., Vulchanova L., Wang J., Li X., Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 1999;11(3):946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 5.Szallasi A., Blumberg P.M., Annicelli L.L., Krause J.E., Cortright D.N. The cloned rat vanilloid receptor VR1 mediates both R-type binding and C-type calcium response in dorsal root ganglion neurons. Mol. Pharmacol. 1999;56(3):581–587. doi: 10.1124/mol.56.3.581. [DOI] [PubMed] [Google Scholar]
- 6.Ward S.M., Bayguinov J., Won K.J., Grundy D., Berthoud H.R. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J. Comp. Neurol. 2003;465(1):121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- 7.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 8.Tominaga M., Caterina M.J., Malmberg A.B., et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 9.Zahner M.R., Li D.P., Chen S.R., Pan H.L. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J. Physiol. 2003;551(Pt 2):515–523. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Z., Han J., Zhao W., et al. TRPV1 activation exacerbates hypoxia/reoxygenation-induced apoptosis in H9C2 cells via calcium overload and mitochondrial dysfunction. Int. J. Mol. Sci. 2014;15(10):18362–18380. doi: 10.3390/ijms151018362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szallasi A., Blumberg P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30(2):515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- 12.De Petrocellis L., Schiano Moriello A. Modulation of the TRPV1 channel: Current clinical trials and recent patents with focus on neurological conditions. Recent Patents CNS Drug Discov. 2013;8(3):180–204. doi: 10.2174/1574889808666131209124012. [DOI] [PubMed] [Google Scholar]
- 13.Bevan S., Hothi S., Hughes G., et al. Capsazepine: A competitive antagonist of the sensory neuron excitant capsaicin. Br. J. Pharmacol. 1992;107(2):544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walpole C.S.J., Bevan S., Bovermann G., et al. The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitant capsaicin and resiniferatoxin. J. Med. Chem. 1994;37(13):1942–1954. doi: 10.1021/jm00039a006. [DOI] [PubMed] [Google Scholar]
- 15.Szallasi A., Blumberg P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51(2):159–212. [PubMed] [Google Scholar]
- 16.Caterina M.J., Rosen T.A., Tominaga M., Brake A.J., Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 17.Muraki K., Iwata Y., Katanosaka Y., et al. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ. Res. 2003;93(9):829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 18.Koch S.E., Gao X., Haar L., et al. Probenecid: Novel use as a non-injurious positive inotrope acting via cardiac TRPV2 stimulation. J. Mol. Cell. Cardiol. 2012;53(1):134–144. doi: 10.1016/j.yjmcc.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwata Y., Katanosaka Y., Arai Y., Kamamura K., Miyatake K., Shigekawa M. A novel mechanism of myocyte degeneration involving Ca2+-permeable growth factor-regulated channel. J. Cell Biol. 2003;161(5):957–967. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwata Y., Ohtake H., Suzuki O., Matsuda J., Komamura K., Wakabayashi S. Blockade of sarcolemmal TRPV2 accumulation inhibits progression of dilated cardiomyopathy. Cardiovasc. Res. 2013;99(4):760–768. doi: 10.1093/cvr/cvt163. [DOI] [PubMed] [Google Scholar]
- 21.Lorin C., Vögeli I., Niggli E. Dystrophic cardiomyopathy: Role of TRPV2 channels in stretch-induced cell damage. Cardiovasc. Res. 2015;106(1):153–162. doi: 10.1093/cvr/cvv021. [DOI] [PubMed] [Google Scholar]
- 22.Bang S., Kim K.Y., Yoo S., Lee S.H., Hwang S.W. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci. Lett. 2007;425(2):120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Mihara H., Boudaka A., Shibasaki K., Yamanaka A., Sugiyama T., Tominaga M. Involvement of TRPV2 activation in intestinal movement through nitric oxide production in mice. J. Neurosci. 2010;30(49):16536–16544. doi: 10.1523/JNEUROSCI.4426-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liedtke W., Choe Y., Marti-Renom M.A., et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103(3):525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q.F., Qian C., Zhao N., et al. Activation of transient receptor potential vanilloid 4 involves in hypoxia/reoxygenation injury in cardiomyocytes. Cell Death Dis. 2017;8(5):e2828. doi: 10.1038/cddis.2017.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinstein J., Lasko V.M., Koch S.E., et al. Novel role of transient receptor potential vanilloid 2 in the regulation of cardiac performance. Am. J. Physiol. Heart Circ. Physiol. 2014;306(4):H574–H584. doi: 10.1152/ajpheart.00854.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch S.E., Nieman M.L., Robbins N., et al. Tranilast blunts the hypertrophic and fibrotic response to increased afterload independent of cardiomyocyte transient receptor potential vanilloid 2 channels. J. Cardiovasc. Pharmacol. 2018;72(1):40–48. doi: 10.1097/FJC.0000000000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katanosaka Y., Iwasaki K., Ujihara Y., et al. TRPV2 is critical for the maintenance of cardiac structure and function in mice. Nat. Commun. 2014;5:3932. doi: 10.1038/ncomms4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naticchioni M., Karani R., Smith M.A., et al. Transient receptor potential vanilloid 2 regulates myocardial response to exercise. PLoS One. 2015;10(9):e0136901. doi: 10.1371/journal.pone.0136901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J., Wang M.H., Wang L., et al. Role of transient receptor potential vanilloid 4 in the effect of osmotic pressure in myocardial contractility in rat. Acta Phys. Sin. 2008;60(2):181–188. [PubMed] [Google Scholar]
- 31.Willette R.N., Bao W., Nerurkar S., et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J. Pharmacol. Exp. Ther. 2008;326(2):443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- 32.Wang L., Wang D.H. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005;112(23):3617–3623. doi: 10.1161/CIRCULATIONAHA.105.556274. [DOI] [PubMed] [Google Scholar]
- 33.Entin-Meer M., Levy R., Goryainov P., et al. The transient receptor potential vanilloid 2 cation channel is abundant in macrophages accumulating at the peri-infarct zone and may enhance their migration capacity towards injured cardiomyocytes following myocardial infarction. PLoS One. 2014;9(8):e105055. doi: 10.1371/journal.pone.0105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sexton A., McDonald M., Cayla C., Thiemermann C., Ahluwalia A. 12-Lipoxygenase-derived eicosanoids protect against myocardial ischemia/reperfusion injury via activation of neuronal TRPV1. FASEB J. 2007;21(11):2695–2703. doi: 10.1096/fj.06-7828com. [DOI] [PubMed] [Google Scholar]
- 35.Huang W., Rubinstein J., Prieto A.R., Thang L.V., Wang D.H. Transient receptor potential vanilloid gene deletion exacerbates inflammation and atypical cardiac remodeling after myocardial infarction. Hypertension. 2009;53(2):243–250. doi: 10.1161/HYPERTENSIONAHA.108.118349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei J., Zhu F., Zhang Y., Duan L., Lei H., Huang W. Transient receptor potential vanilloid subtype 1 inhibits inflammation and apoptosis via the release of calcitonin gene-related peptide in the heart after myocardial Infarction. Cardiology. 2016;134(4):436–443. doi: 10.1159/000444439. [DOI] [PubMed] [Google Scholar]
- 37.Randhawa P.K., Jaggi A.S. Exploring the putative role of TRPV1-dependent CGRP release in remote hind preconditioning-induced cardioprotection. Cardiovasc. Ther. 2017;35(5) doi: 10.1111/1755-5922.12276. [DOI] [PubMed] [Google Scholar]
- 38.Randhawa P.K., Jaggi A.S. Investigating the involvement of glycogen synthase kinase-3β and gap junction signaling in TRPV1 and remote hind preconditioning-induced cardioprotection. Eur. J. Pharmacol. 2017;814:9–17. doi: 10.1016/j.ejphar.2017.07.045. [DOI] [PubMed] [Google Scholar]
- 39.Jiang X.X., Liu G.Y., Lei H., Li Z.L., Feng Q.P., Huang W. Activation of transient receptor potential vanilloid 1 protects the heart against apoptosis in ischemia/reperfusion injury through upregulating the PI3K/Akt signaling pathway. Int. J. Mol. Med. 2018;41(3):1724–1730. doi: 10.3892/ijmm.2017.3338. [DOI] [PubMed] [Google Scholar]
- 40.Wei Z., Wang L., Han J., et al. Decreased expression of transient receptor potential vanilloid 1 impaires the postischemic recovery of diabetic mouse hearts. Circ. J. 2009;73(6):1127–1132. doi: 10.1253/circj.cj-08-0945. [DOI] [PubMed] [Google Scholar]
- 41.Hurt C.M., Lu Y., Stary C.M., et al. Transient receptor potential vanilloid 1 regulates mitochondrial membrane potential and myocardial reperfusion injury. J. Am. Heart Assoc. 2016;5(9):e003774. doi: 10.1161/JAHA.116.003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Y., Heymann H.M., Gross E.R. Non-opioid analgesic use and concerns for impaired organ protection. Br. J. Anaesth. 2018;120(2):403–405. doi: 10.1016/j.bja.2017.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heymann H.M., Wu Y., Lu Y., Qvit N., Gross G.J., Gross E.R. Transient receptor potential vanilloid 1 inhibitors block laparotomy- and opioid-induced infarct size reduction in rats. Br. J. Pharmacol. 2017;174(24):4826–4835. doi: 10.1111/bph.14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bisogno T., Hanus L., De Petrocellis L., et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134(4):845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Petrocellis L., Ligresti A., Schiano Moriello A., et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011;163(7):1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin N., Neeper M.P., Liu Y., Hutchinson T.L., Lubin M.L., Flores C.M. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 2008;28(24):6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durst R., Danenberg H., Gallily R., et al. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2007;293(6):H3602–H3607. doi: 10.1152/ajpheart.00098.2007. [DOI] [PubMed] [Google Scholar]
- 48.Rajesh M., Mukhopadhyay P., Bátkai S., et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010;56(25):2115–2125. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ligresti A., De Petrocellis L., Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016;96(4):1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- 50.Horváth B., Mukhopadhyay P., Haskó G., Pacher P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am. J. Pathol. 2012;180(2):432–442. doi: 10.1016/j.ajpath.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu S., Xu D. Cold stress accentuates pressure overload-induced cardiac hypertrophy and contractile dysfunction: Role of TRPV1/AMPK-mediated autophagy. Biochem. Biophys. Res. Commun. 2013;442(1-2):8–15. doi: 10.1016/j.bbrc.2013.10.128. [DOI] [PubMed] [Google Scholar]
- 52.Chen M., Xin J., Liu B., et al. Mitogen-activated protein kinase and intracellular polyamine signaling is involved in TRPV1 activation-induced cardiac hypertrophy. J. Am. Heart Assoc. 2016;5(8):e003718. doi: 10.1161/JAHA.116.003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aguettaz E., Lopez J.J., Krzesiak A., et al. Axial stretch-dependent cation entry in dystrophic cardiomyopathy: Involvement of several TRPs channels. Cell Calcium. 2016;59(4):145–155. doi: 10.1016/j.ceca.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong Q., Li J., Wu Q.F., et al. Blockage of transient receptor potential vanilloid 4 alleviates myocardial ischemia/reperfusion injury in mice. Sci. Rep. 2017;7:42678. doi: 10.1038/srep42678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peart J.N., Gross E.R., Reichelt M.E., Hsu A., Headrick J.P., Gross G.J. Activation of kappa-opioid receptors at reperfusion affords cardioprotection in both rat and mouse hearts. Basic Res. Cardiol. 2008;103(5):454–463. doi: 10.1007/s00395-008-0726-z. [DOI] [PubMed] [Google Scholar]
- 56.Halestrap A.P., Clarke S.J., Javadov S.A. Mitochondrial permeability transition pore opening during myocardial reperfusion - a target for cardioprotection. Cardiovasc. Res. 2004;61(3):372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 57.Halestrap A.P. Calcium, mitochondria and reperfusion injury: A pore way to die. Biochem. Soc. Trans. 2006;34(Pt 2):232–277. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 58.Halestrap A.P., Clarke S.J., Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim. Biophys. Acta. 2007;1767(8):1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evangelista S. 2014. Capsaicin receptor as target of calcitonin gene-related peptide in the gut. [DOI] [PubMed] [Google Scholar]
- 60.Luo X.J., Liu B., Dai Z., Yang Z.C., Peng J. Stimulation of calcitonin gene-related peptide release through targeting capsaicin receptor: A potential strategy for gastric mucosal protection. Dig. Dis. Sci. 2013;58(2):320–325. doi: 10.1007/s10620-012-2362-6. [DOI] [PubMed] [Google Scholar]
- 61.Rezaeian A.H., Isokane T., Nishibori M., et al. αCGRP and βCGRP transcript amount in mouse tissues of various developmental stages and their tissue expression sites. Brain Dev. 2009;31(9):682–693. doi: 10.1016/j.braindev.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Franco-Cereceda A., Lundberg J.M., Saria A., Schreibmayer W., Tritthart H.A. Calcitonin gene-related peptide: release by capsaicin and prolongation of the action potential in the guinea-pig heart. Acta Physiol. Scand. 1988;132(2):181–190. doi: 10.1111/j.1748-1716.1988.tb08316.x. [DOI] [PubMed] [Google Scholar]
- 63.Poyner D.R., Marshall I. CGRP receptors: Beyond the CGRP1-CGRP2 subdivision. Trends Pharmacol. Sci. 2001;22(5):223. doi: 10.1016/s0165-6147(00)91555-4. [DOI] [PubMed] [Google Scholar]
- 64.Poyner D.R., Sexton P.M., Marshall I., et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Re. 2002;54(2):233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 65.Goto K., Miyauchi T., Homma S., Ohshima N. Calcitonin gene-related peptide in the regulation of cardiac function. Ann. N. Y. Acad. Sci. 1992;657:194–203. doi: 10.1111/j.1749-6632.1992.tb22768.x. [DOI] [PubMed] [Google Scholar]
- 66.Main M.J., Brown J., Brown S., Fraser N.J., Foord S.M. The CGRP receptor can couple via pertussis toxin sensitive and insensitive G proteins. FEBS Lett. 1998;441(1):6–10. doi: 10.1016/s0014-5793(98)01507-5. [DOI] [PubMed] [Google Scholar]
- 67.Chatterjee T.K., Moy J.A., Fisher R.A. Characterization and regulation of high affinity calcitonin gene-related peptide receptors in cultured neonatal rat cardiac myocytes. Endocrinology. 1991;128(6):2731–28. doi: 10.1210/endo-128-6-2731. [DOI] [PubMed] [Google Scholar]
- 68.Li Y.J., Xiao Z.S., Peng C.F., Deng H.W. Calcitonin gene-related peptide-induced preconditioning protects against ischemia-reperfusion injury in isolated rat hearts. Eur. J. Pharmacol. 1996;311(2-3):163–167. doi: 10.1016/0014-2999(96)00426-8. [DOI] [PubMed] [Google Scholar]
- 69.Randhawa P.K., Jaggi A.S. Investigating the involvement of TRPV1 ion channels in remote hind limb preconditioning-induced cardioprotection in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2017;390(2):117–126. doi: 10.1007/s00210-016-1311-x. [DOI] [PubMed] [Google Scholar]