Abstract
Many advances in the knowledge of medical science are due to the observation of an unknown phenomenon that remains an open question. A plausible hypothesis must be demonstrated and proved through a scientific method in order to be accepted by the scientific community and the same results must be reached by following either the same or different techniques. The original case described by Rosenbaum MB et al., in this review triggered a series of anatomic and physiologic investigations with clinical and experimental observations that supported the trifascicular nature of the intraventricular conduction system of the heart and the concept of hemiblocks. The recognition and description of the left fascicular blocks made by the Argentinian School of Electrocardiology bridged an important gap in electrocardiography and many electrocardiograms that could not be explained until that moment could finally be understood. This review intends to redefine reliable criteria for the electrocardiographic and vectorcardiographic diagnosis of left fascicular blocks [hemiblocks]. The anatomy of the left bundle branch is also discussed to better understand the incidence, prevalence, clinical significance and main causes of left anterior and left posterior hemiblock either isolated or associated with right bundle branch block. This review offers the reader a reappraisal of the trifascicular nature of the intraventricular conduction system regarding the anatomy of the left bundle branch system and its pathophysiological and clinical significance.
Keywords: Fascicular blocks, intraventricular conduction system, hemiblocks, electrocardiography, left bundle branch block, right bundle branch block
1. INTRODUCTION
Intraventricular conduction disturbances may occur at different levels: 1. In the branching portion of the His bundle, 2. in the bundle branches; 3. in the main divisions of the bundle branches (fascicular or segmental blocks); 4. in the subdivisions of the main fascicles (arborization blocks) and 5. in the Purkinje network or in the ventricular wall (focal blocks).
It was unfortunate that in Eppinger and Stoerk´s [1] experimental studies on the canine heart, what they called “left bundle branch block” (LBBB), was in fact a block in the right bundle branch (RBBB) in humans. This erroneous nomenclature for the human bundle branch block, which was accepted for many years, was put forward when Barker et al., [2] obtained direct electrocardiographic evidence of a right or left ventricular delay by premature stimulation of one or the other ventricle. The experimental and clinical diagnostic advancement made by Barker [2] and Wilson [3] remains valid to this day.
2. The bundle branches and its fascicles
The right bundle branch (RBB) arises as a compact cord at the end of the branching portion of the His bundle (pseudo bifurcation) and as a long, thin and unique fascicle reaches the base of the right papillary muscle. At this level, it usually gives off two or three terminal ramifications to the lower septum and the free wall of the right ventricle, which are not well defined or constant. Because of a dense meshwork of the Purkinje tissue around the papillary muscle, these subdivisions cannot remain physiologically independent. Consequently, clearly recognizable arborization blocks are unlikely in the right ventricle [4-6]. Conversely, the left bundle branch (LBB) forks shortly after its origin into well-defined fascicles or divisions, which are directed towards the base of the anterior and posterior papillary muscles of the left ventricle. The anterior division travels forward to the anterior papillary muscle crossing the outflow of the left ventricle on the endocardial surface or as false tendons. The posterior division of the LBB, almost always wider and shorter than the anterior one, is distributed to the intermediate and posterior septal areas and on the base of the posterior papillary muscle (Fig. 1A and B). Adjacent to the papillary muscles, the divisions of the LBB spread out in their subsidiary Purkinje networks and false tendons covering the endocardial surface of the interventricular septum and the free wall. Thus, the two divisions of the LBB are amply interconnected by the Purkinje fibers [4-9] (Fig. 1A and B).
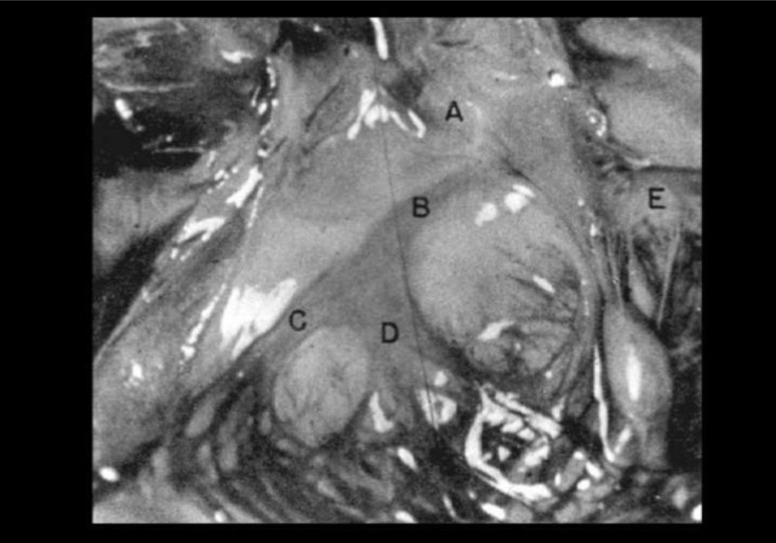
Fig. (1A).
A: Staining of the left bundle branch [LBB], its divisions and the Purkinje network of a child who died at 2 days of age as a consequence of respiratory distress. The LBB system shows the typical configuration. The posterior division (D) is twice as wide as the anterior one (C), which, before reaching the posterior papillary muscle, gives off a distinct strand that covers the mid and posterior third of the lower septum. Both divisions are amply interconnected by the Purkinje network. (A): aorta; (B): LBB; (E): mitral valve. From Spach MS et al. [8]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
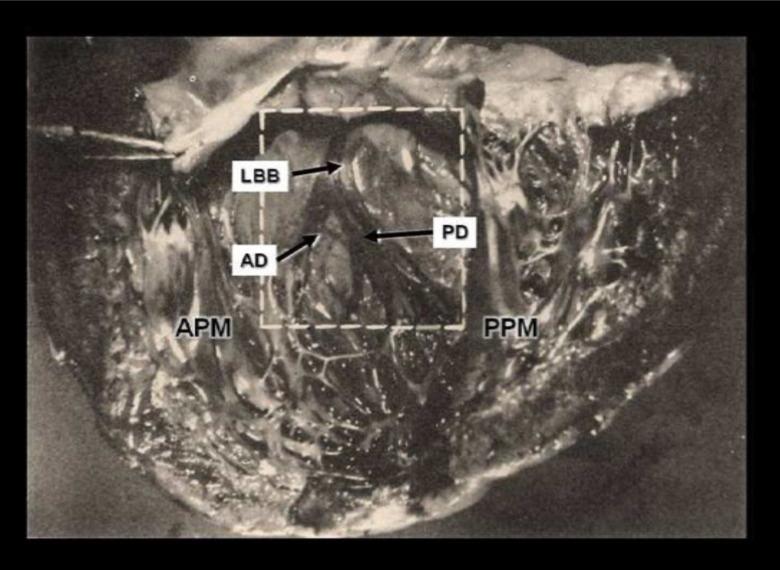
Fig. (1B).
B: Demonstration of the left bundle branch (LBB), stained with iodine solution, of a normal young man’s heart. LBB emerges beneath the aortic valve and descends along the septum. After the short run, it gives origin to the anterior (AD) and posterior (PD) divisions, which are clearly seen heading for their respective papillary muscles. APM: anterior papillary muscle; PPM: posterior papillary muscle. The size and distribution of the divisions of the LBB are strikingly similar to those shown in Figure 1A. From Uhley HN and Rivkin LM [9]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The main divisions of the LBB are clearly independent of each other from the anatomical and physiological point of view and because of this, left fascicular blocks produce definite electrocardiographic entities that were coined as Hemiblocks [4-6]. In the block of the anterior division (left anterior hemiblock - LAH), the area activated with delay comprises the anterosuperior half of the left ventricle and in the block of the posterior division (left posterior hemiblock – LPH), the delay occurs in the posteroinferior half of the left ventricle. In both, the conduction block produces typical electrocardiographic changes. It has been proposed that block in the so-called mid septal fibers arising from the posterior division may also produce a recognizable electrocardiographic pattern [10, 11].
The QRS widening in bundle branch block (BBB) depends, primarily, on the delay with which the ventricular septum is slowly activated from the contralateral ventricle. Conversely, fascicular blocks widen the QRS interval in a much lesser degree since the blocked area is activated via the homolateral Purkinje fibers or false tendons with minimal delay. Thus, despite the remarkable changes in the QRS complex, its duration does not increase greatly [4-6].
3. The Hemiblocks
In the 50’s, Rosenbaum was consulted by a patient with coronary artery disease. The ECG showed an anterior myocardial infarction, RBBB and an AQRS at -75°. A few weeks later, the ECG changed to RBBB with an AQRS at + 110° and in other tracings, both types of QRS complex were seen to alternate with each other (Fig. 2). The presence of permanent RBBB facilitated the interpretation. During both types of QRS, the impulse obligatorily reached the left ventricle first and hence, it must follow intermittently two different pathways within the left ventricle. These pathways were the anterior and posterior divisions. When the impulse followed the posterior division (because the anterior one was blocked), the AQRS direction shifted superiorly to the left to -75°; when the other one was taken (because the posterior division was blocked), the AQRS shifted inferiorly to the right to + 110°. Five similar cases were seen afterward, labeled as RBBB with intermittent LAH and LPH, providing clear-cut evidence of the trifascicular nature of the intraventricular conducting system (ICS) and also, took for granted the discovery of the hemiblocks. Similar records in many patients indicated that LAH alone or associated with RBBB was rather common in coronary artery disease, cardiomyopathies and in Lenègre’s and Lev’s diseases [4-6, 12-14]. From then onwards, the electrocardiographic features of isolated and uncomplicated LAH and LPH were readily recognized. Cases of intermittent LAH or LPH or the analysis of aberrant ventricular conduction in supraventricular premature beats (physiological blocks) greatly contributed to uncover every electrocardiographic change produced by complete or incomplete hemiblocks, either in normal or abnormal conditions [4-7, 15]. These clinical observations were followed by a careful study of the conducting system of 190 mammalian hearts (60 of humans, 67 of canines, 2 of monkeys and different ungulates), which disclosed that the LBB has two main divisions.
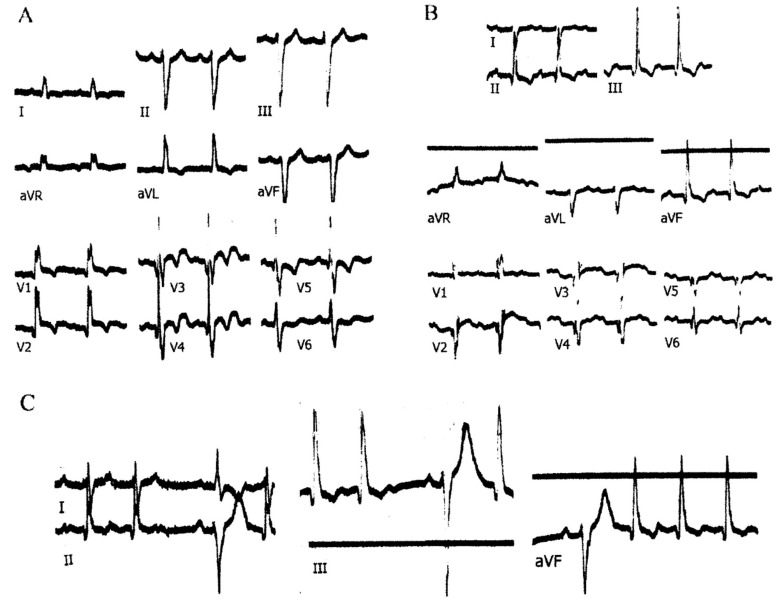
Fig. (2).
Trifascicular block recorded from a 58-year-old man with severe coronary artery disease and an anteroseptal myocardial infarction. A: RBBB with LAH: AQRS -75°; QRS interval 0.13 sec; PR interval 0.16 sec. B: RBBB with LPH; AQRS + 110°; QRS interval 0.13 sec; PR interval 0.26 sec. C: both types of AQRS occur in the same tracing. The PR interval is longer when RBBB with LPH is present. See text for further description.
Our next step was to produce the hemiblocks experimentally in the canine and monkey hearts that included “divisional” LBB and “trifascicular” heart block. Moreover, histopathologic studies of the ICS correlate the electrocardiographic and pathologic findings [5, 12-14]. In addition to these series of anatomical, physiological, clinical and experimental observations supporting the existence of hemiblocks, this concept could be applied to interpret other pathophysiologic and electrocardiographic problems such as: the mechanism of bidirectional tachycardia [4, 6]; the site of origin of ventricular ectopic beats [4, 6]; the mechanism of the narrow ventricular extrasystoles [4, 6, 16]; a new form of LBBB, which was named divisional LBBB; physiologic hemiblocks and bundle branch blocks during aberrant intraventricular conduction of premature supraventricular beats [4-6, 15]; the concomitant occurrence of myocardial infarction or other forms of myocardial necrosis with hemiblocks and the demonstration that they can either conceal or simulate the features of myocardial infarction or myocardial ischemia [4-6, 17-20].
The most reliable and accurate evidence of the effects on the QRS complex produced by the hemiblocks is: 1. the experimental approach [4-6]; 2. the clinical cases in which the conduction disturbance is intermittent or transitory in the divisions of the LBB, and 3. aberrant ventricular conduction of supraventricular premature beats [4-6, 15]. The association of hemiblock with RBBB provides clear evidence of the existence of RBBB with LAH and RBB with LPH, which in turn proves that the LBB operates as bifascicular and the whole intraventricular conduction system, as trisfascicular [4-7].
Fig. (3) illustrates an LAH produced experimentally in a monkey heart (A and B) and the ECG and VCG of a “pure” LAH in the human (C and D). The main changes occur in the limb leads: 1. the first 0.02-sec QRS vector depicts an inferior and rightward direction on the frontal plane (≈ + 120°) responsible for a small Q wave in leads I and aVL and sharp small R waves in II, III and aVF. 2. The main QRS forces are directed superiorly to the left, causing deep S waves in leads II, III and aVF shifting the AQRS to - 60°. The depth of the S waves and the left axis deviation mainly depends on the degree of LAH [5, 15]. These changes are responsible for the presence of a Q1S3 pattern that denotes the counterclockwise rotation along the longitudinal axis. 3. The QRS widening is no greater than 0.02 sec in “pure” LAH (Fig. 3 C). The presence of left myocardial damage may raise the QRS duration. 4. Uncomplicated LAH produces a typical vectorcardiographic frontal loop with small initial forces directed inferiorly to the right and main forces oriented superiorly to the left with a wide open counterclockwise-rotated loop (Fig. 3D); 5. LAH may conceal signs of inferior myocardial ischemia and infarction and may mimic left ventricular hypertrophy (LVH) or lateral infarct and it may conceal signs of LVH or lateral ischemia in the left precordial leads [4-6, 17-20]. The chest leads disclose distinct features in LAH depending on the position of the recording electrodes to the point that they may mimic a septal necrosis or conceal LVH [4-6]. Of note, there are electrocardiographic varieties of LAH depending on noncardiac or cardiac-associated conditions (pregnancy, obesity, emphysema, vertical heart or severe LVH) [4-6, 20].
Fig. (3).
Experimental LAH in a monkey heart. Left panel: A: Control tracing. B: LAH provoked after a gentle injury of the anterior division. The main QRS forces were oriented superiorly to the left and a Q1S3 develops. Right panel: C: The ECG of intermittent LAH of a patient with Steinert disease. In every lead, the first beat shows normal conduction and the second, LAH. Right bottom panel: D: The vectorcardiogram shows normal conduction (top) and LAH (bottom). See the text for further details.
4. Differential diagnosis of LAH
Other causes of left axis deviation, excluding LBB and LAH are LVH, changes of the anatomical position of the heart and extensive inferior myocardial infarction. Electrocardiograms from normal subjects with horizontal hearts, pregnant women and cases of LVH revealed that it is most unusual for these factors to shift the AQRS direction beyond -30°. Thus, it is difficult to draw a reliable line between left axis deviation due to LAH and other causes of left axis deviation and consequently, some overlapping is unavoidable. An axis of -30° may be related to the above mentioned causes or an incomplete degree of LAH [4-6, 15]. Accordingly, an AQRS of -45° can separate the greatest number of cases of LAH from those of left axes unrelated to it. Thus, when referring to LAH in a single electrocardiographic tracing, we must have a QRS direction beyond -45° (with the exception of a broad inferior myocardial infarction). The upper limit for “pure” LAH was found to be around -80° [4-6].
5. Incomplete LAH
As in RBBB and LBBB, incomplete degrees of LAH can be recognized readily during aberrant ventricular conduction of premature supraventricular beats that overlap normal variants of the AQRS. An AQRS at 0° or -20° may correspond to an incomplete degree of LAH, but this is impossible to ascertain in a single electrocardiographic tracing. There are clinical examples of progressive degrees of LAH in which the AQRS shifts through + 50°, + 40°, + 30° covering the whole range to -60°.
6. Incidence and clinical causes of LAH
In 1,658 ECGs from a cardiological service, there were 76 cases of LAH (4.58%), many of which were associated with RBBB; with 53 cases of RBBB (3.19%) and 17 cases of LBBB (1.02%). If we put together LBBB and LAH, it is evident that conduction disturbances are more frequent in the LBB system, which is consistent with the fact that left heart disease is far more common than the right one [4-6].
In a presumably healthy population of 8,915 individuals engaged in civilian flying activities in Argentina, followed from 3 to 42 years (mean 15.3 ± 10.2), 247 cases (22.02%) of LAH were found [21]. To be noted, 95 out of the 247 cases showed LAH in individuals aged from 17 to 29 years old and the highest percentage of cases (54.7%) were in age group 40-59 years. Associated pathologies were found in 43 cases, while heart disease could not be demonstrated in the 204 remaining cases. The striking number (95 cases) of LAH in subjects aged under 29 years was attributed, at least in some of them, to spontaneous closure of membranous septal defects [22], a sequel of myocarditis or a congenital anomaly of the conduction system. In a similar study, in 1,450 healthy pilots from the Israel Aeromedical Center, 15 male pilots were found to have an AQRS of -45° to -60° [23]. The mean age was 21 ± 4 years and none of them had cardiac abnormalities.
In 128 cases of LAH, in patients assisted in service of cardiology, hypertension was observed in 39 cases (30.4%); coronary artery disease in 23 (17.9%); hypertension plus coronary artery disease in 30 (23.4%); Chagasic myocarditis in 12 (9.3%); aortic valvular disease in 8 (7.8%) and of unknown cause in 2 [4-6]. In asymptomatic middle-aged or older, Lenègre’s and Lev’s diseases are suspected, respectively [4-6, 12, 13]. Other causes are dilated cardiomyopathy [4-6] and Chagasic cardiomyopathy [24]. In tricuspid atresia, 70% of the cases are accompanied by LAH due to left ventricular dilation [4-6]. In endocardial cushion defects, LAH is a common finding [25]. Other causes are the anomalous origin of the left coronary artery from the pulmonary trunk [26]; the Noonan syndrome, a pure pulmonic stenosis plus an AQRS between -150° and -120° due to the coexistence of right ventricular hypertrophy [27]; ventricular septal defects [28], single ventricle [29, 30] and aneurysm of the membranous septum [31, 32]. In tricuspid valve replacement, LAH by itself or LAH with RBBB was produced by surgical lesions in the branching portion of the bundle of His [33]. Likewise, in surgical repair of tetralogy of Fallot, RBBB with LAH has been shown as a frequent complication [34, 35].
7. Electrocardiographic features of LPH
The pattern of LPH was characterized by the analysis of cases of RBBB with intermittent LAH and LPH, LPH during aberrant ventricular conduction of supraventricular premature beats, and experimental observations in canine and monkey hearts [4-6]. The following criteria for the diagnosis of LPH are: 1. An AQRS direction of about +120° with an S1 Q3 pattern and normal QRS duration in the absence of right ventricular hypertrophy or a vertical heart, commonly in the presence of severe left ventricular disease. Therefore, LPH must necessarily be a clinical-electrocardiographic diagnosis. A large anterolateral infarction causing the right axis deviation must also be ruled out [4-6, 20]. The electrocardiographic pattern of LPH is the exact mirror image of LAH in the standard leads (Fig. 4). The first forces of LPH (0.02 sec) are oriented superiorly to the left about -50° to -60° producing small R waves in leads I and aVL and small clean Q waves in leads II, III and aVF. This vector is ascribed to the early activation of the anterolateral wall of the left ventricle. The main terminal forces of the QRS complex are oriented inferiorly to the right at about + 120° producing S waves in leads I and aVL and tall R waves in leads II, III and aVF, which correspond to delayed activation of the posteroinferior wall of the left ventricle. The vectorcardiogram is also the mirror image of LAH, disclosing initial forces oriented superiorly to the left, followed by a large wide open clockwise rotated loop, whose main forces are directed inferiorly to the right. Incomplete degrees of LPH have an AQRS direction of less than +120° (+90°, +80° or +70°) [4-6, 15].
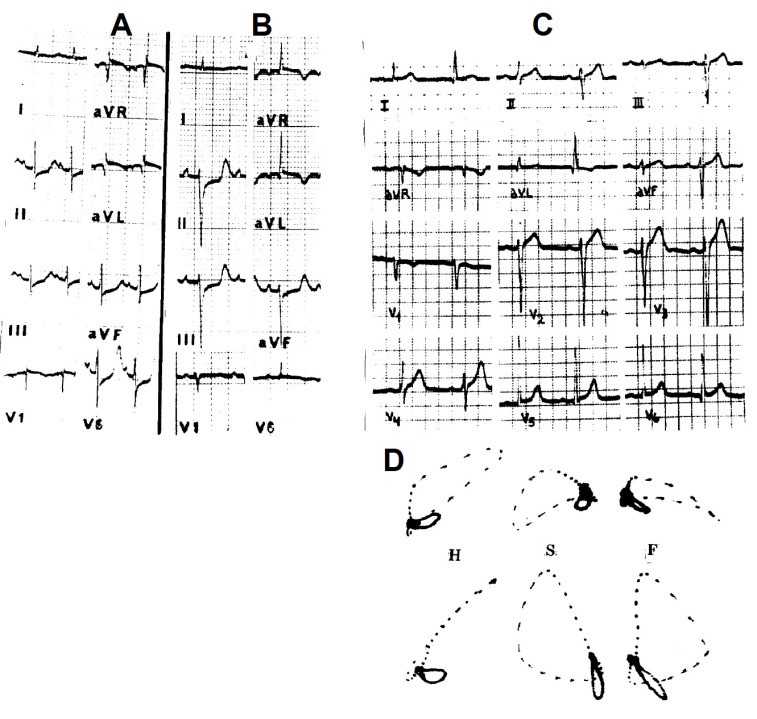
Fig. (4).
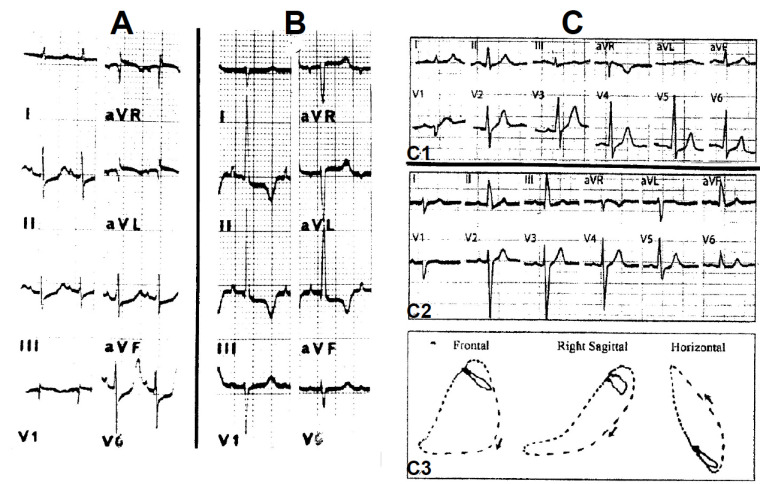
Left panel: Experimental LPH in a monkey heart (same animal as in Figure 3). A: Control tracing obtained after recovery of the previous injury. B: LPH caused by injuring the posterior division of the LBB. The main QRS forces were oriented inferiorly and to the right disclosing an S1Q3 pattern; C: C1: a 61-year-old man without apparent heart disease. The AQRS is ≈ +50° and the ECG tracing is normal. C2: a year later, the AQRS shifted to + 110° and except for the change of the electrical axis, the ECG may be considered normal. C3: The vectorcardiogram depicts the typical wide open clockwise-rotated loop in the frontal plane, whose initial forces are oriented superiorly and to the left and the terminal QRS forces inferiorly and to the right (≈ + 100°). The ECG and vectorcardiogram of LPH are the exact mirror picture of LAH in the standard and unipolar leads.
Since the posterior division of the LBB is the less vulnerable segment of the whole intraventricular conduction system, LPH either isolated or associated with RBBB is much less common than LAH. Therefore, when the lesions are so extensive to alter or interrupt conduction in the posterior division, it is almost axiomatic that the RBB or the anterior division of the LBB or both are also involved. This accounts for the relatively high incidence of AV block in LPH with RBBB, which reflects additional involvement of the anterior division of the LBB. Strong evidence supporting this is that in 9 out of 30 cases of RBBB with LPH, LAH was also documented during follow-up. Thus, LPH may be hidden in many cases of divisional LBBB or complete AV block [4-6].
The main recognizable causes of LPH, either isolated or in association with RBBB, are coronary artery disease and cardiomyopathies [4-6, 24]. In our original material, 9 out of 29 cases (31%), aged between 60 and 68 years, did not disclose any demonstrable etiology and hence, it was assumed that Lenègre’s disease [12] was the most likely possibility. The posterior division has a double blood supply, from both the anterior descending coronary artery and the posterior one. This, together with its thickness and the fact that it is less surrounded by potentially dangerous relationships, explains why this fascicle is the least vulnerable segment of the whole ICS.
8. Electrocardiographic diagnosis and clinical significance of RBBB with LAH or LPH
Whenever an RBBB shows an AQRS oriented superiorly between -60° and -120° with a Q1S3 pattern, the additional presence of LAH should be considered (Fig. 2). The diagnosis of LAH is supported when the first half of the QRS main forces correspond to an LAH. RBBB with LAH was observed in 15 cases of a group of 1,658 patients (0.9%). Within the same group, 53 cases showed RBBB and 76 isolated LAH [4-6]. Altogether, the most common clinical causes of RBB with LAH were coronary artery disease (10%); hypertension (25%); both, coronary disease and hypertension (17.8%), Chagasic myocarditis (27.8%) and Lenegre’s and Lev’s diseases (10.7%). In congenital heart disease, RBBB with LAH is commonly associated with endocardial cushion defects and less commonly with other congenital conditions.
Fig. 2 discloses the main electrocardiographic findings of RBBB with LPH. The AQRS direction is usually at + 120° ranging between +100° and +135°. The initial 0.08 to 0.09 sec of the QRS correspond to the LPH while the terminal forces, oriented approximately between +150° and + 180°, are caused by the RBBB. The presence of RBBB may significantly increase the voltage of R3 [4-6]. Like in LPH alone, right ventricular hypertrophy or vertical heart must be ruled out. As already stated, the main causes of LPH associated with RBBB are coronary artery disease and cardiomyopathies [4-6].
A peculiar form of RBBB with LAH is the so-called “masquerading BBB” usually observed in patients with severe LVH and myocardial damage with focal anterolateral block [4-6, 36-38]. The ECG depicts signs of RBBB in the chest leads and LBBB in the limb leads: S1 and Q1 are very small or absent. Deep S waves in II, III and aVF are present. This pattern implies delayed QRS forces oriented to the left that cancel the late forces of RBBB.
9. A reappraisal of the trifascicular nature of the intraventricular conduction system
The results of anatomical, experimental and clinical investigations performed by the Argentine School of Electrocardiology on intraventricular blocks achieved a marked impact on Latin American cardiology that extended to the international scientific community after the publication of the English and Italian versions of “The Hemiblocks” [4-6]. More than 30 articles followed the publication of these books, making this breakthrough be known worldwide. Of note, between 1970 and 1983, these books and papers were cited in more than 3,000 publications in the international scientific literature (Citation Index).
This international recognition to the concept of hemiblocks and trifascicular blocks was given by the cardiologic community and from that moment, it became firmly established as a historical milestone [39]. The quick and easy terms LAH and LPH [38] convey, with these meliflous words, as Hecht stated [40], the clear concept of block of the anterior and posterior divisions of the LBB, respectively. This terminology has been widely used in clinical and pedagogical context for almost 50 years and represents one of the major accomplishments in clinical electrocardiography [40]. The two divisions of the LBB, together with the RBB form a trifascicular conduction system. If we consider block, either complete or intermittent, occurring in each one of these fascicles in different combinations, there are twelve possibilities of bifascicular blocks and eight of trifascicular ones [4-6].
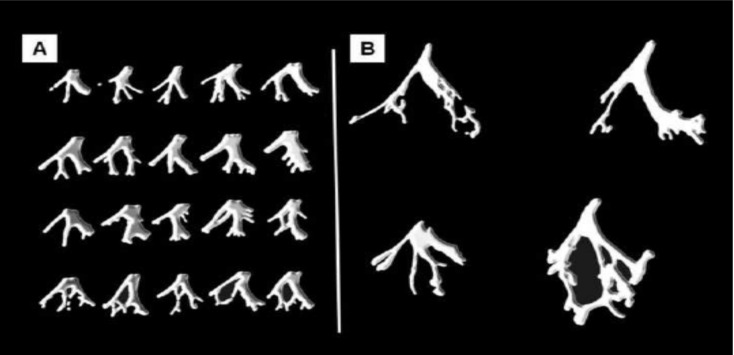
The anatomical study of human hearts by Demoulin and Kulbertus [41, 42] established the presence of a third fascicle that traveled to the mid septal region in about half of the human subjects. This study consisted of histological serial sections of the septum parallel to the atrioventricular ring (Fig. 6). These cuts were shown by a picture drawn to represent “as closely as possible” the location and width of the various left bundle ramifications. Forty-nine hearts were studied following this technique and their conclusions were: “the results obtained confirm the consistent existence of a thin, elongated anterior radiation and a wider posterior one” [42]. However, they emphasized the presence of conspicuous mid-septal fibers. These anatomical findings obtained correlated with the electrophysiological results in that three endocardial areas of the left ventricle were synchronously excited at the beginning of left ventricle excitation [43, 44]. This electrophysiological behaviour is also compatible with our description of the ICS [4-6].
Fig. (6).
A: Diafragmatic sketches of the LBB system in twenty human hearts. The drawings represent “as closely as possible the location and width of the various left bundle ramifications” obtained by serial sections of the septum in the plane parallel to the atrioventricular ring. B: Four human LBB systems dissected and separated from the heart, which represent the main prototypes of our material. In every case, anterior and posterior divisions are observed. The bottom left LBB shows a medial septal fascicle emerging from a wide posterior division, a pattern that can also be observed in the examples of 6.A.
The existence of left mid septal fibers cannot be disregarded and the fact that the presence of conduction troubles at this level cannot be denied, either as an arborization block. However, despite the strongest defenders of the mid septal fascicular block (MSFB), no definite and reliable evidence for its electrocardiographic pattern is unanimously accepted [45]. There are also the contradictory electrocardiographic descriptions of MSFB and the fact that most of the criteria are “hypothetical or based on parameters that can be found under many circumstances” [46]. Prominent anterior forces in the mid-to-late QRS in right precordial leads have been proposed as major criteria for MSFB. However, as pointed out by Bayes de Luna et al., [45], the obstacle that this may present is how to separate degrees of incomplete RBBB from MSFB. An example of this problem is illustrated in Fig. (5), where prominent R waves are observed during incomplete degrees of RBBB. Beyond the precarious and controversial definition of the electrocardiographic changes of MSFB, the experimental production is also full of discrepancies since the results of the provoked ECG patterns were not uniform [45]. Due to all these uncertainties, the guidelines on ventricular conduction from the AHA/ACC/HRS literally state: “the term left septal fascicular block is not recommended because of the lack of universally accepted criteria” [47]. This also explains why articles related to MSFB were titled as questions like: myth or reality? [48], in search of the septal fascicular block [10], quadrifascicular? [49], left median hemiblock - a chimera? [50], meaning that the ECG findings or criteria are not well-defined type of block.
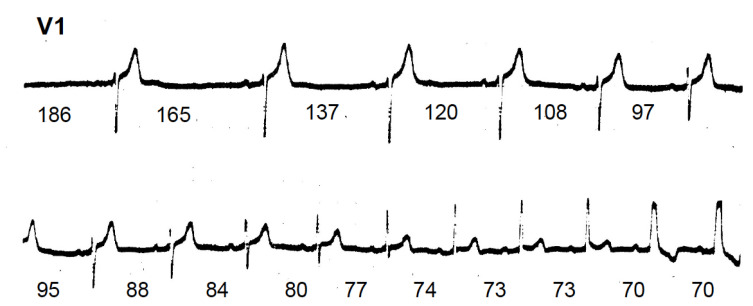
Fig. (5).
Continuous strips of lead V1 obtained during carotid sinus massage from a patient with intermittent RBBB. The numbers indicate the RR intervals in hundreds of a second. After the longer pauses, RBBB entirely disappears and when the intervals get progressively shorter, high degrees of RBBB occur. It is to be noted that before reaching the pattern of complete RBBB, there are four QRS complexes showing prominent anteriorly directed forces with a narrow complex, which could be interpreted as a mid septal fascicular block.
Finally, despite the different methods used for the studies of the ICS performed by well-known pathologists and researchers, it is unquestionable that what is the mode of branching of the LBB, which varies from species to species as well as within the same species, but it always exhibits two main divisions, anterior and posterior heading for the anterior and posterior papillary muscles, respectively [51-66].
CONCLUSION
In conclusion, it is worth remarking that the most reliable method to visualize the system is by its staining with an iodine solution. Only two normal hearts were successfully studied and reported in the medical literature [8, 9] (Fig. 1) and both unquestionably show that the LBB has two main divisions, which are amply interconnected at the level of the papillary muscles. Moreover, it is also clearly observed that the main septal fibers are provided by the posterior division. Unfortunately, it is almost impossible to obtain human hearts rapidly enough [no more than 60 minutes after death] to stain the high-glycogen content of the conducting system in order to clear away the anatomy of the LBB. This method to study the ICS is so accurate that it should bring all inconsistencies and confounding newly formulated concepts to an end.
Acknowledgements
Declared none.
LIST OF ACRONYMS
- BBB
Bundle Branch Block
- ICS
Intraventricular Conduction System
- LAH
Left Anterior Hemiblock
- LBB
Left Bundle Branch
- LBBB
Left Bundle Branch Block
- LPH
Left Posterior Hemiblock
- MSFB
Mid Septal Fascicular Block
- RBB
Right Bundle Branch
- RBBB
Right Bundle Branch Block
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Eppinger H., Stoerk O. Zur Klinik des Electrokardiogramms. Klin. Med. (Mosk.) 1910;71:157–164. [Google Scholar]
- 2.Barker P.S., Mcleod A.G., Alexander J. The excitatory process observed in the exposed human heart. Am. Heart J. 1930;5:720–742. doi: 10.1016/S0002-8703(30)90088-9. [DOI] [Google Scholar]
- 3.Wilson F.N., Mcleod A.G., Barker P.S. The interpretation of the initial deflections of the ventricular complex of the electrocardiogram. Am. Heart J. 1931;6:637–664. doi: 10.1016/S0002-8703(31)90439-0. [DOI] [Google Scholar]
- 4.Rosenbaum M.B., Elizari M.V., Lázzari J.O. Los hemibloqueos. Buenos Aires: Editorial Paidós; 1968. [Google Scholar]
- 5.Rosenbaum MB, Elizari MV, Lázzari JO. The hemiblocks New concepts of intraventricular conduction based on human anatomical, physiological and clinical studies. 1970.
- 6.Rosenbaum M.B., Elizari M.V., Lázzari J.O. Gli Emiblocchi. Padova, Italia: Piccin Editore; 1976. [Google Scholar]
- 7.Elizari M.V. The normal variants in the left bundle branch system. J. Electrocardiol. 2017;50(4):389–399. doi: 10.1016/j.jelectrocard.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Spach M.S., Huang S., Armstrong S.I., Canent R.V., Jr Demonstration of the peripheral conduction system in human hearts. Circulation. 1963;28:333–338. doi: 10.1161/01.CIR.28.3.333. [DOI] [PubMed] [Google Scholar]
- 9.Uhley H.N., Rivkin L.M. Visualization of the left branch of the human atrioventricular bundle. Circulation. 1959;20:419–421. doi: 10.1161/01.CIR.20.3.419. [DOI] [PubMed] [Google Scholar]
- 10.MacAlpin R.N. In search of left septal fascicular block. Am. Heart J. 2002;144(6):948–956. doi: 10.1067/mhj.2002.125503. [DOI] [PubMed] [Google Scholar]
- 11.Pérez Riera A.R., Ferreira C., Ferreira Filho C., et al. Electrovectorcardiographic diagnosis of left septal fascicular block: anatomic and clinical considerations. Ann. Noninvasive Electrocardiol. 2011;16(2):196–207. doi: 10.1111/j.1542-474X.2011.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenègre J. Contribution à l’Étude des Blocs de Branche. Paris: JB Baillière et Fils; 1958. [Google Scholar]
- 13.Lev M. Anatomic basis for atrioventricular block. Am. J. Med. 1964;37:742–748. doi: 10.1016/0002-9343(64)90022-1. [DOI] [PubMed] [Google Scholar]
- 14.Rossi L. Histopathologic features of cardiac arrhythmias. Milano: Casa Editrice Ambrosiana; 1969. [Google Scholar]
- 15.Elizari M.V., Chiale P.A. The electrocardiographic features of complete and partial left anterior and left posterior hemiblock. J. Electrocardiol. 2012;45(5):528–535. doi: 10.1016/j.jelectrocard.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum M.B., Halpern M.S., Nau G.J., Elizari M.V., Lázari J.O. The mechanism of narrow ventricular ectopic beats.. In: Flensten-Jensen Sandøe E, editor. Symposium of Cardiac Arrhythmias. [Google Scholar]
- 17.Benchimol A., editor. The Williams & Wilkins Co. Baltimore, Md., USA: 1973. [Google Scholar]
- 18.Castellanos A., Jr, Myerburg R.J. The Hemiblocks in Myocardial Infarction Appleton Century-Crofts. New York, USA: 1976. [Google Scholar]
- 19.Lévy S. Bundle branch blocks and/or hemiblocks complicating acute myocardial ischemia or infarction. J. Interv. Card. Electrophysiol. 2018;52(3):287–292. doi: 10.1007/s10840-018-0430-3. [DOI] [PubMed] [Google Scholar]
- 20.Elizari M.V., Acunzo R.S., Ferreiro M. Hemiblocks revisited. Circulation. 2007;115(9):1154–1163. doi: 10.1161/CIRCULATIONAHA.106.637389. [DOI] [PubMed] [Google Scholar]
- 21.Canaveris G., Halpern M.S., Elizari M.V. Intraventricular conduction disturbances in civilian flying personnel: left anterior hemiblock. Aviat. Space Environ. Med. 1992;63(4):292–298. [PubMed] [Google Scholar]
- 22.Elizari M.V., Ansaldi M.S., Chiale P.A., et al. Cierre espontáneo de la comunicación interventricular. Una causa ignorada de trastornos de la conducción aurículoventricular e intraventricular. Rev. Argent. Cardiol. 1996;64:165–174. [Google Scholar]
- 23.Krivisky M., Aberbouch L., Shochat I., Ribak J., Tamir A., Froom P. Left anterior hemiblock in otherwise healthy pilots. Aviat. Space Environ. Med. 1988;59(7):651–652. [PubMed] [Google Scholar]
- 24.Rosenbaum M.B. Chagasic myocardiopathy. Prog. Cardiovasc. Dis. 1964;7:199–225. doi: 10.1016/S0033-0620(64)80020-7. [DOI] [PubMed] [Google Scholar]
- 25.Durrer D., Roos J.P., van Dam R.T. The genesis of the electrocardiogram of patients with ostium primum defects (ventral atrial septal defects). Am. Heart J. 1966;71(5):642–650. doi: 10.1016/0002-8703(66)90314-0. [DOI] [PubMed] [Google Scholar]
- 26.Wesselhoeft H., Fawcett J.S., Johnson A.L. Anomalous origin of the left coronary artery from the pulmonary trunk. Its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation. 1968;38(2):403–425. doi: 10.1161/01.CIR.38.2.403. [DOI] [PubMed] [Google Scholar]
- 27.Noonan J.A. Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am. J. Dis. Child. 1968;116(4):373–380. doi: 10.1001/archpedi.1968.02100020377005. [DOI] [PubMed] [Google Scholar]
- 28.Lev M. Conduction system in congenital heart disease. Am. J. Cardiol. 1968;21(5):619–627. doi: 10.1016/0002-9149(68)90259-2. [DOI] [PubMed] [Google Scholar]
- 29.Brink A.J., Neill C.A. The electrocardiogram in congenital heart disease; with special reference to left axis deviation. Circulation. 1955;12(4):604–611. doi: 10.1161/01.CIR.12.4.604. [DOI] [PubMed] [Google Scholar]
- 30.Elliott L.P., Ruttenberg H.D., Eliot R.S., Anderson R.C. Vectorial analysis of the electrocardiogram in common ventricle. Br. Heart J. 1964;26(3):302–311. doi: 10.1136/hrt.26.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heggtveit H.A. Congenital aneurysm of the membranous septum associated with bundle branch block. Am. J. Cardiol. 1964;14:112–117. doi: 10.1016/0002-9149(64)90115-8. [DOI] [PubMed] [Google Scholar]
- 32.Yang S.S., Maranhao V., Ablaza S.G., Morse D.P., Goldberg H. Aneurysm of the membranous portion of the ventricular septum. Am. J. Cardiol. 1969;23(1):83–88. doi: 10.1016/0002-9149(69)90245-8. [DOI] [PubMed] [Google Scholar]
- 33.Aravindakshan V., Elizari M.V., Rosenbaum M.B. Right bundle-branch block and left anterior fascicular block (left anterior hemiblock) following tricuspid valve replacement. Circulation. 1970;42(5):895–902. doi: 10.1161/01.CIR.42.5.895. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum M.B., Corrado G., Oliveri R., Castellanos A., Jr, Elizari M.V. Right bundle branch block with left anterior hemiblock surgically induced in tetralogy of Fallot. Relation to the mechanism of electrocardiographic changes in endocardial cushion defects. Am. J. Cardiol. 1970;26(1):12–19. doi: 10.1016/0002-9149(70)90752-6. [DOI] [PubMed] [Google Scholar]
- 35.Wolff G.S., Rowland T.W., Ellison R.C. Surgically induced right bundle-branch block with left anterior hemiblock. An ominous sign in postoperative tetralogy of Fallot. Circulation. 1972;46(3):587–594. doi: 10.1161/01.CIR.46.3.587. [DOI] [PubMed] [Google Scholar]
- 36.Richman J.L., Wolff L. Left bundle branch block masquerading as right bundle branch block. Am. Heart J. 1954;47:383–393. doi: 10.1016/0002-8703(54)90295-1. [DOI] [PubMed] [Google Scholar]
- 37.Elizari M.V., Baranchuk A., Chiale P.A. Masquerading bundle branch block: a variety of right bundle branch block with left anterior fascicular block. Expert Rev. Cardiovasc. Ther. 2013;11(1):69–75. doi: 10.1586/erc.12.142. [DOI] [PubMed] [Google Scholar]
- 38.Anderson K.P. Left bundle branch block and the evolving role of QRS morphology in selection of patients for cardiac resynchronization. J. Interv. Card. Electrophysiol. 2018;52(3):353–374. doi: 10.1007/s10840-018-0426-z. [DOI] [PubMed] [Google Scholar]
- 39.Uhley H.N. The concept of trifascicular intraventricular conduction: historical aspects and influence on contemporary cardiology. Am. J. Cardiol. 1979;43(3):643–646. doi: 10.1016/0002-9149(79)90025-0. [DOI] [PubMed] [Google Scholar]
- 40.Hecht H.H. 1970. [Google Scholar]
- 41.Demoulin J.C., Kulbertus H.E. Histopathological examination of concept of left hemiblock. Br. Heart J. 1972;34(8):807–814. doi: 10.1136/hrt.34.8.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulbertus H.E., Demoulin J.C. 1976. Pathologial Basis of Concept of Left Hemiblock. [Google Scholar]
- 43.Durrer D., van Dam R.T., Freud G.E., Janse M.J., Meijler F.L., Arzbaecher R.C. Total excitation of the isolated human heart. Circulation. 1970;41(6):899–912. doi: 10.1161/01.CIR.41.6.899. [DOI] [PubMed] [Google Scholar]
- 44.Myerburg R.J., Nilsson K., Gelband H. Physiology of canine intraventricular conduction and endocardial excitation. Circ. Res. 1972;30(2):217–243. doi: 10.1161/01.RES.30.2.217. [DOI] [PubMed] [Google Scholar]
- 45.Bayés de Luna A., Riera A.P., Baranchuk A., et al. Electrocardiographic manifestation of the middle fibers/septal fascicle block: a consensus report. J. Electrocardiol. 2012;45(5):454–460. doi: 10.1016/j.jelectrocard.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Alboni P. Intraventricular Conduction Disturbances. The Hague: Martinus Nihoff Pub.; 1981. [Google Scholar]
- 47.Surawicz B., Childers R., Deal B.J., et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119(10):e235–e240. doi: 10.1161/CIRCULATIONAHA.108.191095. [DOI] [PubMed] [Google Scholar]
- 48.MacAlpin R.N. Left septal fascicular block: myth or reality? Indian Pacing Electrophysiol. J. 2003;3(3):157–177. [PMC free article] [PubMed] [Google Scholar]
- 49.Quadrifascicular H.I. J. Electrocardiol. 2012;45(5):536–538. doi: 10.1016/j.jelectrocard.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 50.de Pádua F., dos Reis D.D., Lopes V.M., et al. Left median hemiblock-a chimera? Adv. Cardiol. 1978;21:242–248. doi: 10.1159/000400459. [DOI] [PubMed] [Google Scholar]
- 51.Tawara S. Das Reizleitunssystem des Saugetierherzens. Jena: G. Fischer; 1906. [Google Scholar]
- 52.Holl M. Makroskopische Darstellung der Atrioventikularen Verbindungsbundels am menschlichen und tierischen Herzen Denkschr D math Nat Kl D kais Akad d Wiss. Viena; 1911. p. 87. [Google Scholar]
- 53.Lewis T. The Mechanism and Graphic Registration of the Heart Beat. London: Shaw Sons Ltd.; 1925. [Google Scholar]
- 54.Mahaim I. Les Maladies Organiques du Faisceau de HisTawara. Paris: Masson et Cie; 1931. [Google Scholar]
- 55.Lev M., Widran J., Erickson E.E. A method for the histopathologic study of the atrioventricular node, bundle, and branches. AMA Arch. Pathol. 1951;52(1):73–83. [PubMed] [Google Scholar]
- 56.Read J.L., Hegre E.S., Russi S. Reaffirmation of the auriculoventricular conduction system in man; the introduction of a unique technic for its serial motion picture reconstruction. Circulation. 1953;7(1):42–51. doi: 10.1161/01.CIR.7.1.42. [DOI] [PubMed] [Google Scholar]
- 57.Titus J.L., Daugherty G.W., Edwards J.E. Anatomy of the normal human atrioventricular conduction system. Am. J. Anat. 1963;113:407–415. doi: 10.1002/aja.1001130305. [DOI] [PubMed] [Google Scholar]
- 58.Lev M. The normal anatomy of the conduction system in man and its pathology in atrioventricular block. Ann. N. Y. Acad. Sci. 1964;111:817–829. doi: 10.1111/j.1749-6632.1964.tb53149.x. [DOI] [PubMed] [Google Scholar]
- 59.Watt T.B., Jr, Murao S., Pruitt R.D. Left axis deviation induced experimentally in a primate heart. Am. Heart J. 1965;70:381–389. doi: 10.1016/0002-8703(65)90186-9. [DOI] [PubMed] [Google Scholar]
- 60.Lev M. The conduction system. Luisada, AA: Development and structure of the Cardiovascular System. Mc Graw-Hill; 1968. [Google Scholar]
- 61.Roberts J.T. The Conduction System.Development and structure of the Cardiovascular System. Mc Graw-Hill; 1961. [Google Scholar]
- 62.Truex R.C. Comparative anatomy and functional considerations of the cardiac conduction system. Paes de Carvalho A, de Mello C, Hoffman B The specialized tissues of the heart. Amsterdam, London, New York: Elsevier Publ Co; 1961. pp. 22–43. [Google Scholar]
- 63.Truex R.C. Anatomy of the specialized tissues of the heart.; 1973. [Google Scholar]
- 64.Sugiura M., Okada R., Hiraoka K., Okawa S. Histological studies on the conduction system in 14 cases of right bundle branch block associated with left axis deviation. Jpn. Heart J. 1969;10(2):121–132. doi: 10.1536/ihj.10.121. [DOI] [PubMed] [Google Scholar]
- 65.Wilson F.N., Johnston F.D., Hill I.G.W. The form of the electrocardiogram in experimental myocardial infarction. IV. Additional observations on the later effects produced by ligation of the anterior descending branch of the left coronary artery. Am. Heart J. 1935;10:1025–1041. doi: 10.1016/S0002-8703(35)90381-7. [DOI] [Google Scholar]
- 66.Hudson R.E.B. Cardiovascular Pathology. London: Edward Arnold Pub Ltd.; 1965. p. 1. [Google Scholar]