Abstract
The fervency for advancement and evolution in percutaneous coronary intervention has revolutionised the treatment of coronary artery disease. Historically, the focus of the interventional cardiologist was directed at the restoration of luminal patency of the major epicardial coronary arteries, yet whilst this approach is evolving with much greater utilisation of physiological assessment, it often neglects consideration of the role of the coronary microcirculation, which has been shown to clearly influence prognosis. In this review, we explore the narrative of the coronary circulation as more than just a simple conduit for blood but an organ with functional significance. We review organisation and physiology of the coronary circulation, as well as the current methods and techniques used to examine it. We discuss the studies exploring coronary artery endothelial function, appreciating that coronary artery disease occurs on a spectrum of disorder and that percutaneous coronary intervention has a latent effect on the coronary circulation with long-term consequences. It is concluded that greater recognition of the coronary artery endothelium and mechanisms of the coronary circulation should further guide revascularisation strategies.
Keywords: Drug coated balloons, endothelial function, vasoreactivity testing, vasomotion, bioresorbable scaffolds, drug eluting stents, microvascular angina, MINOCA, coronary microcirculation, angina, acute coronary syndrome
1. INTRODUCTION
In 1954, Dr. Rudolf Altshul, an anatomist and histologist at the University of Saskatchewan, Canada, reviewed the role of the endothelium stating “while working on problems of arteriosclerosis, I have realised not only how little I knew about the endothelium, but how much I ought to know for the proper understanding of arteriosclerosis” [1]. Decades later, there is a consistently evolving appreciation that endothelial function and its disorders are critical precursors in the development of atherosclerosis and cardiovascular diseases. Given the evidence that endothelial dysfunction has a direct correlation with poor cardiovascular outcomes [2-4], the focus of this review is on the crucial role of the endothelium within the coronary circulation, the pathophysiology of endothelial dysfunction and its clinical relevance.
2. CORONARY ARTERY DISEASE - A HISTORICAL PERSPECTIVE
The first recorded descriptions of angina in modern history date back to 400 B.C. by Hippocrates, who observed that for some patients, cold wind could precipitate their chest pain [5]. The symptom of chest discomfort was linked to exertion by Phillip Melanchton, a Professor of Greek and theologian who, in the early 16th century, detailed attacks of “chest pains” that had affected his friend Martin Luther [6, 7]. It was not until 1768 however, that the term “angina pectoris” was first used by William Heberden, a renowned physician working in London, who, at the time of presenting his seminal paper “Some account of a Disorder of the Breast”, used the description: “Those who are afflicted with it, are seized while they are walking (more especially if it be uphill, and soon after eating) with a painful and most disagreeable sensation in the breast, which seems as if it would extinguish life if it were to increase or to continue; but the moment they stand still, all this uneasiness vanishes” [6-8].
In this famous account, Heberden proposed no explanation as to possible underlying pathophysiology for the phenomena. However, he later received an anonymous letter from a physician who, having read the paper, had recognised Heberden’s description as matching his own symptomology. The letter went on to request that Heberden perform an autopsy on him when he died in order to further understand the disorder. Edward Jenner (best known for later introducing vaccination in 1798) performed the autopsy with John Hunter in 1772 [9], however, as there was no recognition for a causal link between angina and coronary artery disease at the time, the coronary arteries were not assessed in any detail and so, an opportunity to record the association was missed [7, 8]. It was not until 1786, when after performing further autopsies on patients with similar symptoms, that Jenner wrote in his letter to Heberden that coronary artery disease was explicitly linked to angina [10].
Carl Weigert, a German Pathologist, first proposed that the occlusion of the epicardial coronary artery was the cause for Myocardial Infarction in 1880 [11]. Subsequently, James Herrick’s paper from 1912 “Certain clinical features of sudden obstruction of the coronary arteries” popularised the hypothesis. These led to much greater scrutiny of the coronary artery circulation for strategies for diagnosis and treatments of ischaemic heart disease [8, 10]. The management of ischaemic heart disease changed drastically with the development of selective coronary angiography in 1958 by Sones, Judkins, and Amplatz, as a method for identifying coronary stenosis and laying the foundation for novel treatments [12]. The development of coronary artery bypass grafting in the 1960s and 70s by Sabiston, Kolesov, Favaoloro and Effler and percutaneous coronary angioplasty by Andreas Gruntzig in 1977 revolutionised the management of coronary artery disease [12-15].
However, a significant proportion (40%) of patients with symptoms matching Heberden’s classical description of angina, would subsequently be found to have normal coronary artery appearances on angiography [13, 16, 17], indicating that major epicardial coronary stenoses account for only part of the disease spectrum. Initial studies, involving the close examination of canine hearts in the 1960s, demonstrated the microscopic aspects of the coronary circulation leading to greater recognition of the small vessel circulation [13, 18, 19]. In the 1980s, there was a proliferation of research directed at the microcirculation of the human heart, utilising microscopic video techniques to look at the behaviour and movements of the arterial circulation. In 1985, Cannon and Epstein introduced the term “microvascular angina” proposing that the symptoms of angina arose from the small intramural pre-arteriolar coronary arteries [20, 21]. There was some appreciation at the time that whilst the epicardial circulation was clearly important, there was a significant proportion of patients who were debilitated in the longer-term despite angiographically “normal” coronary artery appearances [22]. Cannon further went on to explore patients with normal epicardial artery appearances via invasive angiography, using a technique that involved the insertion of a pigtail catheter in the patient’s left ventricle and a balloon catheter in the pulmonary artery, then cannulating the great cardiac vein and invasively monitoring brachial artery pressures and calculating the coronary circulatory resistance from the derived values [21, 23, 24]. Cannon was able to demonstrate that these patients with anginal symptoms developed increases in vascular resistance on response to certain stimuli (in his particular study, rapid atrial pacing) compared to those who did not have angina [21, 23, 24]. It was this initial work that helped advance recognition of the function of the coronary circulation as more than a passive channel for blood to travel to the heart muscle and instead a system that elicited physiological responses to various stimuli.
3. THE ENDOTHELIUM AND ITS PHYSIOLOGY
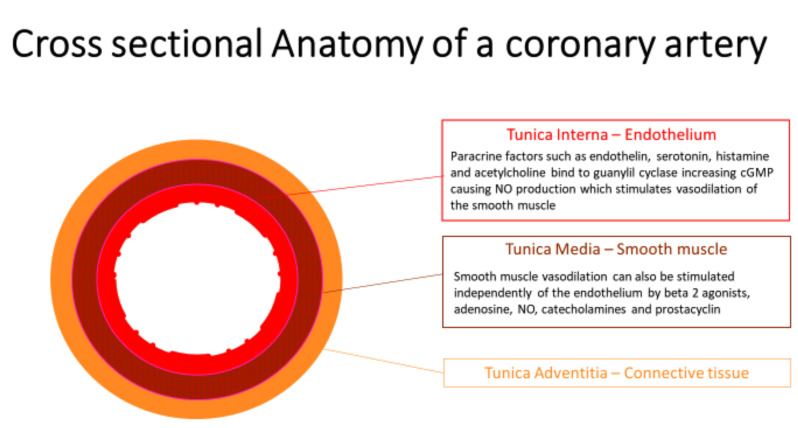
The coronary artery wall is composed of the functional layer called the endothelial lamina, a smooth muscle lamina, and a more architectural, supportive connective tissue lamina (Fig. 1). The endothelium was initially characterised as a simple cellular barrier separating the blood from the interstitial components of the blood vessel, but it has since been demonstrated to be a selectively permeable, metabolically active regulatory interface essential to normal vascular physiology [1, 4, 25, 26].
Fig. (1).
This is an image representation of a cross-section through a coronary artery. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
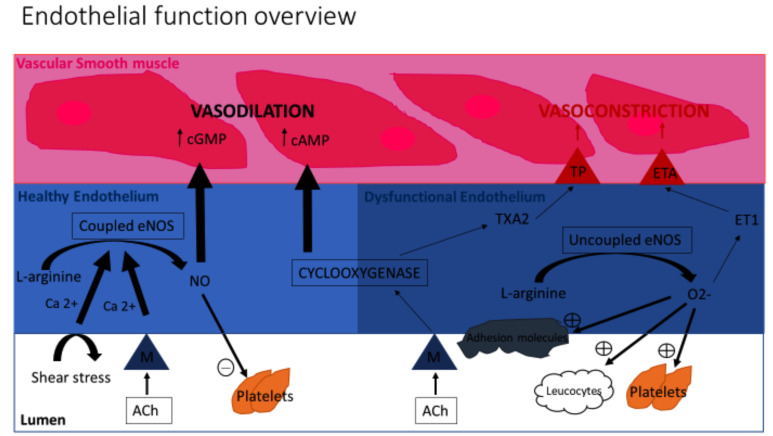
Endothelial cells metabolise L-arginine via an endothelial isoform of nitric oxide synthase to form Nitric Oxide (NO). The NO produced by endothelial cells contributes to maintaining vascular homeostasis by regulating vasomotor tone (normally resulting in vasodilation) and it also serves to inhibit the non-thrombogenic behaviours by acting on platelets and leucocytes (Fig. 2). The NO synthesis can be stimulated by receptor-dependent agonists (acetylcholine and bradykinin) and changes in blood flow. If the endothelium is unhealthy, the endothelial nitric oxide synthase can become uncoupled and actually inactivate NO as well as encouraging oxidative stress.
Fig. (2).
Overview of Endothelial function: An illustration of the processes within the endothelium contributing to Vasodilation and Vasoconstriction. ACH Acetylcholine, NO Nitric Oxide, M Muscarinic receptors, Enos – NO synthase isomer. ET1 Endothelin 1, TXA2 Thromboxane A2, ETA Endothelin A Receptor, TP Thromboxane Prostanoid Receptor. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The endothelium has a number of different functions which include the synthesis and biodegradation of vasoactive substances, buffering of the products of aerobic respiration, transport and metabolism of lipoproteins, secretion and enzymatic remodeling of extracellular matrix components, modulation of the coagulation cascade, elaboration of various growth factors, cytokines and hormone-like substances, as well as the biosynthesis of prostaglandins and other potent autocoids. In addition, endothelial cells perform a reversible adaptive mechanism in response to certain pro-inflammatory cytokines or bacterial endotoxins as part of a response to infection and inflammation [27-30].
4. THE FUNCTIONAL ANATOMY OF THE CORONARY CIRCULATION
In order for the heart muscle to generate the ATP necessary for cardiac pump function, it requires continuous perfusion with oxygenated blood. The healthy, resting coronary blood flow under normal haemodynamic conditions is 0.7 - 1.0mL/min/g, which is maintained by an active and multifactorial homeostatic process [31, 32]. In response to demand under normal circumstances, the coronary circulation can upregulate coronary flow up to five-fold (this potential is called “coronary flow reserve”). The Coronary Flow Reserve (CFR) is a function of the coronary circulation that is measurable as a ratio of hyperaemic flow (usually in response to an agonist like adenosine or dipyridamole) to basal flow [33]. CFR is the net result of the vasodilator capacity of the coronary circulation and can be measured through several methods, including doppler echocardiography and PET [13, 16, 31, 34, 35].
Flow is maintained by a process of “autoregulation”, utilising different stimuli:
Metabolic regulation in response to myocardial oxygen demand (i.e. the demands of the myocardium dictate coronary blood flow).
Shear stress – the endothelium is sensitive to the tractive force exerted by the velocity and viscosity of blood [36, 37].
Neural and bio-humoral regulation via the sympathetic and parasympathetic nervous system (for example: during exercise).
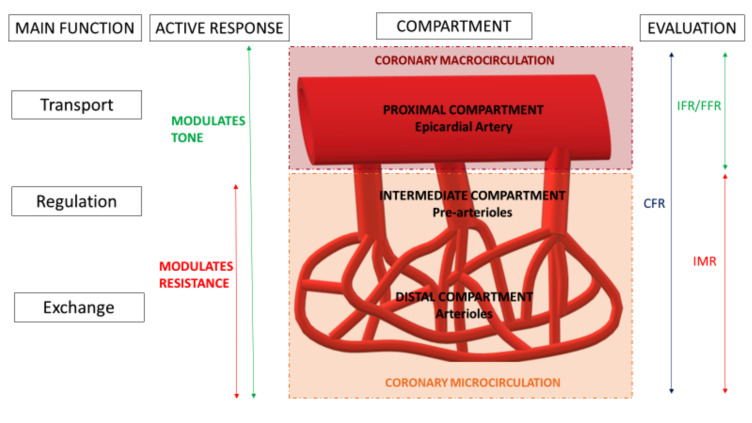
The coronary circulation can be considered as having three major divisions that react to different stimuli in a variety of ways. Their “borders” are transitional and whilst difficult to define histologically or anatomically; the compartments can be characterised based on function; as each compartment is governed by distinct, regulatory mechanisms that alter flow [31, 38]. Conventionally the used nomenclature classifies these divisions as the “proximal”, “intermediate” and “distal compartment”.
The proximal compartment refers to the large, epicardial coronary arteries that are delineated during coronary angiography (with a diameter > 500µm). These epicardial arteries act essentially as capacitance vessels that, in the healthy state, offer no resistance to flow between the aortic and coronary sinus. During systole, the epicardial vessel stretches (with its blood volume increasing up to 25%) generating elastic, potential energy, which is then converted to kinetic energy during diastole. This capacitance response is an active process and modulated by changes in epicardial vessel tone (described as a “vasomotor” response). This vasomotor response is maintained via a balance between a degree of vasoconstriction (of the smooth muscle layer of the vessel) and vasodilatation (directed by the endothelium). This mechanism is, in part, the target of anti-anginal therapy that utilises the bioavailability and action of Nitric Oxide (NO) [39, 40].
The intermediate and distal compartments are referred to, collectively, as the microcirculation, which is the major reservoir for the myocardial blood supply containing 90% of the total myocardial blood volume. The microcirculation behaves in a manner similar to the other vascular beds in the body in that it is these vessels that govern resistance to perfusion [29, 38, 41].
The intermediate compartment is composed of pre-arterioles (diameter 100-500µm), which are extra-myocardial vessels that have a thinner endothelial and smoother muscle layers than the epicardial coronary arteries of the proximal compartment. As mentioned, the compartments are divided by “transitional zones” rather than clearly demarcated borders and such that the proximal segments of the pre-arterioles behave similarly to the epicardial arteries in that they are responsive to flow-dependent dilatation, whereas distally they behave more like the arterioles of the distal compartment in that they are responsive to intravascular pressure changes. The intermediate compartment can be distinguished from the distal due to its independence from the influence of myocardial metabolites.
The distal compartment is composed of arterioles (diameter <100µm), which have an even thinner tunica media (i.e. smooth muscle walls) than the proximal and intermediate compartment vessels. Moreover, the smaller terminal vessels of this compartment may lack a vascular smooth muscle layer entirely (replaced by small, unique cells called “pericytes” which do have some contractile qualities) and the tunica intima may lack an internal elastic membrane as they give rise to the capillary bed [34]. When viewed using plastic casting or ink injections, these vessels appear highly variable in structure as the capillary network is arranged amongst the arterioles and venules [42]. These small vessels run parallel to muscle fibres. The tone and resistance of the distal compartment are governed by metabolites produced by the myocardium and control the flow to the capillary network [34]. Capillaries are essentially composed of two layers, which are an endothelium and a basal lamina [34].
5. DISEASE MECHANISMS OF THE CORONARY CIRCULATION AND ITS SUBTYPES
It is increasingly understood that disease and disorder of the coronary circulation occur via multiple mechanisms that can be divided into different pathogenic subtypes [3, 16, 31, 43, 44].
5.1. Structural Abnormalities Of The Circulation
This subtype includes conditions whereby functional luminal diameter is attenuated which could be due to luminal stenosis, thrombosis, micro-emboli from percutaneous coronary intervention, inherent anomalous coronary vessel anatomy, coronary artery bridges, and aneurysms. There can also be abnormal remodelling and hypertrophy, reducing luminal size as a result of hypertension, aging, or cardiomyopathic processes [34]. This reduction in lumen size was demonstrated by Wienike et al. [45] to have a direct effect on coronary blood flow in a study that involved early uses of the Doppler flow wire and coronary intravascular imaging techniques.
5.2. Extravascular Changes To The Coronary Circulation
There are various influences external to the vascular circulation. These include external compression, shortened diastolic filling, and changes to cardiac metabolism, for example, autonomic dysfunction that follows acute myocardial infarction leading to sympathetic overdrive and vasoconstriction [34, 46]. Complex interactions exist between the autonomic nervous system and the blood vessels. The nerve terminal varicosities release neurotransmitters, which diffuse into and engage vascular cells. Stimulation of the autonomic system induces the release of vasoactive mediators, such as noradrenaline, Adenosine Triphosphate (ATP), and neuropeptide Y that cause vasoconstriction and acetylcholine and Calcitonin Gene Related Peptide (CGRP), which causes vasodilatation [47].
5.3. Systemic, Structural Changes To The Coronary Circulation
This includes sclerosis, extracellular matrix changes, fibrosis, intramyocardial fat infiltration or natural changes, such as aging and menopause [29, 32, 43]. Patients with systemic sclerosis are known to have increased extracellular matrix production leading to the diffuse fibrosis of the skin and internal organs, which reduces compliance and plasticity with impaired remodelling that impedes the flow [48]. Intramyocardial triglyceride deposits (more common in patients with diabetes and obesity) accumulate in the cardiac myocytes causing greater LV mass and greater diastolic dysfunction, which results in greater cardiac demand and work. Intramyocardial triglyceride deposits are also associated with chronic inflammation, which contributes to atherosclerosis [49]. Majerczak et al. [50] looked at arterial stiffness and endothelial function in young athletes who were followed up into old age and demonstrated that despite physical exercise, endothelial function worsened with age as did arterial stiffness. Changes in the vascular matrix (increase in collagen and a decrease in elastin), reduced the function of vascular smooth muscle and bioavailability of NO [31, 50]. There are also changes that occur in sepsis that cause endothelial dysfunction via direct degradation of the endothelium, and are associated with worse outcomes due to enhanced platelet aggregation and vasoconstriction [51, 52].
5.4. Functional Abnormalities of the Coronary Circulation (“Vasomotor Function”)
Vasomotor dysfunction is a disorder of vasomotor function caused by either enhanced vasoconstriction, impaired vasodilation (due to endothelium-dependent or endothelium-independent mechanisms) or a combination/imbalance of these. From a clinical perspective, the endothelium governs a significant part of the vasomotor function, therefore the vasomotor response centres on endothelial function. Endothelial dysfunction is associated with conditions that are widely regarded as precursors to atherosclerosis, including diabetes, obesity, and smoking. It is also demonstrable independently of traditionally associated atherosclerotic risk factors without obstructive epicardial coronary disease via as yet incompletely understood mechanisms (e.g. patients with cardiac syndrome X). Cardiac syndrome X is generally defined for the purpose of clinical practice as the presence of angina-like chest discomfort in the absence of significant epicardial stenosis; though stricter criteria include the presence of ST segment depression (during anginal episodes), an absence of epicardial coronary artery spasm provocation with intracoronary acetylcholine provocation and absence of cardiac or systemic diseases associated with microvascular dysfunction [53]. Patients with syndrome X have shown to have less intrinsic NO bioavailability and have greater impairment of endothelium-dependent and independent cutaneous microvascular function when compared with “normal” controls [17, 53, 54].
Hypertrophic cardiomyopathy is known to cause fibrosis and microvascular dysfunction. Patterns of fibrosis on Cardiovascular MRI have shown to have abnormal flow reserves (i.e. vasomotor responses) [55]. Microvascular dysfunction has a crucial relevance in that it is thought to be an ischaemic substrate for pathological arrhythmia in itself [29]. Furthermore, patients at risk of developing HCM have been identified as having endothelial dysfunction with abnormal vasomotor responses at young ages [56].
6. THE PHYSIOLOGY OF THE NORMAL ENDOTHELIUM
“Vasomotor function” refers to the vasodilatory or vasoconstrictor responses of the three compartments of the coronary circulation to maintain flow (Fig. 3) [2, 4, 21, 44]. Focussed studies on vasomotor activity have shown that the endothelial luminal layer of coronary arteries is a crucial, intrinsic element in regulating this process. A coronary circulation with a damaged or absent endothelium demonstrates a vasomotor function that behaves very differently to a healthy, normal endothelium [57-60].
Fig. (3).
Coronary Vascular bed: A schematic representation of the different elements and functional significance of the different components of the coronary circulation. CFR Coronary Flow Reserve, FFR Fractional Flow Reserve, IFR instantaneous flow reserve, IMR Index of microvascular resistance. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The vasomotor function can be divided into three main processes [31]:
Endothelium-dependent vasodilation. The endothelium responds to various paracrine factors (including endothelin, serotonin, histamine), which stimulate the production of nitric oxide by the endothelium. Nitric oxide binds to guanylyl cyclase, producing cGMP, which causes subsequent vascular smooth muscle relaxation and vasodilation of the artery [31, 32]. This has been demonstrated in studies since the 1980s, most commonly using acetylcholine or analogues which bind to muscarinic receptors and are used for measuring vasomotor responses [61-63].
Endothelium-independent vasodilation. The cell mechanism governing this remains incompletely understood [31]. Common methods of testing have included the use of sodium nitroprusside, papaverine, or glyceryl trinitrate to demonstrate smooth muscle vasodilation in response to nitric oxide [25, 34, 40, 43, 64, 65].
Vasoconstriction. This is commonly demonstrated with the use of vasopressin in studies. Vasopressin has a greater effect on the microcirculation and the wider systemic circulation, with only a limited vasoconstrictive effect on the epicardial coronary arteries [66, 67]. Vasoconstriction has been shown to be excessive in patients with Diabetes, Takutsubo Cardiomyopathy, and Myocarditis, perhaps due to the upregulation of Vasopressin receptors [66]. Vasoconstriction is also considered an important component of “no reflow” phenomenon during Primary PCI [26, 29, 31].
A coronary circulation with a damaged or absent endothelium demonstrates a vasomotor function that behaves very differently to a healthy, normal endothelium. These altered characteristics are summarised in Table 1 [57-60].
Table 1.
Roles of the Endothelium: Comparing the effects of a normal functional endothelium with the adverse mechanisms that occur in endothelial dysfunction.
| Normal Endothelium | Endothelial Dysfunction |
|---|---|
| Vasodilation | Vasoconstriction |
| Thrombolytic | Platelet Aggregation |
| Anti-inflammatory | Inflammation and Proliferative |
| BIOLOGICAL BARRIER | Increased Permeability |
7. ENDOTHELIAL DYSFUNCTION: THE ROLE OF THE ENDOTHELIUM IN ATHEROGENESIS
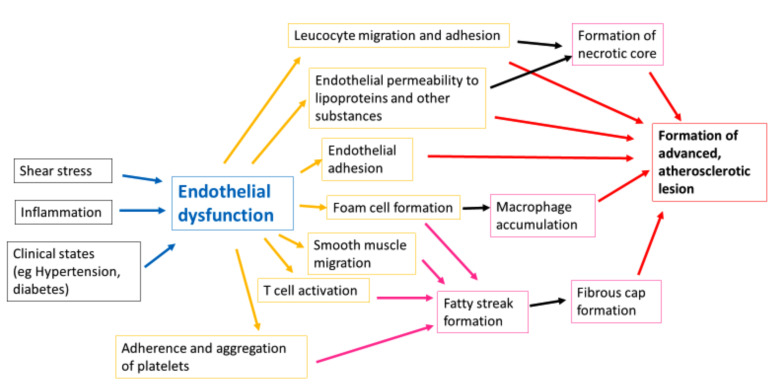
The term “endothelial dysfunction” refers to the broad alterations in endothelial phenotype that may contribute to the clinical expression of atherosclerosis [31, 68, 69]. It can be appreciated by measuring vasomotor responses to stimuli that occur via different mechanisms (Fig. 4). Endothelial dysfunction can lead to increased vascular permeability to lipoproteins as well as the expression of cytokines and adhesion molecules, which are features of atherosclerosis [1, 18, 30, 32]. Furthermore, endothelial dysfunction can perpetuate ischaemia as it facilitates smooth muscle wall proliferation and also produces substances that promote platelet aggregation [30, 70].
Fig. (4).
An illustration of the mechanisms of endothelial dysfunction as a precursor of atherosclerosis. Endothelial dysfunction occurs on a spectrum of disorder with atherosclerosis and whilst there is a degree of continuity to the process, it has a role in the development of atheroma at its various stages. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Endothelial dysfunction is associated with the loss of intrinsic endothelial vasoprotective factors that lead to an imbalance of vasodilation and vasoconstriction with net vasoconstriction contributing to the maladaptive vasomotor response. Acetylcholine usually elicits NO-induced vasodilation, though, in endothelial dysfunction, this is impaired, resulting in lower degrees of dilatation, manifesting as lower coronary blood flow or smaller reductions in systemic vascular resistance. Acetylcholine also has a direct vasoconstrictor effect when acting on smooth muscle cells, which is usually masked by the endothelium mediated smooth muscle dilatation. In the case of endothelial dysfunction, the vasodilation caused by the acetylcholine is attenuated, resulting in net vasoconstriction [27, 39, 60].
The progression of endothelial dysfunction is multifactorial and this occurs as a result of various triggers, such as shear stress, inflammation, and certain clinical states (like diabetes):
7.1. Shear Stress
A common observation in the identification of coronary atheroma is the development of lesions in regions that correlate with arterial branch points and other regions of altered haemodynamics [1, 14, 31]. In the field of experimental fluid mechanics, specialised devices have been used to reconstruct the flow effect over the laminar surface of cultured endothelial cell monolayers in vitro. These studies have demonstrated that laminar shear induces changes in cell morphology, alignment, and cell organisation, suggesting a local risk factor for endothelial cell dysfunction in atherogenesis [14, 37, 71]. Diamond et al. [72] described how shear stress caused transcriptional changes in RNA in cultured human endothelial cells, which directly affected the fibrinolytic pathways as a response to flow. Other studies have confirmed the modulation of DNA in response to haemodynamics. The PREDICTION study [70] was a prospective natural history study in Japan, which included patients with acute coronary syndrome treated with PCI and reassessed at six months follow up with repeat angiography. They measured intravascular flow characteristics to establish shear stress and demonstrate a strong association between disturbed endothelial shear stresses and subsequent lesion progression.
7.2. Inflammation
Inflammation promotes leucocyte adherence and migration via inflammatory cytokines, such as TNF alpha and IL-1, which induce endothelial cells expression of adhesion molecules. This has been shown in patients with systemic lupus erythematosus and rheumatoid arthritis [30, 31, 73]. In endothelial dysfunction, due to chronic inflammation, monocytes transform into macrophages after reaching the intima, which then expresses receptors that accumulate lipids forming foam cells. These continue to form fatty streaks, which are precursors for atheromatous plaques [30].
7.3. Clinical States
It has been generally recognised in diabetes that hyperglycaemia impairs endothelial function. Hyperglycaemia generates Advanced Glycation End products (AGE) that accumulate in the vessel wall and impairs NO activity, and therefore, endothelial function. Once inside the wall, they can also bind to surface receptors amplifying inflammatory responses, as well as stimulate foam cell production, advancing atherosclerosis [27]. The hyperglycaemic state also generates Reactive Oxidative species that have a direct adverse action on soluble Guanylyl cyclase and cyclic GMP Kinase (which mediate smooth muscle relaxation) [61, 74].
Other clinical states known to cause endothelial dysfunction include hypertension (activating the endothelin system), infection (such as sepsis mediated by imbalances in angiopoietin causing increased vessel permeability), obesity (causing a reduction in NO bioavailability due to L-arginine depletion), Vitamin D deficiency (thought to act via mechanisms similar to with hypertension), and aging (due to gradual reductions in NO availability) [49, 75-77]. Whilst their direct mechanisms are incompletely understood, they are thought to increase oxidative stress [61, 74, 78], which leads to leucocyte adhesion and inflammation, lipid deposition, vascular smooth muscle cell proliferation, vasoconstriction, and platelet aggregation, therefore engaging the progression of atherosclerosis and cardiovascular disease [27, 30, 31].
8. CLINICAL RELEVANCE: THE IMPLICATIONS ON PROGNOSIS OF ENDOTHELIAL DYSFUNCTION
There have been studies looking at the prognostic impact of endothelial dysfunction independently of other established risk factors for cardiac events. Ahmadi et al. [60] demonstrated that endothelial dysfunction, in the presence of non-obstructive coronary artery disease, was associated with characteristics of vulnerable plaques when assessed using intravascular ultrasound. Over the last two decades, studies have demonstrated that patients with “unobstructed coronary arteries” (generally luminal stenosis < 30-50% of the diameter) but with demonstrable endothelial dysfunction have increased cardiac events [44, 62, 79, 80].
In the Women’s Ischaemia Syndrome Evaluation (WISE) study [4], patients with abnormal measured coronary dilator responses to intra-coronary acetylcholine had less time free of cardiovascular events (p = 0.004). This was independent of risk factors such as age, hypertension, diabetes, hypercholesterolaemia, tobacco use, and severity of coronary artery disease and fractional luminal cross-sectional area change. In a systematic review and meta-analysis involving six studies including 1192 patients with normal or non-obstructed (< 50% luminal stenosis), Branin et al. [41] found that in those patients with epicardial endothelial-dependent dysfunction, 243 (20.4%) had cardiovascular events with a relative risk of 2.38 (confidence interval 1.74 - 3.25).
Given that endothelial dysfunction is an adverse predictor independent of apparent epicardial obstruction, this would suggest that patients with normal luminography, in the context of a good history of angina, could still benefit from an assessment of their endothelial function as they may still be at risk despite “normal epicardial coronaries”. The data would suggest that there is increased morbidity and mortality associated with these patients and that there is a positive impact to be made with some additional assessment [16, 62]. Reriani et al. [38, 81] followed up 457 patients with chest pain and unobstructed coronary arteries who had coronary vasoreactivity testing with intracoronary acetylcholine. During a mean follow up of 8.4 ± 4.7 years, the patients who were diagnosed and treated for microvascular dysfunction (mainly with beta blockers, aspirin, lipid lowering drugs and nitrates) had a higher quality of life indices. Tagliomonte et al. [82] demonstrated some improvements of transthoracic doppler Coronary Flow Reserve (CFR) in 58 patients with unobstructed coronaries with doses of 500mg Ranolazine twice daily, though the limitation of the study was that participants with luminal stenoses of up to 70% were included. A small double-blinded study by Villano et al. [83] examined 47 patients with microvascular angina and their response to a combination of ivabradine and ranolazine vs. a placebo. There was no significant difference in endothelial function compared to placebo, but the patients had a better quality of life scores and performed better on exercise stress testing with greater time to ST depression and overall performance durations. ACE inhibitors were shown to improve flow-mediated dilation (a measure used as a surrogate for endothelial dysfunction) in patients with hypertension [37], furthermore, there is an established benefit from statin therapy in the modulation of endothelial function [84-86]. A possible conclusion could be that patients with typical symptoms of angina despite normal coronaries would benefit from additional functional testing for reversible ischaemia as a means of guiding treatment.
There are also clinical implications of endothelial function on patients with acute coronary syndromes. The “no reflow” phenomenon is defined as slow coronary artery flow (graded TIMI 0 - 2), and is seen in 5-10% of patients who present with STEMI and is associated with a higher incidence of Major Adverse Cardiovascular Events (MACE) and infarct size on Cardiovascular Magnetic Resonance Imaging (CMR). It is a recognised complication following the restoration of luminal patency, purported to disseminate emboli “downstream” resulting in endothelial dysfunction demonstrable with microcirculatory resistance. [87, 88]. Canine models have demonstrated that microvascular injury in the context of ischaemic contributes to the no-reflow phenomenon. [89]. Electron microscopy of ‘no-reflow’ areas demonstrated swollen intraluminal endothelial protrusions and intraluminal bodies bound to the endothelial membrane, which seemed to obstruct the capillary lumen. Repeated ultrastructural examinations (high magnification cell architecture and biomaterial analysis), after repeated occlusions of the dog model coronaries, showed increased microvascular damage, as well as increasing incidences of microvascular obstruction [90]. The study of no-reflow and microvascular dysfunction overlaps with reperfusion injury. Hausenloy et al. [91] used rat hearts to demonstrate that the initial occlusion causes an interruption of antegrade flow, which induces the inflammatory cascade, causing vasoconstriction as well as platelet aggregation and endothelial edema associated with the no-reflow phenomenon. There have been studies concerned with mitigating the no-reflow process given that this adversely affects the outcomes irrespective of infarct size, although a robust technique has not been identified, thus far, it is clear that the endothelium has a crucial role in this problem [92].
9. METHODOLOGIES FOR THE ASSESSMENT OF ENDOTHELIAL FUNCTION
There are various techniques used to assess endothelial function that utilise either the measurement of vasomotor dilatation/constriction following the application of vasoactive stimuli or the measurement of a surrogate, such as Coronary Blood Flow or vascular resistance (summarised in Table 2 below):
Table 2.
Approaches for the assessment of Endothelial dysfunction: Intracoronary Acetylcholine/Glyceryl Trinitrate (IC ACH/GTN) is given via the intracoronary route and vasomotion can be measured angiographically.
| Invasive Approach | Non-Invasive Approach |
|---|---|
| Vasomotion following IC ACH/GTN Cold Pressor Testing |
Doppler ECHO Stress PET CMR Brachial Reactivity |
| More Risk More Expensive |
Limited Availability Operator Dependence/Variability |
9.1. Invasive Approaches
An invasive assessment can be performed during angiography. The gold standard technique for the assessment of endothelial function is the assessment of coronary flow-mediated dilatation of the epicardial coronary artery in response to intracoronary infusions of ACH [93, 94]. The luminal diameter is measured in two separate angiographic views and fractional change can be calculated from this method. This demonstrates proximal compartment responses but does not reveal changes to the microcirculation.
O’Mering et al. [3, 20] measured intracoronary flow using a Doppler tipped guidewire (0.014-inch FloWire). The tip of the Doppler wire has a piezoelectric transducer that measures peak velocity, which is linearly related to blood flow and can be calculated with the vessel cross-sectional area [45, 95]. They measured the changes in flow, in response to adenosine, which acts as an endothelium-independent vasomotor agonist, inducing hyperaemia and thus giving an estimate of the coronary flow reserve.
Cold Pressor Testing (CPT) is an alternative method for assessing endothelial function. This method involves wrapping an ice pack around the forearm or placing it on the patient’s forehead for two minutes with angiography then repeated. This works via the pathway of sympathetic (and likely also parasympathetic neuro humoral responses) stimulation though this it is postulated that it may be more complex than this as direct trials, comparing this technique with intracoronary acetylcholine, have demonstrated a greater effect on coronary vessel change with CPT [96, 97]. In a normal coronary circulation, sympathetic activation provokes endothelium-dependent vasodilation. However, in endothelial dysfunction, the increase in metabolically mediated flow is offset by adrenergically mediated beta vasoconstriction [98].
9.2. Non-Invasive Approaches
There are currently multiple non-invasive approaches, which usually involve imaging techniques. In studies assessing microvascular function, hyperaemia was elicited by either dipyridamole or adenosine through non-endothelial mechanisms [43, 62]. Techniques for measuring CFR included Transthoracic Doppler ECHO, Stress Positron Emission Tomography (PET), and CMR. Transthoracic Doppler ECHO measures blood flow in the mid-Left Anterior Descending artery, imaged using colour doppler with a high frequency ultrasound probe. Coronary Blood Flow is measured by Pulsed Doppler technique. This could be a largely applicable method that is widely available and inexpensive, however, it is operator-dependent, less accurate, and contingent on good Echocardiographic windows in patients [99, 100]. PET allows quantitative measurements of myocardial blood flow using myocardial dissemination and radioactivity of isotopes [101]. CMR appears to be a robust method that can be used to generate a semi-quantitative assessment of CFR via the Myocardial perfusion reserve index [16, 60, 102]. CMR utilises paramagnetic contrast medium gadolinium to measure myocardial blood flow. Compared with PET, CMR has better spatial resolution and involves no radiation. However, post-acquisition processing is more challenging, artefacts can be a problem and gadolinium should be used cautiously in patients with renal impairment [101]. PET and CMR, however, are not used to assess coronary vasomotor function in routine practice due to limited availability and high cost and are currently restricted in the research arena.
Brachial artery reactivity is another method of assessing endothelium-dependent and endothelium-independent function. Endothelium dependent function can be measured using a high resolution ultrasonography proximal to the antecubital fossa and measuring the increase in the diameter of the brachial artery during reactive hyperaemia, evoked by the release of a cuff inflated to high pressure on the upper arm [37, 79]. This can be repeated after administering GTN to measure endothelium-independent vasodilation.
9.3. Limitations of Provocative Testing
Whilst the action of acetylcholine contributes to both vasoconstriction and vasodilatation, the net change is contingent on the relative smooth muscle cell response. Similarly, responses to certain stimuli, such as the cold in the “cold pressor testing”, may be confounded by adrenergic responses. Furthermore, there may be patients in whom there are global impairments of smooth muscle cell reaction to nitric oxide, in which case, impaired endothelial function may be misattributed [81, 101].
10. THE EFFECT OF PERCUTANEOUS CORONARY INTERVENTION ON ENDOTHELIAL FUNCTION
Percutaneous coronary intervention is a widely adopted strategy for the treatment of coronary artery disease, which continues to advance based on scientific evidence and new developments. Initial strategies, over forty years ago, involved balloon angioplasty (now referred to as “plain old balloon angioplasty” or “POBA”), which were met with great enthusiasm but were limited by complications of acute vessel closure and neointimal proliferation [103, 104]. Bare Metal Stents were developed in the late 1980s as scaffold structures to prevent vessel closure, however, these were limited by potentially fatal Stent Thrombosis (ST) and In-Stent Restenosis (ISR) [105]. First-generation Drug Eluting Stents (DES) were subsequently developed, aiming to reduce these complications and were heralded as a major breakthrough. The drug coating had immunosuppressive properties to prevent the aggregation of inflammatory cells at the site of stent implantation to reduce restenosis rates. Paclitaxel was one of the initial anti-proliferative agents applied to drug eluting stents, which has a local mechanism that inhibits the microtubule network and arrests cell proliferation [106, 107]. Drug eluting stents, with the agent Sirolimus (also known as Rapamycin), were also developed around a similar time. Sirolimus is a cytostatic agent that inhibits serine/threonine-specific protein kinase (mTOR) and also inhibits smooth muscle proliferation. Though further generations of DES showed reduced restenosis rates [65], they were still also associated with ST events. Drug eluting stents also required prolonged periods of dual antiplatelet therapy with a greater associated bleeding risk to the population of patients treated with PCI. This led to the development of Bioresorbable Vascular Scaffolds (BVS) about ten years ago. BVS was initially heralded as the “fourth revolution in interventional cardiology” as they did not require the permanent presence of a metallic foreign body within the artery, and therefore, once the scaffolding disappeared, there would be a restoration of normal coronary luminal anatomy. BVS in humans are reported to resorb within three to five years whilst theoretically liberating the artery of the “cage”, reducing physiological shear stress and restoring pulsatility, cyclical strain, and mechano-transduction [108]. These have been associated with late scaffold thrombosis and other complications that have led to it being licensed for research purposes only [109, 110]. Currently, Drug Coated Balloon Angioplasty is a widely used method to treat ISR. It has been proposed as a technique that does not carry the same level of risk of ST or prolonged, mandated antiplatelet therapy given its absence of scaffold structure [104, 111-113].
There have been various studies looking into the effects of PCI on endothelial function given that intuitively, the presence of the stents have an effect on the coronary artery endothelium and vasomotion (see Table 3) [14]. A mechanism of stent thrombosis includes the presence of the scaffold, not only causing local inflammatory effects but also attenuating endogenous vessel NO levels increasing thrombogenicity of the treated segment [109].
Table 3.
Comparison of the studies looking at vasomotion in patients treated with PCI.
| Author | Number of Patients | Methods | Time scale | Result |
|---|---|---|---|---|
| Komaru et al. [114] | 13 | Patients treated with POBA for stable angina. Measured vasomotion with IC substance P |
Measured the day after angioplasty and three months afterwards | The “day after” there was a vasoconstrictor response. More uniform vasodilator response after three months |
| Vassanelli et al. [58] | 25 | Patients treated with POBA assessed with IC ACH | Three months and six months after treatment | Abnormal vasomotor responses at both three and six months |
| Caramori et al. [115] | 39 | Post-treatment to their LAD artery with POBA and BMS with IC ACH | Six months | Significantly greater vasoconstrictor responses with BMS vs. POBA (-21.8% ± 4.3 vs. -9.5% ± 2.8, p=0.02) |
| Togni et al. [116] | 25 | Treated 11 with BMS and 14 with SES Angiography during exercise |
Six months | Exercise-induced vasoconstriction in SES compared with BMS |
| Kim et al. [28] | 78 | 10 received BMS, 36 received PES, and 39 DES assessed with IC ACH. Measured responses within stents as well as proximally and distally | Six months | Vasoconstriction with DES even proximally and distally that was worse than BMS |
| Gomez-Lara et al. [109] | 59 | Non-diabetic patients randomised to either BVS or Everolimus DES measured response to IC ACH | 13 months | More pronounced in scaffold vasoconstriction in BVS than DES |
| MAGSTEMI [109] | 69 | STEMI patients treated with BVS vs. SES | 12 months | More pronounced vasoconstrictive response with BVS vs. SES |
Abbreviations: BMS = Bare Metal Stents; BVS = Bioresorbable Vascular Scaffolds; IC ACH = Intracoronary Acetylcholine; PES = Paclitaxel Eluting Stents; SES = Sirolimus Eluting Stents.
Komaru et al. [114] looked at the effect of Plain Old Balloon Angioplasty (POBA) on endothelial dysfunction in patients treated for symptoms of stable angina. They used substance P to assess endothelial function and vasomotor response. Substance P is a neuropeptide that operates as a neuromodulator and neurotransmitter and binds to the NK-1 receptor on the vascular endothelium, stimulating cyclic GMP (in a fashion like acetylcholine) to further stimulate nitric oxide release and often vasodilation. This process is similarly attenuated in endothelial dysfunction in a fashion much like acetylcholine. [40]. Komaru et al. [114] assessed 13 patients the day after their POBA and again, after three months. Whilst the day after, they demonstrated a coronary vasoconstrictor response to substance P, there appeared to be a more uniform vasodilator response at three months with the investigators describing this as a “return to normal” [114]. Vassanelli et al. [58] observed 25 patients who received POBA and used intracoronary acetylcholine, demonstrating abnormal vasomotor responses in arteries treated with POBA, three to six months after the initial treatment.
Caramori et al. [115] assessed the endothelial-dependent vasomotor function in a total of thirty-nine patients who had LAD treatment with a mixture of interventions including POBA and bare metal stents (BMS) at least six months prior. Amongst the patients studied, there were 12 patients who had been stented with BMS, 15 with POBA, and 12 received directional atherectomy. Both, their treated LAD and untreated Circumflex (LCx), were assessed with intracoronary acetylcholine. The LAD constricted significantly more when treated by BMS as compared with those treated by POBA (-21.8% ± 4.3 vs. -9.5% ± 2.8, p = 0.02). By multiple regression analysis, stent implantation was the only significant predictor of LAD constriction, and therefore, endothelial dysfunction (p = 0.008).
Togni et al. [116] compared 11 patients treated with BMS and 14 patients treated with sirolimus Drug Eluting Stents (DES). They measured luminal diameter change to assess the coronary vasomotor response to exercise by performing biplane angiography whilst the patients were pedalling supine on a bicycle. Both groups were assessed for more than six months after the initial intervention for de novo coronary lesions. In this study, the vasomotion within the stents (both BMS and DES) was minimal. However, in segments, at intervals 5mm, 10mm, and 20mm, both proximal and distal to the stents, there was exercise-induced vasoconstriction in the sirolimus eluting stents (SES; -12 ± 4% proximally and -15 ± 6% distally) vs. exercise induced vasodilation in the BMS group (+15 ± 3% proximally and +17 ± 4% distally; p < 0.001). Togni et al. [117] later demonstrated similar findings in Paclitaxel eluting stents to SES.
The Caramori study [115] showed vasoconstriction in BMS at the maximal administered doses of acetylcholine, which was at a much high concentration than used in the other studies looking at endothelial dysfunction where vasoconstrictor responses were demonstrated in DES at lower doses. This relative response to acetylcholine relates to the dose-related interaction of the effects of acetylcholine, causing vasodilatation and vasoconstriction with acetylcholine facilitating more vasoconstriction at higher doses [118, 119]. The data would suggest that the anti-proliferative drug had a counterproductive effect on endothelial function, with a tendency to encourage vasoconstriction (evidenced by the lesser attenuation of endothelial function with BMS).
Kim et al. [28] assessed endothelial function using intracoronary acetylcholine, six months after treatment with paclitaxel eluting stents (36 patients) and sirolimus eluting stents (39 patients) and compared these to coronaries treated with BMS (10 patients). They measured coronary vasomotor responses 5mm proximal and 5mm distal to stent implantation. Greater vasoconstriction was seen in both DES groups than BMS, more distally to the stent than proximally. Sirolimus eluting stents showed -24.7 ± 16.8% fractional change to maximum acetylcholine proximally and -70.9 ± 11.5% distally, compared with -23.4 ± 15.7% and 68.7 ± 12.1% in the Paclitaxel eluting stents and - 6.23 ± 8.49% and -21.6 ± 4.04% in the BMS group (proximally p = 0.09 and distally p < 0.001), respectively. These data would further suggest a flow mediated, deleterious effect (given that the effect was greater distally) of the anti-proliferative agent on the drug eluting stents when compared with BMS. Fuke et al. [120] measured the native vasomotor responses prior to Sirolimus eluting stent insertion at portions proximally and distally to the target lesions, and whilst these showed near normal vasomotor responses before the stent was implanted, there were significant vasoconstrictor responses in these same portions when the patients reattended for their repeat angiography at six months. This study provides further evidence that DES has potential long-term adverse effects on local coronary endothelial function.
Kubo et al. [121] looked at eighty patients who were treated for angina with DES and measured brachial artery flow reactivity one week after treatment with drug-eluting stents, with the aim of establishing whether endothelial dysfunction could be a predictor of risk for in-stent restenosis. There was no difference in the target lesion revascularisation between both groups after a 21-month follow up, which showed that low flow-mediated dilatation did not predict restenosis in the treated segment. Low flow-mediated dilation (as a surrogate for endothelial dysfunction) was associated with a higher incidence of cardiovascular events (a composite cardiac death, coronary revascularisation, critical limb ischaemia, and stroke) with a hazard ratio of 2.77 (95% CI 1.23 - 6.19 p = 0.01). It was not the presence of DES that conferred the worse outcome but the presence of poor brachial flow dilatation, indicating that endothelial dysfunction afforded the worse prognosis.
Bioresorbable Vascular Scaffolds (BVS) were considered with interest, given that there would be a theoretical restoration of vessel compliance and vasomotor function [108]. Gogas et al. [122] explored vasomotor function in porcine coronary arteries treated by Everolimus eluting BVS and Everolimus DES. They looked at contraction responses as well as vessel dilatation to Substance P. Endothelial dependent relaxation was tested ex vivo and was found to be 35.91± 24.74% and 1.2 ± 3.79% (p < 0.01) in isolated BVS segments vs. DES, respectively, at two years after implantation [122]. The ABSORB II trial enrolled patients who had de novo disease in one or two lesions (in different epicardial vessels) with evidence of myocardial ischaemia and were randomised to receive either Everolimus eluting BVS or Everolimus DES [123]. They randomised 501 patients and measured vasomotion using only intracoronary nitrate. This could be seen as a disadvantage as the resultant vasodilatation would reflect more the smooth muscle NO capacity rather than giving an account of endothelial response. The vasomotor reactivity was not statistically different at three years (0.047mm [SD ± 0.109] in BVS vs. 0.056mm [SD ± 0.117] in the DES group p= 0.49).
Gomez-Lara et al. [124] studied vasomotor function in 59 non-diabetic patients who were randomised to either BVS or Everolimus DES. These patients were re-evaluated at 13 months following implantation. Vasomotor testing showed a vasoconstrictor response to intracoronary acetylcholine in 75.6% proximally and in 72.2% distally to the peri-scaffold segments. There was no significant difference between the two devices, however, BVS has more pronounced in-scaffold vasoconstriction than the Everolimus DES (60% vs. 27.6%; p < 0.05). The VANISH trial [98] observed 60 patients with single-vessel disease who were randomised to receive a Everolimus BVS or DES and were evaluated one month, one year and three years with Cardiac PET scans using CPT to measure Myocardial blood flow and Coronary Flow reserve. Coronary Flow reserve was actually lower at three years follow up than at one month or one year, implying that neither technique restores vasomotor function [98]. The MAGSTEMI randomised control trial [109] measured vasomotor function in 69 patients treated for STEMI who were randomised to receive a Magnesium based BVS (with a short resorption period < 1year) or Sirolimus DES. In those receiving a BVS, there was a more pronounced vasoconstrictive response to intracoronary acetylcholine compared to the DES (-8.1 ± 3.5% vs. -2.4 ± 1.3% p = 0.003) [109, 125].
Whilst OCT assessment of coronaries, treated by BVS, have shown favourable indications when compared with DES, BVS appears not to offer an advantage in maintaining positive coronary vasomotion [110, 123, 126]. In fact, there appears to be enhanced vasoconstriction in the lesions treated with BVS. Previous assessment have suggested that this is, in part, due to the effects of the resorption process, causing inflammation [109, 124, 127]. Furthermore, BVS has been inhibited by device-related factors such as a higher crossing profile (due to strut thickness), a lack of suitability in calcified lesions and scaffold dismantling, which now means that the European Society Guidelines (2018) do not recommend the use of BVS outside of clinical studies [109, 128].
Given the relatively favourable effects of POBA on the endothelial function when compared with stent/scaffold implantation, it is unfortunate that there is a paucity of study data looking at an endothelial function in patients treated with Drug-Coated Balloon angioplasty (DCB). Plass et al. [59] were unique in that they compared POBA, BMS, DES and DCB angioplasty on porcine coronary arteries. They reviewed vasomotor responses after endothelial injury due to angioplasty at five hours, one day and one month using in vitro techniques measuring changes in tone, by measuring the endothelium dependent and independent vasoreactivity in milliNewtons (mN) or percentage of luminal diameter change. In their assessments, endothelium dependent vasodilation was significantly attenuated five hours after PCI. The control vessel dilated by 49.6 ± 9.5%, was compared with the POBA treated segments which dilated by 9.8 ± 3.7% vs. DCB at 13.4 ± 9.2%, BMS at 5.7 ± 5.3% and DES at 7.6 ± 4.7%. A possible explanation offered for the comparatively favourable responses to the balloon-based treatments is that in the initial phases, additional NO could be made available by the process of microdissections during angioplasty. At one month follow up, endothelium dependent vasodilation to substance P was 68.6 ± 10.0% vs. 76.0 ± 13.1% vs. 78.7 ± 18.3% and 33 ± 7.4% in POBA, DCB, BMS and DES, respectively (p < 0.05). The endothelium independent vasodilation (measured as a response to nitric oxide and sodium nitroprusside) was profoundly impaired one day post PCI and were found to be 0.062 ± 0.045Mn vs. 0.054 ± 0.041Mn and 0.023 ± 0.003mN in DCB, BMS and DES, respectively, compared with controls 0.142 ± 0.047Mn).
The general trend suggests that the physiological homeostasis between the contractile and vasodilatory capacities of the treated vessels was better attenuated in the presence of stents. Furthermore, DCB angioplasty may confer some advantageous effects with respect to endothelial function. The evidence, to date, suggests that by comparison, drug eluting stents have significant vasoconstrictor responses to acetylcholine suggestive of quite marked endothelial dysfunction, when compared with BMS. Furthermore, it would seem that BMS is detrimental to vasomotor function when compared with POBA. It would appear, therefore, that DCB may be a way of bridging the gap in allowing for more preserved endothelial function and vasomotor responses whilst maintaining the benefit of an anti-proliferative in the shorter term to reduce restenosis rates. It is clear that more data, particularly in human coronary arteries, are necessary in order to confirm such an association.
CONCLUSION
The coronary circulation is a diverse and multi-faceted system that whilst incompletely understood is being increasingly appreciated in the wider treatment of cardiovascular disease. The ongoing evolution in the understanding of the impacts of treatments and percutaneous coronary intervention on coronary circulation represent a conceptual shift from the “modus operandi” of ensuring epicardial luminal patency; which may have guided PCI in the past, lending itself to a more comprehensive approach to treating coronary artery disease. Whilst there has been an exuberant uptake of intravascular imaging guided PCI, it is clear that there is data to suggest that there are multifarious functional and physiological indicators that warrant examination as they clearly impact the patient’s outcomes. At present, crucial gaps remain in the greater understanding of coronary artery disease that demand further research and deliberation in order to guide the best possible treatment for patients in the longer term.
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- ACH
Acetyl Choline
- ATP
Adenosine Triphosphate
- BMS
Bare Metal Stents
- BVS
Bioresorbable Vascular Scaffolds
- CFR
Coronary Flow Reserve
- CMR
Cardiac Magnetic Resonance Imaging
- CPT
Cold Pressor Testing
- DCB
Drug Coated Balloon
- DES
Drug Eluting Stents
- cGMP
Cyclic Guanosine Monophosphate
- FFR
Fractional Flow Reserve
- HCM
Hypertrophic Cardiomyopathy
- IC
Intra-coronary
- IL-1
Interleukin 1 Inflammatory Cytokine
- ISR
Instent Restenosis
- IMR
Index of Microvascular Resistance
- LAD
Left Anterior Descending Coronary Artery
- LCx
Left Circumflex Coronary Artery
- NO
Nitric Oxide
- PCI
Percutaneous Coronary Intervention
- PES
Paclitaxel Eluting Stents
- PET
Positron Emission Tomography
- POBA
Plain Old Balloon Angioplasty
- SES
Sirolimus Eluting Stents
- ST
Stent Thrombosis
- TNF alpha
Tumour Necrosis Factor Inflammatory Cytokine
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Gimbrone MA.
- 2.Shechter M., Matetzky S., Prasad M., et al. Endothelial function predicts 1-year adverse clinical outcome in patients hospitalized in the emergency department chest pain unit. Int. J. Cardiol. 2017;240:14–19. doi: 10.1016/j.ijcard.2017.04.101. [DOI] [PubMed] [Google Scholar]
- 3.von Mering G.O., Arant C.B., Wessel T.R., et al. National Heart, Lung, and Blood Institute. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: Results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109(6):722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 4.AlBadri A., Bairey Merz C.N., Johnson B.D., et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J. Am. Coll. Cardiol. 2019;73(6):684–693. doi: 10.1016/j.jacc.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz A.M., Katz P.B. Diseases of the heart in the works of Hippocrates. Br. Heart J. 1962;24:257–264. doi: 10.1136/hrt.24.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan I.A., Mehta N.J. Initial historical descriptions of the angina pectoris. J. Emerg. Med. 2002;22(3):295–298. doi: 10.1016/S0736-4679(01)00489-9. [DOI] [PubMed] [Google Scholar]
- 7.Lanzer C., Lanzer P. Re: Angina pectoris revisited; Exertional angina preceded Martin Luther’s last stretch of a final journey. Eur. Heart J. 2016;37(33):2570. doi: 10.1093/eurheartj/ehw320. [DOI] [PubMed] [Google Scholar]
- 8.Bedford D.E. Book review: The history of coronary artery disease by J.O. Leibowitz, London. Wellcome Institute of the History of Medicine. Med. Hist. 1971;15(2):195–196. doi: 10.1017/S002572730001646X. [DOI] [Google Scholar]
- 9.Fye W.B. Edward Jenner. Clin. Cardiol. 1994;17(11):634–635. doi: 10.1002/clc.4960171115. [DOI] [PubMed] [Google Scholar]
- 10.Olszewski T.M. James Herrick (1861-1954): Consultant physician and cardiologist. J. Med. Biogr. 2018;26(2):132–136. doi: 10.1177/0967772017745701. [DOI] [PubMed] [Google Scholar]
- 11.Smilowitz N.R., Feit F. The history of primary angioplasty and stenting for acute myocardial infarction. Curr. Cardiol. Rep. 2016;18(1):5. doi: 10.1007/s11886-015-0681-x. [DOI] [PubMed] [Google Scholar]
- 12.Mueller R.L., Sanborn T.A. The history of interventional cardiology: Cardiac catheterization, angioplasty, and related interventions. Am. Heart J. 1995;129(1):146–172. doi: 10.1016/0002-8703(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 13.Crea F., Camici P.G., Bairey Merz C.N. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014;35(17):1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Heiden K., Gijsen F.J., Narracott A., et al. The effects of stenting on shear stress: Relevance to endothelial injury and repair. Cardiovasc. Res. 2013;99(2):269–275. doi: 10.1093/cvr/cvt090. [DOI] [PubMed] [Google Scholar]
- 15.Cheng T.O. History of coronary artery bypass surgery--half of a century of progress. Int. J. Cardiol. 2012;157(1):1–2. doi: 10.1016/j.ijcard.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Ford T.J., Corcoran D., Berry C. Stable coronary syndromes: Pathophysiology, diagnostic advances and therapeutic need. Heart. 2018;104(4):284–292. doi: 10.1136/heartjnl-2017-311446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw J., Anderson T. Coronary endothelial dysfunction in non-obstructive coronary artery disease: Risk, pathogenesis, diagnosis and therapy. Vasc. Med. 2016;21(2):146–155. doi: 10.1177/1358863X15618268. [DOI] [PubMed] [Google Scholar]
- 18.Loffler A.B.J. HHS Public Access. Curr. Cardiol. Rep. 2016;8(5):583–592. doi: 10.1002/aur.1474.Replication. [DOI] [Google Scholar]
- 19.Grayson J., Davidson J.W., Fitzgerald-Finch A., Scott C. The functional morphology of the coronary microcirculation in the dog. Microvasc. Res. 1974;8(1):20–43. doi: 10.1016/0026-2862(74)90061-2. [DOI] [PubMed] [Google Scholar]
- 20.Marinescu M., Loffler A., Ouellette M., Smith L., Kramer C., Bourque J. Coronary microvascular dysfunction and microvascular angina: A systematic review of therapies. JACC Cardiovasc. Imaging. 2015;8(2):210–220. doi: 10.1016/j.jcmg.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannon R.O., III, Cattau E.L., Jr, Yakshe P.N., et al. Coronary flow reserve, esophageal motility, and chest pain in patients with angiographically normal coronary arteries. Am. J. Med. 1990;88(3):217–222. doi: 10.1016/0002-9343(90)90145-4. [DOI] [PubMed] [Google Scholar]
- 22.Papanicolaou M.N., Califf R.M., Hlatky M.A., et al. Prognostic implications of angiographically normal and insignificantly narrowed coronary arteries. Am. J. Cardiol. 1986;58(13):1181–1187. doi: 10.1016/0002-9149(86)90378-4. [DOI] [PubMed] [Google Scholar]
- 23.Cannon R.O., III, Quyyumi A.A., Schenke W.H., et al. Abnormal cardiac sensitivity in patients with chest pain and normal coronary arteries. J. Am. Coll. Cardiol. 1990;16(6):1359–1366. doi: 10.1016/0735-1097(90)90377-2. [DOI] [PubMed] [Google Scholar]
- 24.Cannon R.O., III Microvascular angina and the continuing dilemma of chest pain with normal coronary angiograms. J. Am. Coll. Cardiol. 2009;54(10):877–885. doi: 10.1016/j.jacc.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furchgott R.F., Zawadzki J.V.F.F.R. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 26.Widlansky M.E., Gokce N., Keaney J.F., Jr, Vita J.A. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003;42(7):1149–1160. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 27.Park K-HP. 2015.
- 28.Kim J.W., Suh S.Y., Choi C.U., et al. Six-month comparison of coronary endothelial dysfunction associated with sirolimus-eluting stent vs. Paclitaxel-eluting stent. JACC Cardiovasc. Interv. 2008;1(1):65–71. doi: 10.1016/j.jcin.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Selthofer-Relatic K., Mihalj M., Kibel A., et al. Coronary microcirculatory dysfunction in human cardiomyopathies: A pathologic and pathophysiologic review. Cardiol. Rev. 2017;25(4):165–178. doi: 10.1097/CRD.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 30.Ross R. Inflammation or atherogenesis. N. Engl. J. Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 31.Crea F, Lanza GA, Camici PG. Coronary microvascular dysfunction. 2013.
- 32.Mann D.L., Zipes D.P., Libby P., Bonow R.O., Braunwald E. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Single Volume; 2014. [Google Scholar]
- 33.Gould K.L., Johnson N.P. Coronary physiology beyond coronary flow reserve in microvascular angina: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018;72(21):2642–2662. doi: 10.1016/j.jacc.2018.07.106. [DOI] [PubMed] [Google Scholar]
- 34.Fonseca DA, Antunes PE, Cotrim MD. 2016.
- 35.Fonseca D.A., Antunes P.E., Cotrim M.D. Endothelium-dependent vasoactivity of the human internal mammary artery. Coron. Artery Dis. 2014;25(3):266–274. doi: 10.1097/MCA.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 36.Morris P.D., Ryan D., Morton A.C., et al. Virtual fractional flow reserve from coronary angiography: modeling the significance of coronary lesions: Results from the VIRTU-1 (VIRTUal Fractional Flow Reserve From Coronary Angiography) study. JACC Cardiovasc. Interv. 2013;6(2):149–157. doi: 10.1016/j.jcin.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Ghiadoni L., Salvetti M., Muiesan M.L., Taddei S. Evaluation of endothelial function by flow mediated dilation: Methodological issues and clinical importance. High Blood Press. Cardiovasc. Prev. 2015;22(1):17–22. doi: 10.1007/s40292-014-0047-2. [DOI] [PubMed] [Google Scholar]
- 38.Crea F. Coronary microvascular obstruction--a puzzle with many pieces. N. Engl. J. Med. 2015;372(15):1464–1465. doi: 10.1056/NEJMe1501882. [DOI] [PubMed] [Google Scholar]
- 39.Laughlin M.H., Bowles D.K., Duncker D.J. The coronary circulation in exercise training. Am. J. Physiol. Heart Circ. Physiol. 2012;302(1):H10–H23. doi: 10.1152/ajpheart.00574.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karabucak B., Walsch H., Jou Y.T., Simchon S., Kim S. The role of endothelial nitric oxide in the Substance P induced vasodilation in bovine dental pulp. J. Endod. 2005;31(10):733–736. doi: 10.1097/01.don.0000157988.13010.25. [DOI] [PubMed] [Google Scholar]
- 41.Brainin P., Frestad D., Prescott E. The prognostic value of coronary endothelial and microvascular dysfunction in subjects with normal or non-obstructive coronary artery disease: A systematic review and meta-analysis. Int. J. Cardiol. 2018;254:1–9. doi: 10.1016/j.ijcard.2017.10.052. [DOI] [PubMed] [Google Scholar]
- 42.Reese D.E., Mikawa T., Bader D.M. Development of the coronary vessel system. Circ. Res. 2002;91(9):761–768. doi: 10.1161/01.RES.0000038961.53759.3C. [DOI] [PubMed] [Google Scholar]
- 43.Herscovici R., Sedlak T., Wei J., Pepine C.J., Handberg E., Bairey Merz C.N. Ischemia and no obstructive coronary artery disease (INOCA): What is the risk? J. Am. Heart Assoc. 2018;7(17):e008868. doi: 10.1161/JAHA.118.008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merz C.N.B., Kelsey S.F., Pepine C.J., et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J. Am. Coll. Cardiol. 1999;33(6):1453–1461. doi: 10.1016/S0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 45.Wieneke H., von Birgelen C., Haude M., et al. Determinants of coronary blood flow in humans: Quantification by intracoronary Doppler and ultrasound. J. Appl. Physiol. 2005;98(3):1076–1082. doi: 10.1152/japplphysiol.00724.2004. [DOI] [PubMed] [Google Scholar]
- 46.Beltrame J.F., Crea F., Camici P. Advances in coronary microvascular dysfunction. Hear Lung Circ; 2008. [DOI] [PubMed] [Google Scholar]
- 47.Sheng Y., Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int. J. Physiol. Pathophysiol. Pharmacol. 2018;10(1):17–28. [PMC free article] [PubMed] [Google Scholar]
- 48.Zanatta E., Famoso G., Boscain F., et al. Nailfold avascular score and coronary microvascular dysfunction in systemic sclerosis: A newsworthy association. Autoimmun. Rev. 2019;18(2):177–183. doi: 10.1016/j.autrev.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Guzzardi M.A., Iozzo P. Fatty heart, cardiac damage, and inflammation. Rev. Diabet. Stud. 2011;8(3):403–417. doi: 10.1900/RDS.2011.8.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majerczak J., Grandys M., Frołow M., et al. Age-dependent impairment in endothelial function and arterial stiffness in former high class male athletes is no different to that in men with no history of physical training. J. Am. Heart Assoc. 2019;8(18):e012670. doi: 10.1161/JAHA.119.012670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colbert J.F., Schmidt E.P. Endothelial and microcirculatory function and dysfunction in sepsis. Clin. Chest Med. 2016;37(2):263–275. doi: 10.1016/j.ccm.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curzen N.P., Griffiths M.J.D., Evans T.W. Role of the endothelium in modulating the vascular response to sepsis. Clin. Sci. (Lond.) 1994;86(4):359–374. doi: 10.1042/cs0860359. [DOI] [PubMed] [Google Scholar]
- 53.Agrawal S., Mehta P.K., Bairey Merz C.N. Cardiac Syndrome X: Update. Heart Fail. Clin. 2016;12(1):141–156. doi: 10.1016/j.hfc.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Majidinia M., Rasmi Y., Khadem Ansari M.H., Seyed-Mohammadzad M., Saboory E., Shirpoor A. Metoprolol improves endothelial function in patients with cardiac syndrome X. Iran. J. Pharm. Res. 2016;15(3):561–566. [PMC free article] [PubMed] [Google Scholar]
- 55.Petersen S.E., Jerosch-Herold M., Hudsmith L.E., et al. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: New insights from multiparametric magnetic resonance imaging. Circulation. 2007;115(18):2418–2425. doi: 10.1161/CIRCULATIONAHA.106.657023. [DOI] [PubMed] [Google Scholar]
- 56.Fernlund E, Schlegel TT, Platonov PG, Carlson J, Carlsson M, Liuba P. Peripheral microvascular function is altered in young individuals at risk for hypertrophic cardiomyopathy and correlates with myocardial diastolic function. 2015. [DOI] [PubMed]
- 57.Gómez-Hospital J.A., Tenas M.S., Cequier Fillat A., et al. Endothelial function in coronary segments previously treated with balloon angioplasty. Rev. Esp. Cardiol. 2000;53(11):1467–1473. [PubMed] [Google Scholar]
- 58.Vassanelli C., Menegatti G., Zanolla L., Molinari J., Zanotto G., Zardini P. Coronary vasoconstriction in response to acetylcholine after balloon angioplasty: Possible role of endothelial dysfunction. Coron. Artery Dis. 1994;5(12):979–986. doi: 10.1097/00019501-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Plass C.A., Sabdyusheva-Litschauer I., Bernhart A., et al. Time course of endothelium-dependent and -independent coronary vasomotor response to coronary balloons and stents. Comparison of plain and drug-eluting balloons and stents. JACC Cardiovasc. Interv. 2012;5(7):741–751. doi: 10.1016/j.jcin.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 60.Ahmadi N., Ruiz-Garcia J., Hajsadeghi F., et al. Impaired coronary artery distensibility is an endothelium-dependent process and is associated with vulnerable plaque composition. Clin. Physiol. Funct. Imaging. 2016;36(4):261–268. doi: 10.1111/cpf.12220. [DOI] [PubMed] [Google Scholar]
- 61.Higashi Y., Maruhashi T., Noma K., Kihara Y. Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications. Trends Cardiovasc. Med. 2014;24(4):165–169. doi: 10.1016/j.tcm.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Suwaidi J.A., Hamasaki S., Higano S.T., Nishimura R.A., Holmes D.R., Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.CIR.101.9.948. [DOI] [PubMed] [Google Scholar]
- 63.el-Tamimi H., Davies G.J., Crea F., Maseri A. Response of human coronary arteries to acetylcholine after injury by coronary angioplasty. J. Am. Coll. Cardiol. 1993;21(5):1152–1157. doi: 10.1016/0735-1097(93)90239-W. [DOI] [PubMed] [Google Scholar]
- 64.Patik J.C., Christmas K.M., Hurr C., Brothers R.M. Impaired endothelium independent vasodilation in the cutaneous microvasculature of young obese adults. Microvasc. Res. 2016;104:63–68. doi: 10.1016/j.mvr.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Niccoli G., Montone R.A., Lanza G.A., Crea F. Angina after percutaneous coronary intervention: The need for precision medicine. Int. J. Cardiol. 2017;248:14–19. doi: 10.1016/j.ijcard.2017.07.105. [DOI] [PubMed] [Google Scholar]
- 66.Feng J., Liu Y., Singh A.K., et al. Effects of diabetes and cardiopulmonary bypass on expression of adherens junction proteins in human peripheral tissue. United States. Surgery. 2017;••• doi: 10.1016/j.surg.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng J., Chu L.M., Dobrilovic N., Liu Y., Singh A.K., Sellke F.W. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with uncontrolled diabetes mellitus. United States: Surg; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ford T.J., Rocchiccioli P., Good R., et al. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur. Heart J. 2018;39(46):4086–4097. doi: 10.1093/eurheartj/ehy529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.House E.H., Berry C., Balachandran K.P., et al. Structure of the coronary circulation. Eur. Heart J. 2014;28(3):278–291. doi: 10.1093/eurheartj/ehl446. [DOI] [PubMed] [Google Scholar]
- 70.Stone P.H., Saito S., Takahashi S., et al. PREDICTION Investigators Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126(2):172–181. doi: 10.1161/CIRCULATIONAHA.112.096438. [DOI] [PubMed] [Google Scholar]
- 71.Koskinas K.C., Chatzizisis Y.S., Antoniadis A.P., Giannoglou G.D. Role of endothelial shear stress in stent restenosis and thrombosis: Pathophysiologic mechanisms and implications for clinical translation. J. Am. Coll. Cardiol. 2012;59(15):1337–1349. doi: 10.1016/j.jacc.2011.10.903. [DOI] [PubMed] [Google Scholar]
- 72.Diamond S.L., Sharefkin J.B., Dieffenbach C., Frasier-Scott K., McIntire L.V., Eskin S.G. Tissue plasminogen activator messenger RNA levels increase in cultured human endothelial cells exposed to laminar shear stress. J. Cell. Physiol. 1990;143(2):364–371. doi: 10.1002/jcp.1041430222. [DOI] [PubMed] [Google Scholar]
- 73.Ohyama K., Matsumoto Y., Amamizu H., et al. Association of coronary perivascular adipose tissue inflammation and drug-eluting stent-induced coronary hyperconstricting responses in pigs: 18F-fluorodeoxyglucose positron emission tomography imaging study. Arterioscler. Thromb. Vasc. Biol. 2017;37(9):1757–1764. doi: 10.1161/ATVBAHA.117.309843. [DOI] [PubMed] [Google Scholar]
- 74.Maruhashi T., Kihara Y., Higashi Y. Diabetes and endothelial dysfunction. Diabetes and Aging-related Complications; 2017. [Google Scholar]
- 75.Page AV, Liles WC. 2013.
- 76.Bhatta A., Yao L., Xu Z., et al. Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1. Cardiovasc. Res. 2017;113(13):1664–1676. doi: 10.1093/cvr/cvx164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borgi L., McMullan C., Wohlhueter A., Curhan G.C., Fisher N.D., Forman J.P. Effect of Vitamin D on endothelial function: A randomized, double-blind, placebo-controlled trial. Am. J. Hypertens. 2017;30(2):124–129. doi: 10.1093/ajh/hpw135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Juni R.P., Duckers H.J., Vanhoutte P.M., Virmani R., Moens A.L. Oxidative stress and pathological changes after coronary artery interventions. J. Am. Coll. Cardiol. 2013;61(14):1471–1481. doi: 10.1016/j.jacc.2012.11.068. [DOI] [PubMed] [Google Scholar]
- 79.Zalos G., Finkel T., Hill J.M., et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. Obstet. Gynecol. Surv. 2004;58(7):467–468. doi: 10.1097/01.ogx.0000074096.62998.d7. [DOI] [PubMed] [Google Scholar]
- 80.Schächinger V., Zeiher A.M. Atherogenesis--recent insights into basic mechanisms and their clinical impact. Nephrol. Dial. Transplant. 2002;17(12):2055–2064. doi: 10.1093/ndt/17.12.2055. [DOI] [PubMed] [Google Scholar]
- 81.Reriani M., Flammer A.J., Duhé J., et al. Coronary endothelial function testing may improve long-term quality of life in subjects with microvascular coronary endothelial dysfunction. Open Heart. 2019;6(1):e000870. doi: 10.1136/openhrt-2018-000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tagliamonte E., Rigo F., Cirillo T., et al. Effects of ranolazine on noninvasive coronary flow reserve in patients with myocardial ischemia but without obstructive coronary artery disease. Echocardiography. 2015;32(3):516–521. doi: 10.1111/echo.12674. [DOI] [PubMed] [Google Scholar]
- 83.Villano A., Di Franco A., Nerla R., et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am. J. Cardiol. 2013;112(1):8–13. doi: 10.1016/j.amjcard.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 84.Leenders G.J., Smeets M.B., van den Boomen M., et al. Statins promote cardiac infarct healing by modulating endothelial barrier function revealed by contrast-enhanced magnetic resonance imaging. Arterioscler. Thromb. Vasc. Biol. 2018;38(1):186–194. doi: 10.1161/ATVBAHA.117.310339. [DOI] [PubMed] [Google Scholar]
- 85.Hamasaki S., Tei C. Effect of coronary endothelial function on outcomes in patients undergoing percutaneous coronary intervention. J. Cardiol. 2011;57(3):231–238. doi: 10.1016/j.jjcc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Sandhu K., Mamas M., Butler R. Endothelial progenitor cells: Exploring the pleiotropic effects of statins. World J. Cardiol. 2017;9(1):1–13. doi: 10.4330/wjc.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Durante A. Role of no reflow and microvascular obstruction in the prognostic stratification of STEMI patients. Anatol. J. Cardiol. 2018;19(5):346–349. doi: 10.14744/AnatolJCardiol.2018.62343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karimianpour A., Maran A. Advances in coronary no-reflow phenomenon-a contemporary review. Curr. Atheroscler. Rep. 2018;20(9):44. doi: 10.1007/s11883-018-0747-5. [DOI] [PubMed] [Google Scholar]
- 89.Reffelmann T., Kloner R.A. The “no-reflow” phenomenon: Basic science and clinical correlates. Heart. 2002;87(2):162–168. doi: 10.1136/heart.87.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kloner R.A., Ganote C.E., Jennings R.B. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J. Clin. Invest. 1974;54(6):1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hausenloy D.J., Chilian W., Crea F., et al. The coronary circulation in acute myocardial ischaemia/reperfusion injury: A target for cardioprotection. Cardiovasc. Res. 2018;••• doi: 10.1093/cvr/cvy286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kloner RA, King KS, Harrington MG. No-reflow phenomenon in the heart and brain. 2018. [DOI] [PubMed]
- 93.De Bruyne B., Oldroyd K.G., Pijls N.H.J. Microvascular (Dys)function and clinical outcome in stable coronary disease. J. Am. Coll. Cardiol. 2016;67(10):1170–1172. doi: 10.1016/j.jacc.2015.11.066. [DOI] [PubMed] [Google Scholar]
- 94.Layland J., Carrick D., Lee M., Oldroyd K., Berry C. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc. Interv. 2014;7(6):581–591. doi: 10.1016/j.jcin.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 95.Doucette J.W., Corl P.D., Payne H.M., et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85(5):1899–1911. doi: 10.1161/01.CIR.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 96.AlBadri A., Wei J., Mehta P.K., et al. Acetylcholine vs. cold pressor testing for evaluation of coronary endothelial function. PLoS One. 2017;12(2):e0172538. doi: 10.1371/journal.pone.0172538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horigome M., Kumazaki S., Hattori N., et al. Noninvasive evaluation of coronary endothelial function following sirolimus-eluting stent implantation by using positron emission tomography. Cardiology. 2009;114(3):157–163. doi: 10.1159/000226093. [DOI] [PubMed] [Google Scholar]
- 98.Stuijfzand W.J., Schumacher S.P., Driessen R.S., et al. Myocardial blood flow and coronary flow reserve during 3 years following bioresorbable vascular scaffold vs. metallic drug-eluting stent implantation: The VANISH trial. JACC Cardiovasc. Interv. 2019;12(10):967–979. doi: 10.1016/j.jcin.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 99.Walborn A., Rondina M., Mosier M., et al. Diagnostic approach to patients with stable angina and no obstructive coronary arteries. J. Hypertens. 2019;25(4):1460–1467. doi: 10.15420/ecr.2019.22.2. [DOI] [Google Scholar]
- 100.Lanza G.A., Camici P.G., Galiuto L., et al. Gruppo di Studio di Fisiopatologia Coronarica e Microcircolazione, Società Italiana di Cardiologia. Methods to investigate coronary microvascular function in clinical practice. J. Cardiovasc. Med. (Hagerstown) 2013;14(1):1–18. doi: 10.2459/JCM.0b013e328351680f. [DOI] [PubMed] [Google Scholar]
- 101.Lanza G.A. Diagnostic approach to patients with stable angina and no obstructive coronary arteries. Eur. Cardiol. 2019;14(2):97–102. doi: 10.15420/ecr.2019.22.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hofma S.H., van der Giessen W.J., van Dalen B.M., et al. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur. Heart J. 2006;27(2):166–170. doi: 10.1093/eurheartj/ehi571. [DOI] [PubMed] [Google Scholar]
- 103.McKavanagh P., Zawadowski G., Ahmed N., Kutryk M. The evolution of coronary stents. Expert Rev. Cardiovasc. Ther. 2018;16(3):219–228. doi: 10.1080/14779072.2018.1435274. [DOI] [PubMed] [Google Scholar]
- 104.Merinopoulos I., Wickramarachchi U., Wardley J., et al. Day case discharge of patients treated with drug coated balloon only angioplasty for de novo coronary artery disease: A single center experience. Catheter. Cardiovasc. Interv. 2020;95(1):105–108. doi: 10.1002/ccd.28217. [DOI] [PubMed] [Google Scholar]
- 105.Iqbal J., Gunn J., Serruys P.W. Coronary stents: historical development, current status and future directions. Br. Med. Bull. 2013;106:193–211. doi: 10.1093/bmb/ldt009. [DOI] [PubMed] [Google Scholar]
- 106.Nawarskas J.J., Osborn L.A. Paclitaxel-eluting stents in coronary artery disease. Am. J. Health Syst. Pharm. 2005;62(21):2241–2251. doi: 10.2146/ajhp040621. [DOI] [PubMed] [Google Scholar]
- 107.Scheller B., Speck U., Abramjuk C., Bernhardt U., Böhm M., Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation. 2004;110(7):810–814. doi: 10.1161/01.CIR.0000138929.71660.E0. [DOI] [PubMed] [Google Scholar]
- 108.Sotomi Y., Suwannasom P., Tenekecioglu E., et al. Differential aspects between cobalt-chromium everolimus drug-eluting stent and absorb everolimus bioresorbable vascular scaffold: from bench to clinical use. Expert Rev. Cardiovasc. Ther. 2015;13(10):1127–1145. doi: 10.1586/14779072.2015.1089172. [DOI] [PubMed] [Google Scholar]
- 109.Brugaletta S., Cequier A., Alfonso F., et al. MAGnesium-based bioresorbable scaffold and vasomotor function in patients with acute ST segment elevation myocardial infarction: The MAGSTEMI trial: Rationale and design. Catheter. Cardiovasc. Interv. 2019;93(1):64–70. doi: 10.1002/ccd.27825. [DOI] [PubMed] [Google Scholar]
- 110.Ormiston J.A., De Vroey F., Serruys P.W., Webster M.W.I. Bioresorbable polymeric vascular scaffolds: A cautionary tale. Circ. Cardiovasc. Interv. 2011;4(5):535–538. doi: 10.1161/CIRCINTERVENTIONS.111.963710. [DOI] [PubMed] [Google Scholar]
- 111.Jeger R.V., Farah A., Ohlow M.A., et al. BASKET-SMALL 2 Investigators. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet. 2018;392(10150):849–856. doi: 10.1016/S0140-6736(18)31719-7. [DOI] [PubMed] [Google Scholar]
- 112.Merinopoulos I., Gunawardena T., Wickramarachchi U., Ryding A., Eccleshall S., Vassiliou V.S. Percutaneous coronary intervention in the elderly: Are drug-coated balloons the future? Curr. Cardiol. Rev. 2018;14(1):45–52. doi: 10.2174/1573403X14666171226144120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Corballis N.H., Wickramarachchi U., Vassiliou V.S., Eccleshall S.C. Duration of dual antiplatelet therapy in elective drug-coated balloon angioplasty. Catheter. Cardiovasc. Interv. 2019;1-5 doi: 10.1002/ccd.28632. [DOI] [PubMed] [Google Scholar]
- 114.Komaru T., Isoyama S., Sekiguchi N., et al. Coronary angioplasty ameliorates hypoperfusion-induced endothelial dysfunction in patients with stable angina pectoris. J. Am. Coll. Cardiol. 1996;27(1):30–37. doi: 10.1016/0735-1097(95)00441-6. [DOI] [PubMed] [Google Scholar]
- 115.Caramori P.R., Lima V.C., Seidelin P.H., Newton G.E., Parker J.D., Adelman A.G. Long-term endothelial dysfunction after coronary artery stenting. J. Am. Coll. Cardiol. 1999;34(6):1675–1679. doi: 10.1016/S0735-1097(99)00411-8. [DOI] [PubMed] [Google Scholar]
- 116.Togni M., Windecker S., Cocchia R., et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J. Am. Coll. Cardiol. 2005;46(2):231–236. doi: 10.1016/j.jacc.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 117.Togni M., Räber L., Cocchia R., et al. Local vascular dysfunction after coronary paclitaxel-eluting stent implantation. Int. J. Cardiol. 2007;120(2):212–220. doi: 10.1016/j.ijcard.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 118.Knight D.R., Shen Y.T., Young M.A., Vatner S.F. Acetylcholine-induced coronary vasoconstriction and vasodilation in tranquilized baboons. Circ. Res. 1991;69(3):706–713. doi: 10.1161/01.RES.69.3.706. [DOI] [PubMed] [Google Scholar]
- 119.Ludmer P.L., Selwyn A.P., Shook T.L., et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N. Engl. J. Med. 1986;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 120.Fuke S., Maekawa K., Kawamoto K., et al. Impaired endothelial vasomotor function after sirolimus-eluting stent implantation. Circ. J. 2007;71(2):220–225. doi: 10.1253/circj.71.220. [DOI] [PubMed] [Google Scholar]
- 121.Kubo M., Miyoshi T., Oe H., Ohno Y., Nakamura K., Ito H. Prognostic significance of endothelial dysfunction in patients undergoing percutaneous coronary intervention in the era of drug-eluting stents. BMC Cardiovasc. Disord. 2015;15(1):102. doi: 10.1186/s12872-015-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gogas B.D., Benham J.J., Hsu S., et al. Vasomotor function comparative assessment at 1 and 2 years following implantation of the absorb everolimus-eluting bioresorbable vascular scaffold and the xience V everolimus-eluting metallic stent in porcine coronary arteries: Insights from in vivo angiography, ex vivo assessment, and gene analysis at the stented/scaffolded segments and the proximal and distal edges. JACC Cardiovasc. Interv. 2016;9(7):728–741. doi: 10.1016/j.jcin.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 123.Serruys P.W., Chevalier B., Sotomi Y., et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): A 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388(10059):2479–2491. doi: 10.1016/S0140-6736(16)32050-5. [DOI] [PubMed] [Google Scholar]
- 124.Gomez-Lara J., Salvatella N., Romaguera R., et al. Collaborators. A randomized comparison of the coronary vasomotor function and myocardial flow in patients treated with everolimus-eluting bioresorbable scaffolds and everolimus-eluting metallic stents. EuroIntervention. 2020;16(2):e155–e163. doi: 10.4244/EIJ-D-18-01203. [DOI] [PubMed] [Google Scholar]
- 125.Sabaté M., Alfonso F., Cequier A., et al. Magnesium-based resorbable scaffold vs. permanent metallic sirolimus-eluting stent in patients with st-segment elevation myocardial infarction: the magstemi randomized clinical trial. Circulation. 2019;140(23):1904–1916. doi: 10.1161/CIRCULATIONAHA.119.043467. [DOI] [PubMed] [Google Scholar]
- 126.Cassese S., Byrne R.A., Ndrepepa G., et al. Everolimus-eluting bioresorbable vascular scaffolds vs. everolimus-eluting metallic stents: A meta-analysis of randomised controlled trials. Lancet. 2016;387(10018):537–544. doi: 10.1016/S0140-6736(15)00979-4. [DOI] [PubMed] [Google Scholar]
- 127.Ludman PF. Percutaneous coronary intervention. 2018.
- 128.Sousa-Uva M., Neumann F.J., Ahlsson A., et al. ESC/EACTS Guidelines on myocardial revascularization. Eur. J. Cardiothorac. Surg. 2018;2019 doi: 10.1093/ejcts/ezy289. [DOI] [Google Scholar]