Abstract
Objective:
Many patients with coronavirus disease 2019 (COVID-19) need mechanical ventilation secondary to acute respiratory distress syndrome. Information on the respiratory system mechanical characteristics of this disease is limited. The aim of this study is to describe the respiratory system mechanical properties of ventilated COVID-19 patients.
Design, Setting, and Patients:
Patients consecutively admitted to the medical intensive care unit at the University of Iowa Hospitals and Clinics in Iowa City, USA, from April 19 to May 1, 2020, were prospectively studied; final date of follow-up was May 1, 2020.
Measurements:
At the time of first patient contact, ventilator information was collected including mode, settings, peak airway pressure, plateau pressure, and total positive end expiratory pressure. Indices of airflow resistance and respiratory system compliance were calculated and analyzed.
Main Results:
The mean age of the patients was 58 years. 6 out of 12 (50%) patients were female. Of the 21 laboratory-confirmed COVID-19 patients on invasive mechanical ventilation, 9 patients who were actively breathing on the ventilator were excluded. All the patients included were on volume-control mode. Mean [±standard deviation] ventilator indices were: resistive pressure 19 [±4] cmH2O, airway resistance 20 [±4] cmH2O/L/s, and respiratory system static compliance 39 [±16] ml/cmH2O. These values are consistent with abnormally elevated resistance to airflow and reduced respiratory system compliance. Analysis of flow waveform graphics revealed a pattern consistent with airflow obstruction in all patients.
Conclusions:
Severe respiratory failure due to COVID-19 is regularly associated with airflow obstruction.
Keywords: COVID-19, mechanical ventilation, atypical, respiratory mechanics, obstruction, airway resistance
Introduction
In December 2019, the first patients with pneumonia due to a new species of coronavirus (novel coronavirus, now named SARS-CoV-2) were admitted to hospitals in Wuhan, China.1 Since then, SARS-CoV-2 virus has spread across the world, leading to a pandemic of the associated disease, COVID-19. A significant proportion of patients with COVID-19 develop severe respiratory disease and may require admission to the intensive care unit (ICU) and mechanical ventilation.2–4
Patients with COVID-19 pneumonia requiring mechanical ventilation typically fulfill the diagnostic criteria for the acute respiratory distress syndrome (ARDS).5 Some authors pointed out that a substantial fraction of patients with COVID-19 pneumonia exhibit features quite atypical for ARDS. For example, gas exchange may be impaired out of proportion to mechanical abnormalities (“happy hypoxia”)6 and respiratory system compliance is relatively preserved.7 Gattinoni and colleagues have hypothesized 2 distinct phenotypes of lung failure in COVID-19: L-type with low elastance and H-type with high elastance.8 These peculiar features may affect treatment choices, such as levels of positive end expiratory pressure (PEEP) or strategies for delivering lung-protective ventilation. More fundamentally, these characteristics offer clues to pathophysiology of lung failure in COVID-19 pneumonia and could point to new therapies.
In our experience managing patients with COVID-19, we noticed another unusual feature: airflow obstruction was typically evident. Ventilator graphics display pressure and flow waveforms as a function of time, allowing for prompt recognition of this phenomenon. The typical findings of airflow obstruction, including elevated peak airway pressure, abnormal resistive pressure (peak minus plateau airway pressure, Presist), and an abnormal expiratory flow profile (low expiratory flow rate with flow persisting late in the expiratory phase, sometimes with evident end-expiratory flow) were present so often that we hypothesize that airflow obstruction is an integral part of COVID-19-related lung failure.
Materials and Methods
Ethics
The project was approved by the Institutional Review Board (IRB). Waiver of informed consent was granted.
Patient Selection, Setting, and Data Acquisition
Adult subjects with ARDS due to COVID-19 pneumonia ventilated in the medical ICU between April 19 and May 1, 2020 were prospectively identified during usual ICU care. ARDS was defined according to the Berlin criteria. All subjects were passively breathing on volume-control mode at the time of contact. A heat moisture exchange (HME) filter was used in all ventilator circuits. Ventilator settings and patient-ventilator interaction measurements were obtained and documented in the electronic health record (EHR), as were screenshots of ventilator waveforms. The following additional data were collected:
Patient information including age, sex, virologic testing results including SARS-CoV2 and respiratory viral panel, duration of mechanical ventilation, body mass index (BMI), history of prior lung disease, smoking history and prior pulmonary function test results.
Prone vs supine position.
Use of neuromuscular blocking agents (NMB).
Ventilator information including settings (tidal volume (Vt), respiratory rate, fraction of inspired oxygen (FiO2), positive end-expiratory pressure (PEEP)), peak pressure (Ppeak), plateau pressure (Pplat), total PEEP (PEEPtot), and ventilator graphics.
Institutional recommendations were consistent with evidence-based care for ARDS, including low tidal volume ventilation, maintenance of plateau pressures less than 30 cm H2O, driving pressure of 15 cm H2O or less, and conservative fluid therapy. Prone positioning was utilized for severe hypoxemia (e.g. ratio of partial pressure of oxygen on arterial blood gas to fraction of inspired oxygen (PaO2/FiO2) less than 150, or ratio of oxygen saturation on pulse oximetry to FiO2 (SaO2/FiO2) less than 180). NMB was initiated for significant patient-ventilator dyssynchrony. PEEP titration and other aspects of patient management were at the discretion of the ICU attending physician.
Measurements and Definitions
The Pplat and PEEPtot were measured by performing manual inspiratory and expiratory hold maneuvers respectively for at least 0.4 seconds. Airway resistive pressure (Presist) was calculated as the difference between Ppeak and Pplat. Airway resistance was calculated as Ppeak minus Pplat divided by inspiratory flow rate. Driving pressure was calculated as Pplat minus PEEPtot. AutoPEEP was calculated as the difference between PEEPtot and set PEEP. Static respiratory system compliance (Cstat) was calculated as Vt divided by driving pressure.
Statistical Analysis
Quantitative continuous variables were reported as mean ± standard deviation. Categorical variables were reported as counts and percentages. Descriptive statistics were used to summarize clinical data.
Results
Patients, Clinical Characteristics, and Ventilator Settings
Between April 19, 2020 and May 1, 2020, 21 patients were admitted to the medical ICU with ARDS due to COVID-19, 12 of whom were passively ventilated. Patient characteristics are reported in Table 1. The mean age was 58 and 50% (6/12) were men. Most of the subjects were non-smokers (8/12) and had no known history of pulmonary disease (10/12). The average duration between intubation and time of data collection was 1.7 days. Half (6/12) of the patients were receiving NMB at the time of data collection. All were ventilated in the supine position. None were receiving inhaled pulmonary vasodilators or extracorporeal membrane oxygenation (ECMO). One patient received inhaled bronchodilators in the 24 hours preceding data collection. No patients had significant secretions. No prior PFTs were available for any of the subjects. Ventilator settings are summarized in Table 2.
Table 1.
Demographic and Clinical Characteristics of the Patients.
| Patient no. | Age | Sex | BMI | Smoking history | History of COPD/asthma | Other chronic pulmonary diseases | Number of days since intubation |
|---|---|---|---|---|---|---|---|
| Patient 1 | 67 | F | 33.22 | Never smoked | None | None | 2 |
| Patient 2 | 62 | F | 49.04 | Unknown | COPD | None | 3 |
| Patient 3 | 59 | M | 45.64 | Former smoker | None | None | 3 |
| Patient 4 | 64 | M | 32.45 | Never smoked | None | None | 1 |
| Patient 5 | 61 | M | 32.03 | Never smoked | None | None | 2 |
| Patient 6 | 61 | F | 28.12 | Never smoked | Asthma | Ehlers-Danlos syndrome | 2 |
| Patient 7 | 44 | M | 35.26 | Unknown | None | None | 12 |
| Patient 8 | 35 | M | 46.23 | Never smoked | None | None | 3 |
| Patient 9 | 74 | F | 23.56 | Never smoked | None | History of pulmonary embolism | 0 |
| Patient 10 | 78 | M | 27.14 | Never smoked | None | None | 0 |
| Patient 11 | 52 | F | 41.14 | Former smoker | None | None | 2 |
| Patient 12 | 43 | F | 25.09 | Never smoked | None | None | 1 |
| Mean (±SD) | 58 (±13) | 35 (±9) | 3 (±3) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Table 2.
Ventilator Settings Selected by the Attending Physician or Respiratory Therapist.
| Patient no. | Set RR (per minute) | Set Vt (ml) | Set FiO2 (%) | Set PEEP (cm H2O) | Set flow rate (L/min) | Minute ventilation (L/min) |
|---|---|---|---|---|---|---|
| Patient 1 | 34 | 300 | 35 | 14 | 45 | 10 |
| Patient 2 | 30 | 380 | 40 | 12 | 57 | 11 |
| Patient 3 | 32 | 370 | 40 | 12 | 55 | 12 |
| Patient 4 | 32 | 410 | 40 | 8 | 54 | 13 |
| Patient 5 | 32 | 380 | 45 | 12 | 57 | 12 |
| Patient 6 | 30 | 350 | 60 | 14 | 60 | 11 |
| Patient 7 | 36 | 280 | 100 | 15 | 56 | 10 |
| Patient 8 | 24 | 420 | 100 | 15 | 56 | 10 |
| Patient 9 | 32 | 350 | 50 | 10 | 53 | 11 |
| Patient 10 | 32 | 350 | 100 | 12 | 60 | 11 |
| Patient 11 | 30 | 300 | 40 | 14 | 60 | 9 |
| Patient 12 | 32 | 320 | 40 | 14 | 55 | 10 |
| Mean (±SD) | 31 (±3) | 351 (±44) | 58 (±26) | 13 (±2) | 56 (±4) | 11 (±1) |
Abbreviations: FiO2, fraction of inspired oxygen; PEEP, positive end expiratory pressure; RR, respiratory rate; Vt, tidal volume.
Patient-Ventilator Interaction and Ventilator Waveforms
Respiratory system mechanics are shown in Table 3. All patients had increased airway resistance, illustrated by Ppeak and Presist that were significantly elevated at 42.3 [±5.26] cm H2O and 18.8 [±3.60] cm H2O respectively. Calculated airway resistance was markedly increased at 20.4 [±4.13] cm H2O/L/sec. AutoPEEP was minimally elevated at 1 [±1] cm H2O. Driving pressure was 9.92 [±2.75] cm H2O. Static compliance of the respiratory system was 39.0 [±15.6] ml/cm H2O. Examination of the ventilator waveforms, specifically expiratory flow curve, indicated the presence of airflow obstruction (Figures 1 -3).
Table 3.
Ventilator-Derived Indices of Airway Resistance and Respiratory System Compliance.
| Patient no. | Ppeak (cm H2O) | Pplat (cm H2O) | AutoPEEP (cm H2O)* | Presist (cm H2O)* | Airway resistance (cmH2O/L/sec)* | Driving pressure (cm H2O)* | Cstat (ml/cmH2O)* |
|---|---|---|---|---|---|---|---|
| Patient 1 | 45 | 25 | 1 | 20 | 26.4 | 10 | 30 |
| Patient 2 | 44 | 23 | 1 | 21 | 22.2 | 10 | 38 |
| Patient 3 | 37 | 24 | 0 | 13 | 14.4 | 12 | 30.83 |
| Patient 4 | 34 | 19 | 4 | 15 | 16.8 | 7 | 58.57 |
| Patient 5 | 38 | 21 | 4 | 17 | 18 | 5 | 76 |
| Patient 6 | 43 | 27 | 1 | 16 | 16.2 | 12 | 29.16 |
| Patient 7 | 51 | 29 | –1 | 22 | 23.4 | 15 | 18.67 |
| Patient 8 | 48 | 24 | 0 | 24 | 25.71 | 9 | 46.67 |
| Patient 9 | 40 | 20 | 1 | 20 | 22.86 | 9 | 38.89 |
| Patient 10 | 49 | 25 | 1 | 24 | 24 | 12 | 29.17 |
| Patient 11 | 38 | 21 | 0 | 17 | 17 | 7 | 42.86 |
| Patient 12 | 41 | 25 | 0 | 16 | 17.49 | 11 | 29 |
| Mean (±SD) | 42 (±5) | 24 (±3) | 1 (±1) | 19 (±4) | 20 (±4) | 10 (±3) | 39 (±16) |
Abbreviations: Cstat, static compliance; Ppeak, peak pressure; Pplat, plateau pressure; Presist, resistive pressure.
* Calculation of indices: AutoPEEP = intrinsic PEEP – extrinsic PEEP; Presist = Ppeak – Pplat; airway resistance = (Ppeak – Presist)/flow rate; driving pressure = Pplat – intrinsic PEEP; Cstat = Vt/driving pressure.
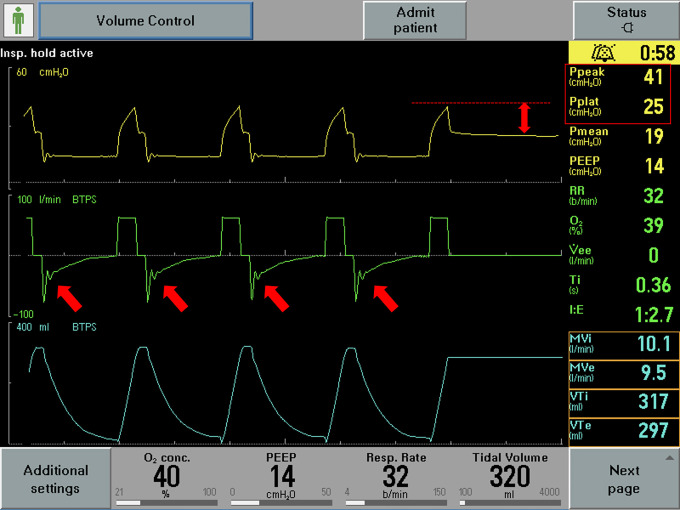
Figure 1.
Representative image of ventilator waveforms. The expiratory flow waveform demonstrates rapid flow deceleration followed by slow return to baseline (arrows). Also note the abnormal Ppeak minus Pplat (double-headed arrow and right-hand side of the pane).
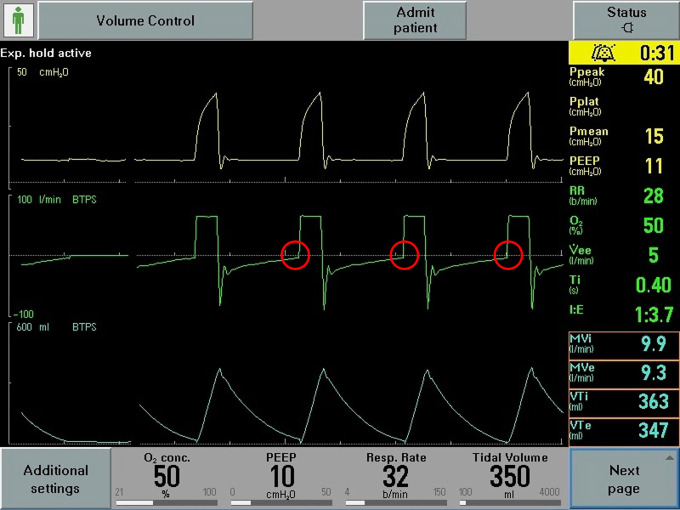
Figure 2.
Representative image of ventilator waveforms. Expiratory flow present at end-expiration (circles).
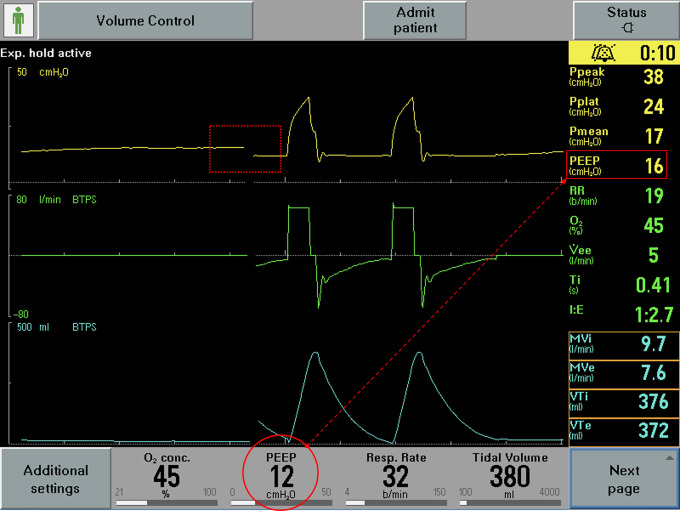
Figure 3.
Representative image of ventilator waveforms. Intrinsic PEEP unmasked by manual expiratory hold maneuver (dotted rectangle; circle at bottom of the pane represents operator set PEEP and rectangle on the right-hand side of the pane represents PEEPtot).
Discussion
In mechanically ventilated patients, breath-by-breath measurement of pressure and flow allows characterization of the mechanical properties of the respiratory system, such as compliance and resistance. Such measurements revealed an unusual finding in our patients with COVID-19 ARDS: airways resistance was elevated in all subjects. This novel finding has not been previously reported to our knowledge. Airways resistance is modestly elevated in ARDS,9 but generally not to the degree seen in our patients.
Our findings show that peak pressures were significantly elevated in all subjects. Further, this was from a combination of both decreased compliance leading to elevated plateau pressures, and increased resistance to airflow. The latter is indicated by the elevated Presist and airway resistance. While decreased compliance would be an expected finding in ARDS secondary to COVID-19, the elevated airway resistance to this degree is unusual and unexpected. Although there was individual variation in the magnitude of resistive indices, they were elevated in all subjects.
There are several challenges which make interpretation of the results difficult—most importantly, lack of a control group. Additionally, we considered the possibility that our findings were due to the effects of the ventilator circuit. We assessed whether the HME filter, used to filter viral particles, was responsible for the abnormal resistance. Older literature reported increased airway resistance with the use of filters in ventilator circuits, especially with high minute ventilation.10,11 The manufacturer of the filter we use (Intersurgical ltd, Inter-ThermTM range, model number: 1341031 S)12 claims that it adds a resistance of 2.7 cmH2O at a flow rate of 60 L/min. The mean flow rate for our subjects was 56 L/min, therefore only a small fraction of the resistive pressure we measured can be accounted for by the filter. Moreover, in one non-study subject, we briefly removed the HME filter to determine its impact, finding little change in Ppeak, Presist, or expiratory flow shape. We also conducted an experiment using the HME filter on a simulator (Michigan Instruments 5600i Dual Adult TTL Training/Test Lung) at 2 different flow rates and found that the filter added a resistance of 2 cmH2O/L/sec at any given flow. The endotracheal tube itself presents a resistive load, but this would be insufficient to explain resistance of sufficient magnitude.13 In our cohort of patients, the smallest size of endotracheal tube used was 7 millimeters (internal diameter). We assume that increased Presist reflects an increase in the Ohmic component of resistance rather than the viscoelastic fraction. We believe it unlikely that substantially increased viscoelastic resistance accounts for our findings since we used a rather brief (0.4 s) end-inspiratory pause. Given the complexity of patient-ventilator interactions, further confirmatory studies are needed to address the issue of airway resistance in patients ventilated with heated humidification systems.
An alternative explanation to account for our finding of slowed expiratory flow is expiratory braking. The post-inspiratory complex, which controls the transition from inspiration to expiration, could act to limit expiratory flow, mimicking expiratory airflow obstruction.14,15 SARS-CoV-2 is a neurotropic virus and could impact the respiratory centers directly. We think this explanation is unlikely since it would not also explain the elevation in inspiratory Presist.
Finally, the mean BMI of patients in our study is 35. Airways resistance is modestly elevated in obesity,16 but this cannot explain the degree of elevation in our patients, nor account for the presence of obstruction in all of them.
We believe it is more likely that increased airways resistance is an intrinsic feature of severe COVID-19 lung disease. There is precedent for viral pneumonias to produce a resistive lesion, most notably with infection due to respiratory syncytial virus.17 The anatomic site of obstruction is uncertain, although some patients have increased bronchomotor tone. Others exhibit airflow obstruction due to neutrophil extracellular traps (NETs), webs of extracellular chromatin, microbicidal proteins, and oxidant enzymes in small airways, as has been seen in RSV and other pneumonias.18,19 Evidence for the presence of NETs in patients with COVID-19, especially in those with more severe disease, has been published. Further, serum from patients with COVID-19 triggers NET release from control neutrophils in vitro.20
Radiographic surveys of patients with COVID-19 have identified airway abnormalities, including bronchial wall thickening.21 These findings are more evident in the most severely afflicted patients.22 Few autopsy studies have been published, but peribronchial lymphocytic accumulation, connective tissue within bronchioles, and granulocytic infiltration of bronchi have been described (in addition to other findings).23,24 Each of these provides a potential structural basis for the physiological findings we describe.
Recognition of airflow obstruction in COVID-19 pneumonia has potential implications for therapy. Heliox lowers the resistive pressure where flow is turbulent,25–27 and can ameliorate respiratory distress in infants with RSV bronchiolitis.28 One case report describes successful treatment of airflow obstruction associated with coronavirus OC43 in a child with high-flow heliox administration.29 Heliox is most effective when the fraction of helium is high, but the modest degree of hypoxemia seen in many COVID-19 patients may allow its use. If NETs play a causal role in the pathogenesis of COVID-19 respiratory failure, aerosolized dornase might bear consideration,30 as has been reported in other subtypes of ARDS.31 Bronchodilator use, preferably via in-line metered dose inhalers, could also be considered, especially when significant airflow obstruction interferes with ventilation (for example, when there is evidence of developing auto-PEEP). Recently, a case series showed excellent tolerability of nebulized in-line dornase and albuterol in mechanically ventilated patients with COVID-19.32
Not long after the initial description of ARDS, John Murray warned against lumping multiple diseases into one syndrome,33 thereby “detracting from important and distinctive differences in pathogenesis, therapy, and prognosis.” Forty-five years later, and only months following Dr. Murray’s death from COVID-19, his words ring true.
Conclusion
Severe respiratory failure due to COVID-19 is regularly associated with airflow obstruction. This may have implications for the pathogenesis of respiratory failure and suggests potential therapies.
Acknowledgments
The study could not have been completed without the expertise of Justin Kuhn, BS, RRT, University of Iowa Hospitals and Clinics, Iowa City, IA. We would like to thank Justin for helping us with downloading and transferring data from patient ventilators and also for providing us with information on ventilator filters.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Dr. Schmidt reports financial relationship in the form of royalty with the following entities: McGraw-Hill, UpToDate, and Elsevier.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Maksym Puliaiev, MD  https://orcid.org/0000-0001-6951-9140
https://orcid.org/0000-0001-6951-9140
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 6. Tobin MJ. Basing respiratory management of coronavirus on physiological principles. Am J Respir Crit Care Med. 2020;201(11):1319–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright PE, Bernard GR. The role of airflow resistance in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1989;139(5):1169–1174. [DOI] [PubMed] [Google Scholar]
- 10. Buckley PM. Increase in resistance of in-line breathing filters in humidified air. Br J Anaesth. 1984;56(6):637–643. [DOI] [PubMed] [Google Scholar]
- 11. Cohen IL, Weinberg PF, Fein IA, Rowinski GS. Endotracheal tube occlusion associated with the use of heat and moisture exchangers in the intensive care unit. Crit Care Med. 1988;16(3):277–279. [DOI] [PubMed] [Google Scholar]
- 12. Intersurgical. Inter-Therm™ and Inter-Therm™ Mini. Published August 2019. Accessed February 26, 2021. https://us.intersurgical.com/content/files/113418/658477610.
- 13. Wright PE, Marini JJ, Bernard GR. In vitro versus in vivo comparison of endotracheal tube airflow resistance. Am Rev Respir Dis. 1989;140(1):10–16. [DOI] [PubMed] [Google Scholar]
- 14. Jonkman AH, de Vries HJ, Heunks LMA. Physiology of the respiratory drive in ICU patients: implications for diagnosis and treatment. Crit Care. 2020;24(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pellegrini M, Hedenstierna G, Roneus A, Segelsjö M, Larsson A, Perchiazzi G. The diaphragm acts as a brake during expiration to prevent lung collapse. Am J Respir Crit Care Med. 2017;195(12):1608–1616. [DOI] [PubMed] [Google Scholar]
- 16. Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87(3):654–660. [DOI] [PubMed] [Google Scholar]
- 17. Hall WJ, Hall CB, Speers DM. Respiratory syncytial virus infection in adults: clinical, virologic, and serial pulmonary function studies. Ann Intern Med. 1978;88(2):203–205. [DOI] [PubMed] [Google Scholar]
- 18. Cortjens B, de Boer OJ, de Jong R, et al. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J Pathol. 2016;238(3):401–411. [DOI] [PubMed] [Google Scholar]
- 19. Cortjens B, de Jong R, Bonsing JG, van Woensel JBM, Antonis AFG, Bem RA. Local dornase alfa treatment reduces NETs-induced airway obstruction during severe RSV infection. Thorax. 2018;73(6):578–580. [DOI] [PubMed] [Google Scholar]
- 20. Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11):e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30(8):4381–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55(6):327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Copin MC, Parmentier E, Duburcq T, Poissy J, Mathieu D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020;46(6):1124–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients With COVID-19. Ann Intern Med. 2020;173(12):1030. [DOI] [PubMed] [Google Scholar]
- 25. Kudukis TM, Manthous CA, Schmidt GA, Hall JB, Wylam ME. Inhaled helium-oxygen revisited: effect of inhaled helium-oxygen during the treatment of status asthmaticus in children. J Pediatr. 1997;130(2):217–224. [DOI] [PubMed] [Google Scholar]
- 26. Manthous CA, Hall JB, Caputo MA, et al. Heliox improves pulsus paradoxus and peak expiratory flow in nonintubated patients with severe asthma. Am J Respir Crit Care Med. 1995;151(2 Pt 1):310–314. [DOI] [PubMed] [Google Scholar]
- 27. Mink SN, Wood LD. How does HeO2 increase maximum expiratory flow in human lungs? J Clin Invest. 1980;66(4):720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liet JM, Ducruet T, Gupta V, Cambonie G. Heliox inhalation therapy for bronchiolitis in infants. Cochrane Database Syst Rev. 2015;(9):Cd006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morgan SE, Vukin K, Mosakowski S, et al. Use of heliox delivered via high-flow nasal cannula to treat an infant with coronavirus-related respiratory infection and severe acute air-flow obstruction. Respir Care. 2014;59(11):e166–170. [DOI] [PubMed] [Google Scholar]
- 30. Earhart AP, Holliday ZM, Hofmann HV, Schrum AG. Consideration of dornase alfa for the treatment of severe COVID-19 acute respiratory distress syndrome. New Microbes New Infect. 2020;35:100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pottecher J, Noll E, Borel M, et al. Protocol for TRAUMADORNASE: a prospective, randomized, multicentre, double-blinded, placebo-controlled clinical trial of aerosolized dornase alfa to reduce the incidence of moderate-to-severe hypoxaemia in ventilated trauma patients. Trials. 2020;21(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weber AG, Chau AS, Egeblad M, et al. Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID-19 patients: a case series. Mol Med. 2020;26(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murray JF. Editorial: the adult respiratory distress syndrome (may it rest in peace). Am Rev Respir Dis. 1975;111(6):716–718. [DOI] [PubMed] [Google Scholar]