Abstract
Objective
Traumatic brain injury (TBI) is a leading cause of epilepsy. Our aim was to characterise the risk of epilepsy in adults after hospitalisation for TBI.
Methods
Register-based cohort study. All individuals aged 18–100 with a first hospitalisation for TBI in the comprehensive national patient register in Sweden between 2000 and 2010 (n=111 947) and three controls per exposed (n=325 881), matched on age and sex were included. Exposed individuals were categorised according to TBI severity. Kaplan-Meier curves were used to estimate the risk of epilepsy and Cox regression to estimate the hazard in univariate or multivariate regression.
Results
The 10-year risk of epilepsy was 12.9% (95% CI 11.7% to 14.1%) for focal cerebral injuries, 8.1% (95% CI 7.5% to 8.7%) for diffuse cerebral injuries, 7.3% (95% CI 6.9% to 7.7%) for extracerebral injuries, 2.8% (95% CI 2.4% to 3.2%) for skull fractures and 2.6% (95% CI 2.4% to 2.8%) for mild TBI. The risk of epilepsy after any TBI was 4.0% (95% CI 3.8% to 4.2%). The corresponding 10-year risk for controls was 0.9% (95% CI 0.9% to 0.9%). The HR increased with a more severe injury, from 3.0 (95% CI 2.8 to 3.2) for mild injury to 16.0 (95% CI 14.5 to 17.5) for focal cerebral injury. Multivariable analyses identified central nervous system (CNS) comorbidities as risk factors, but TBI remained significant also after adjustment for these. Other identified risk factors were male sex, age, mechanical ventilation and seizure during index hospitalisation.
Conclusion
The risk of post-traumatic epilepsy is considerable, also with adjustments for CNS comorbidities.
Introduction
Post-traumatic epilepsy (PTE) accounts for 20% of the symptomatic and 3%–6% of all new-onset epilepsy.1 Previous population-based studies of PTE include a study in Minnesota of patients between 1935 and 1984 and a study of children and young adults in Denmark born 1977–2002, which both demonstrated increased risk of PTE with increasing severity of the brain injury.2 3 An increased risk of epileptic seizures after traumatic brain injury (TBI) was also identified in inhabitants of Stockholm, Sweden, in 2000–2008.4 A recent meta-analysis identified severity of the trauma, male sex, acute symptomatic seizures and comorbidities including alcohol abuse as risk factors, but the literature was considered heterogeneous with regard to many of the investigated risk factors.5 Many of the included studies were case-control investigations, with associated weaknesses regarding temporal associations between exposure to risk factors and outcome. A recent large study based on insurance claims6 found a twofold increased risk of epilepsy after TBI, but there is a paucity of studies from countries with comprehensive patient registers. In the present study, we used national registers to follow a cohort of all adults hospitalised for brain trauma in Sweden between 2000 and 2010 and investigated development of subsequent epilepsy. In addition to estimating the epilepsy risk after different kinds of brain trauma and identifying risk factors, the register-based approach allowed detection of other brain disorders that are important confounders of epilepsy risk.
Material and methods
Registers and cohort
This was a cohort study using Swedish national comprehensive registers based on the personal identification number unique to all Swedish inhabitants; the National Patient Register (NPR) and the Cause of Death Register (CDR), managed by the National Board of Health and Welfare, and the population register managed by Statistics Sweden (the government agency responsible for official statistics in Sweden). Reporting to the NPR and CDR is compulsory for all healthcare providers. The NPR contains information on all hospital inpatient care since 1987 and specialised outpatient care since 2001, with improvements in coverage until 2005. The National Board of Health and welfare identified all patients aged 18 or above with a first hospitalisation for head trauma, defined as occurrence of an International Classification of Disease (ICD)−10 diagnostic code of S06, S02.0–02.1, S02.7 or S02.9 in the years 2000–2010. Statistics Sweden selected three age-matched and sex-matched controls for each exposed, except for 287 exposed where controls could not be identified. On this initial material of 509 684 individuals, we applied the following exclusion criteria: epilepsy before index date (n=11 757), corresponding control, to exposed who had epilepsy before index date (n=19 371), previous trauma in controls (n=6353), age >100 (n=300), death within 30 days of index (n=23 552) and potential reuse of the personal identification number (n=388). From these 115 255 exposed and 335 527 controls, we excluded exposed with codes of both mild trauma/concussion and structural damage (uncertainty regarding severity/potential coding error), with corresponding controls. The final cohort consisted of 111 947 exposed and 325 881 controls.
Definitions
Information on trauma, epilepsy, comorbidities and risk factors was obtained from the NPR. The index trauma was categorised based on the ICD-10 code, as previously described by Christensen, but with a subdivision of the structural injuries.3
Injuries were categorised into mild injury (S060), extracerebral injury (S064, S065, S066), focal cerebral injury (S063), diffuse cerebral injury (S061, S062, S067, S068, S069) or fracture (S020, S021, S027, S029). In the case of several codes, exposed were categorised according to the code inferring highest epilepsy risk. Epilepsy was defined seas occurrence of ICD9 code 345 except 345Q, or ICD10 G40, meeting the definition of probable epilepsy recommended by the International League against Epilepsy.7 Seizure was defined as occurrence of ICD-9 780D or ICD-10 R568. Status epilepticus was defined as occurrence of ICD9 345Q or ICD10 G41. Central nervous system (CNS) comorbidities were defined by occurrence relevant codes; stroke (ICD9: 430–436, or ICD10: I60–I64), brain tumour (ICD 9: 191 225 198D 198E 237F 239G ICD 10: C71 C793 D430 D32 D330) and brain infections (ICD 9: 006F, 013, 036A, 036B, 045–049, 052B, 053A, 053B, 054D, 054H, 055A, 056A, 062–064, 072B, 072C, 094, 136C, 320–325, ICD 10: A06.6, A17, A39, A80–A89, B00.3, B00.4, B01.0, B01.1, B02.0, B02.1, B05.0, B05.1, B06.0, B22.0, B26.1, B26.2, B37.5, B38.4, B43.1, B50.0, B58.2, B60.2, G00–G08, R29.1). In one sensitivity analysis, we identified coregistered overconsumption of alcohol (ICD 9: 291A, 291B, 291C, 291D, 291E, 291W, 291X, 303X, 305A ICD 10: F10.).
We defined risk factors as occurrence of codes for mechanical ventilation (DG020-026 or GBB00 of the index admission), acute symptomatic seizure (R568 or G41 at the index admission). The causes of trauma were categorised by ICD-codes; fall injury W00–W19, transportation V01–99, violence, including suicide X60–Y09, Y35–36, W50–52 and W32–34.
Statistical analyses
All analyses were performed in SPSS V.25, except for the competing risk analysis (proportional subdistribution hazards model) and the multivariable analysis of the overall HR for epilepsy which were performed in SAS, V.9.4. In Kaplan-Meier (KM) and Cox regression analysis of epilepsy risk, time was calculated from the index admission to epilepsy or censoring at death or end of study (31 December 2017). CIs of KM estimates were calculated as 1.96 times SE.
HR of epilepsy was calculated by Cox proportional regression modelling, either univariably or multivariably with adjustment for age, sex and CNS comorbidities (time-updated variables). In the analysis of HR for epilepsy related to trauma mechanism, individuals with only one type of trauma were included (n=82 591), with corresponding controls (n=240 415).
Results
The largest age category was 18–39 years and 59% were male. The mean and median age at index date was 55 and 56, respectively, for both exposed and controls. Mean and median follow-up time was 9 and 10 years for exposed and 10 and 11 years in controls. At the end of follow-up, the mean and median age was 65 and 67, respectively, among the exposed and 65 and 68 years, in controls.
All CNS comorbidities were significantly more common in exposed compared with controls (table 1).
Table 1.
Demographics, severity of injury, pretraumatic and post-traumatic circumstances and comorbidities
| Exposed | Control | ||||
| N | % (95 % CI) | N | % (95 % CI) | ||
| Age | 18–39 | 35 691 | 31.9 (31.6 to 32.2) | 103 908 | 31.9 (31.7 to 32.0) |
| 40–59 | 24 882 | 22.2 (22.0 to 22.5) | 73 198 | 22.5 (22.3 to 22.6) | |
| 60–79 | 27 547 | 24.6 (24.4 to 24.9) | 79 917 | 24.5 (24.4 to 24.7) | |
| 79< | 23 827 | 21.3 (21.0 to 21.5) | 68 858 | 21.1 (21.0 to 21.3) | |
| Sex | Male | 65 633 | 58.6 (58.3 to 58.9) | 190 606 | 58.5 (58.3 to 58.7) |
| Female | 46 314 | 41.4 (41.1 to 41.7) | 135 275 | 41.5 (41.3 to 41.7) | |
| Type of injury | Control | 0 | 0.0 | 325 881 | 100 |
| Mild Injury | 76 329 | 68.2 (67.9 to 68.5) | 0 | 0.0 | |
| Fracture | 6081 | 5.4 (5.3 to 5.6) | 0 | 0.0 | |
| Extracerebral Injury | 16 492 | 14.7 (14.5 to 14.9) | 0 | 0.0 | |
| Diffuse Cerebral Injury | 8778 | 7.8 (7.7 to 8.0) | 0 | 0.0 | |
| Focal Cerebral Injury | 4267 | 3.8 (3.7 to 3.9) | 0 | 0.0 | |
| Seizure during hospitalisation | 363 | 0.3 (0.3 to 0.4) | 0 | 0.0 | |
| Mechanical ventilation | 1367 | 1.2 (1.2 to 1.3) | 27 | 0.0 (0.0 to 0.0) | |
| Stroke | 21 429 | 19.1 (18.9 to 19.4) | 33 614 | 10.3 (10.2 to 10.4) | |
| Cerebral infection | 1315 | 1.2 (1.1 to 1.2) | 2064 | 0.6 (0.6 to 0.7) | |
| Tumour | 1433 | 1.3 (1.2 to 1.3) | 2844 | 0.9 (0.8 to 0.9) | |
Mild cerebral injury was the most common injury and the most common injury mechanism was fall; 67.7% (95% CI 67.4% to 68.0%), followed by transportation accident; 26.0% (95% CI 25.7% to 26.2%), followed by violence; 10.7% (95% CI 10.5% to 10.8%). Multiple injury mechanisms were recorded in 20.2% (95% CI 20.0% to 20.5%) of exposed. The most severe injury type—focal cerebral injury—occurred in 4.3% (95% CI 4.2% to 4.5%) of males, compared with 3.1% (95% CI 3.0% to 3.3%) of the females.
Epilepsy
An epilepsy diagnosis was detected in 4292 individuals in the exposed group (3.8%, 95% CI 3.7% to 3.9%) and in 2934 controls (0.9%, 95% CI 0.9% to 0.9%). In exposed, the median age at the first epilepsy diagnosis was 61 (range: 18–99) and the median time to epilepsy was 1 year (range: 0–17 years). When stratified by type of injury, the cumulative incidence was highest after focal cerebral injury, n=512, 12.0% (95% CI 11.0% to 13.0%), followed by diffuse cerebral injury, n=641, 7.3% (95% CI 6.8% to 7.9%), extracerebral injury, n=967, 5.9% (95% CI 5.5% to 6.2%), fracture, n=181, 3.0% (95% CI 2.6% to 3.4%) and mild injury, n=1991, 2.6% (95% CI 2.5% to 2.7%).
Epilepsy risk
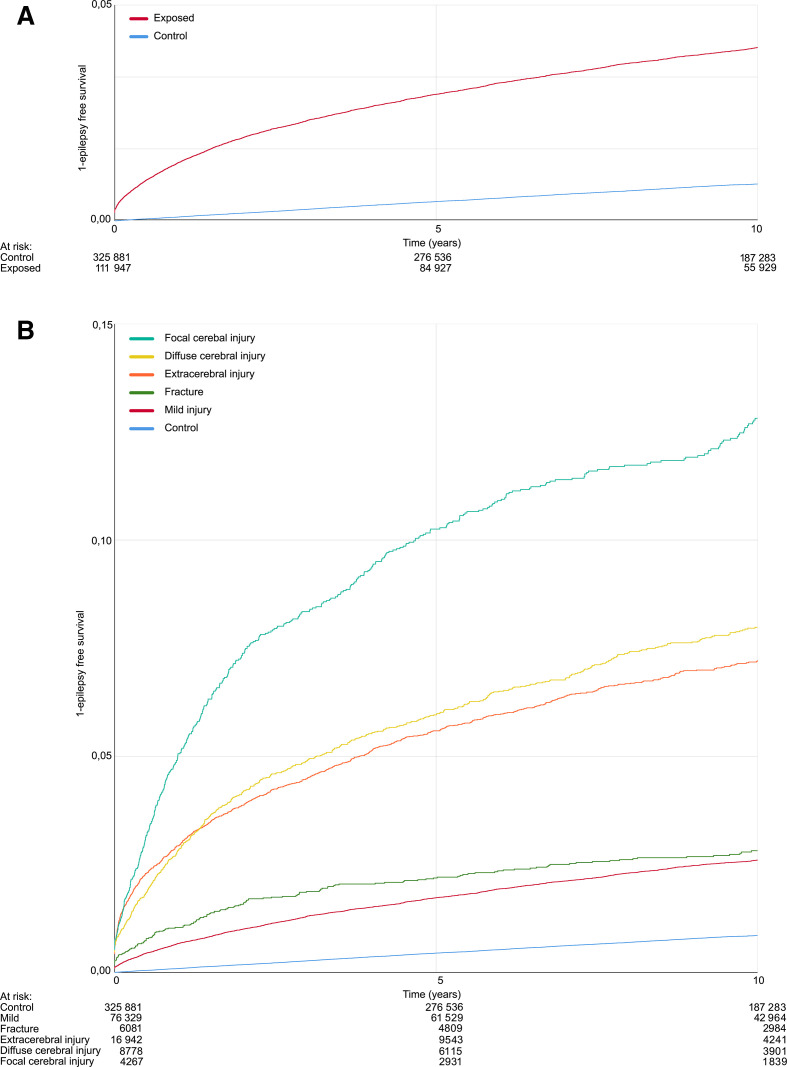
Epilepsy risk was estimated using KM curves (figure 1). The 10-year risk was 4.0% (95% CI 3.8% to 4.2%) after any TBI and 0.9% (95% CI 0.9% to 0.9%) in controls. When exposed were categorised by type of injury, the greatest risk was seen after focal cerebral injury, and the lowest risk seen after mild cerebral injury (table 2).
Figure 1.
Risk of epilepsy. Kaplan-Meier curves of the occurrence of epilepsy after traumatic head injury and controls at risk indicates the remaining of exposed. (A) Divided into controls (blue line) and exposed (red line). (B) Dived into controls (blue line) and severity of the head injury (mild injury—red line, fracture—green line, extracerebral injury—orange line, diffuse cerebral injury—yellow line, focal cerebral injury—turquoise line).
Table 2.
The 10-years Kaplan-Meier estimated risk of epilepsy in % (95% CI)
| Type of injury | % (95% CI) |
| Control | 0.9 (0.9 to 0.9) |
| Any injury | 4.0 (3.8 to 4.2) |
| Mild | 2.6 (2.4 to 2.8) |
| Fracture | 2.8 (2.4 to 3.2) |
| Extracerebral injury | 7.3 (6.9 to 7.7) |
| Diffuse cerebral injury | 8.1 (7.5 to 8.7) |
| Focal cerebral injury | 12.9 (11.7 to 14.1) |
In Cox proportional modelling any injury inferred an unadjusted HR of epilepsy of 4.7 (95% CI 4.4 to 4.9). For the more severe categories, the HR was lower in a multivariable model with adjustments for sex, age and comorbidities. Also with adjustments, the greatest risk of epilepsy was seen after focal cerebral injury (table 3).
Table 3.
Cox proportional HR of epilepsy compared with control, univariably and with multivariable adjustment for age, sex and central nervous system comorbidities, (95% CI)
| Type of injury | Univariable | Multivariable |
| Control | ref | ref |
| Any injury | 4.7 (4.4 to 4.9) | 3.9 (3.7 to 4.1) |
| Mild | 3.0 (2.8 to 3.2) | 2.9 (2.7 to 3.1) |
| Fracture | 3.6 (3.1 to 4.2) | 3.3 (2.8 to 3.8) |
| Extracerebral injury | 9.3 (8.7 to 10.0) | 4.5 (4.2 to 4.9) |
| Diffuse cerebral injury | 9.6 (8.8 to 10.5) | 7.4 (6.8 to 8.1) |
| Focal cerebral injury | 16.0 (14.5 to 17.5) | 10.7 (9.7 to 11.8) |
Risk factors
We finally investigated risk factors for epilepsy among exposed. Type of injury, mechanical ventilation, and seizure during the initial hospitalisation were associated with an increased HR for epilepsy—univariably and with multivariable adjustment (table 4).
Table 4.
HR for epilepsy depending on risk factors among exposed, univariably and with multivariable adjustment for age, sex and central nervous system comorbidities
| Univariable | Multivariable | |
| Male sex | 1.2 (1.1 to 1.3) | 1.2 (1.1 to 1.3) |
| Agecontinuous | 1.0 (1.0 to 1.0) | 1.0 (1.0 to 1.0) |
| Severity of injury: | ||
| Mild | ref | ref |
| Fracture | 1.2 (1.0 to 1.4) | 1.1 (0.9 to 1.3) |
| Extracerebral injury | 3.0 (2.7 to 3.2) | 1.8 (1.6 to 1.9) |
| Diffuse cerebral injury | 3.2 (2.9 to 3.5) | 2.5 (2.3 to 2.7) |
| Focal cerebral injury | 5.2 (4.8 to 5.8) | 3.7 (3.3 to 4.1) |
| Severity | ||
| Mechanical ventilation | 5.6 (4.9 to 6.3) | 2.8 (2.5 to 3.3) |
| Seizure index adm | 10.7 (8.8 to 13.0) | 7.2 (5.9 to 8.8) |
Hazard by injury mechanism
When analysed by injury mechanism, the HR for epilepsy was highest after fall, 4.5 (95% CI 4.2 to 4.8), followed by transportation accident 1.7 (95% CI 1.5 to 1.9), followed by violence 1.3 (95% CI 1.1 to 1.7). The median age at trauma among individuals after fall was 72, mild injury accounted for 65.3% (95% CI 64.9% to 65.7%). In individuals after transportation accident, the median age at trauma was 39, mild injury accounted for 76.8% (95% CI 76.2% to 77.4%). In individuals after violence the median age was 27, mild injury accounted for 77.9% (95% CI 76.9% to 78.9%).
Hazard by age categories
We also analysed the risk of PTE by age categories. Among exposed, the highest HR for epilepsy was seen in individuals between 40 and 79 years of age, univariably and with adjustments for sex, injury severity and comorbidities (table 5).
Table 5.
HR for epilepsy depending on age categories among exposed, univariably and with multivariable adjustment for age, sex and Central nervous system comorbidities
| Age, categorical | ||
| 18–39 | ref | ref |
| 40–59 | 2.3 (2.1 to 2.5) | 1.9 (1.8 to 2.1) |
| 60–79 | 2.6 (2.4 to 2.9) | 1.6 (1.5 to 1.8) |
| 79+ | 1.7 (1.6 to 1.9) | 1.1 (1.0 to 1.2) |
(95% CI).
Sensitivity analyses
We performed several sensitivity analyses. To ensure that the results were not biased by our exclusion of persons with both mild and severe injury codes (potential erroneous coding), we performed an additional analysis with these individuals included and the injury categorised by the most severe code, including 115 255 exposed and 335 527 controls. This analysis showed no significant differences in HRs for different injuries compared with the main analysis (table 3). To assess the potential of reverse causation (a seizure in epilepsy erroneously classified as PTE causing the trauma), we analysed the timing of the first epilepsy diagnosis. The first epilepsy diagnosis was detected in admissions following the trauma in 280 cases with PTE (6.5%, 95% CI 5.8% to 7.3%). The temporal resolution of the register data, in which codes are linked to admission dates, does not allow a more detailed analysis, but most of these cases presumably reflect actual PTE based on seizures occurring after the trauma. The risk of reverse causation is further minimised by our method of excluding individuals with a seizure before the index date; a second seizure could otherwise have caused both trauma and an epilepsy diagnosis, but a first seizure resulting in head injury would typically not result in an epilepsy diagnosis. To assess potential bias by competing risks not being taken into account in the Cox proportional hazard models, we also performed a competing risk analysis with death as competing factor. This analysis showed a similar HR 4.3 (95% CI 4.1 to 4.5), for PTE in exposed as in our main analysis. Finally, we also analysed the impact of overconsumption of alcohol in the risk factors described in table 4. There was no significantly different HR for PTE when entering alcohol overconsumption into the multivariable model.
Discussion
In adults hospitalised for brain trauma in Sweden in 2000–2010, the 10-year risk of subsequent epilepsy was 4.0% (95% CI 3.8% to 4.2%). Unsurprisingly, focal intracerebral injuries carried the largest risk. We also identified several risk factors; age, male sex, acute symptomatic seizures and mechanical ventilation.
To our knowledge, this is the largest and most current nationwide estimate of PTE in adults using comprehensive national registers. The findings are similar to those of another large study, which was based on insurance claims.6 A recent meta-analysis of 20 articles demonstrated heterogeneous results in the literature regarding PTE, with the epilepsy risk in different cohorts ranging from 1.3% to 5.3%.5 In agreement with the meta-analysis and previous investigations, the severity of the head injury was a major determinant of epilepsy risk in our cohort. The risk factors age and male sex have been identified by other investigators.5 6 To an extent, these factors may reflect different injury panorama—males had more severe injuries and the median age was higher among individuals suffering falls, the injury with the greatest PTE risk. Nonetheless, age and sex remained significant also in the multivariable analysis, suggesting that they could independently be associated with increased risk of PTE. Mechanical ventilation probably indicates a more severe injury. Early seizures and severity of injury have also been identified by other investigators as risk factors of PTE.6
We detected a high prevalence of other brain diseases in patients with brain trauma, and in Cox regression, these were seen to influence the risk of epilepsy. Many previous studies have not focused on the impact of comorbidities,2 3 but in a Taiwanese study of 19 336 patients the risk of epilepsy after traumatic head injury was significantly increased also after adjustment for relevant risk factors.8 Several relationship between co-morbidities and trauma are possible. Trauma may be more common in patients with comorbidities, but comorbidities may also arise as consequences of TBI and act as intermediaries with regard to PTE risk. In support of the latter notion, we found that comorbidities had the largest impact on the HR for epilepsy in individuals with extracerebral injury and focal cerebral injury. In these groups, the HR attributed to the trauma may, therefore, be somewhat underestimated. Finally, it is possible that patients with several concomitant brain disorders are more likely to develop PTE, if epileptogenesis occurs because of a threshold effect. Future studies should aim to investigate if the risks of epilepsy after trauma and other brain disorders are additive or multiplicative.
With regard to the trauma mechanism, fall injuries had the greatest HR for PTE, possibly because these cases had more severe TBI and were of a higher age. From a societal perspective, prevention of fall seems to be at least as important as reducing violence and traffic accidents in order to reduce the burden of PTE.
The study was register based, with associated drawbacks and advantages. A particular strength is that NPR registration is mandatory for all specialised healthcare in Sweden.9 Since neurology in Sweden is mainly hospital based, this results in sensitive detection of epilepsy. The ICD-code for epilepsy has >90% accuracy in the Swedish NPR.10 A further validation of the method is that our results were similar to a large Danish study, with a comparable study design, which also found an increased risk of PTE in patients with relatives with epilepsy.3 Our data set did not have multigeneration linkage, preventing us from investigating the contribution of family history to PTE, which could be an additional risk factor. The temporal resolution of register data makes it hard to completely exclude cases of reverse causation, in which the seizure resulting in an epilepsy diagnosis causes the trauma. We excluded anyone with a prior seizure, and the number of PTE diagnoses made during the first day of admission after the trauma was <7%. The seizure on which these PTE diagnoses were made can have occurred at any time before the discharge, so a substantial proportion can be assumed to have reflect PTE, so reverse causation seems limited in our material. The Swedish registers allowed us to exclude pre-existing epilepsy with good certainty, especially towards the end of the study period, and the risk of undetected preexisting epilepsy should be similar in exposed and controls. Our injury severity categories may not have been of sufficient resolution to capture all differences in the injury panorama between sexes and age groups, making it hard to exclude residual confounding. Another potential caveat is that since individuals after TBI are at increased risk of dying compared with controls, competing risks may skew KM and Cox proportional hazard models. We, therefore, performed a competing risk analysis with death as a competing factor as a sensitivity analysis and found the HR for epilepsy not significantly different from our main analysis, suggesting that this effect should be relatively minor. We also performed a sensitivity analysis with regard to the impact of overconsumption of alcohol and found no significant effect on our risk factors. Importantly, there is probably an under-recording of this condition in specialised healthcare and our study design is not optimal for studying the impact of alcohol use on PTE risk.
In conclusion, we found a substantial risk of PTE after head trauma in Sweden. The risk of epilepsy after more severe TBI parallels that seen after stroke.11 This calls for more research on PTE. What is the impact of epilepsy on prognosis, most importantly mortality, in survivors of head trauma? Can PTE be predicted, perhaps with the use of biomarkers? More information is also needed on epilepsy treatment and outcome.
Acknowledgments
Per Ekman
Statistiska Konsultgruppen, Gothenburg
Data management and validation of analysis
Footnotes
Contributors: MK: Data analysis, drafting and revising manuscript for intellectual content. JL: Data interpretation, revising manuscript for intellectual content. JZ: Study conceptualisation, study planning, data analysis, data interpretation, drafting and revising manuscript for intellectual content.
Funding: This study was funded by grants from the Swedish state under the ALF-agreement (ALFGBG-715781 and ALFGBG-784921), Swedish Society of Medicine(SLS-881501), Swedish Society of Medical Research (S18-0040), Linnea and Josef Carlsson foundation (90_20180321_048), The Promobilia foundation (18012), Göteborg Medical Society (GLS-780651), Magnus Bergvall foundation (2017–01990).
Competing interests: JZ has been speaker at non-branded educational events organised by UCB (honoraria) and Eisai (no personal compensation), and investigator in clinical trials sponsored by Bial, UCB, SK life science and GW Pharma as an employee of Sahlgrenska University Hospital (no personal compensation).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available. The data from the Swedish National Patient Register cannot be shared by the authors because of confidentiality laws.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Ethical Review Authority (approval number 612-18). The data was anonymized by the National Board of Health and Welfare
References
- 1. Hakimian S, Kershenovich A, Miller JW, et al. Long-Term outcome of extratemporal resection in posttraumatic epilepsy. Neurosurg Focus 2012;32:E10. 10.3171/2012.1.FOCUS11329 [DOI] [PubMed] [Google Scholar]
- 2. Annegers JF, Hauser WA, Coan SP, et al. A population-based study of seizures after traumatic brain injuries. N Engl J Med 1998;338:20–4. 10.1056/NEJM199801013380104 [DOI] [PubMed] [Google Scholar]
- 3. Christensen J, Pedersen MG, Pedersen CB, et al. Long-Term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet 2009;373:1105–10. 10.1016/S0140-6736(09)60214-2 [DOI] [PubMed] [Google Scholar]
- 4. Mahler B, Carlsson S, Andersson T, et al. Unprovoked seizures after traumatic brain injury: a population-based case-control study. Epilepsia 2015;56:1438–44. 10.1111/epi.13096 [DOI] [PubMed] [Google Scholar]
- 5. Xu T, Yu X, Ou S, et al. Risk factors for posttraumatic epilepsy: a systematic review and meta-analysis. Epilepsy Behav 2017;67:1–6. 10.1016/j.yebeh.2016.10.026 [DOI] [PubMed] [Google Scholar]
- 6. DeGrauw X, Thurman D, Xu L, et al. Epidemiology of traumatic brain injury-associated epilepsy and early use of anti-epilepsy drugs: an analysis of insurance claims data, 2004-2014. Epilepsy Res 2018;146:41–9. 10.1016/j.eplepsyres.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475–82. 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 8. Yeh C-C, Chen T-L, Hu C-J, et al. Risk of epilepsy after traumatic brain injury: a retrospective population-based cohort study. J Neurol Neurosurg Psychiatry 2013;84:441–5. 10.1136/jnnp-2012-302547 [DOI] [PubMed] [Google Scholar]
- 9. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sveinsson O, Andersson T, Carlsson S, et al. The incidence of SUDEP: a nationwide population-based cohort study. Neurology 2017;89:170–7. 10.1212/WNL.0000000000004094 [DOI] [PubMed] [Google Scholar]
- 11. Galovic M, Döhler N, Erdélyi-Canavese B, et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the select score): a multivariable prediction model development and validation study. Lancet Neurol 2018;17:143–52. 10.1016/S1474-4422(17)30404-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available. The data from the Swedish National Patient Register cannot be shared by the authors because of confidentiality laws.