Abstract
Objective
To summarise the available information on efficacy and safety of immunomodulatory agents in SARS-CoV-2 infection.
Methods
As part of a European League Against Rheumatism (EULAR) taskforce, a systematic literature search was conducted from January 2019 to 11 December 2020. Two reviewers independently identified eligible studies according to the Population, Intervention, Comparator and Outcome framework and extracted data on efficacy and safety of immunomodulatory agents used therapeutically in SARS-CoV-2 infection at any stage. The risk of bias was assessed with validated tools.
Results
Of the 60 372 records, 401 articles were eligible for inclusion. Studies were at variable risk of bias. Randomised controlled trials (RCTs) were available for the following drugs: hydroxychloroquine (n=12), glucocorticoids (n=6), tocilizumab (n=4), convalescent plasma (n=4), interferon beta (n=2), intravenous immunoglobulins (IVIg) (n=2) and n=1 each for anakinra, baricitinib, colchicine, leflunomide, ruxolitinib, interferon kappa and vilobelimab. Glucocorticoids were able to reduce mortality in specific subsets of patients, while conflicting data were available about tocilizumab. Hydroxychloroquine was not beneficial at any disease stage, one RCT with anakinra was negative, one RCT with baricitinib+remdesivir was positive, and individual trials on some other compounds provided interesting, although preliminary, results.
Conclusion
Although there is emerging evidence about immunomodulatory therapies for the management of COVID-19, conclusive data are scarce with some conflicting data. Since glucocorticoids seem to improve survival in some subsets of patients, RCTs comparing glucocorticoids alone versus glucocorticoids plus anticytokine/immunomodulatory treatment are warranted. This systematic literature review informed the initiative to formulate EULAR ‘points to consider’ on COVID-19 pathophysiology and immunomodulatory treatment from the rheumatology perspective.
Keywords: therapeutics, inflammation, immune system diseases
Key messages.
What is already known about this subject?
The SARS-CoV-2 pandemic is a global health problem. Aberrant host immune response plays an important role throughout the course of mild, moderate and severe COVID-19.
There is intense investigation to explore the utility of immunomodulatory drugs commonly used in the rheumatology arena as agents that may mitigate against COVID-19 to improve disease prognosis.
What does this study add?
Robust and reliable evidence of the efficacy of immunomodulatory therapies is scarce, but results from randomised controlled trials (RCTs) ruled out any benefit of hydroxychloroquine at any stage of SARS-CoV-2 infection while demonstrating the ability of some glucocorticoids to reduce mortality in specific patient subsets with severe COVID-19.
Data from RCTs on tocilizumab are conflicting, and definite conclusions cannot be drawn at this point in time. Anakinra was not effective in the only available RCT, while baricitinib+remdesevir was effective in specific patient subgroups (patients with non-invasive ventilation) in the only available RCT.
Evidence for several immunomodulatory compounds is scarce, and data from RCTs are required to elucidate their role in the context of different phenotypes of SARS-CoV-2 infection.
How might this impact on clinical practice or future developments?
This systematic literature review evaluated the evidence pertaining to immunomodulatory drugs where there is some evidence for efficacy in severe COVID-19 and a good safety profile thus far.
Further evidence is needed regarding the optimal use and consideration of combination therapies for severe disease in a rapidly evolving arena.
Introduction
SARS-CoV-2 infection encompasses a heterogeneous clinical picture ranging from asymptomatic to multisystem life-threatening manifestations. Although the majority of patients experience only mild to moderate symptoms, a relevant proportion of infected subjects may develop respiratory failure, acute respiratory distress syndrome and death.1 2 The severest forms of COVID-19 pneumonia are associated with severe pulmonary inflammatory responses, including oedema and inflammatory cell infiltration with severe alveolitis and associated pulmonary immunothrombosis. Beside the specific pathogenic effect of SARS-CoV-2, the immune response may be deleterious and excessive since postmortem studies may show excessive immune activation but a paucity of evidence for active viral alveolitis. A vicious circle encompassing the intrapulmonary release of proinflammatory mediators, along with the aberrant activation of immune cells, coagulopathy and histological evidence of haemophagocytosis in patients with more severe COVID-19 demonstrated some features that resembled the macrophage activation syndrome (MAS) also known as secondary haemophagocytic lymphohistocytosis (sHLH).3 4
Rheumatologists routinely use immunomodulatory drugs and are well aware of conditions like MAS/sHLH that may be observed as a complication of autoimmune or inflammatory rheumatic and musculoskeletal diseases (RMDs). On this basis, a large number of immunomodulatory drugs used in rheumatology for years have been investigated in SARS-CoV-2 infection, particularly severe COVID-19. This systematic literature review (SLR) was performed to inform the EULAR taskforce responsible for developing the points to consider (PtC) on COVID-19 pathophysiology and immunomodulatory treatment as viewed from the rheumatology perspective. Specifically, the SLR aimed to summarise the available information on the use of immunomodulatory drugs for the management of SARS-CoV-2 infection at any stage.
Methods
Search methodology
The EULAR task force that developed PtCs on COVID-19 pathophysiology and immunomodulatory treatment from the rheumatology perspective outlined the scope of the systematic literature search, according to the Population, Intervention, Comparator and Outcome approach.5 Based on a set of research questions encompassing the pathogenesis of SARS-CoV-2 infection, its management with immunomodulatory agents and its possible role as trigger of new-onset RMDs, three separate searches (online supplemental text S1−S4) were performed. The searches were performed in MEDLINE, Embase, The Cochrane Database of Systematic Reviews, CENTRAL and CINAHL. The searches on pathogenesis and RMDs were conducted up to 2 November 2020, while the one on immunomodulatory treatment up to 11 December 2020. The PubMed Similar Articles tool was also used, and a crosscheck of the key scientific journals in general medicine and immunology was performed. Non peer-reviewed literature was excluded given this SLR aimed at informing recommendations. However, given the rapid evolution of knowledge on COVID-19 treatment, a parallel hand search of ‘grey literature’ consisting only of RCT not yet published in peer-review journals but accessible in press releases or in extenso in preprint repositories was performed. These not yet published RCTs are presented separately and were not used to inform the PtC. In order to ensure this SLR to be as comprehensive as possible and provide an overview of all evidence (regardless of the level), no restriction to specific study design (eg, randomised controlled trials (RCTs)) was defined. The results of the search focused on the pathogenesis of SARS-CoV-2 infection are published elsewhere.
annrheumdis-2020-219725supp001.pdf (868.1KB, pdf)
Study selection, data collection and assessment of risk of bias (RoB)
Briefly, original research articles of any study design, published in English, in peer-reviewed journals and addressing adults with proven SARS-CoV-2 infection treated with one or more immunomodulatory agent were eligible (online supplemental text S4). Two reviewers (AA and AN) independently assessed titles and abstracts according to the predetermined eligibility criteria, followed by full-text review. The agreement between reviewers, calculated with the Cohen’s kappa, was 0.95. Discrepancies were resolved by discussion. The task force methodologist (PMM) was consulted in the case of uncertainties. Data on patient characteristics, investigated drug administration scheme and comparators and outcomes were extracted. The RoB was assessed using validated tools according to the study design (online supplemental text S5). Only the results pertaining to immunomodulatory therapies are presented here.
Results
Of the 60 372 records yielded by the three searches, 700 were selected for full-text review and seven additional articles were identified by cross-referencing. Of these, 401 articles on 33 therapeutic strategies met the inclusion criteria for the research questions on immunomodulatory treatment of COVID-19 (online supplemental tables S1−S3). Robust evidence was mostly available for moderate to severe/critical COVID-19. The best evidence available for each compound is shown.
Immunomodulatory therapies with evidence on severe (patients on oxygen therapy) or critical (patients in intensive care unit (ICU)) COVID-19
Data from RCTs
A total of 39 RCTs, all at high or unclear RoB, evaluating 13 therapeutic approaches in severe/critical COVID-19 were retrieved by the SLR (online supplemental table S4).
Glucocorticoids
Efficacy
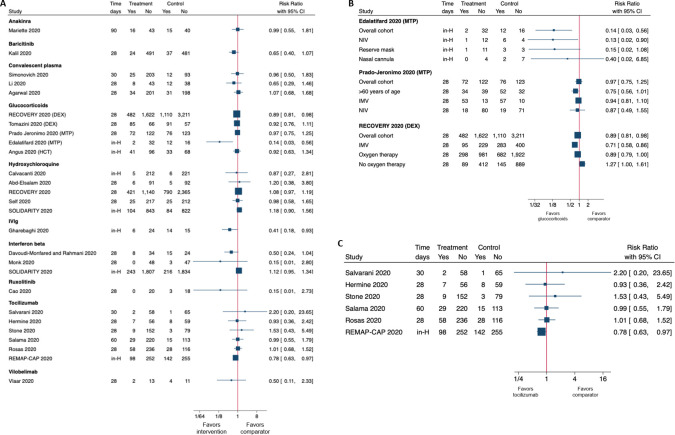
Of the six RCTs on glucocorticoids in severe/critical COVID-19, two investigated dexamethasone (DEX) (one at unclear and one at high RoB), two investigated methylprednisolone (MTP) (one at unclear and one at high RoB) and two investigated hydrocortisone (HCT) (both at unclear RoB). Most of the studies included severe and critical patients with between 15% and 100% of subjects requiring invasive mechanical ventilation (IMV).6–11 In one study at high RoB, none of the patients needed IMV at enrolment.11 This, along with the variability of other inclusion criteria, the use of different compounds (eg, long acting vs short acting) and different schedule may have contributed to the conflicting results for the majority of outcomes in the overall analysis (tables 1 and 2). Conversely, subgroup analyses revealed positive results for two (DEX and MTP) out of three compounds with regard to mortality (figure 1). The study from the RECOVERY Collaborative Group (unclear RoB) enrolled 6425 patients with severe COVID-19 of which 2104 were assigned to receive DEX in addition to standard of care (SOC) and 4321 to receive SOC only.6 The two groups were comparable with regard to need of oxygen therapy/non-invasive or IMV at randomisation. The addition of DEX to SOC reduced mortality but only in patients requiring respiratory support. Likewise, the addition of MTP to SOC in a study at unclear RoB was able to reduce mortality in patients aged 60 years or over.7 HCT failed to show benefit in reducing mortality in both studies.9 10 Importantly, the RECOVERY trial also reported that in patients not receiving oxygen therapy, DEX may have a possible (even if not statistically significant) deleterious effect on mortality (OR=1.22, 95% CI 0.93 to 1.61, p=0.14).6
Table 1.
Effect of immunomodulatory drugs on mortality, assessed by randomised controlled trials, in moderate to severe COVID-19 (with oxygen therapy) and in critical COVID-19 (patients in ICU)
| Study, year, ref | Drug, dosage and administration, N | Timepoint (days)† | Mortality intervention (%) | Mortality SOC (%) | Results | Subgroup analysis | % Absolute risk reduction (95% CI) | Risk of bias |
| Hydroxychloroquine | ||||||||
| Cavalcanti et al 202016 | HCQ 400 mg twice daily for 7 d. n=221. |
Hospital stay | 2.3 | 2.6 | No difference between groups. | None performed for mortality. | 0.3 (−2.9 to 3.6) |
Unclear |
| Abd-Elsalam et al 202013 | HCQ 400 mg twice daily on d1 followed by 200 mg twice daily. n=97. |
28 | 6.2 | 5.2 | No difference between groups. | None performed for mortality. | −1.0 (−8.3 to 6.2) |
High |
| RECOVERY 202018 | HCQ 800 mg at baseline and at 6 hours, then 400 mg starting at 12 hours after the initial dose and then every 12 hours for the next 9 d or until discharge. n=1561. |
28 | 27.0 | 25.0 | No difference between groups. | No significant RR subgrouping by age, gender, race or ethnic group, days since symptoms, oxygen therapy/IMV and baseline risk. | −1.9 (−4.6 to 0.7) |
Unclear |
| Self et al 202020 | HCQ 400 mg twice daily for two doses, then 200 mg twice daily for eight doses. n=242. |
28 | 10.3 | 10.5 | No difference between groups. | No significant RR subgrouping by age, gender, race or ethnic group, days since symptoms. | 0.2 (−5.3 to 5.8) |
Unclear |
| SOLIDARITY 202019 | HCQ 800 mg at baseline and 6 hours, then 400 mg twice daily starting at 12 hours for 10 d. | Hospital stay | 11 | 9.3 | No difference between groups. | No significant differences subgrouping by age, gender, days from hospital admission to randomisation, respiratory support, bilateral lung lesions, smoking, various comorbidities, use of corticosteroids and geographic location. | −1.7 (−4.5 to 1.5) |
Unclear |
| Glucocorticoids | ||||||||
| RECOVERY 20206 | DEX* 6 mg/d. n=2104. |
28 | 22.9 | 25.7 | Lower in the DEX group. | IMV: RR (95% CI) 0.71 (0.58 to 0.85) NNT 8; Oxygen therapy: RR (95% CI) 0.89 (0.79 to 1.0) NNT 35; no oxygen therapy RR (95% CI) 1.27 (0.99 to 1.61) NNT −27. |
2.8 (0.5 to 4.9) |
Unclear |
| Tomazini et al 20208 | DEX* 20 mg/d intravenous for 5 d and then 10 mg/d intravenous for 5 d. n=151. |
28 | 56.3 | 61.5 | No difference between groups. | None performed for mortality. | 5.2 (−5.9 to 16.1) |
High |
| Jeronimo et al 20207 | MTP* 0.5 mg/kg twice daily intravenous for 5d. n=194. |
28 | 37.1 | 38.2 | No difference between groups. | >60 years of age RR (95% CI) 0.75 (0.55 to 1.0) NNT 7. | 1.1 (−8.4 to 10.5) |
Unclear |
| Edalatifard et al 202011 | MTP* 250 mg/day intravenous pulse for 3 d. | Hospital stay | 5.9 | 42.9 | Lower in the MTP group. | NIV: RR (95% CI) 0.13 (0.01–0.90) NNT 2; Reserve mask: RR (95% CI) 0.15 (0.01–1.08) NNT 2; Nasal cannula: RR (95% CI) 0 NNT 5. | 37 (15.9 to 55.5) |
High |
| Angus et al 20209 | HCT* 50 mg intravenous every 6 hours for 7 d. | Hospital stay. | 29.9 | 32.7 | No difference between groups. | None performed for mortality. | 2.7 (−8.9 to 14.7) |
Unclear |
| Convalescent plasma | ||||||||
| Simonovich et al 202043 | Convalescent plasma 1 infusion titre >1:800. n=228. |
30 | 11.0 | 11.4 | No difference between groups. | None performed for mortality. | 0.46 (−6.2 to 8.7) |
Unclear |
| Li et al 202041 | Convalescent plasma 1 infusion 4–13 mL/kg of recipient body weight. n=52. |
28 | 15.7 | 24 | No difference between groups. | No significant differences subgrouping by disease severity. | 8.3 (−7.4 to 23.7) |
High |
| Agarwal et al 202042 | Convalescent plasma 2 doses of 200 mL 24 hours apart. n=235. |
28 | 14.5 | 13.5 | No difference between groups. | None performed for mortality. | −0.93 (−7.2 to 6.6) |
High |
| Tocilizumab | ||||||||
| Salvarani et al 202025 | TCZ 8 mg/kg intravenous within 8 hours from randomisation followed by a second dose after 12 hours. n=60. |
30 | 3.3 | 1.5 | No difference between groups. | None performed for mortality. | −1.8 (−9.9 to 5.5) |
Unclear |
| Hermine et al 202023 | TCZ, 8 mg/kg, intravenous on day 1 and on day 3 if clinically indicated. n=64. |
28 | 11.1 | 11.9 | No difference between groups. | None performed for mortality. | 0.8 (−10.8 to 12.2) |
Unclear |
| Stone et al 202024 | TCZ 8 mg/kg intravenous single dose. n=161. |
28 | 5.6 | 3.7 | No difference between groups. | No significant differences subgrouping by age, gender, ethrnicity, BMI, diabetes, serum IL-6 and therapy with remdesivir. | −1.93 (−7.2 to 5.1) |
Unclear |
| Salama et al 202026 | TCZ intravenous 8 mg/kg one or two doses. n=249. |
60 | 11.6 | 11.8 | No difference between groups. | Lower time to death or IMV in Hispanic or Latino treated with TCZ. No significant differences subgrouping by age, region, use of systemic glucocorticoids or antivirals and total number of drug study dose. | 0.07 (−6.3 to 7.6) |
Unclear |
| Anakinra | ||||||||
| Mariette et al 2020 CORIMUNO-1930 | ANA 200 mg intravenous twice daily at d 1, 2 and 3, then 100 mg twice daily at d 4 and 100 mg/d at d 5. In case of absence of improvement at d4: 400 mg/d at d 4, 5 and 6, 200 mg/d at d 7 and 100 mg/d at d 8. n=59. |
90 | 27.1 | 27.3 | No difference between groups. | No significant differences subgrouping patients by C reactive protein levels or use of corticosteroids. | 0.2 (−15.8 to 16.3) |
Unclear |
| Ruxolitinib | ||||||||
| Cao et al 202036 | RUXO 5 mg twice daily. n=22. |
28 | 0.0 | 14.3 | No difference between groups. | None performed for mortality. | 14.29 (−4.3 to 34.6) |
High |
| Interferon beta | ||||||||
| Davoudi-Monfared et al 2020, Rahmani et al 202033 34 | IFN-beta 250 µg sc eod for 2 weeks. n=46. |
28 | 19 | 38.5 | Lower in the IFN group. | None performed for mortality. | 19.4 (−0.4 to 37.5) |
High |
| Monk et al 202035 | IFN-beta (SNG001) 6 MIU delivered via nebuliser once daily for up to 14 d. n=50. |
28 | 0.0 | 6.0 | No difference between groups. | None performed for mortality. | 6 (−2.3 to 16.2) |
Unclear |
| SOLIDARITY 202019 | IFN-beta patients receiving high-flow oxygen, ventilation or extracorporeal membrane oxygenation: 10 µg/d intravenous for 6 d. Patients not receiving oxygen therapy or receiving low-flow oxygen therapy: 44 µg at baseline d 3 and d 6. |
Hospital stay | 12 | 10.5 | No difference between groups. | No significant differences subgrouping by age, gender, days from hospital admission to randomisation, respiratory support, bilateral lung lesions, smoking, various comorbidities, use of corticosteroids and geographic location. | −1.3 (−3.2 to 0.6) |
Unclear |
| IVIg | ||||||||
| Gharebaghi et al 202039 | IVIg 5 gm 5/day for 3 d. n=30. |
Hospital stay. | 20.0 | 48.3 | Lower in the IVIg group. | None performed for mortality. | 28.3 (4.1 to 48.5) |
High |
| Vilobelimab | ||||||||
| Vlaar et al 202037 | VILO 800 mg/d intravenous up to seven doses. n=15. | 28 | 13.3 | 26.7 | No difference between groups. | Lower mortality in patients intubated within 6 hours after randomisation and treated with VILO. | 13 (−15.8 to 40.4) |
Unclear |
| Baricitinib | ||||||||
| Kalil et al 202031 | BARI 4 mg/day for 14 days or until hospital discharge +remdesivir 200 mg on d 1 followed by 100 mg/d through 10 d or until hospital discharge or death. n=515. |
28 | 4.7 | 7.1‡ | No difference between groups. | Numerically lower in patients with a baseline ordinal score of 5 or 6. | 2.5 (−0.4 to 5.4) |
Unclear |
The study comparator is standard of care unless otherwise stated.
The absolute risk reduction (ARR) represents the proportion of patients who are spared the adverse outcome (in this case death) as a result of having received the experimental rather than the control therapy. The smaller the treatment effect, the lower the ARR. The number needed to treat, NNT (1/ARR), is the number of patients needed to treat to prevent one additional bad outcome (in this case death). A negative NNT corresponds to a negative ARR, that is, a poorer outcome on the active treatment arm, for example an NNT=−10 indicates that if 10 patients are treated with the new/active treatment, one more would have a bad outcome than if they all received the standard treatment.
*Equivalent doses: DEX=0.75 mg; MTP=4 mg; PDN=5 mg; HCT=20 mg.
†The latest follow-up time available is reported.
‡Comparator in this study is remdesivir 200 mg on day 1 followed by 100 mg/d through 10 d or until hospital discharge or death+placebo+SOC.
ANA, anakinra; BMI, body mass index; d, days; twice daily, twice daily; DEX, dexamethasone; eod, every other day; h, hours; HCQ, hydroxychloroquine; HCT, hydrocortisone; ICU, intensive care unit; IFN, interferon; IL, interleukin; IMV, invasive mechanical ventilation; IVIg, intravenous immunoglobulis; MIU, million international unit; MTP, methylprednisolone; NIV, non-invasive ventilation; RR, relative risk; RUXO, ruxolitinib; SC, subcutaneous; SOC, standard of care; VILO, vilobelimab.
Table 2.
Effect of immunomodulatory drugs on invasive and non-invasive ventilation and on oxygen support, assessed by randomised controlled trials, in moderate to severe COVID-19 (with oxygen therapy) and in critical COVID-19 (patients in ICU)
| Outcome | Drug | Author, year, ref | Study groups | Results | Risk of bias |
| Non-invasive or invasive mechanical ventilation | Hydroxychloroquine | Cavalcanti et al 202016 | SOC+PBO SOC+HCQ + AZT |
No difference between groups HCQ OR 1.77 (95% CI 0.81 to 3.87) HCQ+AZT OR 1.15 (95% CI 0.49 to 2.70). | Unclear |
| Abd-Elsalam et al 202013 | SOC SOC+HCQ |
No difference between groups (4.1% vs 5.2%, p=0.75). | High | ||
| RECOVERY 202018 | SOC SOC+HCQ |
Higher progression to IMV in the HCQ group (risk ratio (RR) 1.14; 95% CI 1.03 to 1.27). | Unclear | ||
| Corticosteroids | RECOVERY 20206 | SOC+DEX SOC |
Risk of progression to IMV was lower in the DEX group than in SOC group (RR 0.77; 95% CI 0.62 to 0.95). | Unclear | |
| Jeronimo et al 20207 | SOC+MTP SOC |
No difference across groups day 7 hour 2.6 (95% CI 8.6 to 13.6); p=0.654. | Unclear | ||
| Tomazini et al 20208 | SOC+DEX SOC |
6.6 (95% CI 5.0 to 8.2) in the DEX group versus 4.0 ventilator-free days (95% CI 2.9 to 5.4) in the SOC group (difference: 2.26; 95% CI 0.2 4.38; p=0.04). | High | ||
| Dequin et al 202010 | SOC+HCT SOC+PBO |
Of the 16 patients per group without IMV at baseline, 8 (50%) in HCT group and 12 (75%) in the PBO group required subsequent intubation. | Unclear | ||
| Tocilizumab | Hermine et al 2020 CORIMUNO-1923 | SOC+TCZ SOC |
At day 14, 12% (95% CI 28% to 4%) fewer patients needed NIV or MV or died in the TCZ group than in the SOC group (24% vs 36%, median posterior HR 0.58; 90% credible interval 0.33 to 1.00). | Unclear | |
| Stone et al 24 | SOC+TCZ SOC+PBO |
No difference across groups in the progression to IMV or death. 0.83 (95% CI 0.38 to 1.81; p=0.64). | Unclear | ||
| Anakinra | Mariette et al 2020 CORIMUNO-1930 | SOC+ANA SOC |
No difference across groups. The proportion of patients dead or in need of NIV or IMV on day 14. (47%, vs 51%, HR 1.0 (0.6–1.5). | Unclear | |
| Ruxolitinib (RUXO) | Cao et al 202036 | SOC+RUXO SOC +100 mg vitamin C | No difference between groups in the need of NIV or IMV and if needed in the duration (p=0.633 and p=0.232). | High | |
| Interferon (IFN) beta | Davoudi-Monfared et al 2020, Rahmani et al 202033 34 | SOC+IFN beta SOC | No difference between groups in the need of MV and if needed in the duration. | High | |
| Monk et al 202035 | SOC+IFN beta PBO +SOC | No significant difference between treatment groups in the odds of intubation or the time to intubation. | Unclear | ||
| IVIg | Tabarsi et al 202040 | SOC+IVIg SOC | No difference in need for IMV (p=0.39) (n=21 IVIG vs n=10 control group). | High | |
| Baricitinib | Kalil et al 202031 | BARI+RDV+ SOC. PBO+RDV+SOC | The incidence of progression to death or NIV or MIV was lower in the RDV+BARI (22.5% vs 28.4%; rate ratio: 0.77; 95% CI 0.60 to 0.98), as was the incidence of progression to death or MIV (12.2% vs 17.2%; rate ratio 0.69; 95% CI 0.50 to 0.95). | Unclear | |
| Oxygen support | Hydroxychloroquine | Cavalcanti et al 202016 | SOC+PBO SOC+HCQ + AZT |
No difference between groups HCQ+AZT OR 1.10 (95% CI 0.60 to 2.03) HCQ OR 1.19 (95% CI 0.65 to 2.21). |
Unclear |
| Tocilizumab | Stone et al 202024 | SOC+TCZ SOC+PBO |
The median time to discontinuation of supplemental O2 was 5.0 days (95% CI 3.8 to 7.6) in the TCZ group and 4.9 days (95% CI 3.8 to 7.8) in the placebo group (p=0.69). No difference across groups. | Unclear | |
| Interferon beta 1a | Davoudi-Monfared et al 2020, Rahmani et al 202033 34 | IFN beta+SOC SOC | No difference between groups. | High |
Only studies reporting on the corresponding outcomes are shown.
AZT, azithromycin; BARI, baricitinib; DEX, dexamethasone; HCQ, hydroxychloroquine; HCT, hydrocortisone; ICU, intensive care unit; IFN, interferon; IMV, invasive mechanical ventilation; MTP, methylprednisolone; NIV, non-invasive ventilation; PBO, placebo; RDV, remdesivir; RR, relative risk; RUXO, ruxolitinib; SOC, standard of care; TCZ, tocilizumab.
Figure 1.
Forest plots showing the risk ratio (RR) and 95% CI for mortality in randomised controlled trials divided by intervention. The latest follow-up available is reported in the timing column. Panel A shows RRs in overall cohorts, panel B shows overall cohorts and subgroup analysis in studies assessing glucocorticoids and panel C shows all studies on tocilizumab (including grey literature).
The two studies on DEX yielded conflicting results with regard to the need of IMV; however, a lack of stratification of inpatients with mild to moderate pneumonia receiving oxygen therapy did not allow us to untangle the effect of DEX in patients requiring a low rate of oxygen (1–2 L/min) from the effect in those requiring higher rate (3–15 L/min). In addition, the studies on MTP and HCT assessing the need of IMV7 10 found no beneficial effect of these compounds. One additional study on HCT in patients with COVID-19 requiring oxygen therapy ≥10 L/min (COVID-19 STEROID) emerged from the search of the ‘grey literature’, reporting no benefit of HCT on 28-day all-cause mortality.12
Safety
Only one study identified safety concerns related to glucocorticoids use in severe COVID-19 with a reported increased insulin use at day 7 in patients treated with MTP+SOC compared with SOC.7 The other RCTs reported either no difference between groups8 or descriptive information without statistical assessment of differences (table 3).9–11
Table 3.
Safety of immunomodulatory drugs assessed by randomised controlled trials in moderate-to-severe COVID-19 (with oxygen therapy) and in critical COVID-19 (patients in ICU)
| Drug | Author, year | Study groups | Results | RoB |
| Hydroxychloroquine | Cavalcanti et al 202016 | SOC+PBO SOC+HCQ+AZT |
Prolongation of the corrected QT interval (p=0.04 for HCQ+AZT; p=0.01 for HCQ) and elevation of liver enzyme p=0.02. More SAE and two deaths in HCQ+AZT groups. |
Unclear |
| RECOVERY 202018 | SOC SOC+HCQ |
HCQ group: greater risk of death from cardiac causes (mean (±SE) excess, 0.4±0.2 percentage points) and from non–SARS-CoV-2 infection (mean excess, 0.4±0.2 percentage points). | Unclear | |
| Tang et al 202015 | SOC SOC+HCQ |
21 (30%) patients HCQ vs 7 (9%) patients PBO. | High | |
| Huang et al 202014 | SOC+HCQ SOC |
5 patients, 9 AEs in HCQ group, none in control group. | High | |
| Self et al 202020 | SOC+HCQ SOC |
30 SAEs were reported, including 18 SAEs from 14 patients (5.8%) in the HCQ group and 12 serious adverse events from 11 patients (4.6%) in the control group. | Unclear | |
| Ulrich et al 202021 | SOC+HCQ SOC |
No difference in AEs between the groups. HCQ was associated with a slight increase in mean corrected QT interval, an increased D-dimer, and a trend towards an increased length of stay. | High | |
| Corticosteroids | Jeronimo et al 20207 | SOC+MTP SOC+PBO |
More insulin at day 7 needed in the MTP group. No more sepsis (but antibiotics in the SOC regimen). |
Unclear |
| Tomazini et al 20208 | SOC+DEX SOC |
No difference in AEs between groups. | High | |
| Dequin et al 202010 | SOC+HCT SOC+PBO |
The proportions of bacteraemia were 6.6% in the hydrocortisone group and 11.0% in the placebo group. | Unclear | |
| Edalatifard et al 202011 | SOC+MTP SOC |
2 patients in each group (5.8% and 7.1%) showed SAE. | High | |
| Angus et al 20209 | SOC+HCT SOC |
10 patients (2.6%) with SAE, 9 of whom were in the fixed-dose (n=4) and shock-dependent (n=5) HCT groups. Two events (severe neuromyopathy and fungaemia) occurred in the fixed-dose hydrocortisone group. | Unclear | |
| Convalescent plasma | Simonovich et al 202043 | SOC+convalescent plasma SOC+PBO |
No difference in AEs between groups. | Unclear |
| Li et al 202041 | SOC+convalescent plasma SOC | No difference in AEs between groups. | High | |
| Agarwal et al 202042 | SOC+convalescent plasma. SOC | No difference in AEs between groups. | High | |
| Tocilizumab | Stone et al 202024 | SOC+TCZ SOC+PBO |
Neutropaenia developed in 22 patients in the TCZ group, as compared with only one patient in the placebo group (p=0.002), but serious infections occurred in fewer patients in the TCZ group (13 (8.1%) vs 14 (17.3%); p=0.03). | Unclear |
| Hermine et al 2020 CORIMUNO-1923 | SOC+TCZ SOC |
SAE occurred in 20 (32%) patients in the TCZ group and 29 (43%) in the SOC group (p = 0.21). Serious infections occurred in 2 (3%) patients in the TCZ group and 14 (21%) in the control group. Neutropaenia developed in 4 (6%) in the TCZ group and 0 in the control group. | Unclear | |
| Colchicine | Deftereos et al 202038 | SOC+COL SOC |
Diarrhoea was more frequent in the colchicine group (25 patients(45.5%) versus nine patients (18.0%); p = 0.003). | Unclear |
| Ruxolitinib | Cao et al 202036 | SOC+RUXO SOC |
No differences between groups 15 patients (71.4%) PBO group and 16 (80%) in RUXO group. | High |
| Interferon beta | Davoudi-Monfared et al 2020, Rahmani et al 202033 34 | SOC+IFN beta SOC | No differences between groups (all p>0.05). A total of 47 common AEs in the IFN and 62 in the control group. | High |
| Monk et al 202035 | SOC+IFN beta PBO+SOC | Treatment emergent AEs were more common in the IFN group. | Unclear | |
| Vilobelimab | Vlaar et al 37 | SOC+VIL SOC | Numbers of SAE were similar between groups (60% of patients in the IFX-1 group vs 47% in the control group). | Unclear |
| Baricitinib | Kalil et al 202031 | BARI+RDV+ SOC PBO+RDV+SOC | No difference in AEs between groups. | Unclear |
Only studies reporting on safety are shown.
AE, adverse event; AZT, azithromycin; COL, colchicine; DEX, dexamethasone; HCQ, hydroxychloroquine; HCT, hydrocortisone; IFN, interferon; MTP, methylprednisolone; MV, mechanical ventilation; PBO, placebo; RR, relative risk; RUXO, ruxolitinib; SAE, severe adverse event; SE, standard error; SOC, standard of care; TCZ, tocilizumab; VIL, vilobelimab.
Hydroxychloroquine
Efficacy
Of the nine RCTs on hydroxychloroquine (HCQ) in severe COVID-19, three studies at high RoB did not report any information regarding the proportions of patients requiring oxygen therapy/NIMV/IMV,13–15 two studies reported NIMV/IMV as exclusion criterion16 17 and four studies detailed the proportion of enrolled patients received either oxygen therapy, NIMV or IMV.18–21 The studies assessing mortality,13 16 18–20 three at unclear and one at high RoB, agreed that the addition of HCQ to SOC did not provide any beneficial effect. As far as clinical severity is concerned, HCQ did not reduce the need of IMV,13 16 19 but one RCT at unclear RoB demonstrated a higher risk of progression to IMV in patients treated with HCQ+SOC compared with SOC only18 (tables 1 and 2). From the parallel hand search in the ‘grey literature’, we identified one additional RCT on HCQ that was prematurely discontinued due to inefficacy—the ORCHID trial.22
Safety
Two studies at unclear RoB alerted on safety issues regarding HCQ. Overall, more adverse events occurred in the HCQ-treated groups. One study reported higher frequency of QTc prolongation and elevation in liver enzyme levels in HCQ-treated patients.16 The other study reported a greater risk of death in HCQ-treated patients, either from non-SARS-CoV-2 infections or from cardiac causes, although the incidence of arrhythmias was similar across groups.18 It is important to mention that the schedule of HCQ in the above-mentioned RCTs was higher than that used in rheumatology practice (eg, a stable dose of 800 mg/day or 800 mg/day for a few days followed by 400 mg/day). Furthermore, the combination with other drugs that could prolongate the QT interval such as azithromycin may account for the safety concerns.
Tocilizumab
Efficacy
Three RCTs on tocilizumab (TCZ) at unclear RoB were retrieved.23–25 In all studies, NIV/IMV represented an exclusion criterion; however, only the CORIMUNO-19 trial excluded also hospitalised patients without need of oxygen therapy, focusing only on patients requiring at least 3 L/min oxygen therapy. In this regard, the observed mortality at day 28 in the former two RCTs was rather low (2%–5%), suggesting that they may have enrolled milder patients than CORIMUNO-19. In Stone’s study, 16% of patients did not receive oxygen therapy. While Stone et al 24 and Salvarani et al 25 failed to demonstrate any benefit from the addition of TCZ to SOC for all the outcomes assessed, the CORIMUNO-19 trial demonstrated benefit of adding TCZ to SOC with regard to lower progression to NIV, IMV or death, although day-28 mortality did not differ between groups.
Two additional RCTs on TCZ were identified in the ‘grey literature’. The EMPACTA trial, using the same inclusion criteria as CORIMUNO-19, met the composite primary outcome of death or IMV at day 28 and was published in The New England Journal of Medicine on 17 December 2020.26 Conversely, the COVACTA trial did not show a benefit in terms of clinical improvement or mortality in the overall population. Unlike the above-mentioned studies, NIV/IMV were not an exclusion criteria in COVACTA, and of note, 65%–70% of patients were receiving either of the two.27 However, positive results were reported in a post hoc analysis with a significantly lower proportion of patients experiencing clinical failure in the subgroup not receiving IMV at randomisation (table 4). In patients recently admitted to ICU within 1 day, the REMAP-CAP study was prematurely stopped because of positive results on hospital mortality with TCZ (28% for TCZ vs 35.8% for controls) and on day 90 survival with TCZ: (median HR=1.59 (1.24 to 2.05), probability of superiority of TCZ >99.9%) (table 4).28 Lastly, an RCT reporting that TCZ was not superior to SOC in improving clinical outcomes at 15 days was published on 22 January 2021.29
Table 4.
’Grey literature’ concerning randomised controlled trials
| Drug | Study name | Author, year | Study groups | Efficacy | Safety | Risk of bias |
| Tocilizumab | REMAP-CAP | Gordon et al 2020 | SOC* SOC* +TCZ SOC* +SARI |
Compared with control, median adjusted ORs for hospital survival were 1.64 (95% CrI 1.14, 2.35) for TCZ and 2.01 (95% CrI 1.18 to 4.71) for SARI. TCZ and SARI were effective across all secondary outcomes, including 90-day survival, time to ICU and hospital discharge and improvement in the WHO ordinal scale at day 14. | Nine serious adverse events reported in the TCZ group including one secondary bacterial infection, five bleeds, two cardiac events and one deterioration in vision. Eleven serious adverse events in the control group, four bleeds and seven thromboses. No serious adverse events in the SARI group. | Unclear |
| TCZ | COVACTA | Rosas et al 2020 | SOC† +PBO SOC† +TCZ |
No difference between groups in mortality at day 28 between TCZ (19.7%) and PBO (19.4%) (difference, 0.3% (95% CI −7.6 to 8.2); nominal p=0.94). Post hoc analysis on patients not on IMV: Among patients not receiving MV at randomisation, less patients in the TCZ group experienced any clinical failure at day 28 compared with PBO (29% vs 42.2%) HR 0.614; 95% CI 0.40 to 0.94; nominal p=0.03). |
Serious adverse events occurred in 34.9% of 295 patients in the TCZ arm and 38.5% of 143 in the PBO arm. | Unclear |
*Standard care of each recruiting site. Since participants could be randomised to other interventions within other domains, depending on domains active at the site, patient eligibility and consent (see www.remapcap.org). Randomisation to the corticosteroid domain for COVID-19 closed on 17 June 2020.12 Thereafter, corticosteroids were allowed as per recommended standard of care.
†Standard care per local practice (antiviral treatment, low-dose steroids, convalescent plasma and supportive care) was permitted; however, concomitant treatment with another investigational agent (except antivirals) or any immunomodulatory agent was prohibited.
AE, adverse event; AZT, azithromycin; COL, colchicine; CrI, credibility interval; DEX, dexamethasone; HCQ, hydroxychloroquine; HCT, hydrocortisone; ICU, intensive care unit; MTP, methylprednisolone; PBO, placebo; RUXO, ruxolitinib; SAE, severe adverse event; SARI, sarilumab; SE, standard error; SOC, standard of care; TCZ, tocilizumab.
Safety
The safety profile of TCZ was good, with the study by Stone et al 24 showing fewer serious infections in the TCZ group in spite of an increase rate of neutropaenia.
Anakinra
Efficacy
One RCT assessed anakinra in patients with COVID-19 requiring at least 3 L/min oxygen therapy (CORIMUNO-19) and was published online in The Lancet Respiratory Medicine on 22 January 2021.30 The addition of the drug to SOC failed to improve survival without NIV (including high-flow oxygen) or IMV at day 14 or survival at day 90.
Safety
From a safety perspective, there was a numerical increase of serious infections in the anakinra group.
Baricitinib
Efficacy
At present, the only RCT available on baricitinib in SARS-CoV-2 infection compared remdesevir+baricitinib versus remdesevir+placebo.31 Patients receiving remdesevir+baricitinib had a median time to recovery of 7 days, as compared with 8 days in the remdesevir+placebo group (rate ratio for recovery: 1.16; 95% CI 1.01 to 1.32; p=0.03), which is statistically significant but clinically probably not meaningful, except in the subgroup of patients with a baseline NIV (including high flow oxygen) in whom median time to recovery was 10 days with the combination therapy, as compared with 18 days in the remdesivir only control group (rate ratio for recovery: 1.51; 95% CI 1.10 to 2.08). It is important to note that the global mortality in the ACTT-2 trial was lower (around 5%) than in other trials like the RECOVERY DEX trial (around 20%) that might explain the modest effect size observed in ACTT-2. Interestingly, the ACTT-4, evaluating the combination of baricitinib and remdesivir compared with DEX and remdesivir is currently ongoing.32
Safety
The incidence of adverse events was similar in the two treatment groups.
Other immunomodulatory drugs
Efficacy
The SLR yielded three publications on two RCTs on interferon (IFN) beta,33–35 one on the Janus kinase inhibitor ruxolitinib,36 one on anti-C5a vilobelimab,37 one on colchicine,38 two on IVIg39 40 and three on convalescent plasma.41–43 The studies on vilobelimab and colchicine were at unclear RoB, while all the others were at high RoB. The studies on IFN-beta provided conflicting results on mortality and other clinical outcomes (tables 2 and 3).33–35 No differences on mortality or in the need of IMV were observed in patients treated with ruxolitinib,36 while IVIg reduced mortality in hospitalised patients requiring NIMV/IMV.39 The addition of colchicine to SOC allowed a larger number of patients to achieve cumulative event-free 10-day survival, using a composite outcome including mortality or need of IMV, and a lower number of patients displayed clinical deterioration.38 However, patients with a slightly milder phenotype not requiring IMV were enrolled. On 24 January 2021 the results of the large COLCORONA trial have been released highlighting that colchicine reduced hospitalisation, use of ventilation and mortality.44 Vilobelimab was not effective on any of the outcomes assessed (table 4). All studies on convalescent plasma failed to show any efficacy on 28-day mortality, progression to severe disease42 or clinical improvement at 2841 or 3043 days. On the day of submission of this article, a press release announced that the phase III RUXCOVID study evaluating ruxolitinib+SOC compared with placebo+SOC in patients with COVID-19 did not meet its primary endpoint of reducing the number of hospitalised patients with COVID-19 who experienced severe complications (death, mechanical ventilation or ICU care).45 Finally, a press release on 2 July 2020 reported the failure of a phase III trial assessing sarilumab in critical patients (requiring IMV) with COVID-19,46 while in the above-mentioned REMAP-CAP study (grey literature) assessing TCZ and sarilumab demonstrated efficacy of the latter in improving survival and other outcomes.28
Safety
Ruxolitininb and vilobelimab and convalescent plasma showed a good safety profile. Conversely, data were conflicting for IFN-beta, not reported for IVIg and worse safety profile for colchicine since authors highlighted a higher frequency of diarrhoea in colchicine-treated patients.
Data from prospective or retrospective controlled studies
Prospective controlled studies were identified as best available evidence for eight therapeutic strategies, three of which using a combination of two immunomodulatory drugs (online supplemental table 5).
Glucocorticoids+TCZ
Efficacy
Three studies assessed this therapeutic strategy.47–49 Ramiro et al 47 enrolled patients requiring any kind of oxygen support, reporting that the proportion of patients receiving IMV was higher in the cohort of patients treated with SOC versus those receiving TCZ (15% vs 1%). The treatment protocol included sequential MTP and TCZ, the latter added if lack or clinical response to MTP within 2–5 days. Historical control groups were identified among patients referred to the same centre in the previous month and receiving SOC only. Significant positive effects were observed in the TCZ+MTP group with regard to mortality, IMV, oxygen support, clinical improvement and time to discharge. Of note, day-28 mortality rate in the control group was high (48%).
Likewise, Sanz Herrero et al 49 compared patients receiving TCZ either monotherapy or in combination with MTP and reported that combination therapy was superior to monotherapy in reducing the risk of death. On the contrary, Gupta et al 50 reported that the association between TCZ treatment and mortality was similar in patients having received or not glucocorticoids on ICU admission (HRs (95% CI) 0.68 (0.46 to 0.99) and 0.71 (0.53 to 0.96)), respectively.
Safety
One study at unclear RoB reported that although the overall rate of adverse events was comparable in the treatment groups, there was a trend towards more pulmonary embolism in the TCZ+glucocorticoids group (p=0.059). Arrhythmias occurred less frequently, although not significantly, in the TCZ+glucocorticoids group (p=0.265).47
Glucocorticoids+baricitinib
Efficacy
The combination of baricitinib and glucocorticoids added to SOC was assessed in a study at high RoB.51 Patients with severe COVID-19, half of which were receiving NIV (IMV was an exclusion criterion) received three consecutive days of pulse MTP therapy (80, 125 or 250 mg/day) followed by prednisone at a starting dose of 30 mg/day tapered until discontinuation within 7–10 days. Those receiving only MTP were compared with those receiving also baricitinib from day 3 (2 or 4 mg/day), and the combination therapy (regardless of the baricitinib dose) was linked to more pronounced clinical improvement, a lower use of supplemental oxygen both at discharge and 1 month later was compared with MTP+SOC.
Safety
A number of adverse events occurred in the two treatment groups, including infectious and cardiac adverse events, but the authors did not flag any specific scenario attributable to baricitinib. Of particular interest, occurrence of venous thromboembolism, a class warning for JAK inhibitors, was similar in the two treatment groups.
Other immunomodulatory drugs
A few small prospective studies at variable RoB evaluated mavrilimumab,52 lenzilumab,53 eculizumab,54 sarilumab,55 recombinant human IL-756 and the combination of ruxolitinib+eculizumab,57 ruxolitinib+glucocorticoids58 and cyclosporin+glucocorticoids.59 However, none of them provided solid positive results.
One retrospective controlled study of infliximab at high RoB showed comparable mortality rate and need of IMV in 17 patients with COVID-19 treated with SOC versus seven patients receiving infliximab in addition to SOC. In the ‘grey literature’, we came across other ongoing studies with infliximab (ACTIV-1: NCT04593940 and CATALYST: ISRCTN40580903) and adalimumab (AVID-CC: ISRCTN33260034).60 One retrospective study explored anakinra in combination with glucocorticoids reporting a possible benefit in reducing mortality.61
Data from non-controlled studies
Canakinumab was evaluated in one retrospective non-controlled study and one case report,62 63 tesidolumab was assessed in one retrospective study64 and itolizumab was assessed in a prospective non-controlled study.65 These studies showed favourable, although very preliminary results, that required to be confirmed in controlled studies.
Immunomodulatory therapies with evidence on mild COVID-19 (without oxygen therapy)
Six immunomodulatory strategies were assessed in RCTs at high or unclear RoB enrolling patients with mild to moderate COVID-19 (table 5).
Table 5.
Effect and safety of immunomodulatory drugs assessed in mild COVID-19 (without oxygen support)
| Outcome | Drug | Author, year (ref) | Study design | Study groups | Results | Risk of bias |
| Mortality | Hydroxychloroquine | Lyngbakken et al 202078 | RCT | SOC+HCQ SOC |
No difference between groups. | High |
| Ulrich et al 202021 | RCT | SOC+HCQ SOC |
No difference between groups at day 14 for the composite criteria (death, ICU admission, mechanical ventilation, extracorporeal membrane oxygenation and/or vasopressor use). | High | ||
| Baricitinib | Bronte et al 202074 | Prospective | SOC+BARI SOC |
1/20 (5%) in BARI group versus 25/56 (45%) SOC group (p<0.001). | High | |
| IFN alpha | Wang et al 202071 | Prospective | SOC+IFN alpha-2b SOC |
None of the patients died in any group. | High | |
| Discharge/Time to Hospital Discharge | Hydroxychloroquine | Lyngbakken et al 202078 | RCT | SOC+HCQ SOC |
No difference between groups p by log-rank test=0.71. | High |
| Baricitinib | Cantini et al 202073 | Prospective | SOC+BARI SOC |
Discharge at week 2 occurred in 58% (7/12) of the BARI-treated patients versus 8% (1/12) of controls (p=0.027). | High | |
| Leflunomide | Wang et al 202069 | RCT | SOC+LEF SOC |
No difference between groups 29.0 (IQR 19.3–47.3) days versu 33.0 (IQR 29.3–42.8) days p=0.170. | High | |
| IFN alpha | Wang et al 202071 | Prospective | SOC+IFN alpha-2b SOC |
Shorter time to discharge in the treatment group. Even shorter if early intervention. | High | |
| Negative conversion of SARS-CoV-2 | Hydroxychloroquine | Mitja et al 202066 | RCT | SOC+HCQ SOC | No difference across groups day 3 and day 7. | Unclear |
| Chen et al 202017 | RCT | SOC+HCQ SOC | No difference in time to negative PCR at day 14: 5 days (95% CI 1 to 9 days) and 10 days (95% CI 2 to 12 days) for the HCQ and SOC groups, respectively (p=0.40). | High | ||
| Omrani et al 202068 | RCT | SOC+HCQ SOC | No difference across groups day 6 negative PCR (p=0.821) HCQ+AZT 16/152 (10.5%), HC 19/149 (12.8%), placebo 18/147 (12.2%). Day 14 (p=0.072) HC +AZ 30/149 (20.1%), HC 42/146 (28.8%), placebo 45/143 (31.5%). |
High | ||
| Leflunomide | Hu et al 202070 | RCT | SOC+LEF SOC |
5 days LEF versus 11 days control group (p=0.046). | High | |
| Wang et al 202069 | RCT | SOC+LEF SOC |
No difference between groups HR for negative RT-PCR, 0.70; (95% CI 0.391 to 1.256; p=0.186). |
High | ||
| IFN alpha | Wang et al 202071 | Prospective | SOC+IFN alpha-2b SOC |
Faster in the treatment group. | High | |
| IFN kappa | Fu et al 202072 | RCT | SOC+IFN kappa SOC | Significantly shorter time to viral RNA negative conversion in IFN group. | Unclear | |
| Treatment emergent AEs | Hydroxychloroquine | Mitjà et al 202066 | RCT | SOC+HCQ SOC |
AE in SOC 16/184 (8.7%)<121/169 (72.0%) in HCQ group. | Unclear |
| Skipper et al 202067 | RCT | SOC+HCQ SOC |
AEs with HCQ >PBO at day 5 (43% (92 of 212) versus 22% (46 of 211); p<0.001). GI symptoms in 31% (66 of 212). | Unclear | ||
| Chen et al 202017 | RCT | SOC+HCQ SOC |
No SAE reported. Grades 1 and 2 HCQ-related adverse events included headache (21.1%), dizziness (5.3%), gastritis (5.3%), diarrhoea (5.3%), nausea (5.3%) and photophobia (5.3%). | High | ||
| Omrani et al 202068 | RCT | SOC+HCQ SOC |
No SAE. No association (p=0.708) between study group and development of pneumonia, which was diagnosed in seven participants (1.5%): three (2.0%) in the HC+AZ group, one (0.7%) in the HC group and three (2.0%) in the placebo group. | High | ||
| Ulrich et al 202021 | RCT | SOC+HCQ SOC |
No difference in AEs between the groups. HCQ was associated with a slight increase in mean corrected QT interval, an increased D-dimer and a trend towards an increased length of stay. | High | ||
| Leflunomide | Hu et al 202070 | RCT | SOC+LEF SOC |
ALT and AST reversibly increased LEF group (p=0.049 and p=0.176, respectively). | High | |
| Wang et al 202069 | RCT | SOC+LEF SOC |
No difference in AEs between the groups. | High | ||
| Tocilizumab | Zhao et al 202075 | RCT | SOC+favipiravir SOC+favipiravir +TCZ |
Nine adverse reactions were reported in the combined treatment group, and two adverse reactions were reported in the favipiravir group and the TCZ group, respectively. | High | |
| Baricitinib | Cantini et al 202073 | Prospective | SOC+BARI SOC |
No SAEs. 1 patient with transaminases elevation in the BARI group. | High | |
| Bronte et al 202074 | Prospective | SOC+BARI SOC |
No SAEs. | High | ||
| IFN alpha | Wang et al 202071 | Prospective | SOC+IFN alpha-2b SOC |
No difference in AEs between the groups. | High | |
| IFN kappa | Fu et al 202072 | RCT | SOC+IFN kappa SOC | No SAEs. | Unclear |
Only studies reporting on the corresponding outcome are shown.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AZT, azithromycin; BARI, baricitinib; COL, colchicine; DEX, dexamethasone; GI, gastrointestinal; HCQ, hydroxychloroquine; HCT, hydrocortisone; IFN, interferon; LEF, leflunomide; MTP, methylprednisolone; PBO, placebo; RT-PCR, real time PCR; RUXO, ruxolitinib; SAE, severe adverse event; SAEs, serious adverse events; SE, standard error; SOC, standard of care; TCZ, tocilizumab.
Hydroxychloroquine
Efficacy
Five RCTs evaluated HCQ in mild to moderate COVID-19,17 21 66–68 but none of them demonstrated any benefit with the addition of this drug to SOC (including in milder non-hospitalised patients.66 67
Safety
In line with what was reported from studies in severe COVID-19, the RCTs enrolling mild to moderate COVID-19 highlighted safety concerns for HCQ since a higher number of adverse events were observed in the HCQ-SOC group compared with SOC.
Other immunomodulatory drugs
Two small RCTs at high RoB reported on leflunomide.69 70 One study observed no difference in length of hospital stay,69 while conflicting results were reported by both studies with regard to a possible effect on negative conversion of SARS-CoV-2. Safety concerns were raised by one of the studies with increased liver enzymes in leflunomide-treated patients.70
IFN-alpha71 and IFN-kappa72 reduced the time to negative conversion of SARS-CoV-2 in two studies. Two prospective studies on baricitinib at high RoB provided conflicting results for every assessed outcome and only agreed on the fact that addition of baricitinib to SOC did not worsen the safety profile of the therapeutic strategy.73 74 One small study evaluated TCZ+favipavir demonstrating positive effects on lung inflammation.75
Discussion
Our SLR has shown that despite the large bulk of articles investigating several immunomodulatory drugs for the treatment of SARS-CoV-2 infection, most studies are at high or unclear RoB, and robust evidence on efficacy is available only for a few drugs and for a low number of outcomes. In particular, data from RCTs showed that the addition of HCQ to SOC was not beneficial at any stage of SARS-CoV-2 infection, while glucocorticoids may reduce mortality in some subgroups of patients with moderate, severe or critical COVID-19. The latter evidence is mainly driven by the large RECOVERY trial.6 Regarding TCZ, three available RCT were positive, but three other RCTs are negative. Thus, TCZ could have a place in some specific subgroups that remain to be determined.23 76
The SLR identified a number of pitfalls that prevented the comparison of retrieved studies and constrains results interpretation. First, heterogeneity of inclusion criteria even in studies claiming to assess the same patient subgroup (eg, severe COVID-19) was often observed. In fact, various parameters, such as the partial pressure of oxygen (PaO2)/fractional inspired oxygen ratio, C reactive protein level and peripheral oxygen saturation to cite a few, with different cut-off values, have been used to classify patients contributing to a relevant selection bias. We tried to overcome this issue and harmonise the presentation of results using a framework inspired by one the WHO scales.77
In RCTs, the definition of ‘standard of care’ was also highly variable making data interpretation difficult. Every immunomodulatory drug that has been assessed was added on top of SOC and compared (with a few exceptions) with SOC alone. However, in COVID-19, SOC changed rapidly, and the approaches recommended as SOC in March 2020 were not the same as in the subsequent months. Moreover, other factors such as local/national regulations or recommendations, criteria for hospital admission/IMV or differing drug availability increased study variability even if published within the same timeframe. In addition, in some studies, the treatment, including glucocorticoids, interferon or other immunomodulatory drugs, was left at the discretion of the treating physician, meaning that a subgroup of the intervention group could receive other drugs in a non-standardised manner, subsequently affecting the interpretability of the results.
In prospective observational studies, the main pitfall was that the control groups were often historical and thus not comparable with the studied group, even if adjusted for baseline characteristics, given the rapid evolution in the treatment of the disease. Finally, yet importantly, study outcomes along with the timing of their assessment largely varied across studies.
In conclusion, this SLR informed the EULAR initiative to formulate PtC on COVID-19 pathophysiology and immunomodulatory therapies. However, the results of the present SLR also underscored the need of RCTs with standardised inclusion criteria and outcomes in order to robustly elucidate the effect of immunomodulatory drugs at different stages of SARS-CoV-2 infection and ultimately improve the care and prognosis of affected people. Another important aspect to be further explored is the identification of factors predicting efficacy of the selected drug(s) in a specific population.
Footnotes
Handling editor: Désirée van der Heijde
Twitter: @pedrommcmachado
AA and AN contributed equally.
Contributors: All authors contributed and finally approved the current manuscript.
Funding: This work was funded by European League Against Rheumatism (CLI122). PMM is supported by the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre.
Disclaimer: The views expressed are those of the authors and not necessarily those of the (UK) National Health Service, NIHR or the Department of Health.
Competing interests: XM has received consulting and/or speaker’s fees from BMS, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Novartis, Pfizer, Servier and UCB, all unrelated to this manuscript. DGM has received consulting and/or speaker’s fees from Abbvie, BMS, Celgene, Eli Lilly, Janssen, MSD, Novartis, Pfizer, Roche and UCB, all unrelated to this manuscript. PMM has received consulting and/or speaker’s fees from Abbvie, BMS, Celgene, Eli Lilly, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, all unrelated to this manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 2. Wong CKH, Wong JYH, Tang EHM, et al. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: a systematic review and meta-analysis. Sci Rep 2020;10:19765. 10.1038/s41598-020-74988-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGonagle D, Sharif K, O'Regan A, O’Regan A, et al. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 2020;19:102537. 10.1016/j.autrev.2020.102537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alunno A, Carubbi F, Rodríguez-Carrio J. Storm, Typhoon, cyclone or Hurricane in patients with COVID-19? beware of the same storm that has a different origin. RMD Open 2020;6:e001295. 10.1136/rmdopen-2020-001295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0, 2013. [Google Scholar]
- 6. The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med Overseas Ed 2020:NEJMoa2021436. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 7. Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis 2020. 10.1093/cid/ciaa1177. [Epub ahead of print: 12 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and Ventilator-Free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the Codex randomized clinical trial. JAMA 2020;324:1307. 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 2020;324:E1–13. 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dequin P-F, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 2020;324:1298. 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J 2020;56. 10.1183/13993003.02808-2020. [Epub ahead of print: 24 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324:E1–12. 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abd-Elsalam S, Esmail ES, Khalaf M, et al. Hydroxychloroquine in the treatment of COVID-19: a multicenter randomized controlled study. Am J Trop Med Hyg 2020;103:1635–9. 10.4269/ajtmh.20-0873 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Huang HD, Jneid H, Aziz M, et al. Safety and effectiveness of hydroxychloroquine and azithromycin combination therapy for treatment of hospitalized patients with COVID-19: a Propensity-Matched study. Cardiol Ther 2020;9:523–34. 10.1007/s40119-020-00201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020;369:m1849. 10.1136/bmj.m1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med 2020;383:e119. 10.1056/NEJMx200021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen C-P, Lin Y-C, Chen T-C, et al. A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID-19). PLoS One 2020;15:e0242763. 10.1371/journal.pone.0242763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. RECOVERY Collaborative Group, Horby P, Mafham M, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020;383:2030–40. 10.1056/NEJMoa2022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO Solidarity Trial Consortium . Repurposed antiviral drugs for Covid-19 — interim who solidarity trial results. N Engl J Med Overseas Ed 2020:NEJMoa2023184. 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Self WH, Semler MW, Leither LM, et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA 2020;324:2165. 10.1001/jama.2020.22240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ulrich RJ, Troxel AB, Carmody E, et al. Treating COVID-19 with hydroxychloroquine (teach): a multicenter, double-blind randomized controlled trial in hospitalized patients. Open Forum Infect Dis 2020;7:ofaa446. 10.1093/ofid/ofaa446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernandez AV, Roman YM, Pasupuleti V, et al. Update alert 3: hydroxychloroquine or chloroquine for the treatment or prophylaxis of COVID-19. Ann Intern Med 2020;173:W156–7. 10.7326/L20-1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:32. 10.1001/jamainternmed.2020.6820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020;383:2333–44. 10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:24–31. 10.1001/jamainternmed.2020.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;384:20–30. 10.1056/NEJMoa2030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Furlow B. COVACTA trial raises questions about tocilizumab's benefit in COVID-19. Lancet Rheumatol 2020;2:e592. 10.1016/S2665-9913(20)30313-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. The REMAP-CAP Investigators . Interleukin-6 receptor antagonists in critically ill patients with Covid-19 – preliminary report. medRxiv 2020. 10.1101/2021.01.07.21249390 [DOI] [Google Scholar]
- 29. Veiga VC, Prats J, Farias DLC. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ 2020. 10.1136/bmj.n84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mariette X, Hermine O, Tharaux P-L. Effect of Anakinra vs usual care in adults hospitalized with COVID-19 and mild-to-moderate pneumonia: a randomized clinical trial. Lancet Respir Med 2020. 10.1016/S2213-2600(20)30556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med 2020. 10.1056/NEJMoa2031994. [Epub ahead of print: 11 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. NIH . Adaptive COVID-19 treatment trial 4 (ACTT-4). Available: https://clinicaltrials.gov/ct2/show/NCT04640168
- 33. Davoudi-Monfared E, Rahmani H, Khalili H. Efficacy and safety of interferon beta-1a in treatment of severe COVID-19: a randomized clinical trial. Antimicrob Agents Chemother 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahmani H, Davoudi-Monfared E, Nourian A, et al. Interferon β-1b in treatment of severe COVID-19: a randomized clinical trial. Int Immunopharmacol 2020;88:106903. 10.1016/j.intimp.2020.106903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 2020. 10.1016/S2213-2600(20)30511-7. [Epub ahead of print: 12 Nov 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020;146:137–46. 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vlaar APJ, de Bruin S, Busch M, et al. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol 2020;2:e764–73. 10.1016/S2665-9913(20)30341-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019. JAMA Netw Open 2020;3:e2013136. 10.1001/jamanetworkopen.2020.13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gharebaghi N, Nejadrahim R, Mousavi SJ, et al. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis 2020;20:786. 10.1186/s12879-020-05507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tabarsi P, Barati S, Jamaati H, et al. Evaluating the effects of intravenous immunoglobulin (IVIg) on the management of severe COVID-19 cases: a randomized controlled trial. Int Immunopharmacol 2021;90:107205. 10.1016/j.intimp.2020.107205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 2020;324:460. 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agarwal A, Mukherjee A, Kumar G. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial). BMJ;151:m3939. 10.1136/bmj.m3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med 2020. 10.1056/NEJMoa2031304. [Epub ahead of print: 24 Nov 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. The Canadian . ‘Major breakthrough’ | Large study shows effectiveness of colchicine to treat COVID-19. Available: https://thecanadian.news/2021/01/23/major-breakthrough-large-study-shows-effectiveness-of-colchicine-to-treat-covid-19/
- 45. Novartis . Novartis provides update on RUXCOVID study of ruxolitinib for hospitalized patients with COVID-19, 2020. Available: https://www.novartis.com/news/media-releases/novartis-provides-update-ruxcovid-study-ruxolitinib-hospitalized-patients-covid-19
- 46. SANOFI . Sanofi and Regeneron provide update on Kevzara® (sarilumab) phase 3 U.S. trial in COVID-19 patients, 2020. Available: https://www.sanofi.com/en/media-room/press-releases/2020/2020-07-02-22-30-00
- 47. Ramiro S, Mostard RLM, Magro-Checa C, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the chiC study. Ann Rheum Dis 2020;79:1143–51. 10.1136/annrheumdis-2020-218479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martínez-Urbistondo D, Costa Segovia R, Suárez Del Villar Carrero R, et al. Early combination of tocilizumab and corticosteroids: an upgrade in anti-inflammatory therapy for severe COVID. Clin Infect Dis 2020. 10.1093/cid/ciaa910. [Epub ahead of print: 04 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sanz Herrero F, Puchades Gimeno F, Ortega García P, et al. Methylprednisolone added to tocilizumab reduces mortality in SARS-CoV-2 pneumonia: an observational study. J Intern Med 2020. 10.1111/joim.13145. [Epub ahead of print: 30 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med 2021;181:41–51. 10.1001/jamainternmed.2020.6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, et al. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology 2021;60:399–407. 10.1093/rheumatology/keaa587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. De Luca G, Cavalli G, Campochiaro C, et al. Gm-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol 2020;2:e465–73. 10.1016/S2665-9913(20)30170-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Temesgen Z, Assi M, Shweta FNU, et al. Gm-CSF neutralization with Lenzilumab in severe COVID-19 pneumonia: a case-cohort study. Mayo Clin Proc 2020;95:2382–94. 10.1016/j.mayocp.2020.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Annane D, Heming N, Grimaldi-Bensouda L, et al. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: a proof-of-concept study. EClinicalMedicine 2020;28:100590. 10.1016/j.eclinm.2020.100590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Della-Torre E, Campochiaro C, Cavalli G, et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis 2020;79:1277–85. 10.1136/annrheumdis-2020-218122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Laterre PF, François B, Collienne C, et al. Association of interleukin 7 immunotherapy with lymphocyte counts among patients with severe coronavirus disease 2019 (COVID-19). JAMA Netw Open 2020;3:e2016485. 10.1001/jamanetworkopen.2020.16485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giudice V, Pagliano P, Vatrella A, et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-Related acute respiratory distress syndrome: a controlled study. Front Pharmacol 2020;11:857. 10.3389/fphar.2020.00857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. D'Alessio A, Del Poggio P, Bracchi F, et al. Low-dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia. Leukemia 2020:1–4. 10.1038/s41375-020-01087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Galvez-Romero JL, Palmeros-Rojas O, Real-Ramírez FA, et al. Cyclosporine a plus low-dose steroid treatment in COVID-19 improves clinical outcomes in patients with moderate to severe disease. A pilot study. J Intern Med 2020. 10.1111/joim.13223. [Epub ahead of print: 03 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mahase E. Covid-19: anti-TNF drug adalimumab to be trialled for patients in the community. BMJ 2020;371:m3847. 10.1136/bmj.m3847 [DOI] [PubMed] [Google Scholar]
- 61. Bozzi G, Mangioni D, Minoia F, et al. Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: an observational cohort study. J Allergy Clin Immunol 2020. 10.1016/j.jaci.2020.11.006. [Epub ahead of print: 19 Nov 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ucciferri C, Auricchio A, Di Nicola M, et al. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol 2020;2:e457–8. 10.1016/S2665-9913(20)30167-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Caracciolo M, Macheda S, Labate D, et al. Case report: canakinumab for the treatment of a patient with COVID-19 acute respiratory distress syndrome. Front Immunol 2020;11:1942. 10.3389/fimmu.2020.01942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zelek WM, Cole J, Ponsford MJ, et al. Complement inhibition with the C5 blocker LFG316 in severe COVID-19. Am J Respir Crit Care Med 2020;202:1304–8. 10.1164/rccm.202007-2778LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Caballero A, Filgueira LM, Betancourt J, et al. Treatment of COVID-19 patients with the anti-CD6 antibody itolizumab. Clin Transl Immunology 2020;9:e1218. 10.1002/cti2.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mitjà O, Corbacho-Monné M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild Covid-19: a Randomized-Controlled trial. Clin Infect Dis 2020. 10.1093/cid/ciaa1009. [Epub ahead of print: 16 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19 : a randomized trial. Ann Intern Med 2020;173:623–31. 10.7326/M20-4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Omrani AS, Pathan SA, Thomas SA, et al. Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe Covid-19. EClinicalMedicine 2020;29:100645. 10.1016/j.eclinm.2020.100645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang M, Zhao Y, Hu W, et al. Treatment of COVID-19 patients with prolonged Post-Symptomatic viral shedding with leflunomide -- a single-center, randomized, controlled clinical trial. Clin Infect Dis 2020. 10.1093/cid/ciaa1417. [Epub ahead of print: 21 Sep 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hu K, Wang M, Zhao Y, et al. A small-scale medication of leflunomide as a treatment of COVID-19 in an open-label Blank-Controlled clinical trial. Virol Sin 2020;35:725–33. 10.1007/s12250-020-00258-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang B, Li D, Liu T, et al. Subcutaneous injection of IFN alpha-2b for COVID-19: an observational study. BMC Infect Dis 2020;20:723. 10.1186/s12879-020-05425-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fu W, Liu Y, Liu L, et al. An open-label, randomized trial of the combination of IFN-κ plus TFF2 with standard care in the treatment of patients with moderate COVID-19. EClinicalMedicine 2020;27:100547. 10.1016/j.eclinm.2020.100547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cantini F, Niccoli L, Matarrese D, et al. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect 2020;81:318–56. 10.1016/j.jinf.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bronte V, Ugel S, Tinazzi E. Baricitinib restrains the immune dysregulation in severe COVID-19 patients. J Clin Invest 2020;130:6409–16. 10.1172/JCI141772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhao H, Zhu Q, Zhang C, et al. Tocilizumab combined with favipiravir in the treatment of COVID-19: a multicenter trial in a small sample size. Biomed Pharmacother 2021;133:110825. 10.1016/j.biopha.2020.110825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Parr JB. Time to reassess tocilizumab's role in COVID-19 pneumonia. JAMA Intern Med 2021;181:12. 10.1001/jamainternmed.2020.6557 [DOI] [PubMed] [Google Scholar]
- 77. Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020;20:e192–7. 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lyngbakken MN, Berdal J-E, Eskesen A, et al. A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat Commun 2020;11:5284. 10.1038/s41467-020-19056-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-219725supp001.pdf (868.1KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.