Abstract
Animal models for inflammatory arthritides such as rheumatoid arthritis (RA) and psoriatic arthritis are widely accepted and frequently used to identify pathological mechanisms and validate novel therapeutic strategies. Unfortunately, many publications reporting on these animal studies lack detailed description and appropriate assessment of the distinct histopathological features of arthritis: joint inflammation, cartilage damage and bone erosion. Therefore, the European consortium BeTheCure, consisting of 38 academic and industrial partners from 15 countries, set as goal to standardise the histological evaluation of joint sections from animal models of inflammatory arthritis. The consensual approach of a task force including 16 academic and industrial scientists as well as laboratory technicians has resulted in the development of the Standardised Microscopic Arthritis Scoring of Histological sections (‘SMASH’) recommendations for a standardised processing and microscopic scoring of the characteristic histopathological features of arthritis, exemplified by four different rodent models for arthritis: murine collagen-induced arthritis, collagen–antibody-induced arthritis, human tumour necrosis factor transgenic Tg197 mice and rat pristane-induced arthritis, applicable to any other inflammatory arthritis model. Through standardisation, the SMASH recommendations are designed to improve and maximise the information derived from in vivo arthritis experiments and to promote reproducibility and transparent reporting on such studies. In this manuscript, we will discuss and provide recommendations for analysis of histological joint sections: identification of the regions of interest, sample preparation, staining procedures and quantitative scoring methods. In conclusion, awareness of the different features of the arthritis pathology in animal models of inflammatory arthritis is of utmost importance for reliable research outcome, and the standardised histological processing and scoring methods in these SMASH recommendations will help increase uniformity and reproducibility in preclinical research on inflammatory arthritis.
Keywords: arthritis, experimental, arthritis, rheumatoid, arthritis, psoriatic, synovitis
Introduction
Inflammatory arthritides such as rheumatoid arthritis (RA) and psoriatic arthritis are common systemic inflammatory diseases characterised by synovial inflammation causing structural joint damage and functional disabilities.1 2 Numerous animal models that closely resemble characteristic features found in patients with arthritis are studied worldwide to identify novel pathologenetic mechanisms or to validate novel therapeutic approaches (figure 1).3–5 Based on the complexity of the disease, it is of particular importance to correctly address the effects of therapeutic agents, or other interventions, such as gene knock-ins or knock-outs on the distinct pathophysiological features including synovial inflammation, bone erosion and cartilage damage in these models. Unfortunately, published findings from many animal studies lack detailed description and appropriate assessment of the distinct histopathological features of arthritis: methods of processing and scoring are poorly defined, and often several histological variables are combined into one score, thereby losing power to detect differences and making it impossible to uncouple processes like joint inflammation and destruction. While clinical trial designs are highly regulated,6–8 studies of experimental arthritis are not standardised and every group may have its own methods for histological processing and scoring of joint sections, thereby hampering the combination and comparison of multiple data sets. Therefore, the European consortium BeTheCure, consisting of 38 academic and industrial partners from 15 countries, funded by the Innovative Medicine Initiative, a public–private partnership between the European Union and the European Federation of Pharmaceutical Industries and Associations, set as one of their main goals to standardise the histological evaluation of joint sections from animal models of inflammatory arthritis. A task force team of 16 academic scientists, industrial scientists and laboratory technicians experienced in arthritis models has therefore developed the Standardised Microscopic Arthritis Scoring of Histological sections (‘SMASH’) recommendations for a standardised processing and microscopic scoring of the histopathological features of arthritis, exemplified by four different models, and applicable to any other inflammatory arthritis model. Selected arthritis models included three of the most established systemic mouse models, namely, collagen-induced arthritis (CIA), collagen–antibody-induced arthritis (CAIA) and human tumour necrosis factor transgenic Tg197 mice as well as the rat pristane-induced arthritis (PIA) model.9–16 Importantly, the standardised assessment of these four models allowed us to highlight their differences regarding extent of synovial inflammation, subchondral bone erosion and articular cartilage damage or development of osteophytes, the latter not being untypical for RA but common in spondyloarthritis. These differences, as well as the different pathophysiological mechanisms like the relative contribution of the innate and/or the adaptive immune system to pathogenesis, determine which model suits the investigator’s research question best.3–5 17–19
Figure 1.
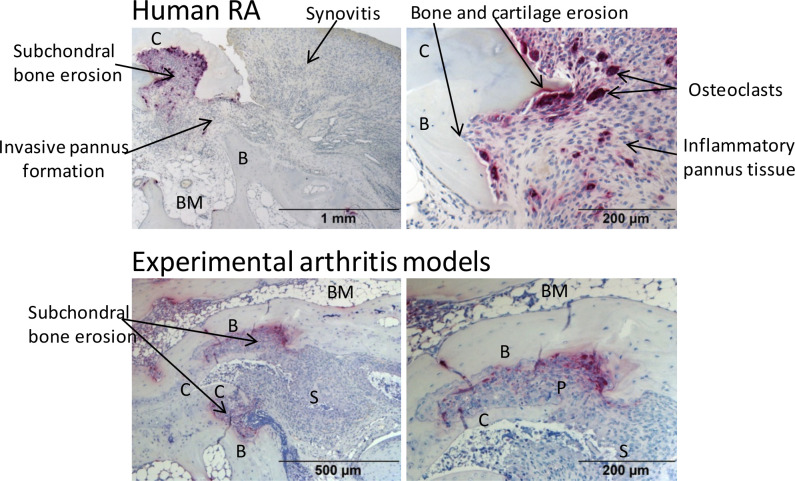
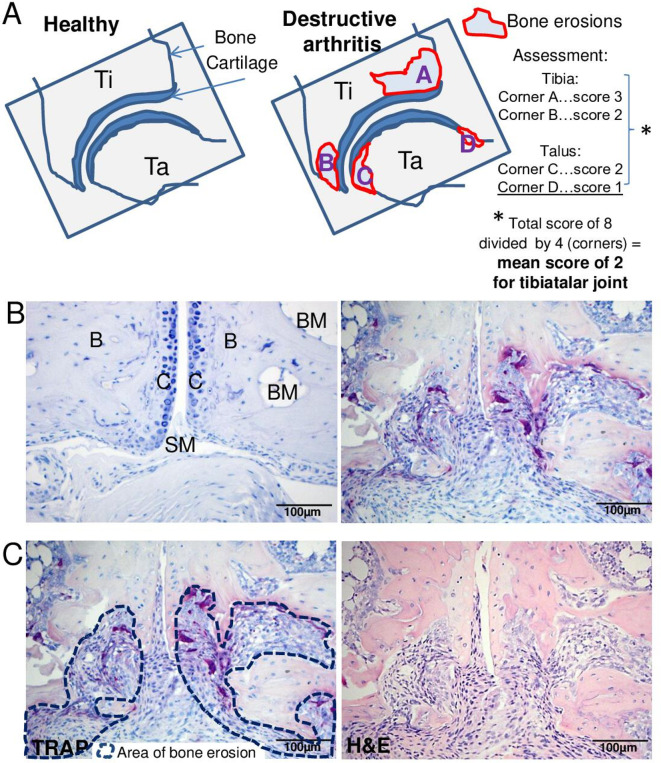
Common histopathological features of inflammatory joint damage in human RA and experimental models. TRAP-stained joint sections indicate synovitis, pannus formation, synovial osteoclast formation, subchondral bone erosions as well as cartilage erosion in human RA (upper row, MTP-1 joint section) and experimental arthritis models (lower row, affected tarsal joint from a 10-week-old Tg197 mouse). Original magnification is 50× (upper left), 100× (upper right; lower left) and 200× (lower right). B, bone; BM, bone marrow; C, cartilage; P, synovial pannus; RA, rheumatoid arthritis; S, synovium; TRAP, tartrate-resistant acid phosphatase.
These SMASH recommendations are primarily aimed at scientists performing histological analysis of joints from arthritis studies in mice and rat. Through standardisation of processing and scoring arthritis pathology, the SMASH recommendations aim to improve and maximise the information derived from in vivo arthritis experiments and to promote reproducibility and transparent reporting on such studies.
Methodology
The BeTheCure task force included 16 academic scientists, industrial scientists and laboratory technicians from eight European countries experienced in histological evaluation of arthritis models. This task force aimed to develop a basic set of recommendations for the histopathological assessment of inflammatory arthritis based on expert opinions and published literature.20–31 Histological procedures and scoring systems have been collected, discussed and defined during Annual BeTheCure meetings, three BeTheCure animal workshops in Stockholm, Athens and Vienna and two expert meetings in Nijmegen and Vienna, which finally resulted in the consensus definition of 30 main recommendations. Recommendations on technical procedures resulted from best practice and knowledge of experts. Recommendations on scoring systems have been finally validated in common microscopic screening rounds by SH, MJV, CG, MIK. Final levels of agreement were assessed by a voting survey (1, disagree, to 10, agree) derived from all task force members (table 1). Mean levels of agreement (SD) were very high for these recommendations (>9/10). Representative images of joint sections were selected from a collection of histological sections from four animal models (CIA, CAIA, Tg197 and PIA) provided by the contributing institutions of the task force members.
Table 1.
Recommendations for standardised processing, scoring and reporting of the histopathology from inflammatory arthritis in mice and rats
| Recommendations | Mean level of agreement (SD) |
| Category 1: Sample selection and orientation | |
| 1) Before starting an animal experiment to test a research hypothesis, a sample size calculation should be performed by defining the primary outcome measure, the anticipated effect size, the SD, the power and significance level. | 9,2 (2,3) |
| 2) To maximise standardisation in the evaluation of histopathology of systemic inflammatory arthritis models, hind paws rather than front paws are recommended for analysis. | 9,0 (2,1) |
| 3) Either sagittal or transverse sections can be used for the evaluation of tarsal and/or ankle joints, as long as a standardised orientation is applied. | 9,1 (2,2) |
| 4) To guarantee optimal morphology, paraffin-embedded joint sections rather than cryo-sections should be used for standardised evaluation of histopathology. | 9,7 (0,8) |
| Category 2: Sample preparation, decalcification and staining procedures | |
| 5) Fixation of isolated paws should be performed in 4%–10% formalin for at least 6 hours at room temperature for mice or overnight at 4°C for rats. | 9,6 (1,3) |
| 6) Decalcification should be done in 14% EDTA solution or in 5% formic acid, and compatibility of decalcification agents should be carefully adjusted to planned staining procedures. | 9,7 (0,6) |
| 7) Conventional histological stainings such as H&E, tartrate-resistant acid phosphatase (TRAP), safranin O (SafO) or toluidine blue (TB) staining are recommended for accurate histological analysis of the various joint pathology features. | 9,6 (0,7) |
| 8) Our recommended staining protocols can be used as basic guidelines and will increase standardisation. | 9,8 (0,5) |
| Category 3: General points to consider for scoring histopathology of inflammatory arthritis | |
| 9) For accurate histological scoring, the distinct histopathological features like synovial inflammation, bone erosion, cartilage destruction, proteoglycan depletion and optionally new bone formation should be evaluated as separate parameters. | 9,7 (0,6) |
| 10) Histological scoring of the hind paws should be evaluated in standardised cutting planes and depths for each specimen, and should cover at least three articular joints of ankle/tarsal bones in sagittal sections or at least six tarsal joints in transversal sections. | 9,8 (0,6) |
| 11) Histopathological analysis should be evaluated in at least two (non-serial) sections, simultaneously stained and obtained scores should be subsequently averaged to result in a single data point per animal. | 9,4 (1,3) |
| 12) Histopathological analysis should preferentially be based on the consensus of two independent observers. | 9,1 (1,4) |
| 13) Analysis should be performed in a blinded manner and can be performed using either a semiquantitative scoring system or a quantitative analysis with appropriate software. | 9,9 (0,5) |
| 14) For standardised semiquantitative assessment of the distinct parameters, joint pathology scores should range from 0 (healthy) to 3 (severe) with in-between grading scores of 0.25–0.5 depending on the level of expertise. | 9,4 (1,3) |
| 15) For standardised quantitative analysis of the distinct parameters, joint pathology should be expressed as area (in mm2 of total region of interest) in the case of synovial inflammation, bone erosion, total cartilage and new bone formation, in percentage (% destained cartilage per total cartilage) or as cell counts (in number of positive cells of total region of interest). | 9,4 (1,4) |
| Category 4: Recommendations for evaluating synovial inflammation | |
| 16) Evaluation of synovial inflammation should be performed in H&E-stained sections with 25× magnification for overview purposes and subsequent 50–100× magnification for detailed scoring. | 9,5 (1,1) |
| 17) The degree of synovial inflammation is recommended to be scored either as semiquantitative or quantitative readout parameter as described under 14) and 15). | 9,8 (0,6) |
| 18) A universal, semiquantitative scoring system for synovial inflammation is proposed as: 0, healthy, one to two cell layers of synovial membrane, no inflammatory infiltrates; 1, three to five cell-layered synovial membrane, mild cellular infiltrate into the synovium and exudate in the joint cavity with low cell density; 2, multilayered synovial membranes, enhanced cellular infiltrates and increased cell density throughout the joints; 3, maximally expanded inflammation filling all joint cavities, hyperplastic synovial tissue with high cell density. | 9,6 (0,8) |
| Category 5: Recommendations for evaluating bone erosion | |
| 19) Evaluation of bone erosion should be performed in H&E or TRAP-stained sections under 25× magnification for overview purposes and subsequent 100× magnification for detailed scoring. | 9,8 (0,6) |
| 20) The degree of bone erosion is recommended to be scored either as semiquantitative or quantitative readout parameter as described under 14) and 15). | 9,4 (1,8) |
| 21) In respect to local varieties of the severity of erosions, semiquantitative analyses of bone erosion should be scored as the average calculated for multiple joint areas within one section. | 9,1 (1,7) |
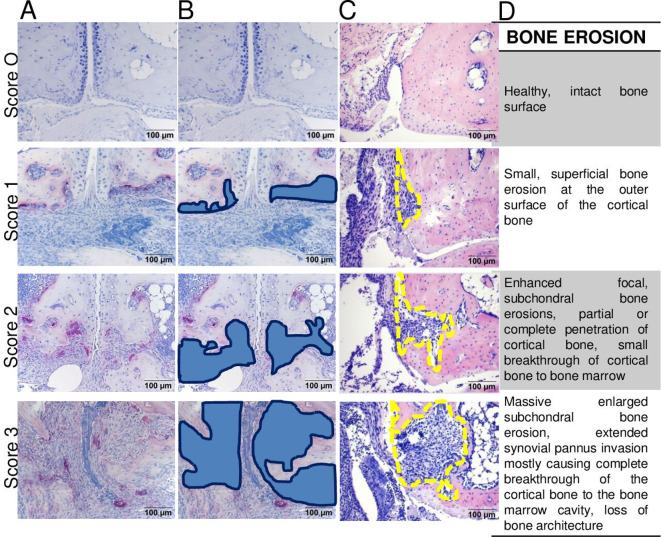
| 22) A universal semiquantitative scoring system for bone erosion is proposed as: 0, healthy, intact bone surface; 1, small focal bone lesions at the surface of cortical bone; 2, enhanced focal, subchondral bone erosions, partial or complete penetration of cortical bone and small breakthrough of cortical bone to bone marrow cavity possible; 3, massive, enlarged erosions of the bone tissue, extended synovial pannus invasion causing complete breakthrough of the cortical bone to the bone marrow cavity, and loss of bone architecture. | 9,5 (1,0) |
| 23) TRAP staining is recommended for further quantification of osteoclasts (as number per total region of interest), where synovial osteoclasts are defined as TRAP+ multinucleated (more than three nuclei) cells within the inflammatory synovial tissue. | 9,8 (0,6) |
| Category 6: Recommendations for evaluating cartilage erosion and proteoglycan loss | |
| 24) Histological scoring of cartilage damage should consist of two major parameters: (1) loss of proteoglycans from the superficial cartilage layer and (2) cartilage erosion of the superficial and/or the deeper calcified cartilage layer. | 9,8 (0,4) |
| 25) Evaluation of cartilage erosion and proteoglycan loss should be performed in SafO or TB-stained sections under 100–200× magnifications for detailed scoring. | 9,7 (0,6) |
| 26) The degrees of cartilage erosion and proteoglycan loss are recommended to be scored either as semiquantitative or quantitative readout parameter as described under 14) and 15). | 9,8 (0,6) |
| 27) In respect to semiquantitative analyses, the severity of cartilage damage should be scored as the average calculated for multiple joint areas within one section. | 9,4 (1,1) |
| Category 7: Data analysis, statistics and reporting | |
| 28) Semiquantitative or quantitative scoring data should be graphically represented, tested for their Gaussian distribution and statistically evaluated by using appropriate parametric or non-parametric tests. | 9,9 (0,3) |
| 29) Representative images of the obtained joint pathology are recommended to be shown to support histological findings. | 9,9 (0,5) |
| 30) To allow standardisation, reproducibility and comparison between different research groups, we recommend to report a minimal dataset to describe the details of the histological procedures and scoring systems, either in the methods section of the main manuscript or as supplemental material in publications. | 9,6 (1,1) |
Results
The BeTheCure task force defined 30 recommendations divided into seven categories for the standardisation of histological processing, evaluation, scoring and reporting of histopathological features from inflammatory arthritis in mice and rats (table 1).
Category 1: sample selection, orientation and regions of interest for mouse and rat hind paw sections
Due to short reproduction times, relatively low costs, easy housing and handling and for ethical reasons, mice and rats are the most commonly used species in animal experiments for arthritis research. Depending on the arthritis model, different joints are affected and can be assessed for histopathological evaluation, including knee joints, carpal, tarsal and/or ankle joints. Knee joints are only recommended for histopathological analysis in gonarthritis models like methylated bovine serum albumin (mBSA)-mediated antigen-induced arthritis or after intra-articular injection of pathogenic mediators like cytokines and ligands like streptococcal cell wall (SCW) fragments into the knee joint. For systemic arthritis models affecting multiple joints, such as CIA, CAIA, Tg197 and PIA, studying the histopathology of the hind paws is the most established and recommended method. Clinical signs of arthritis such as joint swelling and redness in hind paws or loss of grip strength can be non-invasively and longitudinally evaluated during the disease course and can be related to histological outcomes at the end of the study. Histological evaluation of affected front paws demonstrates limitations in standardisation due to lack of consistent cutting planes.
Before starting an animal experiment, appropriate sample size calculation is essential for designing a scientifically conclusive and ethically justifiable study.32 33 Therefore, the primary outcome measure (ie, synovial inflammation, bone erosion or cartilage damage) should be defined for testing the research hypothesis (online supplemental figure S1). Histological sections of the hind paws can be prepared either in transverse or sagittal plane. Preparation of sagittal sections allows for the evaluation of both ankle and tarsal joints including the talocrural (tibia, fibula, talus), subtalar (talocalcaneal), talocalcaneonavicular, calcaneocuboid, cuneocuboid, intercuneiform, cuneonaviculare and tarsometatarsal joints (figure 2A). Sagittal sections can be presented in two variants: in a talus-orientated or calcaneus-orientated section plane. Evaluation of metatarsophalangeal and interphalangeal joints in sagittal sections is not recommended as only a single phalange will be cut using this orientation.
Figure 2.
Regions of interest for histopathological evaluation in sagittal and transverse sections of hind paws. (A) Sagittal section plane of a hind paw can be used to evaluate the ankle and tarsal joints and can be presented as two variants: A more talus-orientated section plane can be used to assess four to five joints and a more calcaneus-orientated section plane to assess four joints. Regions of interest for assessing arthritic features in articular joints are indicated by a blue rectangle. (B) Transverse section plane of a hind paw allows for the evaluation of eight to nine articular joints of tarsal and metatarsal bones. Original magnification of histological images is 25×. Abbreviations marked in the bones of the hind paw: Ca, calcaneus; Cub, cuboid; Cun, cuneiformes; Na, naviculare; MT, metatarsal; Ta, talus; Ti, tibia.
annrheumdis-2020-219247supp001.pdf (84.9KB, pdf)
Preparation of transverse sections of the hind paw allows for the evaluation of eight to nine tarsal joints including calcaneocuboid, cuneonavicular, intercuneiform and tarsometatarsal joints (figure 2B). The evaluation of metatarsophalangeal or interphalangeal joints is not always feasible due to the difficulty in preparing consistent cutting planes.
Category 2: sample preparation of hind paws, decalcification and staining procedures for histological sections
Since frozen joint sections comprising bone tissue are difficult to cut and transfer onto slides, and detailed organisation and morphology is often lost, paraffin embedding is recommended for histological analysis of mouse and rat joints. The following paragraphs provide a detailed guideline for isolation, fixation and decalcification of joint samples for subsequent paraffin embedding.
Sample preparation of hind paws and decalcification of bone tissue
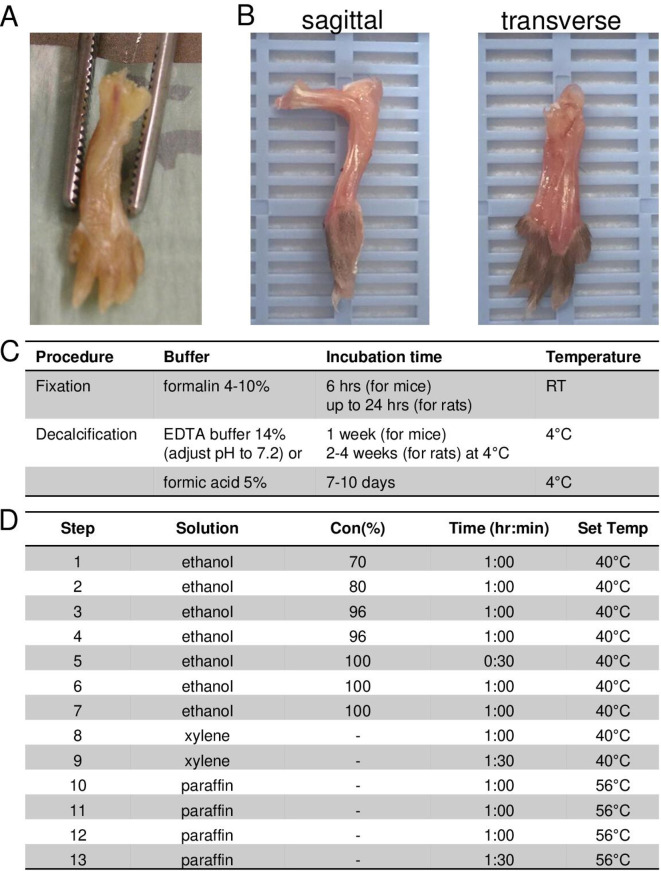
For proper processing of joints for histology, hind paws are cut 2–3 mm above the ankle, nails are removed, and the skin is either randomly incised or completely removed to allow easy penetration of formalin (figure 3A). To improve the workflow, samples can already be positioned into the embedding cassette (figure 3B) before fixation and decalcification steps. Paws are fixed in 4%–10% formalin for at least 6 hours at room temperature (RT) for mice or overnight at 4°C for rats. Caution is advised in case of subsequent immunohistochemical enzymatic staining such as tartrate-resistant acid phosphatase (TRAP), where the formalin fixation period must not exceed 24 hours to avoid disturbances in enzymatic activities or delicate epitope structures. In contrast, longer formalin fixation is not critical for regular H&E, toluidine blue (TB) or safranin O (SafO) stainings. Fixed samples are then decalcified in 14% EDTA solution for at least 1 week (for mice) or 2–4 weeks (for rats) at 4°C, with weekly refreshing of the solution (figure 3C). Alternatively, the use of 5% formic acid as decalcification reagent is possible, with incubation for 7–10 days and refreshing of the solution after 3–4 days (for compatibility of decalcification agents with staining methods see below). A simple way to test the completion of decalcification is to penetrate a bone outside the region of interest with a needle and check whether the tissue has become pliable and soft without crepitus. Samples can be subsequently embedded in paraffin or stored in 70% ethanol for later processing.
Figure 3.
Preparation and positioning of hind paws for sagittal or transverse paraffin-embedded tissue sections. (A) Prepared hind paw of a mouse after skin removal. (B) Proper positioning of isolated hind paws for sagittal (left) and transverse sections (right) into embedding cassette. (C) Fixation and decalcification methods for processing of hind paws for histological analysis. (D) Example of an automated paraffin-embedding protocol. RT, room temperature.
Paraffin-embedding procedure
Samples should be properly positioned into the embedding cassette for subsequent paraffin-embedding allowing appropriate cutting planes (figure 3B). Any standard vacuum infiltration process can be applied for paraffin embedding of paw samples. A representative paraffin-embedding procedure is given in figure 3D.
Standard staining procedures for the evaluation of synovial inflammation, bone erosion and cartilage damage
Paraffin-embedded joints are cut on a regular microtome with disposable blades at a thickness of 2–5 µm. Paraffin sections should be mounted on negatively charged slides to avoid detachment of the specimen during staining procedures. To ensure a reliable scoring of pathology of the entire joint, multiple sections approximately 50–70 µm apart should be used. All sections should be stained simultaneously to avoid variation in colour and intensity. The following standard histological stainings are recommended for histological analysis of joint pathology:
H&E staining: for quantification of synovial inflammation, in particular. H&E is also suitable for detection of bone and cartilage erosions.
TRAP staining: for detection of multinucleated osteoclasts and scoring the extent of bone erosions.
SafO or TB staining: for determination of proteoglycan loss and cartilage erosion.
Deparaffinisation and rehydration of sections
Before staining, paraffin sections are deparaffinised with xylene (2 times 5 min at RT), followed by rehydration through a graded series of 100%, 96% and 70% ethanol, and finally with distilled water (each 5 min at RT).
H&E staining for the evaluation of synovial inflammation
H&E stain is one of the most commonly used stains in histological analyses. An example of an H&E staining procedure is given in table 2. The staining method involves application of hemalum, a complex formed from haematoxylin and alum that colours nuclei blue. The nuclear staining is followed by counterstaining with eosin, which colours eosinophilic structures in various shades of pink (cytoplasm) or red (erythrocytes, eosinophilic granules). H&E staining is routinely used not only to address the presence, distribution and density of cells in tissues but also to give structural information about bone and cartilage. In arthritis assessment, the H&E staining is highly suitable to score the degree of inflammation, visualising the presence of inflammatory cells in the synovial tissue and joint cavities.
Table 2.
HE staining procedure
| H&E staining | |
Reagents:
| |
| Staining procedure (at room temperature): | Time |
| Stain with Meyer’s hemalum working solution | 10 min |
| Rinse in distilled water | 15 s |
| Differentiate in 1%HCl/70%ethanol (under gentle shaking) | 5 s |
| Rinse in running tap water (blue-stained nuclei) | 10 min |
| Incubate with eosin working solution | 15 s |
| Rinse in distilled water | 15 s |
| Dehydrate in 96% ethanol | 5 min |
| Rinse in 100% ethanol | 5 min |
| Incubate in xylene* or xylene substitutes (eg, N-butyle acetate) *xylene covers three isoforms: xylene, xylol or dimethylbenzene |
5 min |
| Seal the slides with permanent mounting medium (eg, Eukitt or Permount) and a coverslip | |
TRAP staining for the identification of bone-resorbing osteoclasts and bone erosions
TRAP is an enzyme with optimal activity in acidic conditions and is frequently used as histochemical marker for osteoclasts. TRAP is expressed by mature osteoclasts as well as its precursors, but its biological function in the latter is still unknown.34 35 The purple TRAP staining in combination with the haematoxylin-blue counterstained nuclei forms a perfect method to identify not only multinucleated osteoclasts but also to easily detect bone sites eroded and invaded by synovial pannus tissue. A TRAP staining protocol is provided in table 3.
Table 3.
TRAP staining procedure
| TRAP staining | |
|
Reagents:
Various kits for TRAP staining are commercially available, like leucocyte acid phosphatase kit from Sigma Diagnostics Cat. No. 387-A Preparation of tartrate solution: mix the following components: 0.25 mL Naphthol AS-BI phosphoric acid, 1.0 mL acetate solution, 0.5 mL tartrate solution add 45 mL distilled water |
|
| Staining procedure | Time |
| Prepare tartrate solution always freshly according to manufacturer instructions | |
| Incubate tartrate solution on specimen at 37°C in a water bath protected from light | 1 hour |
| Preparation of substrate solution: mix following components 0.25 mL fast garnet GBC base solution 0.25 mL sodium nitrite solution |
rest for 2 min at RT |
| Add substrate solution to preincubated slides and develop at 37°C | 2 min |
| Rinse with distilled water | 15 s |
| Counterstain nuclei with Meyer’s haematoxylin (see H&E staining steps 1–3) |
RA, rheumatoid arthritis; TRAP, tartrate-resistant acid phosphatase.
SafO or TB staining for the evaluation of proteoglycan loss and cartilage erosion
SafO and TB are cationic stains that bind to acidic proteoglycans and are often used to study articular cartilage. Standard staining procedures are given in table 4. SafO stains healthy articular cartilage as an intense orange-red colour. Fast green, an acidic substrate, which strongly binds to noncollagenous proteins, is used as counterstain. TB, a blue cationic dye, is an alternative for SafO to stain cartilage proteoglycans. It stains mast cell granules into purple colour and has a different shade of dark blue and purple when bound to cartilage. The intensity of SafO or TB staining is proportional to the proteoglycan content in the cartilage, making these staining highly suitable to evaluate proteoglycan loss from articular cartilage in arthritic joints.
Table 4.
Safranin O and TB staining procedure
| Safranin O staining | |
|
Reagents:
1% HCl in 70% ethanol 1% acetic acid in water Weigert’s iron haematoxylin working solution 0.1% fast green in distilled water 0.1% safranin in distilled water | |
| Staining procedure (at room temperature): | Time |
| Incubate with Weigert‘s haematoxylin working solution | 5 min |
| Differentiate in 1%HCl/70%ethanol (under gentle shaking) | 5 s |
| Rinse in running tap water | 15 s |
| Incubate in 0.1% safranin O | 30 s to 1 min |
| Rinse in 1% acetic acid | 15 s |
| Rinse in running tap water | 15 s |
| Incubate with fast green 0,1% | 5–7 min |
| Rinse in 96% ethanol | 15 s |
| Rinse in 100% ethanol until no colour can be removed | up to 1 min |
| Incubate in xylene | 5 min |
| Seal the slides in permanent mounting medium (eg, Eukitt) | |
| TB staining | |
|
Reagents:
TB stock solution 10×: 1% TB O, 1% natriumtetraborate in water. Stock solution should be filtrated two times before use. 1× TB working solution is prepared in water. | |
| Staining procedure (at room temperature): | Time |
| Incubate in TB working solution (1×) | 8–30 s |
| Rinse in distilled water | 15 s |
| Rinse in 96% ethanol (metachromatic dye becomes visible) | 10–20 s |
| Dehydrate in absolute ethanol | 5 min |
| Incubate in xylene | 5 min |
| Mount with permanent mounting medium (eg, Eukitt) | |
TB, toluidine blue.
Compatibility of decalcification agents with staining methods
There is an ongoing debate whether the different decalcification buffers are compatible with all the staining procedures; indeed, the choice of the decalcification buffer can influence the visual appearance of the staining.36–41 For example, SafO staining results in brighter, different tinctorial staining on formic acid-decalcified sections than when pretreated with EDTA.38 In contrast, TRAP staining is superior and more reliable on EDTA-decalcified sections than on formic acid-decalcified material, since acid decalcifying agents may inhibit enzymatic staining procedures (table 5). Decalcification may result in reduced antigenicity and nucleic acid degradation limiting further molecular analysis.40 42 43 Therefore, immunohistochemistry stainings and spatial transcriptomics may benefit from EDTA rather than formic acid-based decalcification.42 43 The molecular and cellular assessment of synovial, bone or cartilage tissue may also require alternative procedures such as (non)-decalcified cryo-sectioning for immunhistochemistry or tissue digestions of synovial tissue for cellular phenotyping by flow cytometry.44 45
Table 5.
Compatibility of decalcification agents with staining methods
| Compatibility of staining | |||||
| Decalcification | H&E | TRAP | Toluidine blue | Safranin O | IHC |
| EDTA | Highly | Highly | Highly | Less35 | Highly |
| Formic acid | Highly | Less* | Highly | Highly | Not compatible with some epitopes31 36 38 |
*Expert experiences.
IHC, immunohistochemistry; TRAP, tartrate-resistant acid phosphatase.
Category 3: general points to consider for scoring histopathology of inflammatory arthritis
As demonstrated in numerous animal studies,27 29 46–51 some therapeutic agents or genetic interventions can lead to a decoupling of joint inflammation from structural damage, which will not be recognised when applying a method that merges all histological features into one score. Therefore, distinct histopathological features like synovial inflammation, bone erosion, cartilage destruction and proteoglycan depletion should be evaluated as separate parameters. As detailed histological analyses, which are not possible in humans, are one of the main justifications to perform animal studies, researchers also have an ethical obligation to extract as much information as possible from these experiments.
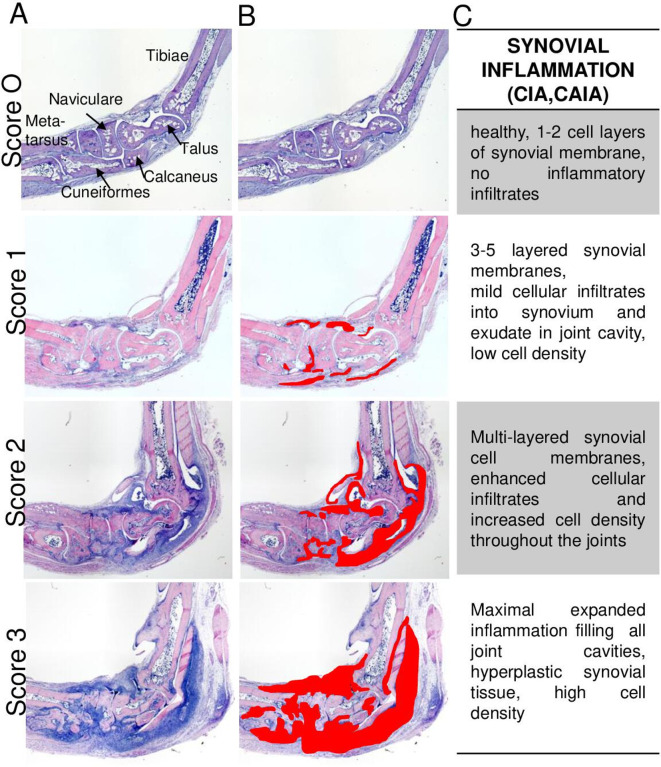
Histopathological analysis should be evaluated in at least two sections (with a minimum distance of 50 µm apart) from the same specimen and should include the same joints in comparable cutting planes and depths. Obtained scores should be subsequently averaged to result in a single data point per animal and should preferentially be based on the consensus of two independent observers. Analysis should be performed in a blinded manner and can be performed using either a semiquantitative scoring system or by quantitative analysis with appropriate software. Of note, both evaluation procedures should cover at least three articular joints of ankle/tarsal bones in sagittal sections and at least six tarsal joints in transversal sections (figure 4A).
Figure 4.
H&E staining used for the assessment of synovial inflammation in sagittal or transverse sections. (A) Rectangles and bold numbers indicate the areas with joints of interest for histopathological evaluation. (B) Representative magnified images of a healthy and an inflamed joint illustrating synovitis and pannus formation. H&E staining (blue nuclei of cells) indicates synovial joint inflammation (green line) characterised by inflammatory cell infiltrates, increase in synoviocytes, thickening of synovial lining and sub-lining as well as invasion of pannus tissue (blue dashed lines). H&E staining also allows morphological discrimination of intact bone surface and bone erosions (loss of bone tissue and eroded bone tissue substituted by invading synovial pannus tissue). Left, intact healthy joint in wild-type mice. Middle and left, inflamed, eroded arthritic joint (here represented by the Tg197 model, 10 weeks of age. Original magnification is 100×. B, bone; BI, bone marrow inflammation; Ca, calcaneus; Cub, cuboid; Cun, cuneiformes; Na, naviculare; MT, metatarsal; S, synovium; SI, synovial inflammation; Ta, talus; Ti, tibia.
Semiquantitative scoring
Histopathological features like synovial inflammation, bone erosion, cartilage erosion and proteoglycan loss are recommended to be scored as separate readout parameters in grading arbitrary scores ranging from 0 (healthy, intact) to 3 (severe) with in-between grading scores of 0.25–0.5 (depending on the level of expertise).
Whereas inflammation is scored as an overall score of the total region of interest per section, and the mean of at least two sections is determined, the severity of bone and cartilage damage may differ between joints within the same section. Therefore, it is recommended to score the degree of damage in each joint or subarea individually and calculate the mean score per section (and subsequently per paw) by dividing the sum of all scores by the number of analysed subareas. Evaluation sheets were created to assist semiquantitative scoring (online supplemental figure S2A–C). Experts will be able to score these readout parameters without counting single sites.
annrheumdis-2020-219247supp002.pdf (2MB, pdf)
Quantitative analysis
Quantitative analysis of histopathological features requires the use of a microscope equipped with a digital camera and connected to a software system that allows marking and determination of areas, surfaces and distances on microscopic images (examples of software systems: OsteoMeasure System from OsteoMetrics, Atlanta, Georgia, USA; Definiens from Definiens AG, Germany; Leica Application Suite from Leica microsystems, Germany; Image J from National Institutes of Health, USA). The investigator screens the region of interest row by row and manually draws a line around the individual histopathological features or marks individual cells on the computer screen (online supplemental figure S3). Drawings are routinely performed in 100× or 200× magnification of the tissue section. Quantitative data from all the fields of interest are automatically added and calculated by the software. Results are given as area (in mm2 per total region of interest) in case of synovial inflammation, bone erosion, total cartilage, destained cartilage or as percentage (proteoglycan loss in %; area of destained cartilage in relation to area of superficial cartilage) or as number of positive cells per total region of interest, like TRAP+multinucleated synovial osteoclasts (in cell numbers).
annrheumdis-2020-219247supp003.pdf (4.7MB, pdf)
Category 4: recommendations for evaluating synovial inflammation
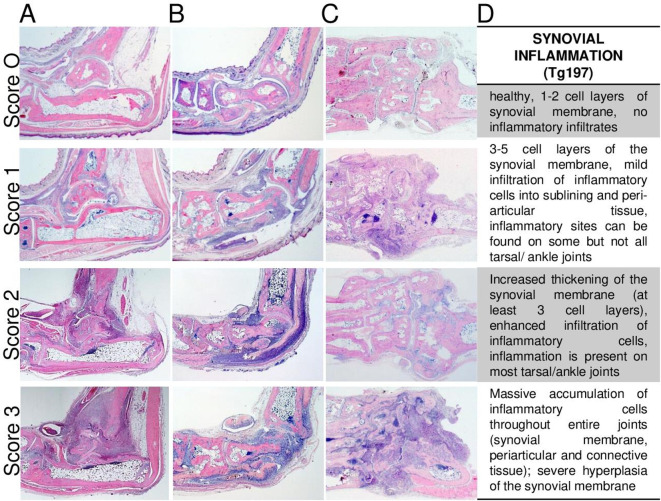
Evaluation of synovial inflammation representing the extent and density of infiltrating inflammatory cells, synovial hyperplasia and pannus invasion can be performed in H&E-stained sagittal or transverse sections from hind paw sections with 25× magnification for overview purposes and with 50–100× magnification for specific scoring purposes (figure 4). Overall semiquantitative scores, ranging from 0 to 3, can be used, or quantitative assessment of the affected area (in mm2 per total region of interest) can be performed as illustrated in sagittal sections from hind paws of CIA (figure 5). Similar presentation and scores of joint inflammation as in CIA are found in CAIA and are, therefore, not defined separately here. Representative images of the different grades of synovial inflammation are also provided in the three variants of tissue section planes from the Tg197 transgenic model (figure 6) and in transverse plane from rat PIA (online supplemental figure S4). A semiquantitative scoring can be commonly described for the various arthritis models as following: 0, healthy, one to two cell layers of synovial membrane, no inflammatory infiltrates; 1, three to five cell-layered synovial membrane, mild cellular infiltrate into the synovium and exudate in the joint cavity with low cell density; 2, multilayered synovial membranes, enhanced cellular infiltrates and increased cell density throughout the joints; 3, maximal expanded inflammation filling all joint cavities, hyperplastic synovial tissue with high cell density. The main differences in joint inflammation between distinct models can be observed in (1) the inflammatory tissue composition with respect to cell type contribution and (2) the extent of extra-articular inflammation, which is, for example, more extended in CIA or CAIA than in the Tg197 model. Of note, this also varies within a model depending on the disease phase that is studied, either early after the arthritis onset, during established disease or during the resolution phase.
Figure 5.
Scoring of synovial joint inflammation in collagen-induced arthritis and collagen-antibody-induced arthritis: (A) Representative H&E-stained sections illustrating the grading of scores for joint inflammation in CIA (days 35 and 42 after immunisation). (B) Quantitative assessment of the area of synovial joint inflammation by manual drawing and contouring the region of interest. (C) Semiquantitative scoring of synovial inflammation from 0 to 3 based on characteristic features including density of infiltrating inflammatory cells, synovial hyperplasia and pannus invasion for synovial inflammation. Original magnification is 25×. CIA, collagen-induced arthritis; CAIA, collagen–antibody-induced arthritis.
Figure 6.
Scoring of synovial joint inflammation in the Tg197 model. Representative H&E images illustrating the grading of scores for joint inflammation in Tg197 mice evaluated using sagittal sections with (A) calcaneus or (B) talus orientation or (C) transverse section of hind paws. Representative histological images are taken from 15 weeks old wild-type and from 10 to 15 weeks old Tg197 mice (A–C). (D) Description of characteristic features enabling semiquantitative scoring of synovial joint inflammation from 0 to 3. Original magnification is 25×.
annrheumdis-2020-219247supp004.pdf (518.1KB, pdf)
On expert level, a separate quantification can be performed of the inflammatory cell mass in infiltrates (within the synovium) and exudates (in the joint cavity), of the synovial hypertrophy and of the invading pannus tissue. Optionally, further inflammatory variables can be assessed including the qualitative analysis and phenotyping of infiltrating and residual synovial cells as well as of bone marrow infiltrates by immunohistochemistry.44 45 52
Category 5: recommendations for evaluating bone erosions
Bone erosions are defined as sites of bone loss that occur through resorption by synovial osteoclasts formed at the cartilage–pannus junction as well as at sites of inflamed synovial tissue adjacent to bone tissue (figure 7).53–55 Evaluation of bone erosions in ankle/tarsal joints can be performed in H&E or TRAP-stained sections under 25× magnification for overview purposes and under 100× magnification for precise scoring purposes. As severity of bone erosion may differ between joints of the same specimen, it is recommended to score each joint (subarea) individually and calculate the mean score per section for at least two sections (figure 7C). Implementing TRAP staining is recommended for identification and quantification of bone-resorbing osteoclasts and the bone sites targeted. H&E staining can be used to determine the extent of bone erosions but does not allow to estimate osteoclasts. Synovial osteoclasts are defined as TRAP+multinucleated (more than three nuclei) cells within the inflammatory synovial pannus tissue. Of note, TRAP+osteoclasts located in the bone marrow are not counted as synovial osteoclasts, although an increased number of these cells may be observed with increasing severity of arthritis. The area of inflammation-mediated bone erosions can be either assessed quantitatively by manual contouring (in mm2 per total region of interest) or semi-quantitatively graded using the following scoring protocol: 0, healthy, intact bone surface; 1, small focal bone lesions at the surface of cortical bone; 2, enhanced focal, subchondral bone erosions, partial or complete penetration of cortical bone and small breakthrough of cortical bone to bone marrow cavity possible; 3, massive, enlarged bone erosion of the bone tissue, extended synovial pannus invasion mostly causing complete breakthrough of the cortical bone to the bone marrow cavity and loss of bone architecture (figure 8). This global scoring can be commonly used for all models; however, some discrepancies on the severity of bone destruction can be found based on different pathophysiological processes of the models, the size of investigated bone (thinner cortical bone of small talus vs thicker cortical bone of tibia or calcaneus), the time point of analysis and the disease progression of the model. For example, the Tg197 model shows the strongest progressive bone destruction phenotype compared with CIA, CAIA or PIA models.16 30 47 56 57
Figure 7.
Evaluation of bone erosions and osteoclasts in arthritic joints. (A) Schematic representation of the bone subareas within a single joint, which should be evaluated for the severity of erosion. Bone erosions should be assessed in each joint separately and finally calculated as mean score from all the investigated joints. (B) TRAP-stained sections identify purple-coloured TRAP +multinucleated bone-resorbing osteoclasts (more than three nuclei) and the occurrence of subchondral bone erosion. Left: intact joint architecture in non-arthritic mice. Right: inflammatory, erosive joint demonstrating the generation of synovial osteoclasts and the formation of an invasive pannus tissue penetrating into subchondral bone areas. (C) Left: manual drawing of the area of subchondral bone erosion (blue dashed lines) in TRAP-stained section for quantitative data assessment on bone erosions (mm2). Right: HE-stained section of the same region. Representative histological images are taken from 15 weeks old wild-type and Tg197 mice (A–C). Original magnification is 200×. B, bone; BM, bone marrow; C, cartilage; SI, synovial inflammation; SP, synovial pannus; TRAP, tartrate-resistant acid phosphatase.
Figure 8.
Scoring of bone erosions in arthritic joints. (A) Representative images from TRAP-stained sections indicating severity scores of bone erosions in small tarsal joints graded from 0 (none) to 3 (severe). Here, erosions are exemplified on sagittal tarsal sections from 8-week-old wild-type and 8-week-old to 12-week-old Tg197 mice. (B) Marked areas defining bone erosion from the same images (blue fields). (C) Labelling of bone erosion in larger bones such as tibia (tibiotalar joint) in HE-stained sections from CIA model (days 35 and 42 after immunisation). (D) Description of characteristic features defining grading scores of local bone erosion from 0 to 3 in the affected joints. Original magnification is 200×. CIA, collagen-induced arthritis; TRAP, tartrate-resistant acid phosphatase.
Category 6: recommendations for evaluating cartilage erosion and proteoglycan loss
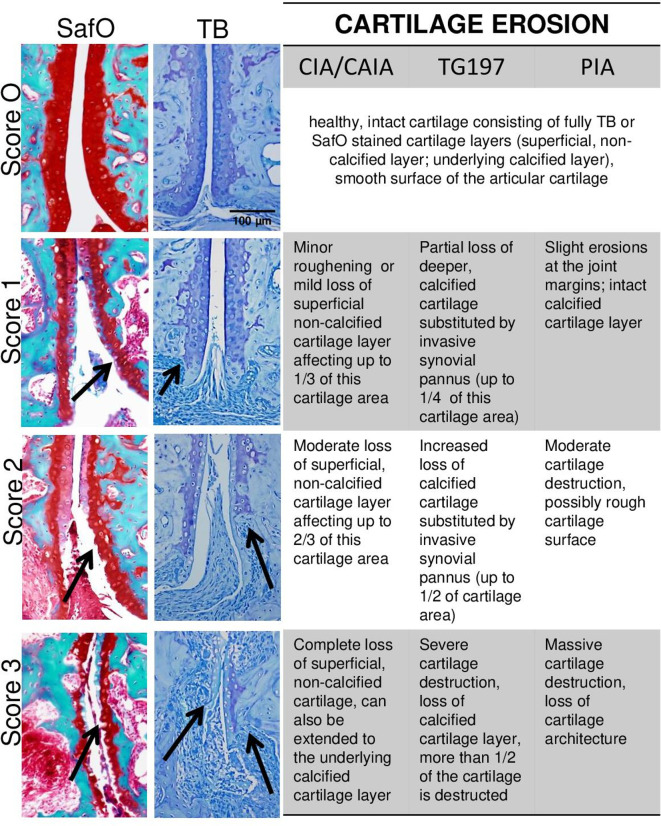
TB or SafO staining is used for the identification of cartilage damage in arthritic mice and rats, which consists of two major features: (1) loss of proteoglycan content from the superficial cartilage layer and (2) cartilage erosion of either the superficial or the deeper calcified cartilage layer or both of them. Under healthy conditions, the murine and rat articular cartilage consists of two layers, which are separated by a tight mark: the superficial, non-calcified layer and the underlying calcified cartilage layer. Inflammatory conditions lead to (1) destaining of the superficial, noncalcified cartilage layer characterised by loss of the dark blue (TB) or red (SafO) staining, indicative of proteoglycan loss and (2) loss of cartilage tissue layers by invading synovial pannus and/or digestive processes coming from the synovial fluid (figure 9). Evaluation of cartilage erosion and proteoglycan loss in ankle/tarsal joints can be performed in SafO or TB-stained sections under 100× magnification. For quantitative assessment, the complete region of interests can be manually contoured to obtain the area of total or destained cartilage tissue (in mm2 per total region of interest). Severity of cartilage damage may differ between joints of the same specimen. With respect to semiquantitative scoring, it is, therefore, recommended to assess the proteoglycan loss and cartilage erosion for each joint (subarea) separately, and finally calculate the mean score from all evaluated joints per section (figure 9A). Due to obvious differences present in inflammation-mediated cartilage damage between the models, scoring grades for proteoglycan loss as well as cartilage erosion ranging from 0 to 3 are separately defined for the models (figures 10 and 11). Of particular notice are the visible differences present in cartilage erosion: Tg197 model predominantly displays erosion of the underlying, calcified cartilage and only during very late stage in the disease degradation of the superficial cartilage is observed. In contrast, early degradation of the superficial cartilage layer is observed in models such as CIA, CAIA and PIA.30 31 58 59
Figure 9.
Evaluation of cartilage damage in arthritic joints. (A) Schematic representation of the cartilage areas within a single joint, which should be evaluated for the severity of cartilage damage. Cartilage damage should be assessed in each joint separately and finally calculated as mean score from all the investigated joints. (B) TB or SafO-stained cartilage in healthy (left) and inflamed joint (right) sections. Healthy, intact mouse articular cartilage consists of two layers that are separated by a tight mark: the dark blue (TB) or red (SafO) stained superficial, non-calcified layer and the underlying stained calcified cartilage layer. Inflammation-mediated loss of proteoglycans is indicated by the loss of blueness (TB) or redness (SafO) of superficial, non-calcified cartilage layer, which can be easily estimated. Cartilage damage can be further characterised by the erosion of the superficial cartilage layer and/or erosion of the underlying calcified cartilage layer invaded by pannus tissue. (C) Labelling of cartilage damage including proteoglycan loss of the superficial cartilage layer (destaining, black line) and cartilage erosion (erosion of underlying calcified cartilage layer-orange line). Subchondral bone erosion areas are indicated by red lines. Images are represented from hind paw sections of a wild-type mouse and Tg197 animals (12 weeks of age). Original magnification is 200×. B, bone; BI, bone marrow infiltrates; BM, bone marrow; C, cartilage; JC, joint cavity; SafO, safranin O; SI, synovial inflammation; SP, invading synovial pannus; TB, toluidine blue.
Figure 10.
Scoring of proteoglycan loss in arthritic joints. Left and middle, representative images of TB and SafO-stained paw sections illustrating the different grading scores (0, none, to 3, severe) of proteoglycan loss in articular cartilage. Proteoglycan loss is represented by hind paw sections from CIA (day 42 after immunisation, SafO) and Tg197 model (8–12 weeks of age, TB), respectively. Right, description of characteristic features defining the grading scores of proteoglycan loss in the different animal models. Eroded areas of superficial layer are evaluated as complete loss. Original magnification is 200×. CIA, collagen-induced arthritis (in mice); CAIA, collagen-antibody-induced arthritis (in mice); PIA, pristane-induced arthritis (in rats); SafO, safranin O; TB, toluidine blue; Tg197, human TNF transgenic mouse.
Figure 11.
Scoring of cartilage erosions in arthritic joints. Left and middle, representative images of SafO and TB stained paw sections illustrating the different grading scores (0, none; to 3, severe) of cartilage erosion in the articular cartilage. Cartilage damage is represented by images from CAIA (day 12 after induction, SafO) and Tg197 model (8–12 weeks of age, TB). Right, description of the characteristic features defining the grading scores of cartilage erosion in the different animal models. Original magnification is 200×. CIA, collagen-induced arthritis (in mice); CAIA, collagen-antibody-induced arthritis (in mice); PIA, pristane-induced arthritis (in rats); SafO, safranin O; Tg197, human TNF transgenic mouse; TB, toluidine blue.
Category 7: data analysis, statistics and reporting
Evaluated data on the histopathological features (synovial inflammation, subchondral bone erosion, number of osteoclasts, proteoglycan loss, cartilage damage) should be graphically represented, checked for Gaussian distribution and statistically evaluated using appropriate parametric or nonparametric tests (online supplemental table S1). Furthermore, representative images of the obtained joint pathology are recommended in publications to support histopathological findings. Finally, to enable standardisation and comparison of data sets between different research groups, we recommend to report in detail on both the histological procedures and scoring systems in publications (online supplemental table S2).
annrheumdis-2020-219247supp005.pdf (62.7KB, pdf)
annrheumdis-2020-219247supp006.pdf (63.2KB, pdf)
Typical histopathological features in different animal models of inflammatory arthritis
Arthritis models are characterised by the development of synovial inflammation, bone erosions and cartilage destruction in various joints; however, the onset, progression and extent of the distinct features vary between different models and induction protocols and also depend on the microbiological status of the animal facility and genetic background and sex of the animals. Therefore, to determine the best time point for histopathological analysis, investigators are strongly advised to perform a pilot study of the animal model to gain insight into the kinetics, severity and variation of the arthritis model at their local animal facility. Histopathologically, variations can be found in terms of (1) sites and extent of cellular influx into the synovium and joint cavities, whereby CIA and CAIA models show the strongest cellular infiltrations, (2) cellular composition of synovial infiltrates and synovial pannus, (3) occurrence of subchondral bone erosions especially observed in Tg197 mice and (4) degradation of the cartilage layers (table 6). Moreover, some arthritis models do not only represent RA characteristics but also develop spondyloarthropathy-like features, namely, new bone formation. It should be noted that except for Tg197 mice, CIA, CAIA and PIA models may display various degrees of new bone formation, sometimes leading to excessive osteophytes in the tarsal/ankle joints (online supplemental figure S5). This feature, if present, should also be quantitatively assessed as additional parameter, for example, through drawing and calculating the area of new bone formation (in mm2 per total region of interest). It is also worth mentioning that these bone appositions mask the bone erosion process, causing an underestimation of bone destruction. Therefore, it is advised to be familiar with the longitudinal changes of the distinct features in the individual model.
Table 6.
Typical histopathological features in different arthritis models
| Animal models | RA features | Non-RA features | Known affected joints | ||
| Inflammation | Bone erosion | Cartilage damage | New bone formation | ||
| Tg197 | ++ | +++ | +++ | Absent | All peripheral joints and spine (i) |
| CIA | +++ | ++ | +++ | +++ | All peripheral joints and spine (i), larynx |
| CAIA | +++ | ++ | ++ | +++ | Front and hind paws; (ii) |
| PIA | +++ | ++ | + | +++ | Front and hind paws, spine (ii) |
(i) Whole body positron emission tomography/computed tomography (PET/CT) imaging indicated systemic arthritis affecting various joints (see references60 61).
(ii) Other joints have not been reported so far.
CAIA, collagen–antibody-induced arthritis; CIA, collagen-induced arthritis; PIA, pristane-induced arthritis; RA, rheumatoid arthritis; Tg197, human TNF transgenic mouse.
annrheumdis-2020-219247supp007.pdf (418.8KB, pdf)
Outlook and conclusion
Hind paws of arthritis models are the most established and most commonly used joints for analysis allowing for a combination of both clinical and histopathological disease parameters; however, systemic arthritis also result in inflammation in other joints such as knees, shoulders and spine in models like CIA or Tg197, as demonstrated by in vivo whole body positron emission tomography/computed tomography (PET/CT) imaging studies.60 61
In conclusion, awareness of the different features of the arthritis pathology in mouse and rat models of inflammatory arthritis is of utmost importance for reliable research outcome, and the standardised histological processing and scoring methods with SMASH recommendations phrased by the BeTheCure consortium will help to increase uniformity and reproducibility in preclinical research on inflammatory arthritis (figure 12).
Figure 12.
Summarised work flow and bullet points of SMASH recommendations. ROI, region of interest; SafO, safranin O; SMASH, Standardised Microscopic Arthritis Scoring of Histological; TB, toluidine blue;TRAP, tartrate-resistant acid phosphatase.
Footnotes
Handling editor: Thomas Pap
Contributors: SH, MJV, MCD, MA, MH, KSN, BN, CG, AF, FA and MIK performed sample collection, screening, and definition of scoring systems. SH and MIK drafted the manuscript with advice from MJV, MCD, FA. All authors made substantial contributions to the conception and design of the work, analysis, and interpretation of data, critical revision and final approval of the recommendations.
Funding: The project was supported from the Innovative Medicines Initiative Joint Undertaking (number 115142, BeTheCure, European Union’s Seventh Framework Programme and EFPIA companies).
Competing interests: MCD, CG and GK report personal fees from Biomedcode, outside the submitted work; and Biomedcode is a service provider of in vivo efficacy studies within the area of inflammatory diseases. NW is employed by Redoxis AB; and Redoxis AB is a service provider selling in vivo studies within the area of autoimmunity and inflammation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017;389:2328–37. 10.1016/S0140-6736(17)31472-1 [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 3. Kollias G, Papadaki P, Apparailly F, et al. Animal models for arthritis: innovative tools for prevention and treatment. Ann Rheum Dis 2011;70:1357–62. 10.1136/ard.2010.148551 [DOI] [PubMed] [Google Scholar]
- 4. Asquith DL, Miller AM, McInnes IB, et al. Animal models of rheumatoid arthritis. Eur J Immunol 2009;39:2040–4. 10.1002/eji.200939578 [DOI] [PubMed] [Google Scholar]
- 5. Benson RA, McInnes IB, Garside P, et al. Model answers: rational application of murine models in arthritis research. Eur J Immunol 2018;48:32–8. 10.1002/eji.201746938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Medicines Agency . Guideline on clinical investigation of medicinal products for the treatment of rheumatoid arthritis; 2017.
- 7. Boers M, Kirwan JR, Gossec L, et al. How to choose core outcome measurement sets for clinical trials: OMERACT 11 approves filter 2.0. J Rheumatol 2014;41:1025–30. 10.3899/jrheum.131314 [DOI] [PubMed] [Google Scholar]
- 8. Radner H, Chatzidionysiou K, Nikiphorou E, et al. 2017 EULAR recommendations for a core data set to support observational research and clinical care in rheumatoid arthritis. Ann Rheum Dis 2018;77:476–9. 10.1136/annrheumdis-2017-212256 [DOI] [PubMed] [Google Scholar]
- 9. Bevaart L, Vervoordeldonk MJ, Tak PP. Collagen-induced arthritis in mice. Methods in molecular biology (Clifton, N. J.) 2010;602:181–92. [DOI] [PubMed] [Google Scholar]
- 10. Holmdahl R, Andersson M, Goldschmidt TJ, et al. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev 1990;118:193–232. 10.1111/j.1600-065X.1990.tb00817.x [DOI] [PubMed] [Google Scholar]
- 11. Brand DD, Latham KA, Rosloniec EF. Collagen-Induced arthritis. Nat Protoc 2007;2:1269–75. 10.1038/nprot.2007.173 [DOI] [PubMed] [Google Scholar]
- 12. Williams RO. Collagen-Induced arthritis as a model for rheumatoid arthritis. Methods Mol Med 2004;98:207–16. 10.1385/1-59259-771-8:207 [DOI] [PubMed] [Google Scholar]
- 13. Hutamekalin P, Saito T, Yamaki K, et al. Collagen antibody-induced arthritis in mice: development of a new arthritogenic 5-clone cocktail of monoclonal anti-type II collagen antibodies. J Immunol Methods 2009;343:49–55. 10.1016/j.jim.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 14. Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. Embo J 1991;10:4025–31. 10.1002/j.1460-2075.1991.tb04978.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tuncel J, Haag S, Hoffmann MH, et al. Animal models of rheumatoid arthritis (I): Pristane-Induced arthritis in the rat. PLoS One 2016;11:e0155936. 10.1371/journal.pone.0155936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am J Pathol 2003;163:1827–37. 10.1016/S0002-9440(10)63542-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caplazi P, Baca M, Barck K, et al. Mouse models of rheumatoid arthritis. Vet Pathol 2015;52:819–26. 10.1177/0300985815588612 [DOI] [PubMed] [Google Scholar]
- 18. Hegen M, Keith JC, Collins M, et al. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann Rheum Dis 2008;;67:1505–15. Nov. 10.1136/ard.2007.076430 [DOI] [PubMed] [Google Scholar]
- 19. van den Berg WB. Lessons from animal models of arthritis over the past decade. Arthritis Res Ther 2009;11:250. 10.1186/ar2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bendele A, McAbee T, Sennello G, et al. Efficacy of sustained blood levels of interleukin-1 receptor antagonist in animal models of arthritis: comparison of efficacy in animal models with human clinical data. Arthritis Rheum 1999;42:498–506. [DOI] [PubMed] [Google Scholar]
- 21. Yang YH, Morand EF, Getting SJ, et al. Modulation of inflammation and response to dexamethasone by Annexin 1 in antigen-induced arthritis. Arthritis Rheum 2004;50:976–84. 10.1002/art.20201 [DOI] [PubMed] [Google Scholar]
- 22. Brenner M, Meng H-C, Yarlett NC, et al. The non-major histocompatibility complex quantitative trait locus Cia10 contains a major arthritis gene and regulates disease severity, Pannus formation, and joint damage. Arthritis Rheum 2005;52:322–32. 10.1002/art.20782 [DOI] [PubMed] [Google Scholar]
- 23. Douni E, Sfikakis PP, Haralambous S, et al. Attenuation of inflammatory polyarthritis in TNF transgenic mice by diacerein: comparative analysis with dexamethasone, methotrexate and anti-TNF protocols. Arthritis Res Ther 2004;6:R65–72. 10.1186/ar1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weinberger A, Halpern M, Zahalka MA, et al. Placental immunomodulator ferritin, a novel immunoregulator, suppresses experimental arthritis. Arthritis Rheum 2003;48:846–53. 10.1002/art.10850 [DOI] [PubMed] [Google Scholar]
- 25. Maia M, de Vriese A, Janssens T, et al. CD248 and its cytoplasmic domain: a therapeutic target for arthritis. Arthritis Rheum 2010;62:3595–606. 10.1002/art.27701 [DOI] [PubMed] [Google Scholar]
- 26. Palmer G, Busso N, Aurrand-Lions M, et al. Expression and function of junctional adhesion molecule-C in human and experimental arthritis. Arthritis Res Ther 2007;9:R65. 10.1186/ar2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Redlich K, Hayer S, Ricci R, et al. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest 2002;110:1419–27. 10.1172/JCI0215582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asquith DL, Miller AM, Reilly J, et al. Simultaneous activation of the liver X receptors (LXRα and LXRβ) drives murine collagen-induced arthritis disease pathology. Ann Rheum Dis 2011;70:2225–8. 10.1136/ard.2011.152652 [DOI] [PubMed] [Google Scholar]
- 29. Stolina M, Schett G, Dwyer D, et al. RANKL inhibition by osteoprotegerin prevents bone loss without affecting local or systemic inflammation parameters in two rat arthritis models: comparison with anti-TNFalpha or anti-IL-1 therapies. Arthritis Res Ther 2009;11:R187. 10.1186/ar2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayer S, Redlich K, Korb A, et al. Tenosynovitis and osteoclast formation as the initial preclinical changes in a murine model of inflammatory arthritis. Arthritis Rheum 2007;56:79–88. 10.1002/art.22313 [DOI] [PubMed] [Google Scholar]
- 31. Koenders MI, Marijnissen RJ, Devesa I, et al. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1β, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum 2011;63:2329–39. 10.1002/art.30418 [DOI] [PubMed] [Google Scholar]
- 32. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol 2020;18:e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother 2013;4:303–6. 10.4103/0976-500X.119726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayman AR, Jones SJ, Boyde A, et al. Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteopetrosis. Development 1996;122:3151–62. [DOI] [PubMed] [Google Scholar]
- 35. Halleen JM, Tiitinen SL, Ylipahkala H, et al. Tartrate-Resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin Lab 2006;52:499–509. [PubMed] [Google Scholar]
- 36. Liu H, Zhu R, Liu C, et al. Evaluation of decalcification techniques for rat femurs using He and immunohistochemical staining. Biomed Res Int 2017;2017:1–6. 10.1155/2017/9050754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mack SA, Maltby KM, Hilton MJ. Demineralized murine skeletal histology. Methods Mol Biol 2014;1130:165–83. 10.1007/978-1-62703-989-5_12 [DOI] [PubMed] [Google Scholar]
- 38. Bogoevski K, Woloszyk A, Blackwood K, et al. Tissue morphology and antigenicity in mouse and rat tibia: comparing 12 different decalcification conditions. J Histochem Cytochem 2019;67:545–61. 10.1369/0022155419850099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jonsson R, Tarkowski A, Klareskog L. A demineralization procedure for immunohistopathological use. EDTA treatment preserves lymphoid cell surface antigens. J Immunol Methods 1986;88:109–14. 10.1016/0022-1759(86)90058-x [DOI] [PubMed] [Google Scholar]
- 40. Savi FM, Brierly GI, Baldwin J, et al. Comparison of different decalcification methods using rat Mandibles as a model. J Histochem Cytochem 2017;65:705–22. 10.1369/0022155417733708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miquelestorena-Standley E, Jourdan M-L, Collin C, et al. Effect of decalcification protocols on immunohistochemistry and molecular analyses of bone samples. Mod Pathol 2020;33:1505–17. 10.1038/s41379-020-0503-6 [DOI] [PubMed] [Google Scholar]
- 42. Schrijver WAME, van der Groep P, Hoefnagel LD, et al. Influence of decalcification procedures on immunohistochemistry and molecular pathology in breast cancer. Mod Pathol 2016;29:1460–70. 10.1038/modpathol.2016.116 [DOI] [PubMed] [Google Scholar]
- 43. Wickham CL, Sarsfield P, Joyner MV, et al. Formic acid decalcification of bone marrow trephines degrades DNA: alternative use of EDTA allows the amplification and sequencing of relatively long PCR products. Mol Pathol 2000;53:336. 10.1136/mp.53.6.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Croft AP, Campos J, Jansen K, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019;570:246–51. 10.1038/s41586-019-1263-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Culemann S, Grüneboom A, Nicolás-Ávila José Ángel, et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 2019;572:670–5. 10.1038/s41586-019-1471-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 2007;13:156–63. 10.1038/nm1538 [DOI] [PubMed] [Google Scholar]
- 47. Danks L, Komatsu N, Guerrini MM, et al. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis 2016;75:1187–95. 10.1136/annrheumdis-2014-207137 [DOI] [PubMed] [Google Scholar]
- 48. Zwerina J, Redlich K, Polzer K, et al. TNF-Induced structural joint damage is mediated by IL-1. Proc Natl Acad Sci U S A 2007;104:11742–7. 10.1073/pnas.0610812104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kato G, Shimizu Y, Arai Y, et al. The inhibitory effects of a RANKL-binding peptide on articular and periarticular bone loss in a murine model of collagen-induced arthritis: a bone histomorphometric study. Arthritis Res Ther 2015;17:251. 10.1186/s13075-015-0753-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Joosten LA, Helsen MM, Saxne T. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. Journal of immunology (Baltimore, Md. 1950) 1999;163:5049–55. [PubMed] [Google Scholar]
- 51. Joosten LA, Lubberts E, Durez P, et al. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum 1997;40:249–60. 10.1002/art.1780400209 [DOI] [PubMed] [Google Scholar]
- 52. Puchner A, Saferding V, Bonelli M, et al. Non-Classical monocytes as mediators of tissue destruction in arthritis. Ann Rheum Dis 2018;77:1490–7. 10.1136/annrheumdis-2018-213250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schett G, Teitelbaum SL. Osteoclasts and arthritis. J Bone Miner Res 2009;24:1142–6. 10.1359/jbmr.090533 [DOI] [PubMed] [Google Scholar]
- 54. Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis 2002;61 Suppl 2:84ii–6. 10.1136/ard.61.suppl_2.ii84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gravallese EM, Harada Y, Wang JT, et al. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 1998;152:943–51. [PMC free article] [PubMed] [Google Scholar]
- 56. Hayer S, Bauer G, Willburger M, et al. Cartilage damage and bone erosion are more prominent determinants of functional impairment in longstanding experimental arthritis than synovial inflammation. Dis Model Mech 2016;9:1329–38. 10.1242/dmm.025460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lubberts E, Oppers-Walgreen B, Pettit AR, et al. Increase in expression of receptor activator of nuclear factor kappaB at sites of bone erosion correlates with progression of inflammation in evolving collagen-induced arthritis. Arthritis Rheum 2002;46:3055–64. 10.1002/art.10607 [DOI] [PubMed] [Google Scholar]
- 58. Hayer S, Pundt N, Peters MA, et al. PI3Kgamma regulates cartilage damage in chronic inflammatory arthritis. Faseb J 2009;23:4288–98. 10.1096/fj.09-135160 [DOI] [PubMed] [Google Scholar]
- 59. Croxford AM, Whittingham S, McNaughton D, et al. Type II collagen-specific antibodies induce cartilage damage in mice independent of inflammation. Arthritis Rheum 2013;65:650–9. 10.1002/art.37805 [DOI] [PubMed] [Google Scholar]
- 60. van der Geest T, Roeleveld DM, Walgreen B, et al. Imaging fibroblast activation protein to monitor therapeutic effects of neutralizing interleukin-22 in collagen-induced arthritis. Rheumatology 2018;57:737–47. 10.1093/rheumatology/kex456 [DOI] [PubMed] [Google Scholar]
- 61. Hayer S, Zeilinger M, Weiss V, et al. Multimodal [18 F]FDG PET/CT Is a Direct Readout for Inflammatory Bone Repair: A Longitudinal Study in TNFα Transgenic Mice. J Bone Miner Res 2019;34:1632–45. 10.1002/jbmr.3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-219247supp001.pdf (84.9KB, pdf)
annrheumdis-2020-219247supp002.pdf (2MB, pdf)
annrheumdis-2020-219247supp003.pdf (4.7MB, pdf)
annrheumdis-2020-219247supp004.pdf (518.1KB, pdf)
annrheumdis-2020-219247supp005.pdf (62.7KB, pdf)
annrheumdis-2020-219247supp006.pdf (63.2KB, pdf)
annrheumdis-2020-219247supp007.pdf (418.8KB, pdf)