Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive disease of unknown cause characterized by relentless scarring of the lung parenchyma leading to reduced quality of life and earlier mortality. IPF is an age-related disorder, and with the population aging worldwide, the economic burden of IPF is expected to steadily increase in the future. The mechanisms of fibrosis in IPF remain elusive, with favored concepts of disease pathogenesis involving recurrent microinjuries to a genetically predisposed alveolar epithelium, followed by an aberrant reparative response characterized by excessive collagen deposition. Pirfenidone and nintedanib are approved for treatment of IPF based on their ability to slow functional decline and disease progression; however, they do not offer a cure and are associated with tolerability issues. In this review, we critically discuss how cutting-edge research in disease pathogenesis may translate into identification of new therapeutic targets, thus facilitate drug discovery. There is a growing portfolio of treatment options for IPF. However, targeting the multitude of profibrotic cytokines and growth factors involved in disease pathogenesis may require a combination of therapeutic strategies with different mechanisms of action.
Keywords: idiopathic pulmonary fibrosis, pathogenesis, disease mechanisms, genomics, single-cell biology, treatment, therapeutic targets, stem cells
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive age-related interstitial lung disease (ILD) of unknown origin with an average life expectancy of 3–5 years after diagnosis if untreated (Lederer and Martinez, 2018; Raghu et al 2018). In Europe alone, approximately 40,000 new cases of IPF are diagnosed each year (Navaratnam et al 2011). The physical, psychologic, and socio-economic burden of IPF is substantial (Diamantopoulos et al 2018), and with the population aging worldwide, the impact of IPF to patients and healthcare providers is expected to steadily increase in the future. IPF is the prototypic progressive fibrosing ILD, but a number of other ILDs can also display a progressive phenotype and a clinical course similar to IPF (Cottin et al 2018).
Although the mechanisms of fibrosis in IPF remain poorly understood, favored concepts of disease pathogenesis involve recurrent subclinical injuries to a genetically predisposed alveolar epithelium, followed by failure of alveolar re-epithelialization and repair (Spagnolo and Cottin 2017). Activated cells within the alveoli release a plethora of cytokines and growth factors that promote the recruitment, proliferation, and differentiation of lung fibroblasts into myofibroblasts, leading to excessive collagen deposition, progressive scarring of the lung parenchyma, and irreversible loss of function (Bellaye et al 2015; Wolters et al 2014). Current evidence-based guidelines for treatment of IPF recommend the use of pirfenidone and nintedanib, two compounds with pleiotropic mechanisms of action (Raghu et al 2015; Spagnolo et al 2015; Spagnolo et al 2018); however, both drugs have limited efficacy in preventing disease progression and improving quality of life, and are also associated with tolerability issues (Galli et al 2017; Bando et al 2016). Lung transplant is the only cure for patients with IPF, but, due to age and comorbidities, this represents a realistic therapeutic option for only a minority of patients (George et al 2019).
These characteristics of IPF indicate the ongoing critical importance of drug discovery efforts. In this review, we critically discuss how cutting-edge research in disease pathogenesis may translate into identification of new therapeutic targets that are needed to facilitate discovery and testing of new medications that can further slow or ideally stop the progression of IPF.
2. Pathogenesis
Whilst inflammatory cells and the mesenchyme were once the primary focus of IPF research, significant progress over the past two decades has led to the current paradigm of IPF pathogenesis, which proposes that, in a genetically susceptible individual, recurrent environmental and/or endogenous injury to alveolar epithelium occurs, with increased cell death, aberrant epithelial repair, and dysregulated epithelial-fibroblast cross-talk promoting persistent mesenchymal activation and extracellular matrix (ECM) deposition (Richeldi et al 2017). The importance of dysregulation of alveolar epithelial cells in the initiation of pulmonary fibrosis has been strengthened by the recent observation that an IPF-associated mutation in the surfactant protein C gene, an alveolar type II (AT2) cell-restricted protein, is sufficient in mice to induce spontaneous lung fibrosis, the first model derived from an IPF-associated gene that has been demonstrated to develop spontaneous lung fibrosis (Nureki et al 2018).
The complexity of IPF biology is demonstrated by the number of cell types and signalling pathways that have now been implicated in disease pathogenesis such as dysregulated epithelial repair, host defence, cell senescence, skewed immune responses including activation of macrophage subsets, fibroproliferative responses linked to aberrant kinase activation, transforming growth factor-β (TGF-β) and its downstream pro-fibrogenic pathways, and developmental pathway reactivation (Richeldi et al 2017). The spatial heterogeneity of IPF further complicates pathogenesis studies, as it is plausible that different biological processes reflecting different disease stages are present in one IPF lung in different areas at the same time. The application of single-cell RNA sequencing (scRNA-seq) approaches to IPF tissue has begun to address these challenges, with recent studies identifying diverse aberrant cell populations in IPF tissue which are now the subject of active investigation to dissect mechanisms underlying their dysregulation and potential therapeutic targeting (Habermann et al 2020; Adams et al 2020; Reyfman et al 2019; Xu 2016; Morse et al 2019; Jones et al 2018).
A number of factors that may underlie dysfunction of AT2 cells in IPF have been proposed including telomere shortening, aberrant mitochondrial bioenergetics, and increased endoplasmic reticulum stress. AT2 cells function as distal epithelial stem cells in the adult lung and so have a key role for regeneration and repair of an injured alveolus (Yu et al 2018; Bueno et al 2015; Naikawadi et al 2016; Korfei et al 2008; Lawson et al 2008). Studies have now identified homeostatic determinants of a progenitor cell niche for AT2 cells including Wnt signalling and hyaluronan (Nabhan et al 2018; Zacharias et al 2018; Liang et al 2016). It has recently been reported that during normal tissue regeneration, prior to terminal maturation into alveolar Type 1 cells, AT2 cells acquire a pre-alveolar type 1 transitional cell state during which time they express senescence-related genes (Kobayashi 2020). Accumulation of pre-alveolar type 1 transitional cell state -like cells have been reported in IPF lungs, suggesting that aberrant differentiation or persistence of this transitional cell senescence state might prevent normal alveolar regeneration and so could promote fibrosis development. Notably, a novel mouse model of conditional p53-dependent AT2 senescence has now been shown to develop spontaneous, progressive pulmonary fibrosis (Yao et al 2020).
Dysfunction of alveolar epithelium is proposed to be a key step in IPF disease initiation, although the factors that determine the persistence and progression of fibrosis remain poorly defined, with both cell intrinsic as well as local microenvironmental factors such as the ECM under active investigation. Until recently, ECM deposition was considered simply the end-stage of fibrosis, but there is now evidence that abnormal ECM deposition itself might modulate disease behaviour, with both altered ECM composition and increased tissue stiffness proposed to dysregulate cell behaviour to induce a positive feedback loop of self-sustaining fibrosis (Booth et al 2012; Parker et al 2014; Morse et al 2019).
IPF has generally been considered an alveolar disease that spares the airways; however, micro computed tomography analyses have now proposed that small airways changes are an early pathologic feature of IPF, identifying thickening of small airway walls together with significant loss of terminal bronchioles within regions of minimal fibrosis (Verleden et al 2020). Consistent with this concept a common gain-of-function MUC5B promoter variant (rs35705950), which is the strongest risk factor for the development of IPF, is over-expressed in bronchoalveolar epithelium and it has been hypothesized that excessive production of MUC5B may either enhance injury at the bronchoalveolar junction due to reduced muco-ciliary clearance or impede repair consequent to disruption of normal regenerative mechanisms in the distal lung (Seibold et al 2011; Schwartz, 2018).
Once considered sterile it is now recognised that the epithelial surface of the respiratory tract is colonized by a complex and dynamic microbiota termed the “lung microbiome”. In recent years studies performing culture-independent microbiological analysis of the lung microbiome have suggested that altered lung microbiome burden, diversity and composition in the airways of patients with IPF may contribute to disease pathogenesis and progression (O’Dwyer et al 2019). Whilst causality for the identified associations has yet to be established, murine model studies are supportive of this concept proposing that lung dysbiosis precedes peak lung injury and can promote alveolar inflammation and aberrant repair (O’Dwyer et al 2019; Molyneaux et al 2014; Molyneaux et al 2017; Han 2014).
2.1. The fibroblastic foci
Histopathologically, IPF is defined by the morphologic pattern of usual interstitial pneumonia, which is characterized by spatial heterogeneity, with areas of marked fibrosis adjacent to normal appearing lung, architectural distortion, microscopic honeycombing (cystic spaces lined by bronchiolar epithelium) and scattered fibroblastic foci (FF), which are believed to represent sites of active fibrogenesis (Cavazza et al 2010; Katzenstein et al 2008; Wuyts et al 2014; Rossi and Spagnolo 2017). FF are enigmatic aggregates of mesenchymal cells within a myxoid appearing matrix, which in 3D have been identified to be heterogeneous structures with large variations in shape and volume, consistent with the concept that they are temporally distinct microscopic foci of dysregulated lung injury and repair (Jones et al 2016). Whilst the mesenchymal cellular populations within FF remain poorly defined, scRNA-seq studies have provided new insights into aberrant cell populations within and adjacent to FF, identifying that the cuboidal epithelial cells overlying FF are aberrant basaloid cells co-expressing basal epithelial, mesenchymal, senescence, and developmental markers (Adams et al 2020), whilst a pro-fibrotic macrophage sub-population highly expressing secreted phosphoprotein 1 has been localised surrounding and within FF (Morse et al 2019). Although speculative, it is plausible that dysregulated cross-talk between these cell populations could represent a core pathway of progressive lung fibrogenesis.
Several studies have focused on functional characterization of FF (Harada 2010; Myllärniemi 2014; Chilosi 2017). Within FF, alveolar epithelial cells (AECs) and myofibroblasts show disruption of the Wnt/beta-catenin pathway, downregulation of phosphatase and tensin homolog, a multi-functional tumor suppressor, and overexpression of gremlin (an oncoprotein) and TFG- β, leading to epithelial-to-mesenchymal transition (Harada et al 2010; Myllärniemi et al 2014; Chilosi et al 2017). AECs and myofibroblasts also overexpress matrix metalloproteinases as well as a number of profibrotic proteins such as Zinc finger E-box-binding homeobox 1 and beta-tubulin III (Chilosi et al 2017; Hill et al 2019). Within the FF, AECs (but not myofibroblasts; Giulio Rossi, personal unpublished observation; Figure 1) overexpress c-MET, a receptor tyrosine kinase, which binds to its ligand, hepatocyte growth factor, to activate a wide range of signaling pathways involved in cell proliferation, motility, migration and invasion (Organ and Tsao, 2011). Therapeutic inhibition of c-MET may potentially interfere with epithelial-to-mesenchymal transition and FF formation in IPF (Tzouvelekis et al 2019).
Figure 1.
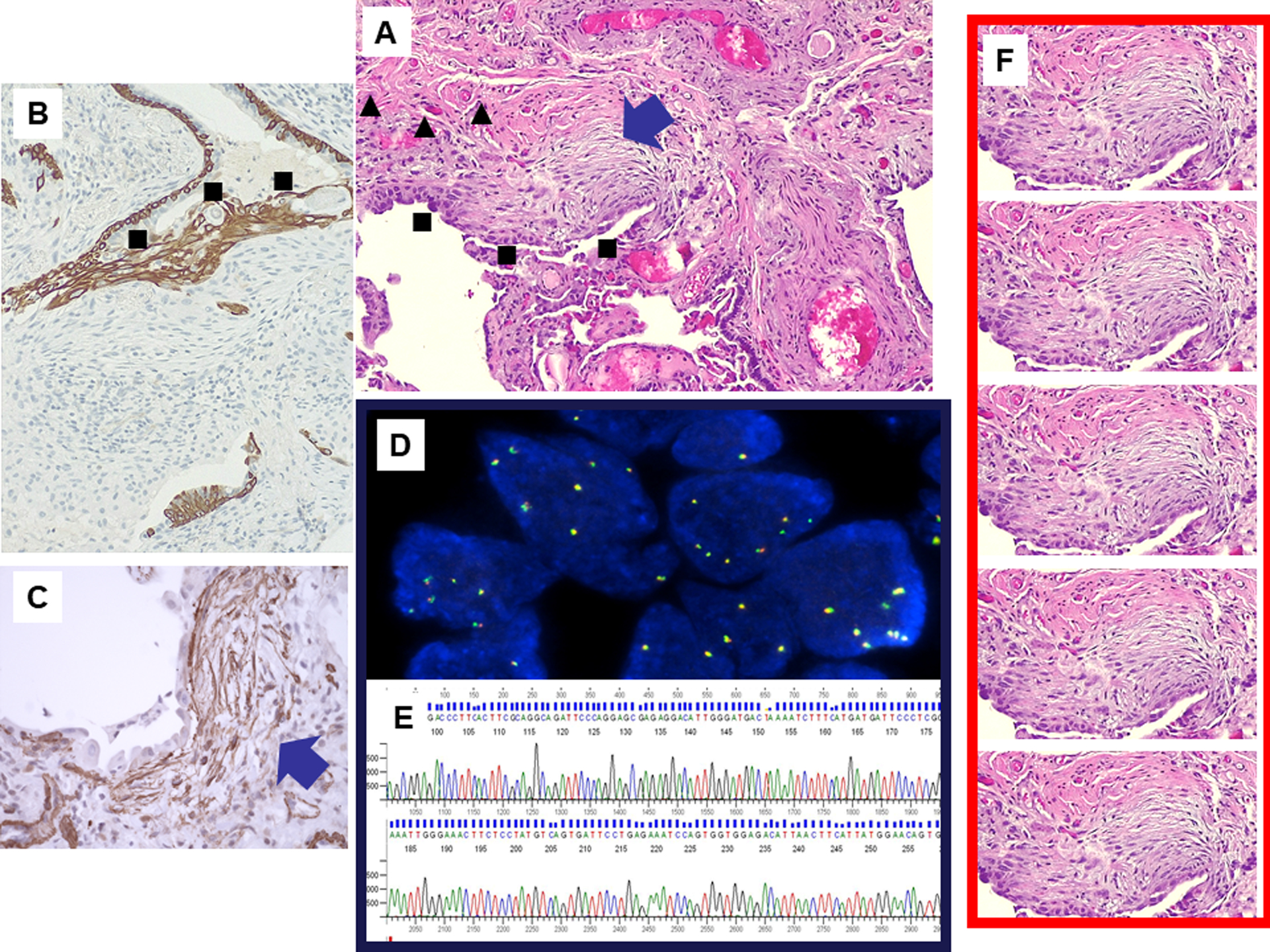
Fibroblastic foci are discrete proliferations of myofibroblasts in myxoid stroma indicative of active fibrosis (blue arrow) often covered by activated alveolar epithelial cells representing the site of acute lung injury (squares) and with peripheral angiogenetic phenomena with multiple small capillaries (triangles) (A, hematoxylin-eosin stain). Fibroblastic foci may be investigated for protein expression by immunohistochemistry (B, up-regulation of c-MET in alveolar epithelial cells; squares; C, upregulation of the epithelial-to-mesenchymal transition protein Twist in myofibroblasts; arrow). Fluorescent in situ hybridization analysis of c-MET (D) and molecular investigation of c-MET mutations (electropherogram, E). Microdissection of fibroblastic foci from transbronchial biopsy allows obtaining an adequate amount of DNA/RNA for selective multiplex genomic and proteomic analysis (F).
3. Establishing a plausible rationale for novel targets
Identifying novel targets for therapy in IPF requires a clear understanding of disease pathogenesis, genetic risk factors, and biomarkers of disease progression. With this foundation, application of traditional and novel drug discovery approaches can be performed to identify therapeutic targets for this progressive disease. The traditional approach to drug discovery is hypothesis-driven with testing of molecular targets first in in vitro and in vivo systems, including animal models. Promising targets are then transitioned into the clinical trial phases in order to demonstrate safety and efficacy (Mahan, 2014). Phase I studies are used to test safety in humans and may also be used to evaluate the best way to administer a drug, including its frequency, dose, and occasionally route of administration. Phase II studies subsequently involve testing people with the disease, to assess either efficacy or safety or both. Finally, more definitive Phase III studies are typically conducted in many centers across several countries with a large enough sample size to detect a meaningful benefit in whatever endpoint provides the optimal balance between clinical relevance and feasibility. Once a target has gone through all of these phases, data are presented to the relevant regulatory authorities to assess effectiveness and to ensure safety and manufacturing quality standards will be met. Only then is a marketing authorization or license issued. Unfortunately, this traditional method is a long and demanding process, with many candidate targets never making it to clinical trials (Moore, 2003). Even those candidates that make it to the trial phases are hampered by cost, recruitment issues, and time.
In recent years, there has been a push for novel methods to identify candidate targets to accelerate drug discovery. High throughput assays, for example, have been proposed in this context (Hughes et al 2011). These assays leverage technology to screen a large number of biological modulators and effectors against specific targets in relevant biological systems. Some novel biological systems being investigated in IPF include organoids (Wilkinson et al 2017) and scar-in-a-jar (Chen et al 2009), among others. Another approach that is gaining traction includes drug repurposing. This strategy consists of using existing drugs and identifying new uses outside the original indications. Attempted examples of this strategy in IPF include metformin (Kheirollahi et al 2019; Rangarajan 2018), and Phosphoinositide 3-kinase / mammalian target of rapamycin inhibitor (Mercer et al 2016; Lukey et al 2019; Woodcock et al 2019). Leveraging off-target effects provides a more cost effective and more efficient way to identify new drugs given that they have already gone through rigorous safety assessments. Last, smarter clinical trial design is being explored (Sessler and Myles, 2020).
Strategies for traditional randomized trials include enriching trial populations for those more likely to progress, using biomarkers to determine who may be more likely to respond to an intervention, or using novel endpoints. Limitations to this approach remain cost, recruitment delays, rigidity of the study protocol, and potentially decreased generalizability outside of the narrow clinical trial population. Other novel trial designs that may avoid some of these limitations without sacrificing validity or study integrity include adaptive, pragmatic, cluster crossover, and stepped wedge trial designs (Sessler and Myles, 2020). For example, adaptive designs use interim data to modify an ongoing trial without compromising validity or introducing bias. There are benefits of this design for rare diseases by improving trial efficiency, but weaknesses can include difficulties in identifying bias and unexpected heterogeneity or effect size.
Ultimately, determining which patient will respond to which drug is the key to drug discovery in IPF. Optimizing clinical trials thus requires a comprehensive understanding of the pathophysiology of IPF, including identification of biomarkers that predict and quantify treatment response.
4. How progress in IPF genomics and other “omics” may inform drug development
Rapidly expanding capacity for, and decreasing cost of, next-generation sequencing technologies has presented a unique opportunity to leverage genetic, genomic, and multi-omic datasets for the pursuit of novel therapeutic targets for IPF and other chronic ILDs.
4.1. Genetic susceptibility to IPF
The role of genetic factors in determining susceptibility to IPF is well established, and inherited factors have been estimated to contribute at least one-third of all IPF risk (Fingerlin et al 2013). Multiple studies now demonstrate that both common (e.g., polymorphisms), and rare/ultra-rare (e.g., mutations) genetic variants are important determinants of IPF risk. Genome-wide association studies have implicated more than 15 common genetic variants as risk factors for IPF (Fingerlin et al 2013; Noth et al 2013; Allen et al 2017; Moore 2019), the most prominent of which is a polymorphism in the promoter region of the gene encoding for the airway mucin MUC5B (Seibold et al 2011; Zhang et al 2011). Compared to other IPF patients, those who carry the MUC5B T allele (which increases IPF risk) develop later onset and less rapidly progressive disease (Peljto et al 2013). Common genetic variants within toll interacting protein, an important regulator of innate immune responses (Zhu et al 2012), have also been associated with IPF susceptibility and prognosis (Noth et al 2013). Moreover, rs3750920, a functional synonymous coding variant that is located within toll interacting protein exon 3, has been associated with different response to N-acetylcysteine (NAC) (Oldham et al 2015). Specifically, NAC therapy was associated with improved survival among subjects carrying an rs3750920 TT genotype but with a trend towards worse survival among those with a CC genotype. If confirmed, these findings would suggest that genetic makeup of individuals with IPF might influence their response to treatment.
Initial studies focused on familial cases of IPF implicated variants in genes related to surfactant processing (Thomas et al 2002; van Moorsel et al 2010; Wang et al 2009; Ono et al 2011) and telomere biology (Armanios et al 2007; Tsakiri et al 2007; Kropski et al 2014; Alder et al 2015; Stuart et al 2015; Cogan et al 2015; Kropski et al 2017; Borie et al 2016). It is now clear that compared to other IPF patients, those with severely shortened peripheral blood telomeres and/or known telomere pathway mutations have earlier-onset and more rapidly progressive disease (Newton et al 2016; Newton et al 2019; Stuart et al 2014). More recently, whole-exome (Petrovski et al 2017; Coghlan et al 2014) and whole-genome-sequencing studies (Dressen et al 2018) have suggested similar patterns of rare genetic variants in sporadic IPF patients. Together, these studies have yielded three major insights: 1) the genetic risk architecture in familial vs. sporadic IPF is similar; 2) IPF risk genes are predominantly expressed in the lung epithelium; and 3) there are likely multiple, genetically distinct IPF endotypes.
Investigation into the functional consequences of IPF-associated genetic variations has led to unexpected convergences that may inform key mechanisms underlying the pathogenesis of IPF. For example, many of the disease-associated surfactant protein mutations lead to improper processing or trafficking of pro-surfactant protein C, which leads to accumulation of misfolding proteins and activation of the unfolded protein response (UPR) (Mulugeta et al 2005; Lawson et al 2011), but it was soon recognized that epithelial UPR activation was a far more prevalent feature of IPF lungs (Korfei et al 2008; Lawson et al 2008) than could be explained by the frequency of surfactant protein mutations. More recently, it has been demonstrated that the MUC5B risk (T) allele (carried by >60% of IPF patients) may contribute to a feed-forward cycle of UPR activation, and escalating MUC5B production is regulated through the UPR-induced alternative splicing of the transcription-factor X-box-protein-1 (Chen et al 2019). In contrast, mutations in telomere-related genes have generally been associated with activation of telomere-DNA damage responses (Alder et al 2015; Chen et al 2015; Naikawadi et al 2016), which can trigger apoptosis or senescence of progenitor cells in the lung epithelium. These genotype-specific disease mechanisms conceptually lend themselves to precision medicine approaches, and underscore the need to consider genetic stratification in preclinical models and in clinical trials of emerging therapeutics.
4.2. Single-cell biology in IPF
The emergence of innovative microfluidic technologies that enable simultaneous profiling of the gene expression programs of thousands of individual cells (single-cell RNA-sequencing, scRNA-seq) (Macosko et al 2015; Klein et al 2015) has led to renewed interest in the use of genomic approaches to elucidate disease mechanisms. There have now been several studies using scRNA-seq in IPF lung tissue, and together they have led to important new insights into disease pathobiology (Xu et al 2016; Reyfman et al 2019; Habermann et al 2020; Adams et al 2020). Among the most striking findings to date are the emergence of cell-states/types in the IPF lung that are rarely observed in the healthy lung. Notable examples of this include a Secreted Phosphoprotein 1 (osteopontin) expressing interstitial macrophage population (Reyfman et al 2019), a Collagen type 15, α−1 expressing peribronchiolar endothelial cell population (Adams et al 2020), a highly-activated fibroblast population characterized by high expression of hyaluronan-synthase-1 (Habermann et al 2020), and a basal-like-cell population expressing a combination of basal cell, alveolar, and mesenchymal programs (Habermann et al 2020; Adams et al 2020).
There are several immediately evident implications for antifibrotic drug development. While fibroblasts are the primary effectors cells that produce the pathologic ECM that is the hallmark of the IPF lung, these datasets have demonstrated that a diversity of fibroblast populations are actively producing pathologic ECM in IPF lungs (Habermann et al 2020; Adams et al 2020; Morse et al 2019). In addition to the well-studied activated myofibroblasts, several other fibroblast populations lacking actin alpha 2/alpha-smooth muscle actin expression and characterized by distinct surface markers, growth-factors/chemokine receptors, and spatial localization are found in the IPF lung. A key implication of this is that an antifibrotic therapy targeting mechanisms only relevant to one of these fibroblast subtypes will likely have a ceiling to its potential efficacy; combination therapies or those targeting mechanisms conserved across fibroblast subtypes may be required for maximal therapeutic benefit. Second, these basal-like cells (“KRT5-/KRT17+” or “aberrant basaloid”) (Habermann et al 2020) represent a previously undescribed cell state that could be therapeutically targeted. These cells are found immediately overlying regions of most active collagen production (Habermann et al 2020), produce collagen and fibronectin themselves, and express TGF-β, connective-tissue growth factor, and a number of other molecular drivers of fibroblast activation (Habermann et al 2020; Adams et al 2020). The origin of these cells is not certain; however, studies using organoid models and genetic lineage tracing indicate that a transcriptomically similar cell program characterizes an intermediate stage of AT1 cell differentiation (Kobayashi et al 2020). Together, these data suggest that failure of AT1 maturation may lead to an accumulation of cells “stuck” in a transitional state that produce key mediators of fibroblast activation through paracrine signaling mechanisms, consistent with data from several mouse models in which AT1 cell differentiation is impaired (Yao et al 2020; Wu et al 2020). Therapies interrupting these signaling loops could hold promise as novel therapeutic strategies. In addition to providing new mechanistic targets for IPF therapies, these high-resolution scRNA-seq datasets can be utilized to advance ongoing therapy development. For example, understanding the cell-type-specific expression profile of the target of a given therapy can be utilized to rationally choose potential biomarkers reflecting target engagement. A role of transcriptomics in ILD diagnosis has been established (Kim et al 2015), and shows promise for prediction of disease outcomes (Herazo-Maya et al 2017). Extension of these and similar approaches to prediction of therapeutic response seems a natural next step.
5. Pharmacological treatment
At present, there is no treatment that can cure IPF. Two drugs, pirfenidone and nintedanib are able to slow disease progression, but neither drug improves or even stabilizes lung function, or improves quality of life, and both therapies have tolerability issues (Kreuter et al 2015).
5.1. Pirfenidone
Pirfenidone (5-methyl-1-phenylpyridin-2[1H]-one) is an orally available, synthetic compound that exerts anti-fibrotic, anti-inflammatory and antioxidant properties through down-regulation of key pro-fibrotic growth factors including TGF-β; inhibition of inflammatory cytokines (e.g., tumor necrosis factor-α) production and release; and reduction of lipid peroxidation and oxidative stress (Iyer et al 1999). Four phase 3 trials have evaluated the efficacy of pirfenidone in patients with IPF (Taniguchi et al 2010; Noble et al 2011; King et al 2014). Overall, these trials showed that pirfenidone slows down disease progression and functional decline in patients with IPF. In addition, pooled analyses and meta-analyses of clinical trials in IPF showed that pirfenidone treatment is associated with a reduced risk of mortality (Nathan et al 2017). Common adverse effects of pirfenidone include gastrointestinal intolerance (e.g., nausea, diarrhea and dyspepsia) and skin reactions (e.g., rash and photosensitivity), which are generally mild to moderate in severity and reversible following dose reduction or temporary drug discontinuation.
5.2. Nintedanib
Nintedanib is an intracellular inhibitor of vascular endothelial growth factor receptor 1–3, fibroblast growth factor receptor 1–3, and platelet-derived growth factor receptor a and b (Wollin et al 2015). By inhibiting these tyrosine kinase receptors, nintedanib interferes with a number of processes that have been implicated in the pathogenesis of IPF, including proliferation and migration of lung fibroblasts, and differentiation of fibroblasts to myofibroblasts. Two parallel 52-week, phase 3 trials consistently showed that nintedanib treatment, compared to placebo, was associated with a significantly reduced risk of disease progression (Richeldi et al 2014). Nintedanib appears also to have a mortality benefit (Canestaro et al 2016). The most frequent side effects associated with nintedanib use are diarrhea (reported by approximately 60% of patients within the first 3 months of treatment) and nausea, which in most cases are of mild or moderate intensity and manageable (Richeldi et al 2014).
6. A novel approach to treatment of IPF – the stem cells
Mesenchymal Stem Cells (MSCs) represent multipotent cells that are easily harvested from many tissues such as adipose tissue, peripheral blood, bone marrow and umbilical cord (Tzouvelekis et al 2018). Several lines of experimental evidence suggest strong anti-fibrotic, anti-inflammatory and immunomodulatory effects for MSCs both through paracrine signaling and potent differentiation capacity (Ortiz et al 2003; Toonkel et al 2013; Germano et al 2009). In particular, pre-clinical studies demonstrated that administration of MSCs was associated with downregulation of TGF-β signaling, significant improvement in lung histopathology, extent of fibrotic lesions as assessed by Aschroft score, lung collagen content, and reduced bronchoalveolar lavage neutrophilia (Kumamoto et al 2009; Moodley et al 2009; Gazdhar et al 2013). Despite relative enthusiasm, there are still major concerns on the therapeutic role of stem cells in IPF mainly arising from experimental data suggesting a detrimental role for MSCs within a pro-fibrotic microenvironment (Barczyk et al 2015).
A previous phase Ib study investigating endobronchial delivery of autologous adipose-derived stem cells in patients with IPF demonstrated an acceptable 5-years safety profile, improvement in parameters of health-related quality and 100% survival rate 2 years following first administration (Tzouvelekis et al 2013; Ntolios et al 2018). In addition, intravenous delivery of placental derived MSCs had an acceptable safety profile in patients with moderately severe IPF (Chambers et al 2014), and functional and morphologic indices, including six-minute walk test, forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide or computed tomography fibrosis score, showed no statistically significant decline during the first 6 months of follow-up (Hostettler et al 2017). Furthermore, the AETHER trial suggested that intravenous administration of allogeneic bone marrow -MSCs in patients with mild-to-moderate IPF (n=9) was safe and promising with regard to slowing down disease progression (Glassberg et al 2017). However, these conclusions should be interpreted with caution, as this study was underpowered for the detection of significant changes in FVC and diffusing capacity of the lung for carbon monoxide; in addition, 2/9 patients died from what appeared to be unrelated causes at 10 weeks and 29 weeks post-infusion, respectively. Results from a recently FDA approved phase 1b randomized, placebo controlled, clinical trial (ReCell) on safety and efficacy of multi-dose intravenous regimens of bone marrow-MSCs in patients with IPF, are greatly anticipated (Tzouvelekis et al 2018). Notably, all the studies on MSCs conducted thus far were severely underpowered and thus firm conclusions cannot be drawn.
Future studies on MSCs are hampered by the 2014 FDA approval of nintedanib and pirfenidone, leading to major delays or even failures to meet enrolment timelines. Finally, many unanswered questions remain with regard to the optimal route of administration (intravenous, endobronchial), the origin of stem cells (bone marrow, adipose- or placental-derived), frequency and dose of therapeutic regimens, and most importantly study design and phenotypic selection of patients (early or advanced disease) who are most likely to benefit from cell-based therapies. Selection of appropriate trial end-points is another important issue, as the majority of previous trials have relied on a minimum number of infusions and a short follow-up period that may limit the ability to identify and quantify meaningful clinical outcomes.
7. A novel approach to drug administration: the inhalation route
Current IPF treatments are administered orally while several compounds in development are given either subcutaneously or intravenously (Table 1; Figure 2). However, of all of the internal organs the lung is the most accessible to topical administration of therapy. Inhaled drugs are attractive for several reasons, the most important being that delivery of treatment directly to the lung has the potential to maximize therapeutic exposure in regions at greatest risk of disease progression, whilst minimizing systemic exposure and thus reducing the potential for adverse effects. Inhaled treatments are already the mainstay for obstructive lung disorders such as asthma and chronic obstructive pulmonary disease, but have been poorly studied in IPF. In part, this reflects concerns regarding the feasibility of delivering inhaled compounds to the distal airspace in lungs distorted by fibrosis. A recent study, using radiolabelled salbutamol, has confirmed that inhaled drug delivery in IPF is feasible but is impacted by severity of fibrosis and particle size (with the optimal size being close to 1.5 microns) (Usmani et al 2018). Another study of a novel compound targeting the integrin avβ6 used positron emission tomography imaging of the integrin to demonstrate effective delivery of the drug to regions of fibrosis (Maher et al 2020). Similarly, a study of an inhaled Galectin-3 inhibitor showed down regulation of the receptor in alveolar macrophages in a dose-dependent manner (Hirani et al 2017). However, the same study identified greater systemic absorption of the drug from the lungs of IPF patients compared with healthy controls. Overall, these data confirm that inhaled administration of drugs is feasible in IPF and that this route may be important in minimizing side effects for patients. This strategy has also been explored with existing anti-fibrotic drugs (Khoo et al 2020; Homma et al 2012). A small Japanese study of patients with advanced IPF suggested that combination therapy with inhaled NAC and oral pirfenidone might reduce the annual rate of decline in FVC and improve progression-free survival compared to pirfenidone alone (Sakamoto et al 2015). Conversely, a recent 48-week, open-label, phase 3 trial of Japanese patients with IPF (n=81) showed that combination treatment with inhaled NAC and oral pirfenidone was associated with more rapid decline in FVC compared to pirfenidone alone (Sakamoto et al 2020).
Table 1.
Selected list of experimental treatments for idiopathic pulmonary fibrosis
| Treatment | Mechanism of action | Phase/Study design | Status | Primary outcome | Trial identifier |
|---|---|---|---|---|---|
| DRUGS TARGETING FIBROBLASTS | |||||
| GLPG1690/ziritaxestat | Autotaxin inhibitor; oral | Phase III; randomized, double-blind, parallel-group, placebo-controlled | Recruiting | Rate of decline in FVC from baseline to week 52 |
NCT03733444 NCT03711162 |
| Pamrevlumab | Fully human monoclonal antibody against CTGF; intravenous | Phase III, randomized, double-blind, placebo-controlled | Recruiting | Change in FVC (litres) from baseline to week 52 | NCT03955146 |
| Pamrevlumab | Fully human monoclonal antibody against CTGF; intravenous | Phase III, randomized, double-blind, placebo-controlled | Recruiting | Proportion of subjects with disease progression (absolute FVC percentage predicted decline ≥10% or death) from baseline to week 52 | NCT04419558 |
| TD139 | Galectin-3 inhibitor; inhaled | Phase IIb, randomized, double-blind, parallel, placebo-controlled | Recruiting | Rate of decline in FVC (mL) from baseline to week 52 | NCT03832946 |
| PLN-74809 | Dual selective inhibitor of αVβ1/αVβ6 inhibitor; oral | Phase IIa, randomized, double-blind, dose-ranging, placebo-controlled | Recruiting | Number of participants with treatment-related AEs and laboratory abnormalities | NCT04396756 |
| PLN-74809 | αvβ1 and αvβ6 selective inhibitor; oral, | Phase IIa, randomized, sequential assignment | Recruiting | Number of participants with a change from baseline in αvβ6 receptor occupancy as measured by PET | NCT04072315 |
| BMS-986278 | LPA antagonist; oral | Phase II, randomized, double-blind, placebo-controlled | Recruiting | Rate of change in percentage predicted FVC from baseline to week 26 | NCT04308681 |
| Jaktinib Dihydrochloride Monohydrate | JAK 1, JAK 2 and JAK 3 inhibitor; oral | Phase II, randomized, double-blind, placebo-controlled | Recruiting | Change in FVC from baseline to week 24 | NCT04312594 |
| Saracatinib | Highly selective Src tyrosine kinase family inhibitor; oral | Phase Ib/IIa, randomized, double-blind, parallel design, placebo-controlled | Recruiting | Safety, tolerability, pharmacokinetics, pharmacodynamics, efficacy (as measured by change in FVC from baseline to week 24 | NCT04598919 |
| DRUGS TARGETING ALVEOLAR MACROPHAGES AND THEIR MEDIATORS | |||||
| rhPTX-2/PRM-151 | TGF-β1 modulator; intravenous | Phase III, randomized, double-blind, placebo-controlled | Recruiting | Absolute change in FVC (mL) from baseline to week 52 | NCT04552899 |
| rhPTX-2/PRM-151 | TGF-β1 modulator; intravenous | Phase III, open-label extension study | Recruiting | Incidence and severity of AEs, and infusion related reactions; percentage of participants permanently discontinuing study treatment due to AEs | NCT04594707 |
| IMMUNOTHERAPIES | |||||
| VAY736/ianalumab | IgG1 monoclonal antibody against BAFF receptor; subcutaneously | Phase II; randomized, subject-, investigator-, sponsor-blinded, placebo-controlled | Recruiting | Change from baseline to week 48 in FVC | NCT03287414 |
| GLPG1205 | GPR84 antagonist; oral | Phase II; randomized, double-blind, placebo-controlled | Completed (results pending) | Change in FVC from baseline to week 26 | NCT03725852 |
| DRUGS TARGETING BROAD PATHWAYS/SIGNALING | |||||
| KD025/SLx-2119 | ROCK2 inhibitor; oral | Phase II, open label | Active, not recruiting | Change in FVC from baseline to week 24; number of subjects experiencing AEs | NCT02688647 |
| ND-L02-s0201/BMS-986263 | HSP47 inhibitor; intravenous | Phase II, randomized, double-blind, placebo-controlled | Recruiting | Number of patients with treatment-related AEs from baseline to week 24 | NCT03538301 |
| CC-90001 | Selective JNK inhibitor; oral | Phase II, randomized, double-blind, placebo-controlled | Recruiting | Change in percentage predicted FVC from baseline to week 24 | NCT03142191 |
| GKT137831/Setanaxib | NOX1 and NOX4 inhibitor; oral | Phase II; randomized, double-blind, placebo-controlled | Not yet recruiting | Surrogate biomarker of oxidative stress by mass spectrometry from baseline to week 24 | NCT03865927 |
| C21 | Angiotensin type 2 receptor agonist; oral | Phase II, open-label, single-arm | Not yet recruiting | Nature and frequency of AEs occurring over the trail period | NCT04533022 |
| SENOLYTICS | |||||
| Dasatinib + Quercetin | Elimination of senescent cells; oral | Phase I, randomized (some patients are randomized to placebo or study drug while others go into open label) | Completed (results pending) | Percentage of pro-inflammatory expressing cells in skin biopsy obtained at baseline and at week 4 | NCT02874989 |
| SMALL INTERFERING RNA | |||||
| TRK-250 | Nucleic acid suppressing expression of TGF-β1 protein at gene expression level; inhaled | Phase I, randomized, double-blind, placebo-controlled, single and multiple dose | Recruiting | Incidence and severity of adverse events up to 7 days after last dose | NCT03727802 |
| STEM CELLS | |||||
| Human autologous lung stem cells | Immunomodulatory, anti-proliferative, and anti-inflammatory; intravenous | Phase I, randomized, open label | Recruiting | Number of patients with AEs and serious AEs | NCT04262167 |
| Human autologous lung stem cells | Immunomodulatory, anti-proliferative, and anti-inflammatory; injected by bronchoscopy | Phase I/Phase II, open label | Recruiting | Change in FVC from baseline to week 48 | NCT02745184 |
| UNDEFINED MECHANISM OF ACTION | |||||
| TD-1058 | Undefined; inhaled | Phase I, randomized, double-blind, parallel-group, placebo-controlled | Recruiting | Number and severity of treatment emergent AEs | NCT04589260 |
Abbreviations: AE: adverse event; AUC: area under the curve; BAFF: B-cell activating factor; BAL: bronchoalveolar lavage; Cmax: maximum observed concentrations; CTGF: connective tissue growth factor; FVC: forced vital capacity; GPR84: G Protein-coupled Receptor 84; HSP: Heat-Shock Protein; JAK: Janus kinase; JNK: c-Jun N-terminal kinase; LPA: Lysophosphatidic acid; mTOR; mammalian target of rapamycin; NOX: Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; pAKT: phosphorylated AKT; PD: pharmacodynamics; PET: positron emission tomography; PI3K: Phosphatidylinositol 3-kinases; ROCK: Rho-associated coiled-coil kinase; TGF-β1: transforming growth factor β1
Figure 2.
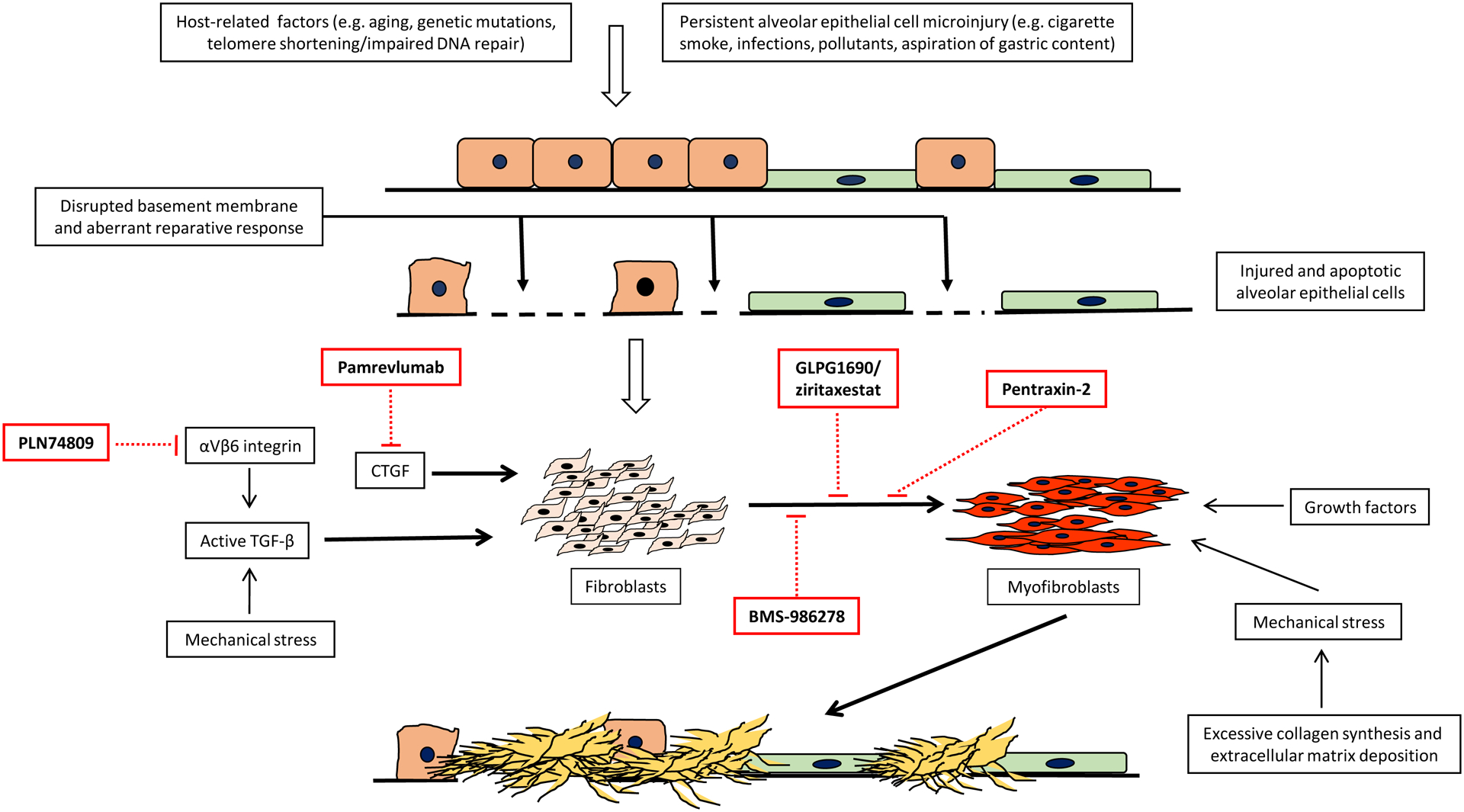
Selected potential targets in IPF
8. Translating preclinical evidence into effective therapies
The IPF literature is littered with negative clinical trials, with many different causes for these results. Early studies of potential IPF therapies suffered from imprecise definitions of major ILD subtypes, with incorrect diagnostic labels given to patients who we would now consider to have different diseases. This resulted in examples of patients with “IPF” who responded to immunomodulatory medications such as prednisone and azathioprine, which was historically a commonly accepted therapy of IPF (American Thoracic society, 2000). Subsequent studies showed significant harm from these same therapies when they were applied to well-characterized patients with what we now define as IPF (Idiopathic Pulmonary Fibrosis Clinical Research Network, 2012), suggesting that previous patients who reportedly benefitted from these therapies likely had other diagnoses (i.e., ILD secondary to connective tissue diseases or hypersensitivity pneumonitis). Ongoing and future studies of IPF are much more rigorous in ensuring a consistent phenotype that is intended to represent a distinct patient population.
Our understanding of IPF biology has also dramatically improved over the last two decades, further supporting abandonment of some therapeutic strategies that were previously assumed to be valid options. Identification of key pathways involved in IPF genesis and progression as described above has ushered in an era in which virtually all major clinical trials now target pathways that are central to IPF. Some of these trials include measurements of simple peripheral blood biomarkers that are intended to reflect disease activity and be responsive to disease modification; however, there is currently no such tool that provides this information for an available IPF therapy. This gap highlights the need for future studies to approach biomarker development and testing in a rigorous manner, with the hopes that this will allow shorter, smaller, and less costly early-phase clinical trials. An ideal biomarker for this purpose would be biologically plausible, able to distinguish IPF from healthy and diseased controls, correlate with disease severity, predict disease progression and response to treatment, and be modified by treatment, with all of these findings then externally validated in a distinct cohort. Although clinically useful biomarkers frequently do not possess all of these features, identifying a biomarker that meets these goals would have incredible benefits for future drug development.
Given their financial costs and the burden placed upon participants, it is imperative that clinical trials pursue appropriate therapeutic targets with drugs that have a high likelihood of success. Fortunately, this is the case for many of the IPF clinical trials that are currently ongoing, and it therefore appears more likely than ever that we will soon have additional medication options for these patients. In addition to assessing more rational and biologically plausible drug targets, it is critical that these large, multinational, and rigorous trials are also used to learn more about the disease by ensuring that participants contribute clinical data and biological samples. This should ideally be done on a large scale and within an open science concept in order to facilitate the next generation of drug development.
9. Conclusions
In the last few years, the dynamic research activity in the pathogenesis of IPF has led to the identification of several therapeutic targets and the development of a large portfolio of novel compounds. Some of these molecules, which are currently being investigated in clinical trials, are likely to reach the clinic in the near future. Nevertheless, the prerequisite for the development of a real cure for IPF is a deeper understanding of the mechanisms underlying disease development.
Conflict of interest form
Dr Spagnolo reports personal fees and institutional grants from PPM Services, Boehringer-Ingelheim and Roche, and personal fees from Chiesi and Galapagos, outside the submitted work. Dr Kropski has received advisory board fees from Boehringer-Ingelheim and Janssen Pharmaceuticals, is on the scientific advisory board of APIE Therapeutics, and has research contracts with Genentech, all outside the submitted work. Dr Jones reports grants from Boehringer Ingelheim, outside the submitted work. Dr Lee has received grants from the NIH and Boehringer Ingelheim, and consulting fees from Galapagos, Boehringer Ingelheim, United Therapeutics, Eleven P15, and Bonac, outside the submitted work. Dr Maher has received grants and personal fees from GlaxoSmithKline R&D and AstraZeneca, and personal fees from Boehringer Ingelheim, Roche, Bayer, Samumed, Galapagos, Celgene, Indalo, Pliant, Blade Therapeutics, Respivant, Novartis and Bristol-Myers Squibb, outside the submitted work. Dr Maher has also received, via his institution, industry-academic funding from and has received consultancy or speakers’ fees from Apellis. Dr Ryerson reports grant funding and personal fees from Boehringer Ingelheim and Hoffmann-La Roche, outside of the submitted work. Dr Rossi, Dr Tzouvelekis and Dr Karampitsakos have nothing to disclose.
Abbreviations
- AECs
alveolar epithelial cells
- AT1
alveolar type 1
- AT2
alveolar type 2
- ECM
Extracellular matrix
- FF
fibroblastic foci
- FVC
forced vital capacity
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- MSCs
Mesenchymal Stem Cells
- TGF-β
Transforming growth factor-β
- UPR
unfolded protein response
References
- Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, et al. (2020). Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Science Advances, 6, eaba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JK, Stanley SE, Wagner CL, Hamilton M, Hanumanthu VS, Armanios M, et al. (2015). Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest, 147, 1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, et al. (2015). Telomere dysfunction causes alveolar stem cell failure. Proceedings of the National Academy of Sciences of the United States of America, 112, 5099–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RJ, Porte J, Braybrooke R, Flores C, Fingerlin TE, Oldham JM, et al. (2017). Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respiratory Medicine, 5, 869–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society. (2000). Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). American Journal of Respiratory and Critical Care Medicine, 161, 646–664 [DOI] [PubMed] [Google Scholar]
- Armanios MY, Chen JJ-L, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. (2007). Telomerase mutations in families with idiopathic pulmonary fibrosis. New England Journal of Medicine, 356, 1317–1326 [DOI] [PubMed] [Google Scholar]
- Bando M, Yamauchi H, Ogura T, Taniguchi H, Watanabe K, Azuma A, et al. (2016). Clinical experience of the long-term use of Pirfenidone for idiopathic pulmonary fibrosis. Internal Medicine, 55, 443–448 [DOI] [PubMed] [Google Scholar]
- Barczyk M, & Schmidt S (2015). Stem Cell-Based Therapy in Idiopathic Pulmonary Fibrosis. Stem Cell Reviews and Reports, 11, 598–620 [DOI] [PubMed] [Google Scholar]
- Bellaye PS, & Kolb M (2015). Why do patients get idiopathic pulmonary fibrosis? Current concepts in the pathogenesis of pulmonary fibrosis. BMC Medicine, 13, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, et al. (2012). Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. American Journal of Respiratory and Critical Care Medicine, 186, 866–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borie R, Tabèze L, Thabut G, Nunes H, Cottin V, Marchand-Adam S, et al. (2016). Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. European Respiratory Journal, 48, 1721–1731 [DOI] [PubMed] [Google Scholar]
- Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, et al. (2015). PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. Journal of Clinical Investigation, 125, 521–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE (2016). Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest, 149, 756–766 [DOI] [PubMed] [Google Scholar]
- Cavazza A, Rossi G, Carbonelli C, Spaggiari L, Paci M, & Roggeri A (2010). The role of histology in idiopathic pulmonary fibrosis: an update. Respiratory Medicine, 104, S11–22 [DOI] [PubMed] [Google Scholar]
- Chambers DC, Enever D, Ilic N, Sparks L, Whitelaw K, Ayres J, et al. (2014). A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology, 19, 1013–1018 [DOI] [PubMed] [Google Scholar]
- Chen CZ, Peng YX, Wang ZB, Fish PV, Kaar JL, Koepsel RR, et al. (2009). The Scar-in-a-Jar: studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well. British Journal of Pharmacology, 158, 1196–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhang K, Chen H, Zhao X, Wang J, Li L, et al. (2015). Telomerase deficiency causes alveolar stem cell senescense-associated low-grade inflammation in lungs. Journal of Biological Chemistry, 290, 30813–30829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Ribeiro CMP, Sun L, Okuda K, Kato T, Gilmore RC, et al. (2019). XBP1S regulates MUC5B in a promoter variant-dependent pathway in idiopathic pulmonary fibrosis airway epithelia. American Journal of Respiratory and Critical Care Medicine, 200, 220–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M, Caliò A, Rossi A, Gilioli E, Pedica F, Montagna L, et al. (2017). Epithelial to mesenchymal transition-related proteins ZEB1, beta-catenin, and beta-tubulin III in idiopathic pulmonary fibrosis. Modern Pathology, 30, 26–38 [DOI] [PubMed] [Google Scholar]
- Cogan JD, Kropski JA, Zhao M, Mitchell DB, Rives L, Markin C, et al. (2015). Rare variants in RTEL1 are associated with familial interstitial pneumonia. American Journal of Respiratory and Critical Care Medicine, 191, 646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan MA, Shifren A, Huang HJ, Russell TD, Mitra RD, Zhang Q, et al. (2014). Sequencing of idiopathic pulmonary fibrosis-related genes reveals independent single gene associations. BMJ Open Respiratory Research, 1, e000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottin V, Hirani NA, Hotchkin DL, Nambiar AM, Ogura T, Otaola M, et al. (2018). Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. European Respiratory Review, 27, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulos A, Wright E, Vlahopoulou K, Cornic L, Schoof N, & Maher TM (2018). The Burden of Illness of Idiopathic Pulmonary Fibrosis: A Comprehensive Evidence Review. Pharmacoeconomics, 36, 779–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressen A, Abbas AR, Cabanski C, Reeder J, Ramalingam TR, Neighbors M, et al. (2018). Analysis of protein-altering variants in telomerase genes and their associations with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respiratory Medicine, 6, 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. (2013). Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nature Genetics, 45, 613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli JA, Pandya A, Vega-Olivo M, Dass C, Zhao H, & Criner GJ (2017). Pirfenidone and Nintedanib for pulmonary fibrosis in clinical practice: Tolerability and adverse drug reactions. Respirology, 22, 1171–1178 [DOI] [PubMed] [Google Scholar]
- Gazdhar A, Susuri N, Hostettler K, Gugger M, Knudsen L, Roth M, et al. (2013). HGF Expressing Stem Cells in Usual Interstitial Pneumonia Originate from the Bone Marrow and Are Antifibrotic. PLoS ONE, 8, e65453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George PM, Patterson CM, Reed AK, & Thillai M (2019). Lung transplantation for idiopathic pulmonary fibrosis. Lancet Respiratory Medicine, 7, 271–282 [DOI] [PubMed] [Google Scholar]
- Germano D, Blyszczuk P, Valaperti A, Kania G, Dirnhofer S, Landmesser U, et al. (2009). Prominin-1/CD133+ lung epithelial progenitors protect from bleomycin-induced pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 179, 939–949 [DOI] [PubMed] [Google Scholar]
- Glassberg MK, Minkiewicz J, Toonkel RL, Simonet ES, Rubio GA, DiFede D, et al. (2017). Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest, 151, 971–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann AC, Gutierrez AJ, Bui LT, Yahn SI, Winters NI, Calvi C. l., et al. (2020). Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Science Advances, 6, eaba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, et al. (2014). Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respiratory Medicine, 2, 548–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Nabeshima K, Hamasaki M, Uesugi N, Watanabe K, & Iwasaki H (2010) Epithelial-mesenchymal transition in human lungs with usual interstitial pneumonia: quantitative immunohistochemistry. Pathology International, 60, 14–21 [DOI] [PubMed] [Google Scholar]
- Herazo-Maya JD, Sun J, Molyneaux PL, Li Q, Villalba JA, Tzouvelekis A, et al. (2017). Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: an international multicentre, cohort study. Lancet Respir Med, 5, 857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Jones MG, Davies DE, & Wang Y (2019). Epithelial-mesenchymal transition contributes to pulmonary fibrosis via aberrant epithelial/fibroblastic cross-talk. Journal of Lung Health and Diseases, 3, 31–35 [PMC free article] [PubMed] [Google Scholar]
- Hirani N, Mackinnon A, Nicod L, Walker J, Ford P, Schambye H, et al. (2017). Td139, A Novel Inhaled Galectin-3 Inhibitor For The Treatment Of Idiopathic Pulmonary Fibrosis (ipf). Results From The First In (ipf) Patients Study. American Journal of Respiratory and Critical Care Medicine, 195, A7560 [Google Scholar]
- Homma S, Azuma A, Taniguchi H, Ogura T, Mochiduki Y, Sugiyama Y, et al. (2012). Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology, 17, 467–477 [DOI] [PubMed] [Google Scholar]
- Hostettler KE, Gazdhar A, Khan P, Savic S, Tamo L, Lardinois D, et al. (2017). Multipotent mesenchymal stem cells in lung fibrosis. PLoS ONE, 12, e0181946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JP, Rees S, Kalindjian SB, & Philpott KL (2011). Principles of early drug discovery. British Journal of Pharmacology, 162, 1239–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idiopathic Pulmonary Fibrosis Clinical Research Network, Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. (2012). Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. New England Journal of Medicine, 366, 1968–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SN, Gurujeyalakshmi G, Giri SN (1999). Effects of Pirfenidone on Transforming Growth Factor-β Gene Expression at the Transcriptional Level in Bleomycin Hamster Model of Lung Fibrosis. Journal of Pharmacology and Experimental Therapeutics, 291, 367–373 [PubMed] [Google Scholar]
- Jones MJ, Fabre A, Schneider P, Cinetto F, Sgalla G, Mavrogordato M, et al. (2016). Three-dimensional characterization of fibroblast foci in idiopathic pulmonary fibrosis. Journal of Clinical Investigation Insight, 1, e86375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MG, Andriotis OG, Roberts JJ, Lunn K, Tear VJ, Cao L, et al. (2018). Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis. Elife, 7, 36354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein AL, Mukhopadhyay S, & Myers JL (2008). Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases. Human Pathology, 39, 1275–1294 [DOI] [PubMed] [Google Scholar]
- Kheirollahi V, Wasnick RM, Biasin V, Vazquez-Armendariz AI, Chu X, Moiseenko A, et al. (2019). Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nature Communications, 10, 2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo JK, Montgomery AB, Otto KL, Surber M, Faggian J, Lickliter JD et al. (2020). A Randomized, Double-Blinded, Placebo-Controlled, Dose-Escalation Phase 1 Study of Aerosolized Pirfenidone Delivered via the PARI Investigational eFlow Nebulizer in Volunteers and Patients with Idiopathic Pulmonary Fibrosis. Journal of Aerosol Medicine and Pulmonary Drug Delivery, 33, 15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Diggans J, Pankratz D, Huang J, Pagan M, Sindy N, et al. (2015). Classification of usual interstitial pneumonia in patients with interstitial lung disease: assessment of a machine learning approach using high-dimensional transcriptional data. Lancet Respiratory Medicine, 3, 473–482 [DOI] [PubMed] [Google Scholar]
- Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, et al. (2015). Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell, 161, 1187–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Tata A, Konkimalla A, Katsura H, Lee RF, Ou J, et al. (2020). Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nature Cell Biology, doi: 10.1038/s41556-020-0542-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, et al. (2008). Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 178, 838–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter M, Bonella M, Wijsenbeek M, Maher TM, Spagnolo P (2015). Pharmacological treatment of idiopathic pulmonary fibrosis: current approaches, unsolved issues and future perspectives. BioMed Research International, 2015, 329481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropski JA, Mitchell DB, Markin C, Polosukhin VV, Choi L, Johnson JE, et al. (2014). A novel dyskerin (DKC1) mutation is associated with familial interstitial pneumonia. Chest, 146, e1–e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropski JA, Reiss S, Markin C, Brown K. k., Schwartz DA, Schwarz MI, et al. (2017). Rare genetic variants in PARN are associated with pulmonary fibrosis in families. American Journal of Respiratory and Critical Care Medicine, 196, 1481–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto M, Nishiwaki T, Matsuo N, Kimura H, & Matsushima K (2009). Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. European Respiratory Journal, 34, 740–748 [DOI] [PubMed] [Google Scholar]
- Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, et al. (2008). Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. American Journal of Physiology-Lung Cellular and Molecular Physiology, 294, L1119–1126 [DOI] [PubMed] [Google Scholar]
- Lawson WE, Cheng D-S, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, et al. (2011). Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proceedings of the National Academy of Sciences of the United States of America, 108, 10562–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer DJ, & Martinez FJ (2018). Idiopathic pulmonary fibrosis. New England Journal of Medicine, 378, 1811–1823 [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, et al. (2016). Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nature Medicine, 22, 1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukey PT, Harrison SA, Yang S, Man Y, Holman BF, Rashidnasab A, et al. (2019). A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. European Respiratory Journal, 53, 1801992. [DOI] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, et al. (2015). Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell, 161, 1202–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan V (2014). Clinical Trial Phases. International Journal of Clinical Medicine, 5, 1374–1383 [Google Scholar]
- Maher TM, Simpson JK, Porter JC, Wilson FJ, Chan R, Eames R, et al. (2020). A positron emission tomography imaging study to confirm target engagement in the lungs of patients with idiopathic pulmonary fibrosis following a single dose of a novel inhaled αvβ6 integrin inhibitor. Respir Res, 21, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer PF, Woodcock HV, Eley JD, Platé M, Sulikowski MG, Durrenberger PF, et al. (2016). Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax, 71, 701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, et al. (2014). The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 190, 906–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux PL, Willis-Owen SAG, Cox MJ, James P, Cowman S, Loebinger M, et al. (2017). Host-Microbial Interactions in Idiopathic Pulmonary Fibrosis. American Journal of Respiratory and Critical Care Medicine, 195, 1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, et al. (2009). Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. American Journal of Pathology, 175, 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Blumhagen RZ, Yang IV, Walts A, Powers J, Walker T, et al. (2019). Resequencing Study Confirms That Host Defense and Cell Senescence Gene Variants Contribute to the Risk of Idiopathic Pulmonary Fibrosis. American Journal of Respiratory and Critical Care Medicine, 200, 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SW (2003). An overview of drug development in the United States and current challenges. Southern Medical Journal, 96, 1244–1255 [DOI] [PubMed] [Google Scholar]
- Morse C, Tabib T, Sembrat J, Buschur KL, Bittar HT, Valenzi E, et al. (2019). Proliferating SPP1/MERKT-expressing macrophages in idiopathic pulmonary fibrosis. European Respiratory Journal, 54, 1802441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, & Beers MF (2005). A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. American Journal of Respiratory Cell and Molecular Biology, 32, 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllärniemi M, Vuorinen K, Pulkkinen V, Kankaanranta H, Aine T, Salmenkivi K, et al. (2008). Gremlin localization and expression levels partially differentiate idiopathic interstitial pneumonia severity and subtype. Journal of Pathology, 214, 456–463 [DOI] [PubMed] [Google Scholar]
- Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, & Desai TJ (2018). Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science, 359, 1118–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, et al. (2016). Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. Journal of Clinical Investigation, 1, e86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan SD, Albera C, Bradford WZ, Costabel U, Glaspole I, Glassberg MK, et al. (2017). Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respiratory Medicine, 5, 33–41 [DOI] [PubMed] [Google Scholar]
- Navaratnam V, Fleming KM, West J, Smith JJ, Jenkins RG, Fogarty A et al. (2011). The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax, 66, 462–467 [DOI] [PubMed] [Google Scholar]
- Newton CA, Batra K, Torrealba J, Kozlitina J, Glazer CS, Aravena C, et al. (2016). Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. European Respiratory Journal, 48, 1710–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton CA, Oldham JM, Ley B, Anand V, Adegunsoye A, Liu G, et al. (2019). Telomere length and genetic variant associations with interstitial lung disease progression and survival. European Respiratory Journal, 48, 53, 1801641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. (2011). Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet, 77, 1760–1769 [DOI] [PubMed] [Google Scholar]
- Noth I, Zhang Y, Ma SF, Flores C, Barber M, Yuang H, et al. (2013). Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respiratory Medicine, 1, 309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntolios P, Manoloudi E, Tzouvelekis A, Bouros E, Steiropoulos P, Anevlavis A, et al. (2018). Longitudinal outcomes of patients enrolled in a phase Ib clinical trial of the adipose-derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Clinical Respiratory Journal, 12, 2084–2089 [DOI] [PubMed] [Google Scholar]
- Nureki SI, Tomer Y, Venosa A, Katzen J, Russo SJ, Jamil S, et al. (2018). Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. Journal of Clinical Investigation, 128, 4008–4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Falkowski NR, et al. (2019). Lung Microbiota Contribute to Pulmonary Inflammation and Disease Progression in Pulmonary Fibrosis. American Journal of Respiratory and Critical Care Medicine, 199, 1127–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham JM, Ma SF, Martinez FJ, Anstrom KJ, Raghu G, Schwartz DA, et al. (2015). TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine,192, 1475–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Tanaka T, Ishida M, Kinoshita A, Fukuoka J, Takaki M, et al. (2011). Surfactant protein C G100S mutation causes familial pulmonary fibrosis in Japanese kindred. European Respiratory Journal, 38, 861–869 [DOI] [PubMed] [Google Scholar]
- Organ SL, & Tsao M-S. (2011). An overview of the c-MET signaling pathway. Therapeutic Advances in Medical Oncology, 3, S7–S19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. (2003). Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America, 100, 8407–8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, et al. (2014). Fibrotic extracellular matrix activates a profibrotic positive feedback loop. Journal of Clinical Investigation, 124, 1622–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, et al. (2013). Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. Journal of the American Medical Association, 309, 2232–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovski S, Todd J. l., Durheim MT, Wang Q, Chien JW, Kelly FL, et al. (2017). An exome sequencing study to assess the role of rare genetic variation in pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 196, 82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. (2015). An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. American Journal of Respiratory and Critical Care Medicine, 192, e3–19. Erratum in: American Journal of Respiratory and Critical Care Medicine, 192, 644 [DOI] [PubMed] [Google Scholar]
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. (2018). Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. American Journal of Respiratory and Critical Care Medicine, 198, e44–e68 [DOI] [PubMed] [Google Scholar]
- Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, et al. (2018). Metformin reverses established lung fibrosis in a bleomycin model. Nature Medicine, 24, 1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, et al. (2019). Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 199, 1517–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L, Collard HR, & Jones MG (2017). Idiopathic pulmonary fibrosis. Lancet, 389, 1941–1952 [DOI] [PubMed] [Google Scholar]
- Rossi G, & Spagnolo P (2017). Biopsy in idiopathic pulmonary fibrosis: back to the future. Expert Review in Respiratory Medicine, 11, 679–684 [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Muramatsu Y, Satoh K, Ishida F, Kikuchi N, Sano G, et al. (2015). Effectiveness of combined therapy with pirfenidone and inhaled N-acetylcysteine for advanced idiopathic pulmonary fibrosis: a case-control study. Respirology, 20, 445–452 [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Kataoka K, Kondo Y, Kato M, Okamoto M, Mukae H, et al. (2020). Pirfenidone plus inhaled N-acetylcysteine for idiopathic pulmonary fibrosis: a randomised trial. European Respiratory Journal, 2020 Jul 23;2000348. doi: 10.1183/13993003.00348-2020 [DOI] [PubMed] [Google Scholar]
- Schwartz DA (2018). Idiopathic Pulmonary Fibrosis Is a Genetic Disease Involving Mucus and the Peripheral Airways. Annals of the American Thoracic Society, 15, S192–S197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. (2011). A common MUC5B promoter polymorphism and pulmonary fibrosis. New England Journal of Medicine, 364, 1503–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessler DI, & Myles PS (2020). Novel Clinical Trial Designs to Improve the Efficiency of Research. Anesthesiology, 132, 69–81 [DOI] [PubMed] [Google Scholar]
- Spagnolo P, Maher TM, Richeldi L (2015). Idiopathic pulmonary fibrosis: Recent advances on pharmacological therapy. Pharmacology and Therapeutics, 152, 18–27 [DOI] [PubMed] [Google Scholar]
- Spagnolo P, & Cottin V (2017). Genetics of idiopathic pulmonary fibrosis: from mechanistic pathways to personalised medicine. Journal of Medical Genetics, 54, 93–99 [DOI] [PubMed] [Google Scholar]
- Spagnolo P, Tzouvelekis A, & Bonella F (2018). The Management of Patients With Idiopathic Pulmonary Fibrosis. Frontiers in Medicine (Lausanne), 5, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart DB, Lee JS, Kozlitina J, Noth I, Devine MS, Glazer CS, et al. (2014). Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respiratory Medicine, 2, 557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart BD, Choi J, Zaidi S, Xing C, Holohan B, Chen R, et al. (2015). Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nature Genetics, 47, 512–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. (2010). Pirfenidone in idiopathic pulmonary fibrosis. European Respiratory Journal, 35, 21–29 [DOI] [PubMed] [Google Scholar]
- Thomas AQ, Lane K 3rd Phillips J, Prince M, Markin C, Speer M, et al. (2002). Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. American Journal of Respiratory and Critical Care Medicine, 165, 1322–1328 [DOI] [PubMed] [Google Scholar]
- Toonkel RL, Hare JM, Matthay MA, & Glassberg MK (2013). Mesenchymal stem cells and idiopathic pulmonary fibrosis. Potential for clinical testing. American Journal of Respiratory and Critical Care Medicine, 188, 133–140 [DOI] [PubMed] [Google Scholar]
- Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. (2007). Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proceedings of the National Academy of Sciences of the United States of America, 104, 7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, Oikonomou A, et al. (2013). A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Journal of Translational Medicine, 11, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouvelekis A, Toonkel R, Karampitsakos T, Medapalli K, Ninou I, Aidinis V, et al. (2018). Mesenchymal Stem Cells for the Treatment of Idiopathic Pulmonary Fibrosis. Front Med (Lausanne), 5, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouvelekis A, Gomatou G, Bouros E, Trigidou R, Tzilas V, & Bouros D (2019). Common pathogenic mechanisms between idiopathic pulmonary fibrosis and lung cancer. Chest, 156, 383–391 [DOI] [PubMed] [Google Scholar]
- Usmani OS, Biddiscombe MF, Yang S, Meah S, Oballa E, Simpson JK, et al. (2018). The topical study of inhaled drug (salbutamol) delivery in idiopathic pulmonary fibrosis. Respir Res, 19, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Moorsel CHM, van Oosterhout MFM, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, et al. (2010). Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a Dutch cohort. American Journal of Respiratory and Critical Care Medicine, 182, 1419–1425 [DOI] [PubMed] [Google Scholar]
- Verleden SE, Tanabe N, McDonough JE, Vasilescu DM, Xu F, Wuyts WA, et al. (2020). Small airways pathology in idiopathic pulmonary fibrosis: a retrospective cohort study. Lancet Respiratory Medicine, 8, 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, et al. (2009). Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. American Journal of Human Genetics, 84, 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DC, Alva-Ornelas JA, Sucre JM, Vijayaraj P, Durra A, Richardson W, et al. (2017). Development of a Three-Dimensional Bioengineering Technology to Generate Lung Tissue for Personalized Disease Modeling. Stem Cells Translational Medicine, 6, 622–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. (2015). Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. European Respiratory Journal, 45, 1434–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters PJ, Collard HR, & Jones KD (2014). Pathogenesis of idiopathic pulmonary fibrosis. Annual Review of Pathology, 9, 157–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock HV, Eley JD, Guillotin D, Platé M, Nanthakumar CB, Martufi M, et al. (2019). The mTORC1/4E-BP1 axis represents a critical signaling node during fibrogenesis. Nature Communications, 10, 6. Author correction in Nature Communications 2020, 11, 4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Yu Y, Huang H, Hu Y, Fu S, Wang Z, et al. (2020). Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell, 180, 107–121 [DOI] [PubMed] [Google Scholar]
- Wuyts WA, Cavazza A, Rossi G, Bonella F, Sverzellati N, & Spagnolo P (2014). Differential diagnosis of usual interstitial pneumonia: when is it truly idiopathic? European Respiratory Review, 23, 308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, et al. (2016). Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. Journal of Clinical Investigation Insight, 1, e90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Guan X, Carraro G, Parimon T, Liu X, Huang G, et al. (2020). Senescence of alveolar stem cells drives progressive pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, September 29. doi: 10.1164/rccm.202004-1274OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, et al. (2018). Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nature Medicine, 24, 39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, et al. (2018) Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature, 555, 251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Noth I, Garcia JGN, & Kaminski N (2011). A common MUC5B promoter polymorphism and pulmonary fibrosis. New England Journal of Medicine, 364, 1576–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Wang L, Luo X, Zhang Y, Ding Q, Jiang X, et al. (2012). Tollip, an intracellular trafficking protein, is a novel modulator of the transforming growth factor-β signaling pathway. Journal of Biological Chemistry, 287, 39653–39663 [DOI] [PMC free article] [PubMed] [Google Scholar]


