Abstract
Background
Soluble suppression of tumorigenesis-2 (sST2) was reported to be associated with cognitive performance and risk of incident stroke. However, the impact of sST2 on cognitive function after ischemic stroke is unclear. We aimed to assess the association of sST2 and cognitive impairment at 3 months in acute ischemic stroke patients.
Methods
Baseline plasma sST2 levels were measured in 619 ischemic stroke patients (mean age: 60.0 ± 10.5 years) from 7 participating hospitals of the China Antihypertensive Trial in Acute Ischemic Stroke. Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) were used to assess cognitive status. Cognitive impairment was defined as a MoCA score < 23 or MMSE score < 27. The association between sST2 and cognitive impairment was evaluated by logistic regression analysis.
Results
325 (52.5%) or 323 (52.2%) participants developed cognitive impairment according to MoCA or MMSE. After adjustment for age, sex, education, and other covariates, the odds ratio for the highest vs lowest quartile of sST2 was 2.38 (95% CI, 1.42–4.00) and 1.82 (95% CI 1.09–3.03) risk of cognitive impairment defined by MoCA and MMSE score, respectively. Incorporation sST2 into a model containing conventional risk factors significantly improved reclassification.
Conclusions
Elevated plasma sST2 levels were significantly associated with post-stroke cognitive impairment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-021-02288-6.
Keywords: Ischemic stroke, sST2, Cognitive impairment, Montreal cognitive assessment, Mini-mental state examination
Background
Stroke is a major cause of serious disability and death [1], as well as the common cause of acquired cognitive impairment [2]. Post-stroke cognitive impairment (PSCI) has been reported to be associated with unfavorable prognosis, including major disability, mortality, stroke recurrence and poorer quality of life [3–5]. Thus, novel and reliable predictors are clearly needed for early identification of patients at higher risk of PSCI.
Heart-brain axis has a greater role in the procession of cognitive impairment and dementia [6–8], available population-based evidence reported suboptimal cardiac function or abnormally elevated cardiac biomarkers, such as N-terminal pro-B-type natriuretic peptide (NT-proBNP) or high-sensitivity cardiac Troponin T (hs-cTnT), were associated with worse cognitive performance [9–13], suggesting cardiac biomarkers may be used to identify individuals at higher risk of cognitive impairment. However, whether the predictive roles of cardiac biomarkers persist in the setting of cerebrovascular disease was less consistent [7]. Soluble suppression of tumorigenesis-2 (sST2), another cardiac stress biomarker of promoting cardiomyocyte hypertrophy and fibrosis, is considered to be an important biomarker of heart failure. Recently, the Framingham Offspring showed that higher sST2 levels were associated with increased risk of incident stroke and subclinical vascular brain injury [14]. However, the association of sST2 and cognitive impairment in patients with ischemic stroke remains to be addressed.
Therefore, we aimed to prospectively assess the relationship between plasma sST2 levels in the acute phase of ischemic stroke and PSCI at 3 months using the data derived from the China Antihypertensive Trial in Acute Ischemic Stroke (CATIS).
Materials and methods
Study design and population
The CATIS trial, a single-blind, blinded end-points randomized clinical trial, was designed to evaluate whether immediate blood pressure (BP) reduction would reduce death and major disability at 14 days or hospital discharge. The study design and main results had been described previously [15]. The enrollment criteria were as follows: (1) first-ever ischemic stroke; (2) age ≥ 22 years; (3) time from onset to admission within 48 h; (4) systolic BP between 140 and 220 mmHg. The exclusion criteria were as follows: (1) BP ≥220/120 mmHg; (2) treated with intravenous thrombolytic therapy; (3) severe heart failure, acute myocardial infarction or unstable angina, atrial fibrillation, aortic dissection, cerebrovascular stenosis, resistant hypertension, or in a deep coma. Finally, 4071 patients with first-ever ischemic stroke within 48 h of onset and elevated systolic BP were recruited. The present prospective observational study was a pre-planned ancillary study of CATIS trial, which was designed to test cognitive function at 3 months. In the ancillary study, acute ischemic stroke patients from 7 randomly selected participating hospitals were consecutively recruited for neurological evaluations. Each of the 7 participating hospitals planned to recruit 80–100 patients. During the period of August 2009 to November 2012, 660 participants were enrolled. After further excluded participants without available cognitive evaluations (n = 22) or sST2 data (n = 19), 619 participants were finally included in the present analysis (Supplementary Figure 1).
This study protocol was approved by the ethical committee at Soochow University in China and Tulane University in the United States, as well as ethical committees at the 7 participating hospitals, in compliance with the Declaration of Helsinki. All participants provided written informed consent.
Data collection
Fasting blood samples were drawn within 24 h of patients’ hospital admission, and were frozen − 80 °C until testing. The concentrations of plasma sST2 and serum high-sensitive C-reactive protein (hsCRP) were measured by commercially available immunoassays (R&D Systems, Minneapolis, MN). The intra-assay and inter-assay coefficients of variation for sST2 were below 5.5 and 2.4%. Similarly, the intra-assay and inter-assay coefficients of variation for hsCRP were below 8.4 and 1.6%. Laboratory technicians who performed measurements were blinded to clinical features and outcomes of patients.
Baseline data regarding demographic characteristics, clinical features, medical history, and prior use of medications were collected using a standard questionnaire at the time of enrollment. The National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale (mRS) were applied to assess stroke severity by trained neurologists. Trial of Org 10,172 in Acute Stroke Treatment criteria was used to classify the ischemic stroke subtypes as large-artery atherosclerosis(thrombotic), cardiac embolism (embolic) and small-vessel occlusion (lacunar), according to the symptoms and imaging data of the patients by experienced neurologists [16]. BP measurements were performed by trained nurses according to a standard protocol adapted from procedures recommended by the American Heart Association.
Outcome assessment
Cognitive function at 3 months after stroke was evaluated by trained neurologists using the Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE). Prior studies found that people whose education ≤12 years tended to perform worse on the MoCA test [17]. Thus, 1 point was additionally added for participants with education ≤12 years on their crude MoCA score (if < 30) to correct for education effects. Lower scores indicate worse cognitive function, and PSCI was defined as a MoCA score of less than 23 [18, 19] or MMSE score of less than 27 [20].
Statistical analysis
Baseline characteristics were summarized according to the quartiles of plasma sST2 levels. Means with standard deviation(SD) or median with interquartile range were used for continuous variables, and the generalized linear regression models were further used to test for trend across the sST2 quartiles. Frequency with percentage was used for categorical variables, and the Cochran-Armitage trend χ2 tests were used to test for trend.
First, we performed generalized linear regression models to test the association between plasma sST2 levels and continuous MoCA and MMSE score. Second, both MoCA and MMSE score were categorized into two groups (without versus with PSCI). Logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the association of plasma sST2 levels with the incident of PSCI. According to baseline characteristics, previous studies and professional knowledge, three models were used with an increasing level of adjustment. Model 1 was adjusted for age, sex and education level (illiteracy, primary, high school and college or higher). Model 2 further adjusted for current smoking, alcohol drinking, time from onset to randomization, systolic BP, baseline NIHSS and mRS score, medical history (hypertension, diabetes mellitus, hyperlipidemia, and coronary heart disease), use of antihypertensive medications, randomized treatment, ischemic stroke subtype (thrombotic, embolic and lacunar), anticoagulant treatment and hypoglycemic treatment. Available evidence suggested that elevated sST2 levels were involved in pro-inflammatory response [21, 22], and inflammation may be an intermediary in the relation of sST2 levels to cognitive impairment. In consideration of adjustment for a risk factor that is in the causal pathway from the exposure to outcome will reduce or even remove the effect of interest, we further conducted a separate analysis with hsCRP included in a multivariable-adjusted model based on the model 2. We tested for linear trends across the median of sST2 quartiles.
Moreover, Hosmer Lemeshow χ2 statistic was used to assess the calibration of the model with or without sST2. C statistic, net reclassification index (NRI) and integrated discrimination improvement (IDI) were applied to evaluate the predictive ability of plasma sST2 beyond conventional risk factors model, which including age, sex, education level, systolic BP, baseline NIHSS, baseline mRS, hypertension, diabetes mellitus, ischemic stroke subtype, hsCRP, anticoagulant treatment and hypoglycemic treatment. In addition, random forest regression model, one of the most robust ensemble machine learning methods for classification and regression, was used to assess the importance of potential predictors. “Mean Decrease Accuracy” is a type of importance measure and the value is larger suggests that the predictor is more important. Multiple imputation for missing data was performed using the Markov chain Monten Carlo method (Supplementary Table 1). All two-sided P values < 0.05 were considered to be statistically significant. Statistical analysis was conducted using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics
A total of 619 patients (434 men and 185 women) with a mean (SD) age of 60.0 (10.5) years were enrolled in the present study. The median of plasma sST2 level was 163.51 pg/mL (interquartile range, 117.60–238.77 pg/mL). The baseline characteristics across the quartiles of sST2 levels were summarized in Table 1. Compared to the patients in the lowest quartile of sST2 levels, those in the higher quartile were tended to be male and older, to have higher baseline NIHSS and mRS score, to have higher level of hsCRP, and to have shorter time from onset to randomization and lower proportion of antihypertensive medications use.
Table 1.
Baseline Characteristics of Participants According to Plasma soluble ST2 (sST2) quartiles
| Characteristicsa | sST2, pg/mL | p trend | |||
|---|---|---|---|---|---|
| < 117.60 | 117.60–163.51 | 163.51–238.77 | ≥238.77 | ||
| No. of subjects | 155 (25.0) | 154 (24.9) | 156 (25.2) | 154 (24.9) | |
| Demographic features | |||||
| Age, y | 58.5 ± 10.3 | 59.9 ± 9.6 | 59.2 ± 10.3 | 62.5 ± 11.2 | 0.003 |
| Male sex, n (%) | 88 (56.8) | 102 (66.2) | 121 (77.6) | 123 (79.9) | < 0.001 |
| Education, n (%) | |||||
| Illiteracy | 8 (5.2) | 15 (9.7) | 10 (6.4) | 18 (11.7) | 0.10 |
| Primary | 61 (39.4) | 64 (41.6) | 53 (34.0) | 56 (36.4) | 0.34 |
| High school | 75 (48.4) | 68 (44.2) | 80 (51.3) | 72 (46.8) | 0.90 |
| College or higher | 11 (7.1) | 7 (4.6) | 13 (8.3) | 8 (5.2) | 0.83 |
| Current cigarette smoking, n (%) | 55 (35.5) | 56 (36.4) | 61 (36.5) | 53 (34.4) | 0.67 |
| Current alcohol drinking, n (%) | 51 (32.9) | 52 (33.8) | 57 (36.4) | 53 (34.9) | 0.63 |
| Clinical features | |||||
| Time from onset to randomization, h | 12.0 (5.0–24.0) | 12.0 (5.0–24.0) | 12.0 (6.0–24.0) | 6.6 (4.0–24.0) | 0.02 |
| Baseline systolic BP, mm Hg | 168.3 ± 17.5 | 166.0 ± 15.2 | 166.6 ± 16.6 | 169.0 ± 17.2 | 0.64 |
| Baseline diastolic BP, mm Hg | 98.5 ± 9.6 | 97.8 ± 9.7 | 98.2 ± 9.4 | 98.6 ± 11.4 | 0.85 |
| Baseline NIHSS score | 4.0 (3.0–7.0) | 4.0 (2.0–6.0) | 4.0 (2.0–7.0) | 6.0 (3.0–9.0) | < 0.001 |
| Baseline modified Rankin Scale score | 3.0 (2.0–3.0) | 3.0 (2.0–4.0) | 3.0 (1.0–3.0) | 3.0 (2.0–4.0) | < 0.001 |
| High-sensitive C-reactive protein, mg/L | 1.3 (0.6–3.7) | 1.8 (0.7–4.9) | 2.1 (0.9–4.5) | 3.8 (1.4–10.0) | < 0.001 |
| Medical history, n (%) | |||||
| Hypertension | 122 (78.7) | 120 (77.9) | 123 (78.9) | 111 (72.1) | 0.21 |
| Hyperlipidemia | 14 (9.0) | 10 (6.5) | 8 (5.1) | 10 (6.5) | 0.32 |
| Diabetes mellitus | 30 (19.4) | 23 (15.0) | 30 (19.2) | 21 (13.6) | 0.34 |
| Coronary heart disease | 12 (7.7) | 20 (13.0) | 16 (10.3) | 18 (11.7) | 0.41 |
| Family history of stroke | 33 (21.3) | 22 (14.3) | 22 (14.1) | 24 (15.6) | 0.19 |
| Use of antihypertensive drugs | 75 (48.4) | 70 (45.5) | 67 (43.0) | 58 (37.7) | 0.05 |
| Ischemic stroke subtype, n (%) | |||||
| Thrombotic | 101 (65.2) | 101 (65.6) | 105 (67.3) | 95 (61.7) | 0.61 |
| Embolic | 4 (2.6) | 5 (3.3) | 5 (3.2) | 9 (5.8) | 0.15 |
| Lacunar | 50 (32.3) | 48 (31.2) | 46 (29.5) | 50 (32.5) | 0.95 |
| Receiving immediate BP reduction | 67 (43.2) | 81 (47.4) | 83 (53.2) | 72 (46.8) | 0.53 |
| Anticoagulant treatment | 35 (22.6) | 32 (20.8) | 36 (23.1) | 48 (31.2) | 0.07 |
| Hypoglycemic treatment | 23 (14.8) | 28 (18.2) | 32 (20.5) | 22 (14.3) | 0.96 |
Abbreviations: BP blood pressure, NIHSS National Institute of Health Stroke Scale
aContinuous variables are expressed as mean ± standard deviation or median (interquartile range). Categorical variables are expressed as frequency (%)
Association between plasma sST2 and PSCI
At 3-month follow-up, the median (interquartile range) score of MoCA and MMSE were 22.0 (18.0–26.0) and 26.0 (22.0–29.0), respectively. There were significantly decreasing trends in MoCA and MMSE scores as sST2 increased from the lowest quartile to the highest quartile (Fig. 1). 325 (52.5%) or 323 (52.2%) participants developed PSCI according to MoCA or MMSE, respectively. The incidences of PSCI increased from the lowest quartile (sST2 < 117.60 pg/mL) to the highest quartile (sST2 ≥ 238.77 pg/mL) (Table 2).
Fig. 1.
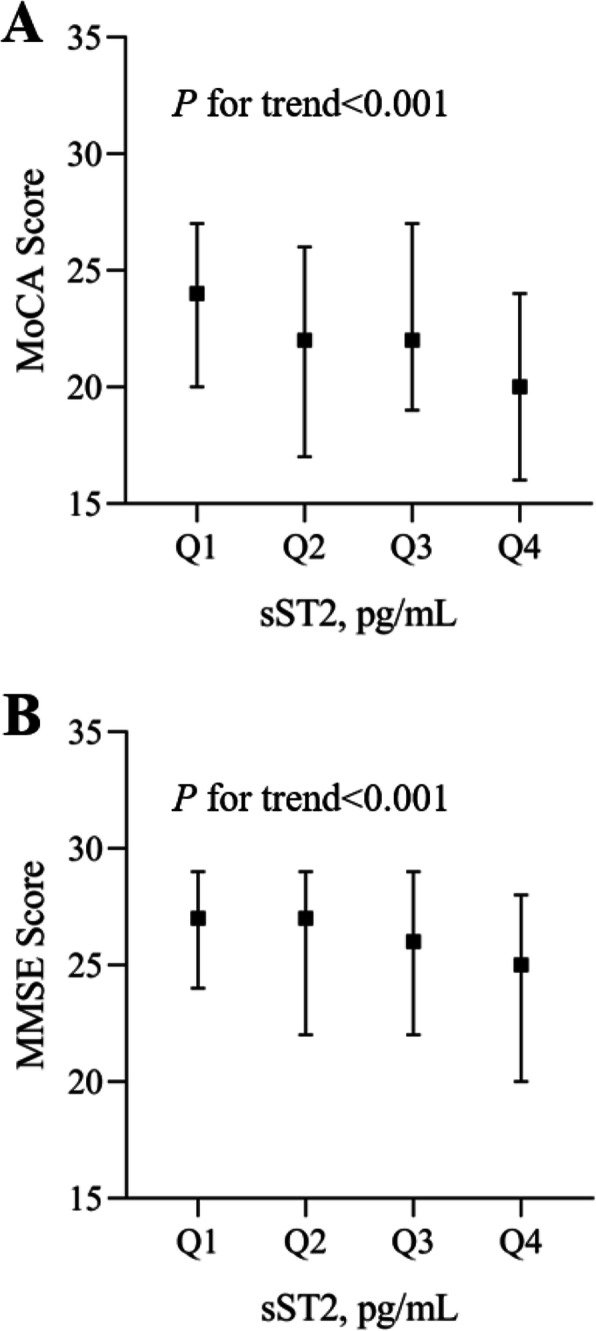
MoCA and MMSE score in acute ischemic stroke patients by sST2 quartiles. Panel a MoCA score; Panel b MMSE score. MoCA: Montreal Cognitive Assessment; MMSE: Mini-Mental State Examination; sST2: soluble suppression of tumorigenicity 2
Table 2.
ORs and 95% CIs for the Risk of post-stroke cognitive impairment According to sST2 quartiles
| sST2, pg/mL | p value for trend | ||||
|---|---|---|---|---|---|
| < 117.60 | 117.60–163.51 | 163.51–238.77 | ≥238.77 | ||
| Median | 89.75 | 139.13 | 196.13 | 349.14 | |
| Cognitive impairment: MoCA | |||||
| Cases, n (%) | 63 (40.7) | 79 (51.3) | 81 (51.9) | 102 (66.2) | < 0.001 |
| Model 1 | 1.00 | 1.48 (0.93–2.35) | 1.56 (0.98–2.48) | 2.53 (1.55–4.12) | < 0.001 |
| Model 2 | 1.00 | 1.57 (0.96–2.55) | 1.64 (1.01–2.68) | 2.35 (1.40–3.93) | 0.002 |
| Model 3 | 1.00 | 1.58 (0.97–2.56) | 1.65 (1.01–2.70) | 2.38 (1.42–4.00) | 0.002 |
| Cognitive impairment: MMSE | |||||
| Cases, n (%) | 69 (44.5) | 73 (47.4) | 81 (51.9) | 100 (64.9) | < 0.001 |
| Model 1 | 1.00 | 1.04 (0.66–1.66) | 1.28 (0.80–2.04) | 1.93 (1.19–3.14) | 0.003 |
| Model 2 | 1.00 | 1.09 (0.67–1.76) | 1.31 (0.81–2.12) | 1.76 (1.06–2.92) | 0.021 |
| Model 3 | 1.00 | 1.10 (0.68–1.78) | 1.33 (0.82–2.16) | 1.82 (1.09–3.03) | 0.016 |
MoCA score of < 23 or MMSE score of < 27 indicates cognitive impairment
Model 1: adjusted for age, sex, and education level;
Model 2: adjusted for model 1 and further adjusted for current smoking, alcohol drinking, time from onset to randomization, systolic blood pressure, baseline National Institutes of Health Stroke Scale score, baseline modified Rankin Scale score, medical history (hypertension, diabetes mellitus, hyperlipidemia, and coronary heart disease), use of antihypertensive medications, randomized treatment, ischemic stroke subtype, anticoagulant treatment and hypoglycemic treatment
Model 3: adjusted for model 2 and further adjusted for hsCRP
Abbreviations: sST2 soluble suppression of tumorigenicity 2, MoCA Montreal Cognitive Assessment, MMSE Mini-Mental State Examination, OR odds ratio
After adjustment for age, sex, education level, current smoking, alcohol drinking, time from onset to randomization, systolic BP, baseline NIHSS and mRS score, medical history (hypertension, diabetes mellitus, hyperlipidemia, and coronary heart disease), use of antihypertensive medications, randomized treatment, ischemic stroke subtype, anticoagulant treatment and hypoglycemic treatment (model 2), the odds of developing PSCI (defined by MoCA score) increased significantly with elevated baseline sST2 levels (p for trend = 0.002). This significant association remained when further adjustment for hsCRP levels (model 3). Compared to patients in the lowest quartile of sST2, those in the highest quartile had 2.38-fold (95% CI 1.42–4.00) risk of PSCI. Similar independent association was also observed when PSCI was defined according to MMSE score, the corresponding adjusted OR (95% CI) of PSCI was 1.82 (1.09–3.03) for patients in the highest quartile of sST2 in comparison with those in the lowest quartile of sST2 (Table 2).
Incremental predictive value of plasma sST2
The Hosmer Lemeshow test showed that model calibration was adequate after adding plasma sST2 to the basic model containing conventional risk factors (MoCA p = 0.20; MMSE p = 0.74). Incorporation sST2 into the basic model also significantly improved the risk reclassification performance (continuous NRI 18.5%; IDI 1.6%; both p < 0.01) when PSCI was defined by MoCA score (Table 3). Similar incremental predictive value of sST2 was observed when PSCI was evaluated by MMSE score.
Table 3.
Reclassification Statistics (95% CI) for post-stroke cognitive impairment by plasma sST2 Among Participants
| C statistic | Calibration statistic | NRI (Continuous) | IDI | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | p value | χ2 | p value | Estimate (95% CI), % | p value | Estimate (95% CI), % | p value | |
| Cognitive impairment: MoCA | ||||||||
| Conventional model | 0.678 (0.640 to 0.715) | 6.12 | 0.63 | Reference | Reference | |||
| Conventional model + sST2 (quartiles) | 0.691 (0.653 to 0.727) | 0.07 | 11.10 | 0.20 | 18.5 (3.0 to 33.9) | < 0.01 | 1.6 (0.6 to 2.6) | < 0.01 |
| Cognitive impairment: MMSE | ||||||||
| Conventional model | 0.672 (0.633 to 0.708) | 1.42 | 0.99 | Reference | Reference | |||
| Conventional model + sST2 (quartiles) | 0.683 (0.644 to 0.719) | 0.11 | 5.19 | 0.74 | 17.8 (2.1 to 33.4) | < 0.01 | 1.0 (0.2 to 1.8) | < 0.01 |
MoCA score of < 23 or MMSE score of < 27 indicates cognitive impairment
Conventional model included age, sex, education level, systolic blood pressure, baseline National Institutes of Health Stroke Scale score, baseline modified Rankin Scale score, hypertension, diabetes mellitus, ischemic stroke subtype, hsCRP, anticoagulant treatment and hypoglycemic treatment
Abbreviations: sST2 soluble suppression of tumorigenicity 2, CI confidence interval, IDI integrated discrimination index, MoCA Montreal Cognitive Assessment, NRI net reclassification improvement
Additionally, to test the contribution of potential predictors in relation to PSCI, mean decrease in accuracy was calculated. Moreover, we found that sST2 was superior to NT-proBNP in predicting PSCI, according to the receiver operating characteristic curves (Supplementary Figure 2). The random forest regression models suggested that baseline plasma sST2 was one of the promising predictors for PSCI (Fig. 2).
Fig. 2.
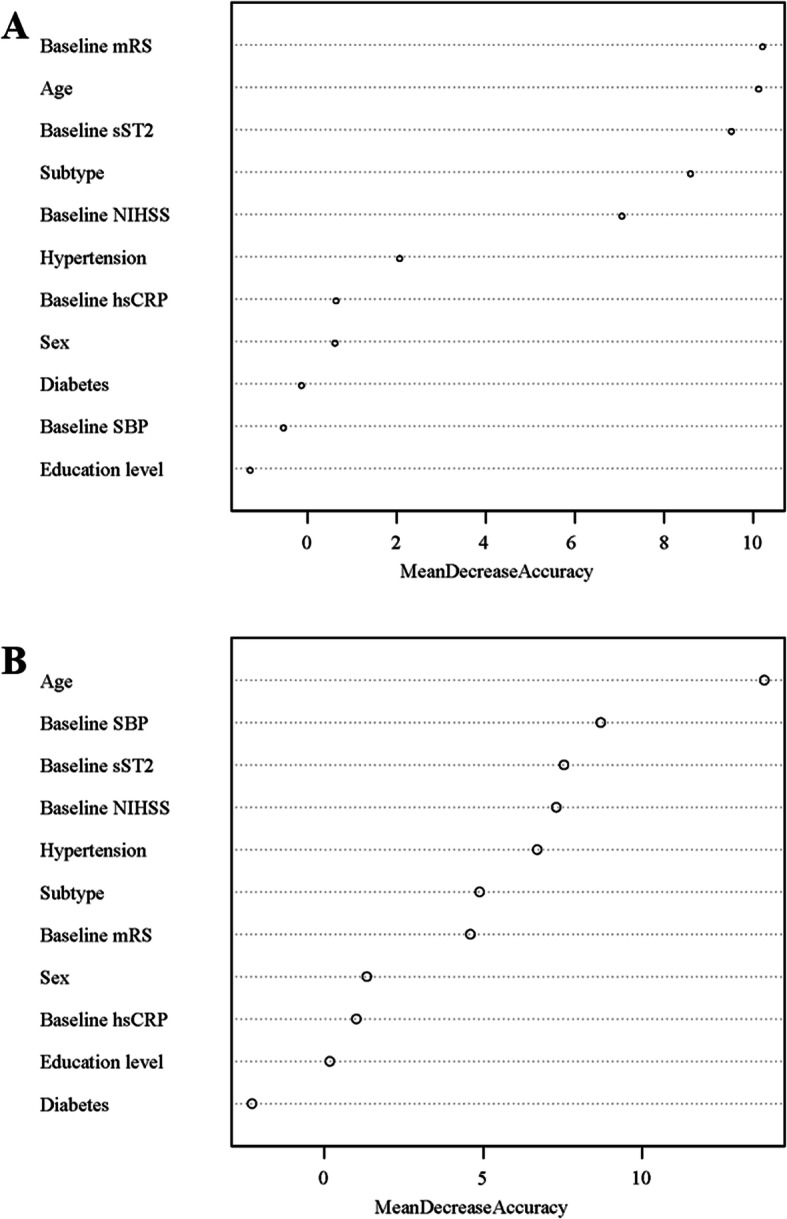
Potential predictors importance analyses for Post-stroke cognitive impairment. Panel a Montreal Cognitive Assessment (MoCA) score < 23; Panel b Mini-Mental State Examination (MMSE) score < 27. mRS: modified Rankin Scale score; sST2: soluble suppression of tumorigenicity 2; NIHSS: National Institutes of Health Stroke Scale score; hsCRP: high-sensitive C-reactive protein; SBP: systolic blood pressure. Mean Decrease Accuracy: a type of importance measure and the value is larger suggests that the predictor is more important
Discussion
This prospective study using data from CATIS trial found that higher plasma sST2 levels were associated with increased risk of PSCI, independently of potential confounders including education, stroke severity and medical history. The significant association remained when further controlled inflammatory biomarker. In addition, adding sST2 levels into the model containing conventional risk factors statistically improved the predictive ability, as evidenced by NRI and IDI statistic. Furthermore, sST2 was one of the promising predictors for PSCI. These findings provided population-based evidence of plasma sST2 as a potential biomarker in predicting PSCI.
Emerging evidence from epidemiological studies support that sST2 is of diagnostic and prognostic value in the setting with various cardiovascular diseases, including heart failure, coronary artery disease, and ischemic stroke [14, 23–26]. For example, the Linz Stroke Unit Study conducted in acute ischemic stroke patients reported a higher level of sST2 in decedents than survivors [24]. Furthermore, Wolcott et al. demonstrated that sST2 was an independent predictor of short-term mortality, functional outcome and hemorrhagic transformation in patients with ischemic stroke [25]. Of note, patients with cardiovascular diseases are at higher risk of experiencing cognitive decline. However, clinical studies designed to specifically investigate the association between sST2 and cognitive impairment, especially in the condition of ischemic stroke, are sparse.
A small study of 18 mild cognitive impairment patients and 17 healthy controls showed that serum sST2 levels were significantly higher in patients with mild cognitive impairment than controls [27]. Furthermore, Andersson et al. using the data from the Framingham Offspring Study reported a cross-sectional association between sST2 concentrations and cognitive impairment, and they found participants in the highest quartile of sST2 had significantly lower brain volumes and poorer delayed performance on the visual reproduction test than those in the lowest quartile [14]. Similarly, previous studies also suggested significant associations between other cardiac biomarkers with neurological disorders. For example, a cross-sectional analysis of 860 ischemic stroke patients suggested that hs-cTnT was associated with the severity of white matter lesions, which was considered as a predictor of poorer cognitive function [28, 29]. Recently, the PROSCIS-B (Prospective Cohort With Incident Stroke Berlin) study reported that higher hs-cTnT was associated with higher prevalence of cognitive impairment at baseline and lower Telephone Interview for Cognitive Status-modified during 3-year follow-up in patients with mild-to-moderate ischemic stroke [20]. The present study, to our knowledge, was the first longitudinal study to directly characterize the relationship of sST2 and PSCI, extending the connection of heart and brain to the patients with ischemic stroke.
The mechanisms underlying the sST2-PSCI association are still unclear, but several potential pathophysiological pathways have been proposed. Cardiac dysfunction was implicated in various pathological conditions, including hemodynamic stress, cerebral hypoperfusion, neuroinflammation, cardiac arrhythmias, and hypercoagulation, and then may further lead to cognitive impairment [6, 7]. Moreover, prior studies showed that cerebral small vessel disease (CSVD) and brain atrophy had relationship with cognitive dysfunction [30, 31]. As a serum cardiac marker, sST2 could indicate the load of the CVSD [7] and elevated sST2 was associated with lower brain volumes [14], which might affect cognitive function. Furthermore, interleukin 33 was found to be neuroprotective in experimental stroke models [32], and the administration of interleukin 33 could reduce cognitive decline [27]. Inflammation might be the potential mechanisms. CRP, a typical inflammatory marker, can induce other proinflammatory factors and was associated with an increased risk of stroke [33, 34]. However, after additionally adjusting hsCRP in the Model 3, the significant relationships remained. Further studies are required to clarify related mechanism.
Several lines of evidence suggested that age, sex, education attainment, admission BP, stroke severity, medical history, ischemic stroke subtype, and inflammation were associated with cognitive status in the general population or participants with cardiovascular disease [35–39]. In the present study, the sST2-PSCI relationship remained after adjustment for these established risk factors, indicating sST2 independently contributed to the risk of PSCI. This might relate to plasma sST2 reflecting heart and brain injury, and its specificity for cardiac function might distinguish plasma sST2 from other blood-based biomarkers with predictive value for PSCI reflecting other biological processes, such as tHcy, RF, MMP-9 and TIMP-1 [40–42].
In addition, incorporating sST2 into a model with known risk factors statistically improved reclassification for PSCI prediction. Moreover, sST2 was one of the promising predictors for PSCI. Therefore, the evaluation of the association between sST2 and PSCI had important clinical significance given the high prevalence and heavy disease burden of PSCI. These findings, coupled with the evidence that sST2 was of prognosis value in ischemic stroke patients, imply the clinical usefulness of sST2 measurement to identify patients at high risk of PSCI and provide novel therapeutic interventions. Future well-designed clinical trials aimed to test the effect of inhibition of sST2 treatment on cognitive impairment among ischemic stroke patients are warranted.
Our study was based on a subsample of the well-performed CATIS trial with standardized protocol and rigid quality control procedures, enabling us to provide a more comprehensive and valid assessment of the association between plasma sST2 levels with PSCI. However, our study has several limitations. First, patients with BP ≥220/120 mmHg or treatment with intravenous thrombolytic therapy at admission were not included in the CATIS trial. These limited the generalizability of our findings to all acute ischemic strokes. Second, plasma sST2 levels were only measured once at admission, we could not explore its dynamic changes over time and the effect on PSCI. Third, we did not collect the information of pre-stroke cognitive status due to lack of feasibility. However, we included NIHSS score at admission in the multivariate model, which had a subset cognitive dysfunction evaluation and had almost the same diagnostic value as the MMSE (area under the ROC curve values of 0.78 and 0.84, respectively) [43]. Finally, the data of brain and cardiac imaging, such as the site or type of acute ischemic lesions, left atrial volume or left ventricular dysfunction were also not recorded. Hence, we could not further control these factors.
Conclusions
In conclusion, elevated concentrations of plasma sST2 levels were significantly associated with cognitive impairment in acute ischemic stroke patients, independently of established risk factors. Our findings provided additional information for early identifying patients at increased risk of PSCI.
Supplementary Information
Additional file 1: Supplementary Figure 1. Study participant flow chart. Supplementary Figure 2. Comparison of ROC curves for sST2 vs NT-proBNP in predicting PSCI. Supplementary Table 1. Missing variable in the study. Supplementary Table 2. Baseline characteristics of acute ischemic stroke patients.
Acknowledgements
We thank the study participants and their relatives and the clinical staff at all participating hospitals for their support and contribution to this project.
Abbreviations
- sST2
Soluble suppression of tumorigenesis-2
- MoCA
Montreal Cognitive Assessment
- MMSE
Mini-Mental State Examination
- PSCI
Post-stroke cognitive impairment
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- hs-cTnT
High-sensitivity cardiac Troponin T
- CATIS
China Antihypertensive Trial in Acute Ischemic Stroke
- BP
Blood pressure
- hsCRP
High-sensitive C-reactive protein
- NIHSS
National Institutes of Health Stroke Scale
- mRS
Modified Rankin Scale
- ORs
Odds ratios
- CIs
Confidence intervals
- NRI
Net reclassification index
- IDI
Integrated discrimination improvement
- SD
Standard deviation
- CSVD
Cerebral small vessel disease
Authors’ contributions
Yonghong Z, TX and CZ designed the study and wrote the protocol. RZ, YL, XB, AW, JZ and ZJ collected and researched data. Yinwei Z, CF, QZ and CZ managed the literature searches and analyses, and wrote the first draft of the manuscript. The author(s) read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81773522 and 81903387), the Natural Science Foundation of Jiangsu Province (Grant No. BK20190818), the Suzhou Science and Technology Project (Grant No: SYS2019023) and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study protocol was approved by the ethical committee at Soochow University in China and Tulane University in the United States, as well as ethical committees at the 7 participating hospitals, in compliance with the Declaration of Helsinki. All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yinwei Zhu, Chongquan Fang and Qi Zhang contributed equally to this work.
Contributor Information
Tan Xu, Email: xutan@suda.edu.cn.
Chongke Zhong, Email: ckzhong@suda.edu.cn.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, et al. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet (London, England) 2014;383(9913):245–254. doi: 10.1016/S0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang EY, Amiesimaka O, Harrison SL, Green E, Price C, Robinson L, Siervo M, Stephan BC. Longitudinal effect of stroke on cognition: a systematic review. J Am Heart Assoc. 2018;7(2):e006443. doi: 10.1161/JAHA.117.006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swartz RH, Bayley M, Lanctot KL, Murray BJ, Cayley ML, Lien K, Sicard MN, Thorpe KE, Dowlatshahi D, Mandzia JL, et al. Post-stroke depression, obstructive sleep apnea, and cognitive impairment: rationale for, and barriers to, routine screening. Int J Stroke. 2016;11(5):509–518. doi: 10.1177/1747493016641968. [DOI] [PubMed] [Google Scholar]
- 4.Mijajlovic MD, Pavlovic A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen HB, Hermann DM, Assayag EB, Richard E, Thiel A, et al. Post-stroke dementia - a comprehensive review. BMC Med. 2017;15(1):11. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon HS, Lee D, Lee MH, Yu S, Lim JS, Yu KH, Oh MS, Lee JS, Hong KS, Lee EJ et al. Post-stroke cognitive impairment as an independent predictor of ischemic stroke recurrence: PICASSO sub-study. J Neurol. 2020;267(3):688-93. [DOI] [PubMed]
- 6.van der Velpen IF, Yancy CW, Sorond FA, Sabayan B. Impaired cardiac function and cognitive brain aging. Can J Cardiol. 2017;33(12):1587–1596. doi: 10.1016/j.cjca.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 7.van der Velpen IF, Feleus S, Bertens AS, Sabayan B. Hemodynamic and serum cardiac markers and risk of cognitive impairment and dementia. Alzheimers Dement. 2017;13(4):441–453. doi: 10.1016/j.jalz.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Hooghiemstra AM, Leeuwis AE, Bertens AS, Biessels GJ, Bots ML, Brunner-La Rocca HP, Greving JP, Kappelle LJ, van Oostenbrugge RJ, van Rossum AC, et al. Frequent cognitive impairment in patients with disorders along the heart-brain Axis. Stroke. 2019;50(12):3369–3375. doi: 10.1161/STROKEAHA.119.026031. [DOI] [PubMed] [Google Scholar]
- 9.Eggermont LH, de Boer K, Muller M, Jaschke AC, Kamp O, Scherder EJ. Cardiac disease and cognitive impairment: a systematic review. Heart. 2012;98(18):1334–1340. doi: 10.1136/heartjnl-2012-301682. [DOI] [PubMed] [Google Scholar]
- 10.Cushman M, Callas PW, McClure LA, Unverzagt FW, Howard VJ, Gillett SR, Thacker EL, Wadley VG. N-terminal pro-B-type natriuretic peptide and risk of future cognitive impairment in the REGARDS cohort. J Alzheimers Dis. 2016;54(2):497–503. doi: 10.3233/JAD-160328. [DOI] [PubMed] [Google Scholar]
- 11.Veugen MGJ, Henry RMA, Brunner-La Rocca HP, Dagnelie PC, Schram MT, van Agtmaal MJM, van der Kallen CJH, Sep SJS, van Boxtel MPJ, Bekers O, et al. Cross-sectional associations between cardiac biomarkers, cognitive performance, and structural brain changes are modified by age. Arterioscler Thromb Vasc Biol. 2018;38(8):1948–1958. doi: 10.1161/ATVBAHA.118.311082. [DOI] [PubMed] [Google Scholar]
- 12.Schneider AL, Rawlings AM, Sharrett AR, Alonso A, Mosley TH, Hoogeveen RC, Ballantyne CM, Gottesman RF, Selvin E. High-sensitivity cardiac troponin T and cognitive function and dementia risk: the atherosclerosis risk in communities study. Eur Heart J. 2014;35(27):1817–1824. doi: 10.1093/eurheartj/ehu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagata T, Ohara T, Hata J, Sakata S, Furuta Y, Yoshida D, Honda T, Hirakawa Y, Ide T, Kanba S, Kitazono T, Tsutsui H, Ninomiya T. NT-proBNP and risk of dementia in a general Japanese elderly population: the Hisayama study. J Am Heart Assoc. 2019;8(17):e011652. doi: 10.1161/JAHA.118.011652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson C, Preis SR, Beiser A, DeCarli C, Wollert KC, Wang TJ, Januzzi JL, Jr, Vasan RS, Seshadri S. Associations of circulating growth differentiation Factor-15 and ST2 concentrations with subclinical vascular brain injury and incident stroke. Stroke. 2015;46(9):2568–2575. doi: 10.1161/STROKEAHA.115.009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, Tong W, Liu C, Xu T, Ju Z, Peng Y, Peng H, Li Q, Geng D, Zhang J, Li D, Zhang F, Guo L, Sun Y, Wang X, Cui Y, Li Y, Ma D, Yang G, Gao Y, Yuan X, Bazzano LA, Chen J, CATIS Investigators Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311(5):479–489. doi: 10.1001/jama.2013.282543. [DOI] [PubMed] [Google Scholar]
- 16.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 18.Carson N, Leach L, Murphy KJ. A re-examination of Montreal cognitive assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018;33(2):379–388. doi: 10.1002/gps.4756. [DOI] [PubMed] [Google Scholar]
- 19.Swartz RH, Cayley ML, Lanctot KL, Murray BJ, Smith EE, Sahlas DJ, Herrmann N, Cohen A, Thorpe KE. Validating a pragmatic approach to cognitive screening in stroke prevention clinics using the Montreal cognitive assessment. Stroke. 2016;47(3):807–813. doi: 10.1161/STROKEAHA.115.011036. [DOI] [PubMed] [Google Scholar]
- 20.Broersen LHA, Siegerink B, Sperber PS, von Rennenberg R, Piper SK, Nolte CH, Heuschmann PU, Endres M, Scheitz JF, Liman TG. High-Sensitivity Cardiac Troponin T and Cognitive Function in Patients With Ischemic Stroke. Stroke. 2020;51(5):1604–7. [DOI] [PubMed]
- 21.De la Fuente M, MacDonald TT, Hermoso MA. The IL-33/ST2 axis: role in health and disease. Cytokine Growth Factor Rev. 2015;26(6):615–623. doi: 10.1016/j.cytogfr.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Matilla L, Ibarrola J, Arrieta V, Garcia-Peña A, Martinez-Martinez E, Sádaba R, Alvarez V, Navarro A, Fernández-Celis A, Gainza A, et al. Soluble ST2 promotes oxidative stress and inflammation in cardiac fibroblasts: an in vitro and in vivo study in aortic stenosis. Clin Sci (London, England : 1979) 2019;133(14):1537–1548. doi: 10.1042/CS20190475. [DOI] [PubMed] [Google Scholar]
- 23.Altara R, Ghali R, Mallat Z, Cataliotti A, Booz GW, Zouein FA. Conflicting vascular and metabolic impact of the IL-33/sST2 axis. Cardiovasc Res. 2018;114(12):1578–1594. doi: 10.1093/cvr/cvy166. [DOI] [PubMed] [Google Scholar]
- 24.Dieplinger B, Bocksrucker C, Egger M, Eggers C, Haltmayer M, Mueller T. Prognostic value of inflammatory and cardiovascular biomarkers for prediction of 90-day all-cause mortality after acute ischemic stroke-results from the Linz stroke unit study. Clin Chem. 2017;63(6):1101–1109. doi: 10.1373/clinchem.2016.269969. [DOI] [PubMed] [Google Scholar]
- 25.Wolcott Z, Batra A, Bevers MB, Sastre C, Khoury J, Sperling M, Meyer BC, Walsh KB, Adeoye O, Broderick JP, Kimberly WT. Soluble ST2 predicts outcome and hemorrhagic transformation after acute stroke. Ann Clin Transl Neurol. 2017;4(8):553–563. doi: 10.1002/acn3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian X, Guo Y, Wang X, Pei L, Wang X, Wu J, Sun S, Li Y, Ning M, Buonanno FS, Xu Y, Song B. Serum soluble ST2 is a potential long-term prognostic biomarker for transient Ischaemic attack and Ischaemic stroke. Eur J Neurol. 2020;27(11):2202–2208. doi: 10.1111/ene.14419. [DOI] [PubMed] [Google Scholar]
- 27.Fu AK, Hung KW, Yuen MY, Zhou X, Mak DS, Chan IC, Cheung TH, Zhang B, Fu WY, Liew FY, et al. IL-33 ameliorates Alzheimer's disease-like pathology and cognitive decline. Proc Natl Acad Sci U S A. 2016;113(19):E2705–E2713. doi: 10.1073/pnas.1604032113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Rennenberg R, Siegerink B, Ganeshan R, Villringer K, Doehner W, Audebert HJ, Endres M, Nolte CH, Scheitz JF. High-sensitivity cardiac troponin T and severity of cerebral white matter lesions in patients with acute ischemic stroke. J Neurol. 2019;266(1):37–45. doi: 10.1007/s00415-018-9085-3. [DOI] [PubMed] [Google Scholar]
- 29.Yatawara C, Ng KP, Chander R, Kandiah N. Associations between lesions and domain-specific cognitive decline in poststroke dementia. Neurology. 2018;91(1):e45–e54. doi: 10.1212/WNL.0000000000005734. [DOI] [PubMed] [Google Scholar]
- 30.Litak J, Mazurek M, Kulesza B, Szmygin P, Litak J, Kamieniak P, Grochowski C. Cerebral Small Vessel Disease. Int J Mol Sci. 2020;21(24):9729. [DOI] [PMC free article] [PubMed]
- 31.Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. 2010;9(9):895–905. doi: 10.1016/S1474-4422(10)70164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo Y, Zhou Y, Xiao W, Liang Z, Dai J, Weng X, Wu X. Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 2015;1597:86–94. doi: 10.1016/j.brainres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Traxler D, Zimmermann M, Simader E, Veraar CM, Moser B, Mueller T, Mildner M, Dannenberg V, Lainscak M, Jug B, Ankersmit HJ. The inflammatory markers sST2, HSP27 and hsCRP as a prognostic biomarker panel in chronic heart failure patients. Clin Chim Acta. 2020;510:507–514. doi: 10.1016/j.cca.2020.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol. 2016;12(10):594–604. doi: 10.1038/nrneurol.2016.125. [DOI] [PubMed] [Google Scholar]
- 35.Wang A, Liu J, Meng X, Li J, Wang H, Wang Y, Su Z, Zhang N, Dai L, Wang Y, Wang Y. Association between oxidized low-density lipoprotein and cognitive impairment in patients with ischemic stroke. Eur J Neurol. 2018;25(1):185–191. doi: 10.1111/ene.13497. [DOI] [PubMed] [Google Scholar]
- 36.Lande MB, Kupferman JC. Blood pressure and cognitive function in children and adolescents. Hypertension. 2019;73(3):532–540. doi: 10.1161/HYPERTENSIONAHA.118.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palta P, Carlson MC, Crum RM, Colantuoni E, Sharrett AR, Yasar S, Nahin RL, DeKosky ST, Snitz B, Lopez O, et al. Diabetes and cognitive decline in older adults: the Ginkgo evaluation of memory study. J Gerontol A Biol Sci Med Sci. 2017;73(1):123–130. doi: 10.1093/gerona/glx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makin SD, Turpin S, Dennis MS, Wardlaw JM. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiatry. 2013;84(8):893–900. doi: 10.1136/jnnp-2012-303645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krogh J, Benros ME, Jorgensen MB, Vesterager L, Elfving B, Nordentoft M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav Immun. 2014;35:70–76. doi: 10.1016/j.bbi.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Zhong C, Bu X, Xu T, Guo L, Wang X, Zhang J, Cui Y, Li D, Zhang J, Ju Z, et al. Serum matrix metalloproteinase-9 and cognitive impairment after acute ischemic stroke. J Am Heart Assoc. 2018;7(1):e007776. doi: 10.1161/JAHA.117.007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Z, Zhong C, Guo D, Bu X, Xu T, Guo L, Liu J, Zhang J, Li D, Zhang J, Ju Z, Chen CS, Chen J, He J, Zhang Y. Multiple biomarkers covering several pathways improve predictive ability for cognitive impairment among ischemic stroke patients with elevated blood pressure. Atherosclerosis. 2019;287:30–37. doi: 10.1016/j.atherosclerosis.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 42.Ge J, Li R, Yuan P, Che B, Bu X, Shao H, Xu T, Ju Z, Zhang J, Zhang Y, Zhong C. Serum tissue inhibitor of metalloproteinase-1 and risk of cognitive impairment after acute ischaemic stroke. J Cell Mol Med. 2020;24(13):7470–7478. doi: 10.1111/jcmm.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cumming TB, Blomstrand C, Bernhardt J, Linden T. The NIH stroke scale can establish cognitive function after stroke. Cerebrovasc Dis. 2010;30(1):7–14. doi: 10.1159/000313438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Figure 1. Study participant flow chart. Supplementary Figure 2. Comparison of ROC curves for sST2 vs NT-proBNP in predicting PSCI. Supplementary Table 1. Missing variable in the study. Supplementary Table 2. Baseline characteristics of acute ischemic stroke patients.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


