Abstract
Objective
This study aimed to investigate the role of sex hormones in postmenopausal women with vestibular migraine.
Methods
This observational study included 242 female patients with vestibular migraine who were postmenopausal during April 2017 to December 2019. Serum levels of sex hormones, including estradiol, progesterone, testosterone, follicle-stimulating hormone, luteinizing hormone, and prolactin, were determined by radioimmunoassay. The duration and frequency (every month) of onset of vestibular migraine were recorded. The degree of vestibular migraine was measured by the visual analogue scale.
Results
Serum levels of estradiol, progesterone, and testosterone were significantly lower, while serum prolactin levels were significantly higher in postmenopausal patients with vestibular migraine compared with healthy controls. Serum estradiol levels were negatively correlated with the duration, frequency, and severity of onset of vestibular migraine. Patients with higher serum estradiol levels showed a longer disease-free survival time.
Conclusion
Sex hormones are correlated with vestibular migraine in postmenopausal women. Additionally, estradiol levels are correlated with the duration, frequency, and severity of onset of vestibular migraine, as well as the disease-free survival time.
Keywords: Sex hormone, postmenopausal woman, vestibular migraine, estradiol, prolactin, vertigo
Introduction
The relationship between vertigo and headache has been known for a long time, but it has only been systematically studied in the most recent 20 years.1–4 Vestibular migraine (VM) was defined as recurrent vertigo episodes in patients with a history of migraine or other clinical features of migraine by the Headache Classification Committee of the International Headache Society in 2013.5 VM is a frequent cause of intermittent vertigo and may also cause symptoms such as nausea, vomiting, and motion sensitivity or visual vertigo in patients with severe VM.6–9 VM has an incidence of approximately 1%, but only approximately 20% of patients obtain an accurate diagnosis of this condition.10–12
Most knowledge of VM has been obtained in the recent decade.13 Therefore, the mechanism of VM, as well as the relationships of VM with age, sex, and other demographic features, are still unclear. Migraines usually have a higher incidence, frequency, duration, and disability of attacks in women than in men, which might be related to the hormonal milieu and its modulation of neuronal and vascular reactivity.14 Nonobese men with migraine show markedly increased serum estradiol levels, indicating a relationship between sex hormones and migraine.15 For postmenopausal women, sex hormones and many other factors have changed at this stage of life.16–18 However, whether these changes are correlated with VM is unknown.
In the present study, we aimed to investigate the clinical significance of serum sex hormones in postmenopausal women with VM. This research might provide more clinical evidence for postmenopausal women with VM.
Methods and materials
Subjects
This prospective, observational study included women aged older than 45 years with VM who visited our hospital during April 2017 to December 2019. All patients were postmenopausal. The postmenopausal condition was defined as the absence of menstruation for more than 1 year. The diagnosis of VM was according to the criteria of the International Classification of Headache Disorders (ICHD), 3rd edition (beta version, 2013) as follows:5 A) if ≥5 times of onset and meeting the criteria of C and D; B) a history of or current migraine with/without aura; C) moderate or severe vestibular symptoms lasting from 5 minutes to 72 hours; D) at least half of onset is related to unilateral, pulsatile, moderate, or severe headache, which can be aggravated in everyday activity, or headache with photophobia, fear of sound, and visual aura; and E) headache that cannot be better explained by other vestibular diseases or diagnosis by the International Classification of Headache Disorders (ICHD), 3rd edition. Exclusion criteria were as follows: 1) patients who were not postmenopausal women; 2) patients with benign paroxysmal positional vertigo (Meniere’s disease); 3) patients with basilar migraine, transient ischemic attack, vestibular paroxysm, mental vertigo, or cerebellar infarction; and 4) patients who used endogenous hormones within 3 months before the study. All patients also received a bedside examination of neurotology, including an examination of eye movement, position, and balance, a hearing test, and laboratory tests, including pure tone audiometry and dual temperature audiometry. All patients who met the inclusion criteria were enrolled and no additional sample size calculation was performed.
Additionally, 200 healthy postmenopausal women who had a physical examination at the same period as patients with VM were enrolled as controls. These healthy women showed normal physical examination items, and individuals who had severe migraine or vertigo within 1 year before the study were excluded. Written informed consent was obtained from all patients. This study followed the relevant EQUATOR Guidelines for the reporting of health research.19 The present study was approved by the Ethics Committee of the First Hospital of Changsha in December 2016 (approval no: 2016-051).
Measurement of serum sex hormones
Blood samples were collected from all participants on the day of treatment. Briefly, 5 mL of cubital venous blood samples were collected in tubes with the anticoagulant EDTA. Samples were then centrifuged at 5000×g for 5 minutes at 4°C. The collected serum samples were stored at −80°C. Serum levels of the sex hormones estradiol, progesterone, testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin were determined by radioimmunoassay using radioimmunoassay kits (Shanghai Xinfan Biotechnology Co., Ltd., Shanghai, China) strictly according to the manufacturer’s instructions. All experiments were performed in triplicate.
Data collection
Demographic data and clinical characteristics of all patients were collected. The duration and frequency (every month) of onset of VM were recorded. The degree of VM was measured by the visual analogue scale (VAS). The relationships among serum sex hormones and symptoms, medical history, and characteristics were analyzed. All patients were followed up for 1 year after admission. The disease-free time was defined as all days in 1 year without onset for every patient, and this was followed every month by telephone or outpatient visit.
Statistical analysis
Continuous data are expressed as mean ± standard deviation. Normal distribution of continuous data was confirmed by Kolmogorov–Smirnov and Shapiro–Wilk analysis. The chi-square test was used to compare counts and rates. Comparison between two groups of continuous data was performed using the Student’s t-test. Correlations among serum sex hormones and clinical characteristics were analyzed using Pearson’s analysis. The Kaplan–Meier curve was constructed for analysis of 1-year disease-free survival time. For analysis of independent factors of 1-year disease-free time of VM, logistic regression with the stepwise method was used. A P value < 0.05 was considered to be statistically significant. All calculations were performed using IBM SPSS 20.0 (IBM Corp., Armonk, NY, USA).
Results
Serum levels of sex hormones in postmenopausal patients with VM and healthy individuals
This study included 242 postmenopausal patients with VM, with a mean age of 54.48±5.93 years and a mean body mass index of 21.02±1.76 kg/m2. There was no significant difference in age or body mass index between patients and controls (Table 1). Among all patients, symptoms of migraine were precursory in 214 (88.43%) cases and non-precursory in 28 (11.57%) cases. The mean age of onset of migraine was 51.36±6.03 years and that for vertigo was 51.54±6.05 years.
Table 1.
Basic characteristics of all participants.
| Variables | Postmenopausal women with vestibular migraine (n = 242) | Postmenopausal healthy controls (n = 200) | P value |
|---|---|---|---|
| Age, years | 54.48 ± 5.93 | 55.02 ± 6.27 | 0.353 |
| BMI, kg/m2 | 21.02 ± 1.76 | 21.06 ± 1.82 | 0.800 |
| Migraine type, n (%) | – | – | |
| Precursory | 214 (88.43) | ||
| Non-precursory | 28 (11.57) | ||
| Recent time of onset, years | – | – | |
| Migraine | 51.36 ± 6.03 | ||
| Vertigo | 51.54 ± 6.05 | ||
| Migraine history | 187 (77.27) | ||
| VAS score | 4.03 ± 1.54 | ||
| Onset frequency, times/month | 8.64 ± 2.61 | ||
| Onset duration, n (%) | |||
| <5 minutes | 49 (20.25) | ||
| 5–60 minutes | 64 (26.45) | ||
| 1–24 hours | 68 (28.10) | ||
| 24–72 hours | 43 (17.77) | ||
| >72 hours | 18 (7.44) | ||
| Symptoms at onset, n (%) | |||
| Headache | 192 (79.34) | ||
| Fear of light | 186 (76.86) | ||
| Fear of sound | 171 (70.66) | ||
| Type of vertigo, n (%) | |||
| Spontaneity | 181 (74.79) | ||
| Positional type | 39 (16.12) | ||
| Visual induction | 48 (19.83) | ||
| Head movement induction | 41 (16.94) | ||
Data are mean ± standard deviation or n (%).
BMI, body mass index; VAS, visual analogue scale.
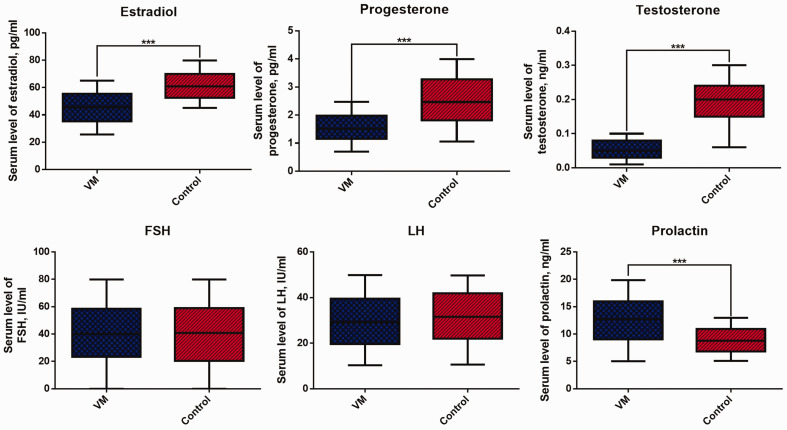
Serum levels of estradiol, progesterone, and testosterone were significantly lower, while serum prolactin levels were significantly higher in postmenopausal patients with VM compared with controls (all P < 0.05, Figure 1). However, there was no significant difference in FSH or LH levels between patients and controls.
Figure 1.
Serum sex hormones in patients with postmenopausal vestibular migraine and healthy individuals. ***P < 0.001.
FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Relationship between serum sex hormones and the duration, frequency, and severity of onset of VM
We analyzed the relationships between serum sex hormones and clinical characteristics. We found that only estradiol levels were correlated with the duration, frequency, and severity of onset of VM in a negative manner (all P < 0.001, Table 2). These results indicated that estradiol might be correlated with clinical characteristics of VM.
Table 2.
Pearson’s analysis for correlation of serum sex hormones with the duration, frequency, and severity of onset of vestibular migraine.
| Estradiol | Progesterone | Testosterone | FSH | LH | Prolactin | ||
|---|---|---|---|---|---|---|---|
| Duration of onset | Pearson correlation | −0.223 | 0.018 | −0.096 | β0.061 | −0.076 | −0.112 |
| P | <0.001 | 0.779 | 0.135 | 0.344 | 0.232 | 0.080 | |
| Frequency of onset | Pearson correlation | −0.311 | 0.053 | 0.003 | −0.003 | 0.118 | 0.034 |
| P | <0.001 | 0.405 | 0.961 | 0.959 | 0.066 | 0.591 | |
| VAS score | Pearson correlation | −0.582 | 0.039 | 0.067 | 0.027 | 0.126 | −0.022 |
| P | <0.001 | 0.543 | 0.297 | 0.669 | 0.050 | 0.731 |
FSH, follicle-stimulating hormone; LH, luteinizing hormone; VAS, visual analogue scale.
Relationships between estradiol levels and clinical characteristics of VM
The patients were then divided into two groups of high/low estradiol groups by the mean value of serum estradiol (45.43±11.41 pg/mL). Patients with low serum estradiol levels showed higher VAS scores, a higher frequency of onset, and longer duration of onset than those with high serum estradiol levels (all P < 0.05, Table 3). However, no significant differences were found in other characteristics. These findings indicated that serum estradiol levels might be mainly related to disease severity of postmenopausal patents with VM.
Table 3.
Clinical characteristics of vestibular migraine in patients with high/low estradiol levels.
| Variables | High estradiol levels (n = 126) | Low estradiol levels (n = 116) | P value |
|---|---|---|---|
| Age, years | 54.00 ± 6.23 | 55.00 ± 5.58 | 0.195 |
| BMI, kg/m2 | 21.16 ± 1.81 | 20.87 ± 1.71 | 0.206 |
| Migraine type, n (%) | 0.192 | ||
| Precursory | 115 (91.27) | 99 (85.34) | |
| Non-precursory | 11 (8.73) | 17 (14.66) | |
| First time of onset, years | |||
| Migraine | 51.02 ± 6.39 | 51.74 ± 5.61 | 0.356 |
| Vertigo | 51.15 ± 6.44 | 51.96 ± 5.60 | 0.302 |
| VAS score | 3.02 ± 1.13 | 5.13 ± 1.12 | <0.001 |
| Onset frequency, times/month | 7.73 ± 1.65 | 9.62 ± 3.08 | <0.001 |
| Onset duration, n (%) | 0.008 | ||
| <5 minutes | 29 (23.02) | 20 (17.24) | |
| 5–60 minutes | 40 (31.75) | 24 (20.69) | |
| 1–24 hours | 38 (30.16) | 30 (25.86) | |
| 24–72 hours | 16 (12.70) | 27 (23.28) | |
| >72 hours | 3 (2.38) | 15 (12.93) | |
| Symptoms at onset, n (%) | 0.669 | ||
| Headache | 102 (80.95) | 90 (77.59) | |
| Fear of light | 95 (75.40) | 91 (78.45) | |
| Fear of sound | 82 (65.08) | 89 (76.72) | |
| Type of vertigo, n (%) | 0.784 | ||
| Spontaneity | 84 (66.67) | 97 (83.62) | |
| Positional type | 22 (17.46) | 17 (14.66) | |
| Visual induction | 23 (18.25) | 25 (21.55) | |
| Head movement induction | 20 (15.87) | 21 (18.10) | |
| Progesterone, pg/mL | 1.51 ± 0.49 | 1.56 ± 0.47 | 0.434 |
| Testosterone, ng/mL | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.563 |
| FSH, IU/mL | 40.51 ± 23.37 | 40.15 ± 21.58 | 0.903 |
| LH, IU/mL | 28.25 ± 11.65 | 30.96 ± 11.34 | 0.069 |
| Prolactin, ng/mL | 12.56 ± 4.42 | 12.61 ± 4.21 | 0.917 |
Data are mean ± standard deviation or n (%).
BMI, body mass index; VAS, visual analogue scale; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Correlation of serum estradiol levels with 1-year disease-free survival time of VM
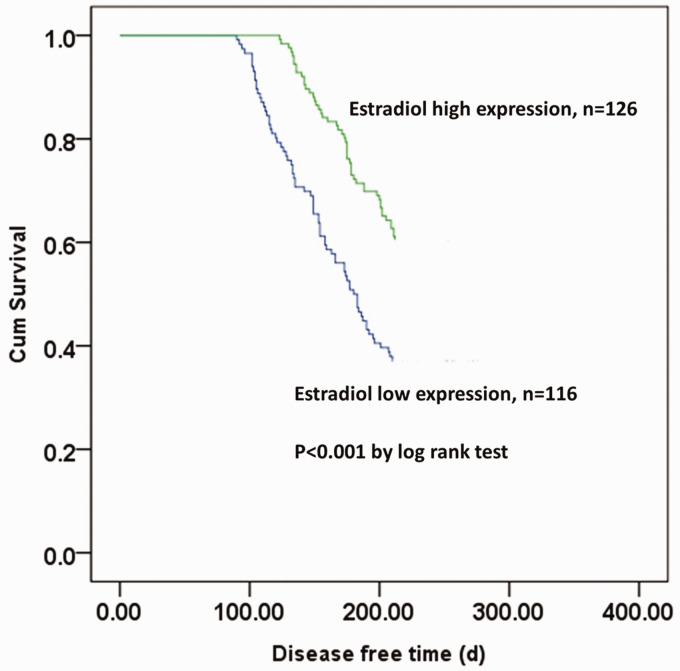
We analyzed the relationship between serum estradiol levels and 1-year recurrence of postmenopausal VM. Recurrence of VM was found in all patients within 1 year after first admission in this study. The Kaplan–Meier curve showed that patients with higher serum estradiol levels demonstrated a significantly longer disease-free survival time (P < 0.001, Figure 2). The mean disease-free survival time of patients was 212.48±69.65 days. Patients with a disease-free survival time of ≥212.48 days were defined as long disease-free survival and patients with a disease-free survival time of < 212.48 days were defined as short disease-free survival. Only the VAS score was an independent risk factor for 1-year disease-free survival (Table 4).
Figure 2.
Kaplan–Meier curve for recurrence of vestibular migraine in postmenopausal patients.
Table 4.
Risk factors associated with 1-year recurrence of vestibular migraine by logistic multivariate regression analysis.
| Wald | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| VAS score | 5.815 | −0.265 | 0.766 (0.618–0.951) | 0.015 |
| Frequency of onset | 1.036 | 0.055 | 1.057 (0.949–1.176) | 0.308 |
| Duration of onset | 0.042 | −0.024 | 0.976 (0.775–1.228) | 0.837 |
| Estradiol | 1.103 | 0.015 | 1.015 (0.986–1.046) | 0.293 |
| Progesterone | 2.394 | 0.428 | 1.535 (0.891–2.642) | 0.121 |
| Testosterone | 1.217 | 5.111 | 281.654 (0.0125–6317409.743) | 0.269 |
| Prolactin | 0.455 | 0.021 | 1.021 (0.959–1.087) | 0.499 |
CI, confidence interval; VAS, visual analogue scale.
Discussion
Because VM is a newly defined disease, knowledge of VM is still limited. In a recent retrospective study, Liu et al showed that female patients older than 65 years had a higher risk of developing benign paroxysmal positional vertigo, and there was a lower incidence of vertigo in patients who took estrogen for menopausal syndromes.20 This finding indicated that sex hormones might be associated with vertigo. However, to date, no studies have focused on correlations between sex hormones and VM. In this study, we found that lower estradiol levels predicted a longer duration, higher frequency, and greater severity of VM, as well as a lower recurrence risk of VM.
Despite the relatively short study period, several studies have shown some characteristics of VM. Russo et al found that patients with VM had significantly increased thalamic activation, which was positively correlated with the frequency of migraine onset.21 Bednarczuk et al showed that patients with VM had upregulated reflexive and perceptual vestibular thresholds, which were further increased by visual motion exposure.22 In another study, Obermann et al showed that patients with VM had a reduction in gray matter volume in the superior, inferior, and middle (MT/V5) temporal gyrus.23 However, more research is still required to identify features of VM.
Relationships among sex, sex hormones, headache, and vertigo have been found in several studies. Delaruelle et al investigated the relationship between sex hormones and primary headaches, and found that migraine was associated with a fluctuation in estrogen levels in the different reproductive stages in women’s lifetime.24 Smith et al also demonstrated that women had a higher risk of developing vestibular disorders, especially after menopause.25 A nationwide home-based study in Turkey showed that the incidence of migraine was almost 2.8:1 for women and men, and postmenopausal women might have more severe symptoms.26 With regard to hormones and related factors, calcitonin gene-related peptide is an important factor, which can be regulated by fluctuations in ovarian steroid hormone (mainly estrogen) levels.27 Estrogen can alleviate neurogenic inflammation through modulation of calcitonin gene-related peptide and progesterone shows dual effects on these neuropeptides in different sites associated with migraine pain.28 In our study, we mainly focused on sex hormones in postmenopausal women with VM. We observed that patients with lower estradiol levels showed a longer duration, higher frequency, and greater severity of VM. These results are consistent with the studies mentioned above, indicating that estradiol might contribute to improvement of migraine pain, while a reduction in estradiol levels aggravates migraine.
This study has some limitations. The sample size was limited and the study was from a single center. The molecular mechanism between estradiol and VM is unclear. Further studies are required to clarify the association between estradiol and VM.
In conclusion, our study shows that serum levels of estradiol, progesterone, and testosterone are remarkably lower, while serum prolactin levels are higher in postmenopausal women with VM than in healthy women. Additionally, serum estradiol levels are correlated with the duration, frequency, and severity of onset of VM, as well as with a lower risk of recurrence of VM.
Footnotes
Data availability: All data are available by proper request from the authors.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The study was funded by the Changsha Financial Enterprise Index 2017125 (No. kq1706002).
Author contributions: Bo Tang wrote the manuscript and conducted the experiments; Xiaojun Yu designed the study and reviewed the manuscript; Wei Jiang collected the data and conducted analysis; Chuang Zhang performed statistical analysis and reviewed the manuscript; Tao Zhan and Yuqin He collected data and reviewed the manuscript.
ORCID iD: Xiaojun Yu https://orcid.org/0000-0002-9855-601X
References
- 1.Shimizu T. Headache and Vertigo. Brain and nerve. 2020; 72: 303–309. [DOI] [PubMed] [Google Scholar]
- 2.Neuhauser H, Leopold M, Von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001; 56: 436–441. [DOI] [PubMed] [Google Scholar]
- 3.Lampl C, Rapoport A, Levin M, et al. Migraine and episodic vertigo: a cohort survey study of their relationship. J Headache pain. 2019; 20: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komiyama S, Murofushi T, Yoshimura E. A case of cerebellar arteriovenous malformation presented with vertigo, hearing loss, and headache. Acta Oto-Laryngologica Case Reports. 2017; 2: 86–88. doi.org/10.1080/23772484.2017.1319735 [Google Scholar]
- 5.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders 3rd edition (beta version). Cephalalgia. 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 6.Brevern MV, Lempert T. Vestibular Migraine: Treatment and Prognosis. Semin Neurol. 2020; 40: 083–086. [DOI] [PubMed] [Google Scholar]
- 7.Lauritsen CG, Marmura MJ. Current Treatment Options: Vestibular Migraine. Curr Treat Options Neurol. 2017; 19: 38. [DOI] [PubMed] [Google Scholar]
- 8.Formeister EJ, Rizk HG, Kohn MA, et al. The epidemiology of vestibular migraine: a population-based survey study. Otol Neurotol. 2018; 39: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 9.Power L, Shute W, McOwan B, et al. Clinical characteristics and treatment choice in vestibular migraine. J Clin Neurosci. 2018; 52: 50–53. [DOI] [PubMed] [Google Scholar]
- 10.Huang TC, Wang SJ, Kheradmand A. Vestibular migraine: an update on current understanding and future directions. . Cephalalgia. 2020; 40: 107–121. [DOI] [PubMed] [Google Scholar]
- 11.Alghadir AH, Shahnawaz A. Effects of Vestibular Rehabilitation in the Management of a Vestibular Migraine: A Review. Front Neurol. 2018; 9: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F, Ma T, Che X, et al. The efficacy of venlafaxine, flunarizine, and valproic acid in the prophylaxis of vestibular migraine. Front Neurol. 2017; 8: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013; 12: 706–715. [DOI] [PubMed] [Google Scholar]
- 14.Allais G, Chiarle G, Sinigaglia S, et al. Gender-related differences in migraine. Neurol Sci. 2020; 41(Suppl 2):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Oosterhout WPJ, Schoonman GG, van Zwet EW, et al. Female sex hormones in men with migraine. Neurology. 2018; 91: e374–e381. [DOI] [PubMed] [Google Scholar]
- 16.Zhao D, Guallar E, Ouyang P, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Card. 2018; 71: 2555–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim G-W, Park K, Jeong G-W. Effects of sex hormones and age on brain volume in post-menopausal women. J Sex Med. 2018; 15: 662–670. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Zhang W, Wang Y, et al. Novel associations between sex hormones and diabetic vascular complications in men and postmenopausal women: a cross-sectional study. Cardiovasc Diabetol. 2019; 18: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman DG, Simera I, Hoey J, et al. EQUATOR: reporting guidelines for health research. Open Med. 2008; 2: e49–e50. [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D-H, Kuo C-H, Wang C-T, et al. Age-related increases in benign paroxysmal positional vertigo are reversed in women taking estrogen replacement therapy: a population-based study in Taiwan. Front Aging Neurosci. 2017; 9: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo A, Marcelli V, Esposito F, et al. Abnormal thalamic function in patients with vestibular migraine. Neurology. 2014; 82: 2120–2126. [DOI] [PubMed] [Google Scholar]
- 22.Bednarczuk NF, Bonsu A, Ortega MC, et al. Abnormal visuo-vestibular interactions in vestibular migraine: a cross sectional study. Brain. 2019; 142: 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obermann M, Wurthmann S, Steinberg BS, et al. Central vestibular system modulation in vestibular migraine. Cephalalgia. 2014; 34: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 24.Delaruelle Z, Ivanova TA, Khan S, et al. Male and female sex hormones in primary headaches. J Headache pain. 2018; 19: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith PF, Agrawal Y, Darlington CL. Sexual dimorphism in vestibular function and dysfunction. J Neurophysiol. 2019; 121: 2379–2391. [DOI] [PubMed] [Google Scholar]
- 26.Akarsu EO, Baykan B, Ertaş M, et al . Sex differences of migraine: Results of a nationwide home-based study in Turkey. Noro Psikiyatr Ars. 2019; 57: 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labastida-Ramírez A, Rubio-Beltrán E, Villalón CM, et al. Gender aspects of CGRP in migraine. Cephalalgia. 2019; 39: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cetinkaya A, Kilinc E, Camsari C, et al. Effects of estrogen and progesterone on the neurogenic inflammatory neuropeptides: implications for gender differences in migraine. Exp Brain Res. 2020; 238: 2625–2639. [DOI] [PubMed] [Google Scholar]