Abstract
Background
Cerebral venous thrombosis (CVT) is easily missed or misdiagnosed in clinical settings because of its high variability in terms of symptoms and radiological findings. Herein, we aimed to explore a promising modality for confirming presumed CVT in the hope to uncover its superior diagnostic performance to conventional imaging modalities.
Case presentation: The patient complained of intolerable pain in her forehead and left eye. Her lumbar puncture opening pressure was 140 mmH2O, and her cerebrospinal fluid composition was normal. No marked abnormalities were observed in routine brain images, including non-contrast computed tomography, magnetic resonance imaging, and contrast-enhanced magnetic resonance venography. However, chronic mural thrombi in the lumen of the left cortical veins, transverse/sigmoid sinus, and superior sagittal sinus were identified in magnetic resonance black-blood thrombus imaging (MRBTI) maps.
Conclusions
MRBTI can be used to directly and non-invasively visualize thrombi, and may thus be a promising tool over alternative routine techniques for confirming the diagnosis of CVT.
Keywords: Cerebral venous thrombosis; chronic; magnetic resonance black-blood thrombus imaging; neuroimaging; diagnostic imaging, case report
Background
Cerebral venous thrombosis (CVT) refers to thrombosis in the cerebral veins and dural sinuses. It is a stroke subtype that mainly occurs in young and middle-aged individuals.1 Currently, a delayed or missed diagnosis remains very common in clinical settings because CVT has non-characteristic symptoms and signs as well as ambiguous features in routine imaging maps. Moreover, it is challenging to identify the stages of CVT based on routine neuroimaging. This can lead to considerable delays in treatment, thus resulting in poor clinical outcomes and even life-threatening consequences.
Clinically, a CVT diagnosis mainly relies on radiological signs, in which digital subtraction angiography (DSA) was once recognized as the gold standard. However, with advances in imaging modalities, DSA is not routinely adopted in outpatient clinics because of its invasive nature; it is generally reserved for hospitalized patients who are being considered for thrombolysis or stenting. Magnetic resonance imaging (MRI) combined with contrast-enhanced magnetic resonance venography (CE-MRV) have also been accepted as first-line imaging modalities for a CVT diagnosis.2 In addition, non-contrast cranial computed tomography (NCCT) is preferred in emergency rooms for its wide availability and rapid speed.2 However, use of the aforementioned imaging modalities for CVT confirmation depends heavily on indirect signals, including cerebral parenchymal changes (e.g., edema, venous infarction, or hemorrhage) and filling defects caused by thrombi, rather than on the direct visualization of thrombi. Accordingly, these indirect imaging features may result in diagnostic pitfalls because a host of anatomic variations, such as prominent arachnoid granulations and intra-sinus septa, can also cause fluid void signals and mimic venous thrombi.2
In the present report, an optimized technique for CVT detection, magnetic resonance black-blood thrombus imaging (MRBTI), was used in a case at our hospital. This magnetic resonance sequence provides the direct and non-invasive visualization of thrombi in cerebral veins and sinuses. Moreover, MRBTI can be used to distinguish the probable ages of thrombi (acute, subacute, or chronic) to provide important references for customized treatment. Previous studies have confirmed the high sensitivity of MRBTI for investigating thrombi in the context of acute ischemic stroke,3 myocardial infarction,4 and deep venous thrombosis.5,6 In the present study, the accuracy and superiority of MRBTI for confirming CVT in the chronic stage was demonstrated for the first time.
Case presentation
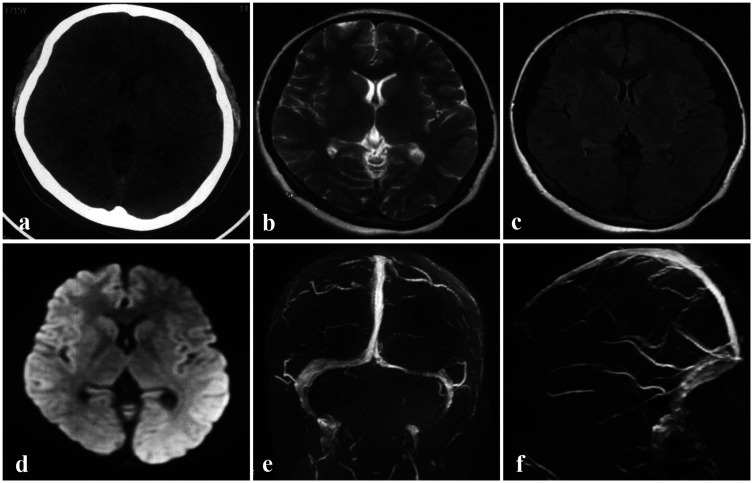
A 15-year-old female patient complained of distension-like pains in her forehead and left eye for more than 1 month and was hospitalized for diagnosis and customized treatment. She had previously experienced a recurrent headache for nearly 1 year (according to her description, the baseline numeric rating scale [NRS] score = 8), with vomiting, and underwent mannitol intravenous infusion at a local hospital. However, no obvious abnormalities were observed in her baseline brain computed tomography (CT), MRI, or magnetic resonance venography (MRV) maps, which were imaged in local hospital, except for a slim left transverse sinus (TS) (Figure 1).
Figure 1.
Brain images obtained at a local hospital prior to admission. a–d: No cerebral parenchymal lesions were observed on NCCT (a), T2WI (b), FLAIR (c), or DWI (d). With the exception of the left slender TS, no other abnormalities were observed in the MRV maps (e–f).
DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; MRV, magnetic resonance venography; NCCT, non-contrast computed tomography; T2WI, T2-weighted imaging; TS, transverse sinus.
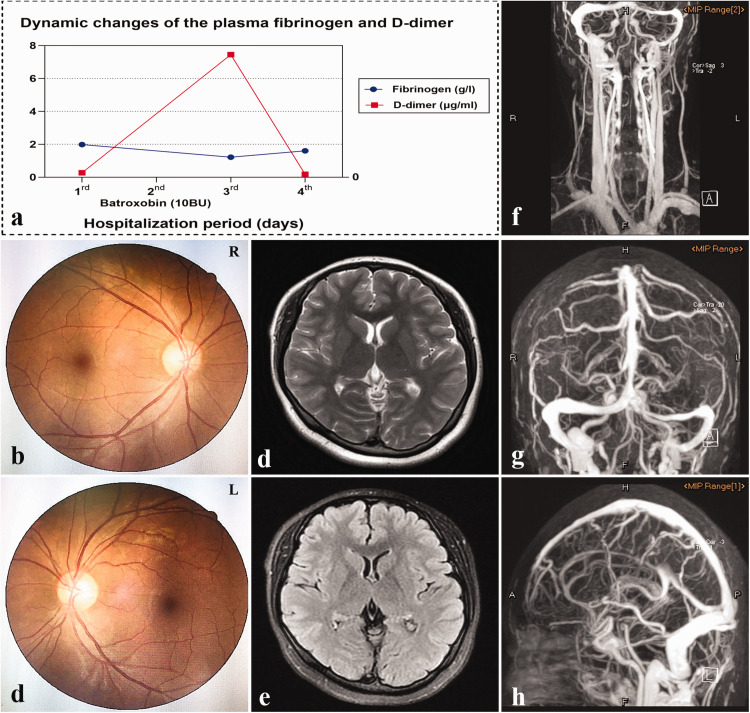
Her blood tests showed no marked hypercoagulability, with normal plasma fibrinogen (FIB), D-dimer, antithrombin III, protein C, protein S, and homocysteine. In addition, her baseline FIB and D-dimer (measured on day 1, prior to medical treatment) were 1.98 g/L and 0.27 μg/mL, respectively. Twenty-four hours after being administered 10 BU batroxobin (a defibrinogenating agent that is able to degrade FIB into water-soluble fibrin, and hence promote the lysis of the newly formed thrombi7) once via intravenous infusion, the patient’s plasma FIB decreased to 1.22 g/L and her D-dimer elevated slightly to 7.45 μg/mL. These findings indicated that her presumed thrombi did not respond sensitively to batroxobin-mediated thrombolysis (Figure 2a). Generally, batroxobin increases the levels of plasma D-dimer to >20 μg/mL in acute or subacute CVT through its thrombolytic effects.8 In addition, ophthalmic examinations by a senior oculist revealed that the patient’s vision, visual field, and optic nipples were all in good condition (Figure 2b and c). A glaucoma diagnosis was also ruled out because her bilateral eyelid pressure was below the cut-off value of 21 mmHg.
Figure 2.
Dynamic plasma fibrinogen and D-dimer, baseline fundoscopy examinations, and MRI and CE-MRV images during hospitalization. a: Dynamic plasma fibrinogen and D-dimer before and after batroxobin treatment. b, c: Bilateral fundus photographs revealed no papilledema (Frisen score = 0). d, e: No parenchymal lesions were observed in T2WI (d) or FLAIR (e). f–h: The left TS appeared slender and the venous vasculature of the entire brain was surrounded by dilated collaterals on the CE-MRV maps.
CE-MRV, contrast-enhanced magnetic resonance venography; FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance imaging; T2WI, T2-weighted imaging; TS, transverse sinus.
Similar to the initial MRI and MRV images, no changes were observed in the brain MRI combined with CE-MRV images performed in our hospital, except for the slender left TS and tortuous venous collaterals (Figure 2d–h). Additionally, her lumbar puncture opening pressure was 140 mmH2O, and her cerebrospinal fluid composition was normal. On the basis of the existing indications, her presumed CVT diagnosis remained relatively uncertain and controversial.
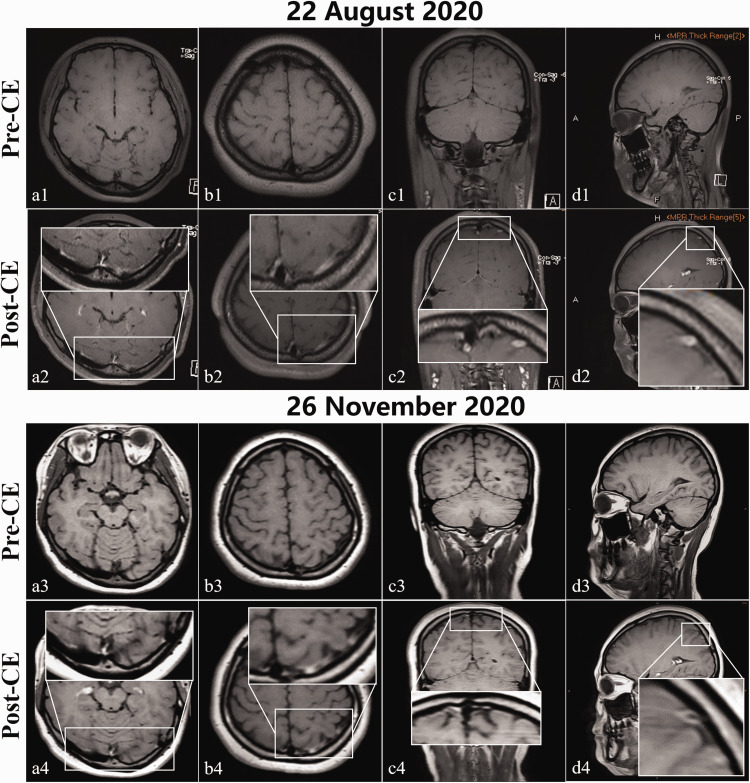
To confirm the diagnosis, the patient underwent MRBTI, which revealed hyperintense signals on the contrast-enhanced sequence distributed in the left cortical veins, transverse/sigmoid sinus, and superior sagittal sinus (Figure 3a2–c2), indicative of chronic CVT.5,9 DSA was ultimately not performed in this patient because DSA was considered to provide limited therapeutic assistance for this case, and because the patient and her parents refused DSA for its associated risk as well as for economic reasons. Furthermore, because cortical venous thrombosis carries a high risk of epilepsy,10 a digital electroencephalogram was performed; however, no epileptic waves were monitored. Finally, the patient underwent tailored oral anticoagulant therapy (warfarin 2.75 mg/day) with the international normalized ratio (INR) maintained between 2.0 and 3.0. She obtained a good clinical outcome and was discharged from the hospital. At the 1-month telephone follow-up, her distension-like pains were markedly relieved (NRS = 0–2). At 3 months post-anticoagulation, the patient’s follow-up MRBTI revealed that the chronic mural thrombi were almost completely diminished compared with her baseline MRBTI maps (Figure 3). Note that the remaining enhancement in the follow-up MRBTI maps may stem from thrombosis-associated venous wall inflammation. Correspondingly, she achieved full recovery from her refractory headache (NRS = 0). Given that the patient had unknown etiology, she was advised to continue with oral anticoagulants and outpatient follow-up on a regular basis.
Figure 3.
Baseline (22 August 2020) and 3-month follow-up (26 November 2020) MRBTI maps. Hyperintensities within the left cortical veins, transverse/sigmoid sinus, and superior sagittal sinus on baseline CE-MRBTI sequences in axial (a2 and b2), coronal (c2), and sagittal (d2) sections were considered by a senior radiologist to be chronic venous thrombi. These hyperintensities had almost completely disappeared in the corresponding follow-up CE-MRBTI maps (a4–d4). The remaining hyperintensities in the left transverse/sigmoid sinus (b4) may be thrombosis-related venous wall inflammation. The shallow or disappeared cerebral sulci and fissures with unclear matter margins that were observed in the baseline CE-MRBTI (a1–d2) were deeper and more clearly observed in the follow-up CE-MRBTI (a3–d4), with well-defined matter borders.
CE-MRBTI, contrast-enhanced magnetic resonance black-blood thrombus imaging; MRBTI, magnetic resonance black-blood thrombus imaging; post-CE, post-contrast enhancement; pre-CE, pre-contrast enhancement.
Discussion
Several key points were revealed in this case report. First, a diagnosis of CVT previously depended on indirect signals imaged using routine modalities; even the gold standard DSA is no exception. Second, compared with MRBTI, MRI together with CE-MRV may be less able to screen some chronic or cortical venous thrombosis. Third, MRBTI can detect thrombi at different stages in a direct and non-invasive manner, which makes it a robust alternative to routine imaging modalities.
MRBTI techniques, namely T1-weighted three-dimensional variable-flip-angle turbo spin-echo sequences, can not only isolate thrombi from suppressed background signals using its inherent blood-nulling capability and black-blood effect,11 but can also image thrombosis-triggered inflammatory changes in vessel walls.12,13 Furthermore, CVT periods may be estimated with MRBTI using the signal intensity of a thrombus, which changes over time with its varying methemoglobin content.5 Specifically, chronic thrombi appear as hyperintense signals on post-CE maps and hypo- or iso-intense signals on pre-CE maps, whereas for acute thrombi, the opposite is true. Moreover, MRBTI offers high spatial resolution and provides a large anatomical coverage area in any chosen plane and thickness after reformation. To the best of our knowledge, no previous report has highlighted the performance of MRBTI versus other routine imaging modalities for confirming a presumed CVT diagnosis.
MRBTI versus NCCT
NCCT frequently serves as a preferred tool in the emergency department for acute CVT cases because it is rapid and widely available. Unfortunately, it is not a reliable way to confirm CVT, and diagnostic confidence may be compromised if NCCT is used as the sole examination method.14 For example, if NCCT is not performed in the acute CVT phase, false negatives may be produced because of the absence of typical signs; that is, the dense delta or cord signs.14 Furthermore, thrombi cannot be discriminated from the normal flow in patients with anemia who have low blood attenuation as a result of decreased hemoglobin.15 Similarly, NCCT may lead to false positives in patients with polycythemia vera or in children or young adults because they often have high hemoglobin or hematocrit values.16 Therefore, despite the superiority of NCCT in emergent situations, MRBTI has advantages over NCCT for confirming CVT because of its greater diagnostic accuracy and direct visualization properties. This is true despite the time it takes, its cost, and the potential gadolinium-mediated nephrotoxicity.
MRBTI versus MRI combined with CE-MRV
The diagnostic value of MRI for CVT has been proposed for almost two decades.17 To date, MRI combined with CE-MRV is accepted as the most advantageous imaging modality for CVT diagnosis.2 High-quality multiplane and spatial resolutions make MRI a better technique than NCCT for imaging parenchymal lesions secondary to CVT. However, not all cases of CVT, or even acute thrombotic events, result in parenchymal changes. In addition, in cases with normal MRI findings, venous flow interruption caused by the thrombi and associated collaterals may be captured by CE-MRV. Nevertheless, CE-MRV can lack the ability to identify thrombosed cortical veins and chronic, partially recanalized thrombi,18,19 as shown in the present case. In contrast, MRBTI can overcome the technical deficits of MRI and CE-MRV, and was a major contributor to the final diagnosis in the present case study. Moreover, a plethora of venous variations, such as prominent arachnoid granulations, hypoplastic sinuses, and intra-septa, may be misinterpreted as venous thrombi on CE-MRV images. These diagnostic pitfalls can be resolved by MRBTI because of its ability to display a direct overview of intralumen.11 Of note, the greater accuracy of MRBTI versus CE-MRV for evaluating the degree of CVT recanalization was demonstrated in our previous study.20
MRBTI versus DSA
DSA is an invasive roadmap imaging method that provides dynamic patterns of cerebral vasculature in areas where the iodinated contrast and catheter or sheath are delivered. It is also an imaging method that can be used to identify thrombi through indirect blood flow signals. However, this method may lead to a broad spectrum of complications, ranging from contrast extravasation or subarachnoid hemorrhage to femoral hematoma, part of which may require the discontinuation of anticoagulation agents and thus delay CVT therapy. Noninvasive MRBTI is an essential solution to these limitations and a preferable option to DSA for CVT screening, as presented in the present case. For the aforementioned reasons, despite DSA currently being the gold standard for CVT diagnosis, it may be suboptimal in both safety and cost considerations.
Moreover, MRBTI may be used to predict thrombus age,5,9 and thrombolysis is effective for fresh thrombi with rich fibrin content, but not for old thrombi with high collagen levels.9,21 Therefore, MRBTI is likely to be a technique of reference value for assessing whether or not catheter-directed thrombolysis is needed, hence protecting individuals with chronic CVT from unnecessary endovascular interventions. Such a complementary use for preoperative evaluations, predating DSA procedures, is another merit of MRBTI.
Notably, the diagnostic value of MRBTI was investigated and established based on a single case in the present study. It is therefore necessary to further confirm its value in a large patient cohort, preferably as a multi-center study. Also of note, MRBTI is performed as a clinical routine method in cases with presumptive CVT in our hospital, which has greatly boosted our diagnostic accuracy of CVT and decreased the rate of DSA.
Conclusions
MRBTI can be used to directly and non-invasively monitor thrombi at different stages within the lumen of veins or sinuses. It may thus be a better choice than DSA, especially in situations where CVT is highly suspected but conventional imaging techniques fail to reach a conclusive diagnosis. Conversely, DSA should be reserved for cases in which intravascular therapy is advocated.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211017001 for Magnetic resonance black-blood thrombus imaging can confirm chronic cerebral venous thrombosis: a case report and literature review by Xiaoqin Wu, Jingkun Sun, Zhiying Chen, Yuchuan Ding and Ran Meng in Journal of International Medical Research
Acknowledgements
We would like to thank the patient and doctors who participated in this study for their cooperation.
Footnotes
Ethics statement: The study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University (2020-106). Written informed consent for the publication of the patient’s clinical details and images was obtained from the patient’s guardians prior to the data being collected.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was sponsored by the National Key R&D Program of China (2017YFC1308401), the National Natural Science Foundation (81371289), and the Beijing Natural Science Foundation (7212047).
Author contributions: Conception and design: XW and RM; administrative support: YD and RM; provision of study materials or patients: XW and RM; collection and assembly of data: XW, JS, and ZC; data analysis and interpretation: XW and RM; manuscript writing: all authors; final approval of manuscript: all authors.
ORCID iD: Ran Meng https://orcid.org/0000-0001-8452-8758
Supplemental material: Supplemental material for this article is available online.
References
- 1.Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med 2005; 352: 1791–1798. DOI: 10.1056/NEJMra042354. [DOI] [PubMed] [Google Scholar]
- 2.Saposnik G, Barinagarrementeria F, Brown RD, Jr, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 1158–1192. DOI: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 3.Jang W, Kwak HS, Chung GH, et al. Three-dimensional black-blood contrast-enhanced MRI improves detection of intraluminal thrombi in patients with acute ischaemic stroke. Eur Radiol 2018; 28: 3840–3847. DOI: 10.1007/s00330-018-5323-4. [DOI] [PubMed] [Google Scholar]
- 4.Holtackers RJ, Van De Heyning CM, Nazir MS, et al. Clinical value of dark-blood late gadolinium enhancement cardiovascular magnetic resonance without additional magnetization preparation. J Cardiovasc Magn Reson 2019; 21: 44. DOI: 10.1186/s12968-019-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie G, Chen H, He X, et al. Black-blood thrombus imaging (BTI): a contrast-free cardiovascular magnetic resonance approach for the diagnosis of non-acute deep vein thrombosis. J Cardiovasc Magn Reson 2017; 19: 4. DOI: 10.1186/s12968-016-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao H, Guan X, Peng K, et al. Time-efficient and contrast-free magnetic resonance imaging approach to the diagnosis of deep vein thrombosis on black-blood gradient-echo sequence: a pilot study. Quant Imaging Med Surg 2021; 11: 276–289. DOI: 10.21037/qims-19-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang MJ, Qi CY, Chen XY, et al. Effects of batroxobin treatment on the survival of random skin flaps in rats. Int Immunopharmacol 2019; 72: 235–242. DOI: 10.1016/j.intimp.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Ding J, Zhou D, Hu Y, et al. The efficacy and safety of batroxobin in combination with anticoagulation on cerebral venous sinus thrombosis. J Thromb Thrombolysis 2018; 46: 371–378. DOI: 10.1007/s11239-018-1718-y. [DOI] [PubMed] [Google Scholar]
- 9.Saha P, Andia ME, Modarai B, et al. Magnetic resonance T1 relaxation time of venous thrombus is determined by iron processing and predicts susceptibility to lysis. Circulation 2013; 128: 729–736. DOI: 10.1161/circulationaha.113.001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutinho JM, Gerritsma JJ, Zuurbier SM, et al. Isolated cortical vein thrombosis: systematic review of case reports and case series. Stroke 2014; 45: 1836–1838. DOI: 10.1161/strokeaha.113.004414. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Duan J, Fan Z, et al. Early detection and quantification of cerebral venous thrombosis by magnetic resonance black-blood thrombus imaging. Stroke 2016; 47: 404–409. DOI: 10.1161/strokeaha.115.011369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treitl KM, Maurus S, Sommer NN, et al. 3D-black-blood 3T-MRI for the diagnosis of thoracic large vessel vasculitis: a feasibility study. Eur Radiol 2017; 27: 2119–2128. DOI: 10.1007/s00330-016-4525-x. [DOI] [PubMed] [Google Scholar]
- 13.Wakefield TW, Strieter RM, Wilke CA, et al. Venous thrombosis-associated inflammation and attenuation with neutralizing antibodies to cytokines and adhesion molecules. Arterioscler Thromb Vasc Biol 1995; 15: 258–268. DOI: 10.1161/01.atv.15.2.258. [DOI] [PubMed] [Google Scholar]
- 14.Selim M, Caplan LR. Radiological diagnosis of cerebral venous thrombosis. Front Neurol Neurosci 2008; 23: 96–111. DOI: 10.1159/000111372. [DOI] [PubMed] [Google Scholar]
- 15.Buyck PJ, De Keyzer F, Vanneste D, et al. CT density measurement and H:H ratio are useful in diagnosing acute cerebral venous sinus thrombosis. AJNR Am J Neuroradiol 2013; 34: 1568–1572. DOI: 10.3174/ajnr.A3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Healy JF, Nichols C. Polycythemia mimicking venous sinus thrombosis. AJNR Am J Neuroradiol 2002; 23: 1402–1403. [PMC free article] [PubMed] [Google Scholar]
- 17.Macchi PJ, Grossman RI, Gomori JM, et al . High field MR imaging of cerebral venous thrombosis. J Comput Assist Tomogr 1986; 10: 10–15. DOI: 10.1097/00004728-198601000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Meckel S, Glücker TM, Kretzschmar M, et al. Display of dural sinuses with time-resolved, contrast-enhanced three-dimensional MR venography. Cerebrovasc Dis 2008; 25: 217–224. DOI: 10.1159/000113859. [DOI] [PubMed] [Google Scholar]
- 19.Dormont D, Sag K, Biondi A, et al. Gadolinium-enhanced MR of chronic dural sinus thrombosis. AJNR Am J Neuroradiol 1995; 16: 1347–1352. [PMC free article] [PubMed] [Google Scholar]
- 20.Ding JY, Pan LQ, Hu YY, et al. Batroxobin in combination with anticoagulation may promote venous sinus recanalization in cerebral venous thrombosis: a real-world experience. CNS Neurosci Ther 2019; 25: 638–646. DOI: 10.1111/cns.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross RL, Beck AW. Fibrinogen and catheter-directed thrombolysis. Semin Vasc Surg 2014; 27: 182–195. DOI: 10.1053/j.semvascsurg.2015.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211017001 for Magnetic resonance black-blood thrombus imaging can confirm chronic cerebral venous thrombosis: a case report and literature review by Xiaoqin Wu, Jingkun Sun, Zhiying Chen, Yuchuan Ding and Ran Meng in Journal of International Medical Research