Abstract
CONSPECTUS: Metals are partners for an estimated third of the proteome and vary in complexity from mononuclear centers to organometallic cofactors. Vitamin B12 or cobalamin represents the epitome of this complexity and is the product on an assembly line comprising some 30 enzymes. Unable to biosynthesize cobalamin, mammals rely on the dietary provision of this essential cofactor that is needed by just two enzymes, one each in the cytoplasm (methionine synthase) and in the mitochondrion (methylmalonyl-CoA mutase). Brilliant clinical genetics studies on patients with inborn errors of cobalamin metabolism spanning several decades, had identified at least seven genetic loci in addition to the two encoding B12 enzymes (see graphic). While cells are known to house a cadre of chaperones dedicated to metal trafficking pathways that contain metal reactivity and confer targeting specificity, the seemingly supernumerary chaperones in the B12 pathway had raised obvious questions as to the rationale for their existence.
With the discovery of the genes underlying cobalamin disorders, our laboratory has been at the forefront of ascribing functions to B12 chaperones and elucidating the intricate redox-linked coordination chemistry and protein-linked cofactor conformational dynamics that orchestrate the processing and translocation of cargo along the trafficking pathway. These studies have uncovered novel chemistry that exploits the innate chemical versatility of alkyl-cobalamins, i.e., the ability to form and dismantle the cobalt-carbon bond using homolytic or heterolytic chemistry. And they have revealed the practical utility of the dimethylbenzimidazole tail, an appendage unique to cobalamins and absent in the structural cousins, porphyrin, chlorin and corphin, as an instrument for facilitating cofactor transfer between active sites.
In this review, we navigate the chemistry of the B12 trafficking pathway, from its point of entry into cells, through lysosomes, and into the cytoplasm where incoming cobalamin derivatives with a diversity of upper ligands, are denuded by the β-ligand transferase activity of CblC to the common cob(II)alamin intermediate. The broad reaction and lax substrate specificity of CblC also enables conversion of cyanocobalamin (technically, vitamin B12 i.e., the form of the cofactor in one-a-day supplements), to cob(II)alamin. CblD then hitches up with CblC via a unique Co-sulfur bond to cob(II)alamin at a bifurcation point leading to the cytoplasmic methylcobalamin or the mitochondrial 5’-deoxyadenosylcobalamin branches. Mutations at loci upstream of the junction point typically affect both branches leading to homocystinuria and methylmalonic aciduria, whereas mutations in downstream loci lead to one or the other disease. Elucidation of the biochemical penalties associated with individual mutations are providing molecular insights into the clinical data and, in some instances, identifying which cobalamin derivative(s) might be therapeutically beneficial.
Our studies on B12 trafficking are revealing strategies for cofactor sequestration and mobilization from low to high affinity and low to high coordination number sites that are in turn, regulated by protein dynamics that construct ergonomic cofactor binding pockets. While these B12 lessons might be broadly relevant to other metal trafficking pathways, much remains to be learned. The review concludes by identifying some of the major gaps and challenges that are needed to complete our understanding of B12 trafficking.
Graphical Abstract
INTRODUCTION
The discovery of cobalamin or vitamin B12 in 1948 by the groups of Karl Folkers at Merck and Lester Smith at Glaxo4,5 was the culmination of a >20 year search for the anti-pernicious anemia factor described by Whipple, Minot and Murphy6,7. This was followed by elucidation of the crystal structure of vitamin B12 by Dorothy Hodgkin in 19568. Chemically, B12 is a tetrapyrrole that is distinguished by a central cobalt ion coordinated to four nitrogen atoms (Fig.1A). A nucleotide tail extends from the edge of a pyrrole ring and terminates in 5,6-dimethylbenzimidazole (DMB), which serves as the lower or α-axial ligand to the cobalt ion in solution at physiological pH. This tail is missing in other tetrapyrrolic cofactors, i.e., porphyrin, chlorin and corphin. While the role of trans effects from the bulky DMB base on cobalt reactivity was extensively investigated, ironically, the tail is pried away from the cobalt in many B12 proteins, suggesting an alternate role. In a subset of proteins that bind B12 in the “base-off” state (the base referring specifically to DMB), the α-ligand is donated by the protein and is often the histidine residue in a DXHXXG motif9 (Fig. 1B–D). The upper or β-axial ligand position is the site of biological activity and the methyl and 5’ -deoxyadenosyl ligands are found in methylcobalamin (MeCbl) and 5’ -deoxyadenosylcobalamin (AdoCbl), respectively.
Figure 1.

A. Chemical structure of cobalamin in which R represents the upper ligand while DMB occupies the lower axial position.B,C,D. Alternate conformations in which the DMB is found in the base-on (B), base-off (C) or base-off/His-on (D) states. (E) The Co-carbon bond in MeCbl is cleaved heterolytically in the presence of a thiolate, generating cob(I)alamin and a thioether as in MS or cleaved homolytically in AdoCbl, leading to cob(II)alamin and the 5’-deoxyadenosyl radical (dAdo•).
In mammals, only two enzymes catalyze B12-dependent reactions: MeCbl-dependent methionine synthase (MS) and AdoCbl-dependent methylmalonyl-CoA mutase (MCM).10 The dual susceptibility of the Co-carbon bond in alkylcob(III)alamins to heterolytic and homolytic cleavage is key to their recruitment by enzymes that catalyze chemically daunting transformations (Fig. 1E). The supernucleophilic cob(I)alamin and paramagnetic cob(II)alamin products of the heterolytic and homolytic reactions, respectively, support SN2 versus radical chemistry.
The full B12 assembly line comprising ~30 enzymes is restricted in distribution and is found in some archaea and bacteria.11 While some prokaryotes salvage corrinoids from the environment and elaborate them into cobalamins, animals lack the capacity for de novo B12 biosynthesis. Instead, they acquire this essential cofactor from the diet and to a limited extent, from gut microbes.12 Following intestinal absorption and release into circulation, a process that depends on several B12 binding and transport proteins, a dedicated cadre of chaperones, ferry and process the cofactor as it journeys through three compartments to find its way to the two client enzymes: cytoplasmic MS and mitochondrial MCM.13–15 Mutations in the trafficking proteins are inherited as autosomal recessive disorders and lead to either isolated or combined homocystinuria and methylmalonic aciduria due to a deficiency in MS or MCM, or both.16 Careful clinical genetics studies on cobalamin deficiency patients provided early clues to the existence of an elaborate trafficking pathway and led to the identification of nine culpable genetic loci (cblA-G, cblJ and mut).
The supernumerary chaperones in B12 trafficking raise obvious questions as to their roles. The cofactor’s large size and its perimetric propionamide and acetamide groups, which help tether it via electrostatic interactions to active sites, pose challenges for its facile translocation between proteins. Further, redox-linked coordination preferences impose geometric constraints and significantly influence cobalamin redox potentials,17 raising questions as to whether these features are gainfully exploited for cofactor movement and for containing reactivity. In the biologically relevant oxidation states, the preferred coordination geometry varies from 6-coordinate (6-c) for cob(III)alamin to 5-c and 4-c for cob(II)alamin and cob(I)alamin, respectively.18 In this review, we discuss the bioinorganic chemistry of B12 trafficking with a focus on studies from our laboratory that have led to their elucidation.
INTRACELLULAR ENTRY OF B12
Lysosomal processing and base-on to base-off transition
The B12 cargo bound to transcobalamin II (TCII) in circulation, is recognized by the transcobalamin receptor and endocytosed into the lysosome (see conspectus graphic).19 The crystal structure of TCII with bound aquocobalamin (H2OCbl), reveals that B12 is in the “base-on” state with a histidine occupying the β-ligand position.20 The histidine does not however, preclude binding of other cobalamins with stronger β-ligands like MeCbl and AdoCbl, which are found in circulation, as well as cyanocobalamin (CNCbl or vitamin B12).21 The lack of β-ligand specificity represents an as yet unrealized opportunity for using TCII to target cancer cells that overexpress the cognate TCII receptor to deliver cytotoxic or imaging agents conjugated to cobalamin.22
Inside the lysosome, proteases release cobalamin from the femtomolar grip of TCII. Mutations in either the cblF or cblJ locus, which encode lysosomal proteins needed for the cofactor’s egress into the cytoplasm, cause a functional intracellular deficiency.23,24 The cblJ locus encodes a homodimeric ATP-binding cassette transporter protein, ABCD4, which is postulated to power the transmembrane movement of cobalamin by ATP hydrolysis. A cryo-EM structure of the human protein revealed a cytosolic nucleotide binding domain and a transmembrane segment that was captured in the lysosomal-open state.25 The cblF locus encodes LMBD1, which apparently stabilizes and targets ABCD4 to the lysosome.24,26
The ABCD4 structure does not provide insights into whether the cofactor is transported with or without DMB coordination. We have speculated that the acidic pH of the lysosome, which is estimated to be 4.5-5,27 favors the base-off state, and that the cofactor remains base-off and protein-bound upon exiting this compartment.13 Maintaining the base-off state could be advantageous for freeing up a cobalt coordination site as well as the tail, to serve as molecular handles for moving the cofactor between active sites.
CYTOPLASMIC JOURNEY OF B12 TO METHIONINE SYNTHASE
The β-ligand transferase activity of CblC
Full-length CblC (also known as MMACHC) comprises 282 amino acids and does not have an identifiable mitochondrial targeting sequence although it was included in the MitoCarta database.28 We have proposed that CblC receives the cobalamin cargo as it enters the cytoplasm. The lack of β-ligand specificity in TCII is mirrored in CblC, which accommodates a diversity of cobalamin derivatives.29 CblC is tasked with converting a medley of B12 derivatives into a common cob(II)alamin intermediate that is subsequently apportioned to meet cellular needs for MeCbl versus AdoCbl.30,31 CblC represents a hotbed of mutations in the B12 trafficking pathway and its deficiency leads to combined homocystinuria and methylmalonic aciduria32 consistent with its role upstream of the cytoplasmic and mitochondrial branchpoint.
Crystal structures of CblC33–36 revealed that it belongs to the flavin nitroreductase superfamily and binds cobalamin in a base-off state with the DMB tail held in a hydrophobic cleft (Fig. 2A). The commodious active site pocket explains how CblC accommodates a range of β-ligands.29 A cluster of arginine residues (Arg-161, -206 and -230 in the human sequence) make crucial contacts with glutathione (GSH), a co-substrate (Fig. 2B).35 Although CblC lacks the canonical flavin binding site seen in the nitroreductase superfamily, it can use reduced flavin as an electron source in the decyanation reaction.33 The affinity of CblC for FMN increases 3-fold in the presence of cobalamin,33 which induces a loop movement in a region that is homologous to the flavin binding site in other superfamily members (Fig. 2A).
Figure 2.

Structure and mechanism of CblC. A. Structure of human CblC (PDB:3SC0) with MeCbl (red). The N-terminal core and the C-terminal cap domains are in navy and green respectively. The cyan loop moves towards the active site upon cobalamin binding, creating what could be a putative flavin binding site. B. Close-up of the active site (PDB:5UOS) showing interactions between GSH (blue) and arginine residues. C. Reaction mechanism and the thiol oxidase activity of CblC showing the alternate fates of the 5-c cob(II)alamin intermediate. X = CN, H2O or NO2; R= Me or dAdo.
The reactions catalyzed by CblC are described by equations 1–5, where “R” (equation 1) is an alkyl or thiolato group and GSR, the corresponding thioether or disulfide.
| [1] |
| [2] |
| [3] |
| [4] |
| [5] |
The dealkylation reaction catalyzed by CblC (equation [1]) involves an SN2 attack by the thiolate of GSH on the alkyl or thiolato group, cleaving the Co-carbon bond heterolytically.31 The resulting cob(I)alamin product is rapidly oxidized to 5-c cob(II)alamin with a water ligand as revealed by EPR spectroscopy. The redox potential of base-off H2O-cob(III)alamin/cob(II)alamin (+510 mV) is 310 mV more positive than for the base-on couple,17 which stabilizes CblC-bound cob(II)alamin against oxidation to H2OCbl by O2.36 However, CblC-bound cob(II)alamin is susceptible to oxidation by superoxide due to the difference in the redox potentials for the H2O2/O2•− (+940 mV) versus the O2/O2•− (−330 mV) couples,37 and sets up a chemical vulnerability to futile thiol oxidation cycles (Fig. 2C).
The reductive decyanation reaction (equation [2]) catalyzed by CblC involves homolytic cleavage of Co-carbon bond eliminating cyanide and forming cob(II)alamin.30 Hence, while the initial products of the decyanation and dealkylation reactions are distinct, both lead to cob(II)alamin in the cellular milieu. GSH serves as a one electron donor in the decyanation reaction and in the reductase reactions (equations [3] and [4]).38,39 The thiol oxidase activity and consequent glutathione disulfide (GSSG) formation is particularly robust with the Caenorhabditis elegans CblC,38 leading to concomitant O2 scrubbing and the unprecedented stabilization of cob(I)alamin in an initially aerobic reaction mixture (Fig. 2C).40 The stabilization of cob(I)alamin is remarkable since it is a supernucleophile with a nucleophilic reactivity constant of 14.4.41
There are dual entry points to the thiol oxidase cycle via dealkylation and reductive elimination reactions, which lead to the common 5-c cob(II)alamin (Fig. 2C, steps i/ii or vii). Cob(II)alamin can be further oxidized to H2OCbl by superoxide generated in situ thereby exiting the cycle (step viii), which is the preferred route in human CblC.42 Alternatively, the H2O ligand is exchanged by a chloride anion (present in buffers to stabilize the protein), forming an unusual chloro-cob(II)alamin intermediate (step iii).43 A second ligand exchange is postulated to lead to glutathionyl-cob(II)alamin, which following oxidation and dethiolation steps, regenerates cob(I)alamin (steps iv-vi). The affinity for chloride (Kact(app)= 76 ± 4 mM) relative to its intracellular concentration (4-15 mM) reveals how the spurious thiol oxidase activity is curtailed in cells and is an example of a catalytic potential that is suppressed under cellular conditions. Arg-161 in human CblC (Fig. 2B) is postulated to play a key role in favoring cob(II)alamin oxidation and dampening the thiol oxidase activity. The clinical mutations R161G and R161Q on the other hand, unleash the thiol oxidase activity and could contribute to the oxidative stress associated with CblC deficiency disease.42
Non-natural antivitamin B12 derivatives inhibit the β-ligand transferase activity of CblC, inducing B12 deficiency in mice.44 The aryl- (4-ethylphenyl-cobalamin) and alkynyl- (2-phenylethynyl-cobalamin, 2,4-difluorphenylethynyl-cobalamin) derivatives are exceptionally stable to heat and nucleophilic substitution, while the alkynyl-cobalamins are additionally stable to photolysis. The antivitamin derivatives that have been tested so far, bind with nanomolar affinity to CblC, and are resistant to Co-carbon bond cleavage by GSH.35,45 The therapeutic potential of antivitamin B12 derivatives as antiproliferative agents remains to be developed.
A novel interprotein Co-sulfur coordination complex between CblC and CblD
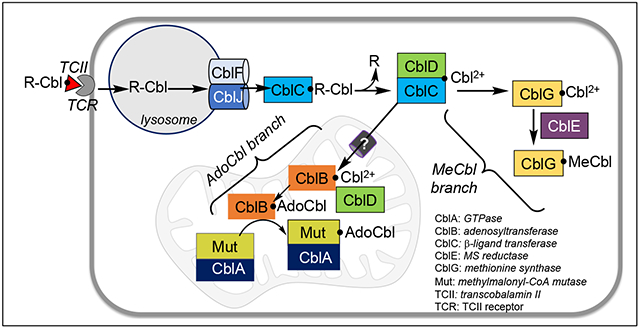
Full-length CblD (also known as MMADHC) comprises 296 amino acids with the putative leader sequence extending from residues 1-11.46 Mutations in the N- and C-terminal regions of CblD uniquely impact the AdoCbl and MeCbl branches, respectively, while mutations in the central region, impact both (Fig. 3A).46 Internal translation initiation sites at Met-62 and Met-116 allow bypassing of clinical mutations between residues 1-115 and continued support of the MeCbl branch.47 The precise role of CblD in regulating B12 traffic and the structural basis of how the C-terminal domain uniquely supports the cytoplasmic branch remain to be elucidated. On the other hand, the role of the N-terminal domain in uniquely regulating the mitochondrial branch is explained in part by its housing the leader sequence (Fig. 3A). The relative AdoCbl versus MeCbl pool size is influenced by CblD as exemplified by an increase in AdoCbl and a corresponding decrease in MeCbl levels when the inefficient natural leader is replaced by a strong targeting sequence.47
Figure 3.
Organization and structure of human CblD. A. Schematic representation showing locations of the mitochondrial leader sequence (MLS) and the alternate initiation sites at Met-62 and Met-116, leading to proteins of varied lengths (arrows). Mutations in the N-terminal region (1-115) result in isolated methylmalonic aciduria (MMA), mutations near the C-terminus (197-259) lead to isolated homocystinuria (HC), while mutations in the central region lead to both. B Structure of CblD (PDB: 6X8Z) starting at residue 133 (denoted as N) with thiolato-cob(III)alamin bound at Cys-261. The secondary structure elements in which mutations lead to isolated homocystinuria are shown in green C. Surface representation of CblD showing the location of individual residues (yellow) in the 197-259 region (green) that lead to isolated homocystinuria when mutated.
The crystal structure of CblD missing the N-terminal third of the protein, which is predicted to be largely disordered, revealed that it most closely resembles CblC.48,49 We have recently obtained a structure of human CblD in which cobalamin is tethered via a novel 2.2 Å long Co-sulfur bond to Cys-261 (Fig. 3B),3 forming a base-on thiolato-cob(III)alamin complex. A map of the secondary structure elements in which mutations lead to the isolated homocystinuria phenotype, identifies the surface that is critical for the MeCbl branch and includes half of the B12-coodinating β-hairpin loop (Fig. 3B,C).
CblD forms a complex with CblC albeit only when cob(II)alamin is bound (Fig. 2C, step ix); the first 115 residues in CblD are dispensable for this interaction.50 Under aerobic conditions, the resulting CblC-CblD complex forms stoichiometrically and the spectrum reveals formation of base-off thiolato-cob(III)alamin.3 The Co-sulfur bond in the CblC-CblD thiolato-cob(III)alamin complex has been estimated to be 2.57 Å by EXAFS. The thiolato-cob(II)alamin intermediate in the CblC-CblD complex can be stabilized under anaerobic conditions and has been characterized by EPR spectroscopy.3 Complex formation with CblD is precluded if the β-ligand site is not vacant as in CblC-bound MeCbl, highlighting the critical role of coordination chemistry. Furthermore, β-ligand exchange between CblC-bound H2OCbl and the Cys-261 thiolate on CblD is not observed. The CblC-CblD complex is also formed if B12 is initially bound as thiolato-cob(III)alamin to CblD. However, the weak affinity of CblD for H2OCbl and the slow binding kinetics render formation of the CblC-CblD complex via this route unlikely to be physiologically relevant.3 In contrast, CblC binds alkylcobalamins with high affinity and catalyzes their dealkylation,40 setting up an alternative route for complex formation with CblD. Interestingly, a subset of clinical mutations in CblD decrease the rate of thiolato-cob(II)alamin oxidation in the CblC-CblD complex,48 suggesting a functional role for this reaction in trafficking.
Coordination chemistry controls cofactor reactivity in methionine synthase
Large gaps exist in our understanding of how cobalamin is transferred from the CblC-CblD complex to MS (or CblG51,52), the cytoplasmic target of the B12 trafficking pathway. The well characterized E. coli MS serves as a model for understanding the reaction mechanism of the human enzyme whose primary role is to turn over 5-methyl-tetrahydrofolate (CH-3-THF), which enters cells from circulation, to THF, which supports one-carbon metabolism.53 This conversion is achieved in two steps in which the methyl group is transiently transferred to cob(I)alamin forming MeCbl (Fig. 4A). The susceptibility of cob(I)alamin to oxidative interception sets up a metabolic liability and the dependence on a reactivation system comprising the diflavin oxidoreductase, MS reductase (MSR or CblE), and S-adenosylmethionine (AdoMet).54
Figure 4.

Structure and reaction catalyzed by MS. A. Scheme showing the catalytic (black) and reactivation (blue) cycles of MS. The base-off state of cobalamin is denoted by the downward arrow. B. MS is multi-domainal and the double headed arrows labeled [1]-[3] represent the interactions between the individual substrate and the B12 domain that enable the three methyl transfer reactions shown in (A). C. Structure of the B12 and activation domains of E. coli MS (PDB 3IVA). The four-helix cap segment within the B12 domain (light blue) moves out of the way when the AdoMet domain is bound. S-adenosylhomocysteine (AdoHcy), cob(II)alamin and the histidine and tyrosine residues that govern coordination state changes, are shown in stick display.
Human MS is a 140 kDa monomeric protein and like its E. coli ortholog, is predicted to be modular in organization.10 The N-terminal homocysteine and CH3-THF domains engage alternately with the B12 domain to enable the methyl transfer steps involved in the catalytic cycle while the C-terminal AdoMet and B12 domains engage in the reactivating methyl transfer cycle (Fig. 4B). Cobalt oxidation-linked coordination state changes are critical for orchestrating this complex molecular juggling act.
Cobalamin is bound in a base-off conformation and during the catalytic cycle, alternates between 4-c cob(I)alamin and 6-c MeCbl, respectively (Fig. 4A). The α-ligand position is occupied by the histidine in the DxHxxG motif in the His-on/base-off state (Fig. 1A).9 In the inactive cob(II)alamin state, the histidine ligand is rotated away and engaged in stabilizing contacts between the B12 and AdoMet domains (Fig. 4C).55 This change in the coordination environment leads to displacement of the cobalt by 2.9 Å and the appearance of a β-axial water ligand.55 The AdoMet domain belongs to the flavin nitroreductase subfamily that includes CblC33 and CblD.48 AdoMet binding induces a conserved tyrosine residue to wedge in and lengthen the β-axial Co2+-H2O bond, stabilizing 4-c cob(II)alamin and parking the water ligand via hydrogen bonding interactions between the tyrosine and an adjoining glutamate (also conserved in human MS).56 Formation of 4-c cob(II)alamin facilitates its reduction to square planar cob(I)alamin, and represents a potential safety valve for averting futile electron transfer in the absence of the methyl donor.
The cob(II)alamin/cob(I)alamin redox potential for E. coli MS increases from −526 mV57 to −490 mV56 when the α-axial histidine is substituted by a β-axial water. The corresponding value for the human MS is not known. The redox potential for the FMN semiquinone/hydroquinone couple in human MSR is in the −175 to −227 mV range depending on the polymorphic variant.58 The unfavorable reduction of cob(II)alamin to cob(I)alamin is kinetically coupled to an exergonic methylation reaction.57
MSR transfers reducing equivalents from NADPH via FMN and FAD to cob(II)alamin bound to MS (Fig. 4A).54 Mutations in MS and MSR lead to isolated homocystinuria, i.e. without accompanying methylmalonic aciduria.16 The two common polymorphic variants in MSR, I22M and S175L exhibit only subtle differences in the kinetics of electron transfer that are ascribed to differences in their relative affinities for MS.58,59 Interestingly, the Met-22 background is essential for the phenotypic expression of the pathogenic V56M mutation, revealing how a single nucleotide polymorphism can modulate disease presentation.60 In human fibroblasts harboring MSR mutations, MS is present predominantly in the holoenzyme form,61 indicating that MSR does not play a role in cofactor loading onto MS.
MITOCHONDRIAL JOURNEY TO METHYLMALONYL-COA MUTASE
The path for cobalamin entry into the mitochondrion is not known. In the matrix, adenosyltransferase (ATR, also known as MMAB or CblB) and CblA (or MMAA), a G-protein chaperone, are recruited for the synthesis and delivery of AdoCbl to the target enzyme, MCM (or Mut) (see conspectus graphic). Mutations in any of these three proteins lead to isolated methylmalonic aciduria while sparing the MeCbl branch of the trafficking pathway.16
Loop dynamics control cobalt coordination and reactivity in adenosyltransferase
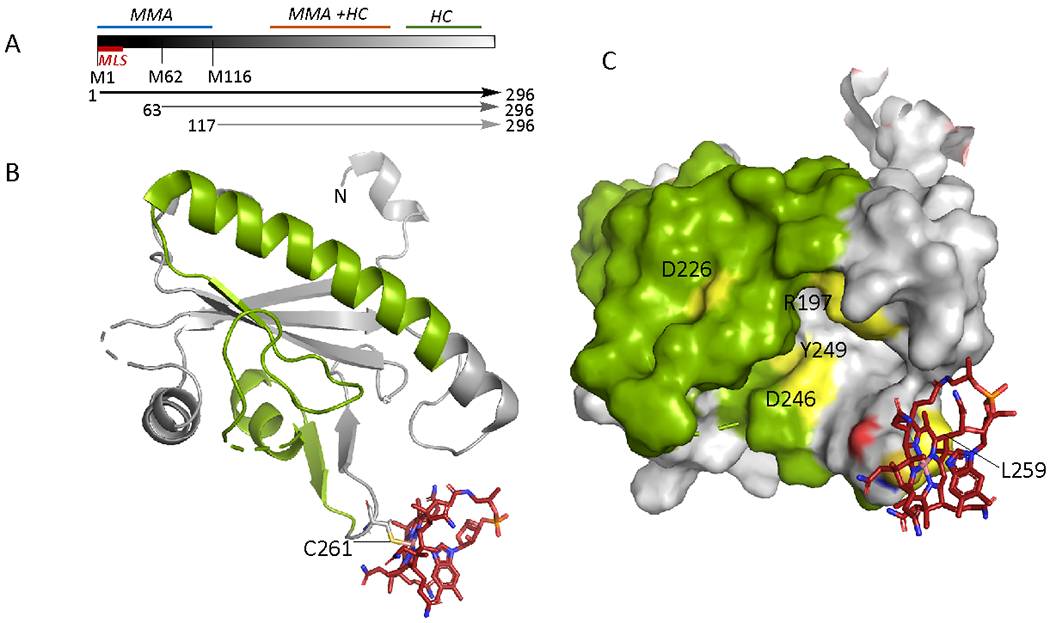
ATR, an 81 kDa homotrimer with active sites housed at the subunit interfaces (Fig. 5A),62 catalyzes the adenosylation of cob(I)alamin by ATP. A common polymorphic variation in ATR, Met-239 versus Lys-239 leads to variants that exhibit very similar overall reaction kinetics.63 Human ATR was the first chaperone that was shown to bind the cofactor in the base-off state,64,65 mirroring the mitochondrial target MCM,66 which led us to propose, and later to demonstrate, that it is both an enzyme for AdoCbl synthesis and an escort for its targeted delivery.67,68 We subsequently described an additional role for ATR in repair i.e., by accepting cob(II)alamin bound to inactive MCM.69
Figure 5.
Structures of ATR. A. Structure of human ATR (PDB:2IDX) with ATP (orange sticks) bound. The 3 subunits are shown in different colors and the purple spheres represent Mg2+. The N-terminal 24 residues following the leader sequence were deleted to aid crystallization. B, C. Structure (B) and close-up (C) of cob(II)alamin bound to Mtb ATR in the presence of PPPi (PDB: 6WH5). The N-terminal 3-28 amino acids (surface display) form a cup around cob(II)alamin, forcing the DMB tail into a side pocket. D,E. Structure (D) and close-up (E) of AdoCbl bound to Mtb ATR (PDB: 6WGS). The N-terminus is only partially ordered (surface display), which allows the DMB tail to be tucked underneath the corrin ring. The positioning of Phe-117 precludes coordination of cobalt by water (C) or DMB (E).
In its twin roles as an enzyme and an escort, ATR is tasked with: (i) easing the thermodynamically difficult reduction of cob(II)alamin to cob(I)alamin, (ii) facilitating the transfer of AdoCbl to MCM, and (iii) averting the transfer of cob(II)alamin to apo-MCM while accepting cob(II)alamin from inactive MCM for repair (Fig. 6A). Coordination chemistry and ATR loop dynamics are critical for supervising these diverse functions.2 ATP binding organizes a high affinity cob(II)alamin pocket in human ATR.62 In the Mycobacterium tuberculosis (Mtb) ATR structure with cob(II)alamin and inorganic triphosphate (PPPi) bound,2 the N-terminus residues spanning 3-28 become ordered forming the roof and floor of the B12 binding pocket and splaying the DMB tail in a side pocket (Fig. 5B,C). A conserved phenylalanine, Phe-117 (Mtb numbering), below the α-face disallows water coordination. EPR spectroscopy reveals that cob(II)alamin is 5-c (with a β-axial H2O ligand) in the presence of PPPi but 4-c in the presence of ATP. 2,65 The square planar geometry of cob(II)alamin raises the redox potential, facilitating formation of 4-c cob(I)alamin, in a step preceding the adenosylation reaction (Fig. 6A). In the crystal structure, the water on the β-face is parked away from the cobalt via a hydrogen-bonding interaction with a phosphate group in PPPi, in contrast to the 5-c cob(II)alamin state in the same crystals seen by EPR spectroscopy. The presence of 4-c cob(II)alamin in the crystal structure suggests that reduction to cob(I)alamin might have occurred in the X-ray beam. The parking structure for a labile water ligand in Mtb ATR is reminiscent of that seen in MS56 and could be a general strategy for facile control of coordination geometry with a weak ligand.
Figure 6.

Functions of ATR and structure and mechanism of MCM. A. Reaction mechanism and roles of ATR. B. The structure of human MCM (PDB:2XIJ) highlighting AdoCbl (red), malonyl-CoA (orange), substrate- (grey) and the B12- (pink) domains in each monomer. C. Reaction mechanism of MCM.
In the presence of AdoCbl, the N-terminal 12 residues are disordered and the active site is only partially organized around B12 (Fig. 5D,E). This allows the DMB tail to move back towards the corrin ring although Phe-117 (Mtb numbering) still precludes direct coordination and enforces an unfavorable 5-c geometry for AdoCbl. In comparison to cob(II)alamin, which is buried in the active site, AdoCbl is relatively surface exposed. This difference in solvent exposure could be germane to promoting AdoCbl transfer to MCM but precluding cob(II)alamin transfer (Fig. 5B). The role of the DMB tail as an instrument for facilitating cofactor transfer to MCM is supported by the failure of ATR to transfer adenosylcobinamide with a truncated tail.2
Remarkably, if ATR is unable to transfer AdoCbl to MCM, it catalyzes Co-carbon bond homolysis in an unconventional reversal of the Co-carbon bond making reaction that uses SN2 chemistry (Fig. 6A). Reformation of cob(II)alamin, which binds 12-fold more tightly than AdoCbl, is postulated to serve as a cofactor sequestration strategy.62 The other product of this reaction is hydroperoxyadenosine, which is formed in the presence of molecular O2.
A G-protein coordinates cofactor movement to and from MCM
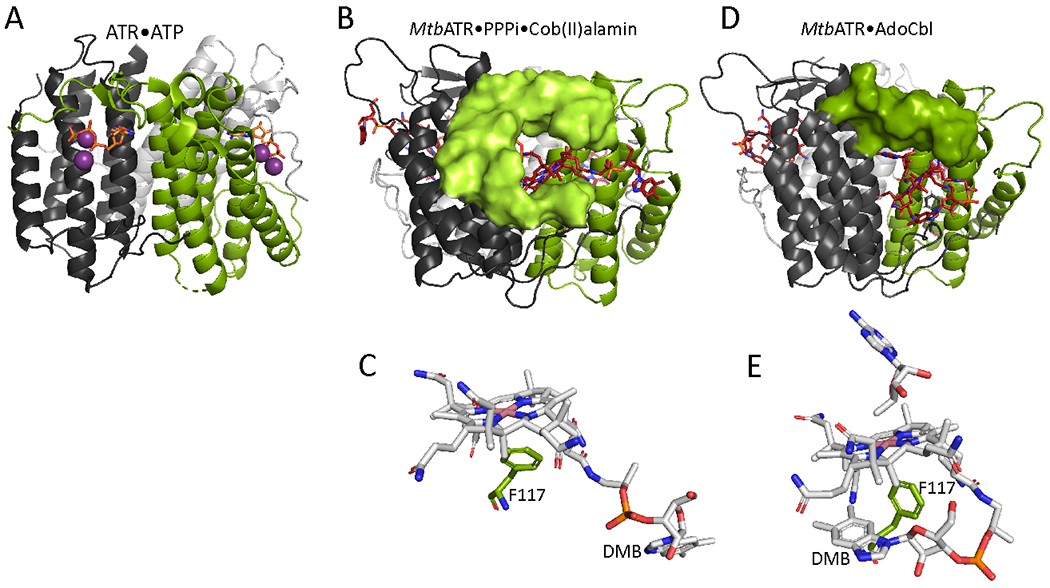
The transfer of AdoCbl from ATR to MCM and of cob(II)alamin in the reverse direction, is gated by CblA, a 92 kDa G-protein chaperone (Fig. 6A). Human MCM (Fig. 6B) and CblA are homodimers and early molecular insights into CblA were derived from our studies on the homologous protein in Methylobacterium extorquens 69–71 and on IcmF,72 in which the G-protein is fused to isobutyryl-CoA mutase73 (Fig. 7). CblA is a member of the P-loop GTPase superfamily and has the signature switch I and II loops that undergo nucleotide-sensitive conformational changes that are important for signaling.74,75 A third conformationally plastic switch III loop is also important for bidirectional signaling to MCM.76 In IcmF, the nucleotide binding site hinges the cobalamin and G-domains, which move as a rigid body with respect to the substrate binding domain.72,77 The G-domain regulates equilibration of the cobalamin domain between open and closed states. In the open state, captured with cob(II)alamin bound (Fig. 7A), the loop bearing the histidine ligand and the B12 binding pocket are solvent accessible.
Figure 7.
IcmF structures captured in open and closed states. The structure of an IcmF protomer (PDB:5CJT) is shown; the structured dimer interface region (418-579) has been omitted for clarity. A. In the open state, cob(II)alamin is bound in the B12 (yellow) domain, which along with the G-protein (blue) domain is held away from the substrate domain (green). B. With AdoCbl (red) and substrate (isobutyryl-CoA) bound, the B12 and G-protein domains move as a rigid body towards the substrate domain. GDP (sticks) and Mg2+ (spheres) are in pink.
Presumably, this conformation enables cofactor offloading to ATR. In the closed state, captured with AdoCbl and isobutyryl-CoA bound, the cofactor is buried deep in the active site and is proximal to the substrate, in a catalytically ready conformation (Fig. 7B). The G-domain interacts with both the substrate and cobalamin domains. The mechanism by which GTP binding and hydrolysis energy are transduced to gate cofactor movement between MCM and ATR is unknown.
Unlike the bacterial CblA and MCM homologs, which interact predominantly in a 2MCM:1CblA stoichiometry, the human proteins exist in a mixture of oligomeric states, interconverting between linear and annular forms in a nucleotide sensitive manner.78 The intrinsic GTPase activity of human CblA is enhanced ~50-fold in the presence of MCM.78 The potential regulatory import of the various MCM-CblA complexes is unknown.
MCM is a 166 kDa homodimer that binds AdoCbl in the base-off/His-on state and catalyzes the 1,2 rearrangement of methylmalonyl-CoA to succinyl-CoA (Fig. 6C). The reaction is initiated by homolysis of the Co-carbon bond as has been postulated for other AdoCbl-dependent isomerases, and the hydrogen atom transfer occurs via quantum tunneling.79 We recently captured the elusive primary deoxyadenosyl radical as an addition product using the substrate decoy, itaconyl-CoA,1 a derivative of the immunometabolite, itaconate.80 The radical rearrangement reaction leads to the occasional loss of the deoxadenosyl moiety and therefore, failure to complete the catalytic cycle.69 The resulting inactive cob(II)alamin state is transferred to ATR for repair in a reaction that is driven by CblA-catalyzed GTP hydrolysis (Fig. 6A).69
FUTURE PERSPECTIVES
Over the past 15 years, biophysical studies on B12 trafficking proteins have furnished rich insights into how the individual components multitask as enzymes and escorts as they assimilate the cofactor into its biologically active forms and ferry it to two target proteins. These studies have also unearthed novel chemistry enabled by protein architectural dynamics, which exert exquisite control over redox-linked coordination geometry and ultimately, cobalamin reactivity. These exciting advances are balanced by large gaps in our understanding of how cobalamin exits the lysosome or enters the mitochondrion, the role of CblD and possibly CblC, in the mitochondrial branch, the mechanism of forward translocation of the tightly bound cobalamin from the CblC-CblD complex, and the intricate interprotein signalling that coordinates cofactor loading onto MS and MCM and off-loading from MCM. The interprotein complexes formed during B12 trafficking, some transient (e.g. between ATR and MCM) and others stable (e.g. between CblC and CblD), have eluded structural characterization so far and their elucidation should be richly informative. Finally, a vast and largely unexplored interface is whether and how the cofactor demands of the microbiome influence host B12 metabolism and whether the host in turn, can leverage the diverse microbial B12 pool for its own needs.
KEY REFERENCES.
Ruetz, M.; Campanello, G. C.; Purchal, M.; Shen, H.; McDevitt, L.; Gouda, H.; Wakabayashi, S.; Zhu, J.; Rubin, E. J.; Warncke, K.; Mootha, V. K.; Koutmos, M.; Banerjee, R. Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science 2019, 366, 589-593.1 The elusive 5’-deoxyadenosyl radical generated via Co-carbon bond homolysis in coenzyme B12 was captured and characterized structurally.
Mascarenhas, R.; Ruetz, M.; McDevitt, L.; Koutmos, M.; Banerjee, R. Mobile loop dynamics in adenosyltransferase control binding and reactivity of coenzyme B12. Proc Natl Acad Sci U S A 2020, 117, 30412-30422.2 The role of protein dynamics in differentially controlling reactivity and access to the inactive versus active B12 cofactor was demonstrated via a series of crystallographic snapshots.
Li, Z.; Mascarenhas, R.; Twahir, U. T.; Kallon, A.; Deb, A.; Yaw, M.; Penner-Hahn, J.; Koutmos, M.; Warncke, K.; Banerjee, R. An Interprotein Co-S Coordination Complex in the B12-Trafficking Pathway. J Am Chem Soc 2020, 142, 16334-16345.3 The crystal structure of CblD coordinated to B12 via a Co-S bond was reported and the novel interprotein Co-S bond in the CblC-CblD complex was established by various spectroscopic methods.
ACKNOWLEDGEMENTS
This work was supported by the NIH (DK45776).
Biographies
Biographies
Ruma Banerjee obtained her PhD in biochemistry from Rensselaer Polytechnic Institute, NY and postdoctoral training in biophysics at the University of Michigan. Her independent academic career began in 1991 at the University of Nebraska-Lincoln, where she was the founding director of the Redox Biology Center. In 2007, she relocated to Michigan Medicine. Her research program focuses on the enzymes, coenzymes and metabolic pathways that support and interact with the sulfur network in mammals. She is the co-Director of the NIH-funded MOSAIC program in the ASBMB.
Harsha Gouda obtained his BS and MS degrees from the Indian Institute of Science Education and Research, Pune. He is currently pursuing his PhD studies in Prof. Banerjee’s laboratory where he is investigating cobalamin trafficking.
Shubhadra Pillay is a Postdoctoral Fellow at Michigan Medicine. She obtained a PhD in structural biology from Nanyang Technological University, Singapore and was a postdoctoral fellow in biophysics at the Max Plank Institute for Biophysical Chemistry, Göttingen. Her research focuses on understanding protein conformational dynamics to gain insights into mechanisms that cause diseases.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Ruetz M; Campanello GC; Purchal M; Shen H; McDevitt L; Gouda H; Wakabayashi S; Zhu J; Rubin EJ; Warncke K; Mootha VK; Koutmos M; Banerjee R Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science 2019, 366, 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Mascarenhas R; Ruetz M; McDevitt L; Koutmos M; Banerjee R Mobile loop dynamics in adenosyltransferase control binding and reactivity of coenzyme B12. Proc Natl Acad Sci U S A 2020, 117, 30412–30422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Li Z; Mascarenhas R; Twahir UT; Kallon A; Deb A; Yaw M; Penner-Hahn J; Koutmos M; Warncke K; Banerjee R An Interprotein Co-S Coordination Complex in the B12-Trafficking Pathway. J Am Chem Soc 2020, 142, 16334–16345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Smith EL Purification of anti-pernicious anaemia factors from liver. Nature 1948, 161, 638. [DOI] [PubMed] [Google Scholar]
- (5).Rickes EL; Brink NG; Konivszy FR; Wood TR; Folkers K Crystalline vitamin B12. Science 1948, 107, 396–397. [DOI] [PubMed] [Google Scholar]
- (6).Whipple GH; Robscheit-Robbins FS Blood regeneration in severe anemia: favorable influence of liver, heart and skeletal muscle in diet. Am. J. Physiol. 1926, 72, 408–418. [Google Scholar]
- (7).Minot GR; Murphy WP Treatment of pernicious anemia by special diet. JAMA 1926, 87, 470–476. [Google Scholar]
- (8).Hodgkin DC; Kamper J; Mackay M; Pickworth JW; Trueblood KN; White JG Structure of vitamin B12. Nature (London) 1956, 178, 64–66. [DOI] [PubMed] [Google Scholar]
- (9).Drennan CL; Huang S; Drummond JT; Matthews RG; Ludwig ML How a protein binds B12: A 3.0 Å X-ray structure of B12-binding domains of methionine synthase. Science 1994, 266, 1669–1674. [DOI] [PubMed] [Google Scholar]
- (10).Banerjee R; Ragsdale SW The many faces of vitamin B12: Catalysis by cobalamin-dependent enzymes. Ann. Rev. Biochem. 2003, 72, 209–247. [DOI] [PubMed] [Google Scholar]
- (11).Roth JR; Lawrence JG; Bobik TA Cobalamin (coenzyme B12): synthesis and biological significance. Annu Rev Microbiol 1996, 50, 137–181. [DOI] [PubMed] [Google Scholar]
- (12).Degnan PH; Taga ME; Goodman AL Vitamin B12 as a modulator of gut microbial ecology. Cell Metab 2014, 20, 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Banerjee R B12 trafficking in mammals: A case for coenzyme escort service. ACS Chem Biol 2006, 1, 149–159. [DOI] [PubMed] [Google Scholar]
- (14).Banerjee R; Gherasim C; Padovani D The Tinker, Tailor, Soldier in intracellular B12 Trafficking. Cur Op Chem Biol 2009, 13, 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Gherasim C; Lofgren M; Banerjee R Navigating the B12 road: assimilation, delivery and disorders of cobalamin. J Biol Chem 2013, 288, 13186–13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Watkins D; Rosenblatt DS Inborn errors of cobalamin absorption and metabolism. Am J Med Genet C Semin Med Genet 2011, 157C, 33–44. [DOI] [PubMed] [Google Scholar]
- (17).Lexa D; Saveant J-M The electrochemistry of vitamin B12. Acc. Chem. Res. 1983, 16, 235–243. [Google Scholar]
- (18).Gruber K; Puffer B; Krautler B Vitamin B(12)-derivatives-enzyme cofactors and ligands of proteins and nucleic acids. Chem Soc Rev 2011, 40, 4346–4363. [DOI] [PubMed] [Google Scholar]
- (19).Youngdahl-Turner P; Rosenberg LE; Allen RH Binding and uptake of transcobalamin II by human fibroblasts. J Clin Invest 1978, 61, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wuerges J; Garau G; Geremia S; Fedosov SN; Petersen TE; Randaccio L Structural basis for mammalian vitamin B12 transport by transcobalamin. Proc Natl Acad Sci U S A 2006, 103, 4386–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wuerges J; Geremia S; Fedosov SN; Randaccio L Vitamin B12 transport proteins: crystallographic analysis of beta-axial ligand substitutions in cobalamin bound to transcobalamin. IUBMB Life 2007, 59, 722–729. [DOI] [PubMed] [Google Scholar]
- (22).Clardy SM; Allis DG; Fairchild TJ; Doyle RP Vitamin B12 in drug delivery: breaking through the barriers to a B12 bioconjugate pharmaceutical. Expert Opin Drug Deliv 2011, 8, 127–140. [DOI] [PubMed] [Google Scholar]
- (23).Coelho D; Kim JC; Miousse IR; Fung S; du Moulin M; Buers I; Suormala T; Burda P; Frapolli M; Stucki M; Nurnberg P; Thiele H; Robenek H; Hohne W; Longo N; Pasquali M; Mengel E; Watkins D; Shoubridge EA; Majewski J; Rosenblatt DS; Fowler B; Rutsch F; Baumgartner MR Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nat Genet 2012, 44, 1152–1155. [DOI] [PubMed] [Google Scholar]
- (24).Rutsch F; Gailus S; Miousse IR; Suormala T; Sagne C; Toliat MR; Nurnberg G; Wittkampf T; Buers I; Sharifi A; Stucki M; Becker C; Baumgartner M; Robenek H; Marquardt T; Hohne W; Gasnier B; Rosenblatt DS; Fowler B; Nurnberg P Identification of a putative lysosomal cobalamin exporter altered in the cblF defect of vitamin B12 metabolism. Nat Genet 2009, 41, 234–239. [DOI] [PubMed] [Google Scholar]
- (25).Xu D; Feng Z; Hou WT; Jiang YL; Wang L; Sun L; Zhou CZ; Chen Y Cryo-EM structure of human lysosomal cobalamin exporter ABCD4. Cell Res 2019, 29, 1039–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Fettelschoss V; Burda P; Sagne C; Coelho D; De Laet C; Lutz S; Suormala T; Fowler B; Pietrancosta N; Gasnier B; Bornhauser B; Froese DS; Baumgartner MR Clinical or ATPase domain mutations in ABCD4 disrupt the interaction between the vitamin B12-trafficking proteins ABCD4 and LMBD1. J Biol Chem 2017, 292, 11980–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Mindell JA Lysosomal acidification mechanisms. Annu Rev Physiol 2012, 74, 69–86. [DOI] [PubMed] [Google Scholar]
- (28).Calvo SE; Clauser KR; Mootha VK MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res 2016, 44, D1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Hannibal L; Kim J; Brasch NE; Wang S; Rosenblatt DS; Banerjee R; Jacobsen DW Processing of alkylcobalamins in mammalian cells: A role for the MMACHC (cblC) gene product. Mol Genet Metab 2009, 97, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kim J; Gherasim C; Banerjee R Decyanation of vitamin B12 by a trafficking chaperone. Proc. Natl. Acad. Sci. U.S.A 2008, 105, 14551–14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kim J; Hannibal L; Gherasim C; Jacobsen DW; Banerjee R A human vitamin B12 trafficking protein uses glutathione transferase activity for processing alkylcobalamins. J Biol Chem 2009, 284, 33418–33424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lerner-Ellis JP; Anastasio N; Liu J; Coelho D; Suormala T; Stucki M; Loewy AD; Gurd S; Grundberg E; Morel CF; Watkins D; Baumgartner MR; Pastinen T; Rosenblatt DS; Fowler B Spectrum of mutations in MMACHC, allelic expression, and evidence for genotype-phenotype correlations. Hum Mutat 2009, 30, 1072–1081. [DOI] [PubMed] [Google Scholar]
- (33).Koutmos M; Gherasim C; Smith JL; Banerjee R Structural basis of multifunctionality in a vitamin B12-processing enzyme. J Biol Chem 2011, 286, 29780–29787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Froese DS; Krojer T; Wu X; Shrestha R; Kiyani W; von Delft F; Gravel RA; Oppermann U; Yue WW Structure of MMACHC reveals an arginine-rich pocket and a domain-swapped dimer for its B12 processing function. Biochemistry 2012, 51, 5083–5090. [DOI] [PubMed] [Google Scholar]
- (35).Ruetz M; Shanmuganathan A; Gherasim C; Karasik A; Salchner R; Kieninger C; Wurst K; Banerjee R; Koutmos M; Krautler B Antivitamin B12 Inhibition of the Human B12 -Processing Enzyme CblC: Crystal Structure of an Inactive Ternary Complex with Glutathione as the Cosubstrate. Angew Chem Int Ed Engl 2017, 56, 7387–7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Li Z; Shanmuganathan A; Ruetz M; Yamada K; Lesniak NA; Krautler B; Brunold TC; Koutmos M; Banerjee R Coordination chemistry controls the thiol oxidase activity of the B12-trafficking protein CblC. J Biol Chem 2017, 292, 9733–9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Muir Wood P The redox potential of the system oxygen--superoxide. FEBS Lett 1974, 44, 22–24. [DOI] [PubMed] [Google Scholar]
- (38).Li Z; Gherasim C; Lesniak NA; Banerjee R Glutathione-dependent One-electron Transfer Reactions Catalyzed by a B12 Trafficking Protein. J Biol Chem 2014, 289, 16487–16497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Mascarenhas R; Li Z; Gherasim C; Ruetz M; Banerjee R The human B12 trafficking protein CblC processes nitrocobalamin. J Biol Chem 2020, 295, 9630–9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Li Z; Lesniak NA; Banerjee R Unusual aerobic stabilization of Cob(I)alamin by a B12-trafficking protein allows chemoenzymatic synthesis of organocobalamins. J Am Chem Soc 2014, 136, 16108–16111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Schrauzer GN; Deutsch E; Windgassen RJ The Nucleophilicity of Vitamin B12s. J. Am. Chem. Soc. 1968, 90, 2441–2442. [DOI] [PubMed] [Google Scholar]
- (42).Gherasim C; Ruetz M; Li Z; Hudolin S; Banerjee R Pathogenic mutations differentially affect the catalytic activities of the human B12-processing chaperone CblC and increase futile redox cycling. J Biol Chem 2015, 290, 11393–11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Li Z; Greenhalgh ED; Twahir UT; Kallon A; Ruetz M; Warncke K; Brunold TC; Banerjee R Chlorocob(II)alamin Formation Which Enhances the Thiol Oxidase Activity of the B12-Trafficking Protein CblC. Inorg Chem 2020, 59, 16065–16072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Mutti E; Ruetz M; Birn H; Krautler B; Nexo E 4-ethylphenyl-cobalamin impairs tissue uptake of vitamin B12 and causes vitamin B12 deficiency in mice. PLoS One 2013, 8, e75312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Ruetz M; Gherasim C; Gruber K; Fedosov S; Banerjee R; Kräutler B Access to organometallic arylcobaltcorrins through radical synthesis: 4-ethylphenylcobalamin, a potential “antivitamin B(12)”. Angew Chem Int Ed Engl 2013, 52, 2606–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Coelho D; Suormala T; Stucki M; Lerner-Ellis JP; Rosenblatt DS; Newbold RF; Baumgartner MR; Fowler B Gene identification for the cblD defect of vitamin B12 metabolism. N Engl J Med 2008, 358, 1454–1464. [DOI] [PubMed] [Google Scholar]
- (47).Stucki M; Coelho D; Suormala T; Burda P; Fowler B; Baumgartner MR Molecular mechanisms leading to three different phenotypes in the cblD defect of intracellular cobalamin metabolism. Hum Mol Genet 2012, 21, 1410–1418. [DOI] [PubMed] [Google Scholar]
- (48).Yamada K; Gherasim C; Banerjee R; Koutmos M Structure of Human B12 Trafficking Protein CblD Reveals Molecular Mimicry and Identifies a New Subfamily of Nitro-FMN Reductases. J Biol Chem 2015, 290, 29155–29166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Deme JC; Miousse IR; Plesa M; Kim JC; Hancock MA; Mah W; Rosenblatt DS; Coulton JW Structural features of recombinant MMADHC isoforms and their interactions with MMACHC, proteins of mammalian vitamin B12 metabolism. Mol Genet Metab 2012, 107, 352–362. [DOI] [PubMed] [Google Scholar]
- (50).Gherasim C; Hannibal L; Rajagopalan D; Jacobsen DW; Banerjee R The C-terminal domain of CblD interacts with CblC and influences intracellular cobalamin partitioning Biochemie 2013, 95, 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Li YN; Gulati S; Baker PJ; Brody LC; Banerjee R; Kruger WD Cloning, mapping and RNA analysis of the human methionine synthase gene. Hum. Molec. Genetics 1996, 5, 1851–1858. [DOI] [PubMed] [Google Scholar]
- (52).Leclerc D; Campeau E; Goyette P; Adjalla CE; Christensen B; Ross M; Eydoux P; Rosenblatt DS; Rozen R; Gravel RA Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum. Molec. Genet. 1996, 5, 1867–1874. [DOI] [PubMed] [Google Scholar]
- (53).Banerjee RV; Matthews RG Cobalamin-dependent methionine synthase. FASEB J. 1990, 4, 1450–1459. [DOI] [PubMed] [Google Scholar]
- (54).Olteanu H; Banerjee R Human methionine synthase reductase, a soluble P-450 reductase-like dual flavoprotein, is sufficient for NADPH-dependent methionine synthase activation. J Biol Chem 2001, 276, 35558–35563. [DOI] [PubMed] [Google Scholar]
- (55).Datta S; Koutmos M; Pattridge KA; Ludwig ML; Matthews RG A disulfide-stabilized conformer of methionine synthase reveals an unexpected role for the histidine ligand of the cobalamin cofactor. Proc Natl Acad Sci U S A 2008, 105, 4115–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Koutmos M; Datta S; Pattridge KA; Smith JL; Matthews RG Insights into the reactivation of cobalamin-dependent methionine synthase. Proc Natl Acad Sci U S A 2009, 106, 18527–18532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Banerjee RV; Harder S, R.; Ragsdale, S. W.; Matthews, R. G. Mechanism of reductive activation of cobalamin-dependent methionine synthase: an electron paramagnetic resonance spectroelectrochemical study. Biochemistry 1990, 29, 1129–1137. [DOI] [PubMed] [Google Scholar]
- (58).Olteanu H; Wolthers KR; Munro AW; Scrutton NS; Banerjee R Kinetic and thermodynamic characterization of the common polymorphic variants of human methionine synthase reductase. Biochemistry 2004, 43, 1988–1997. [DOI] [PubMed] [Google Scholar]
- (59).Olteanu H; Munson T; Banerjee R Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human methionine synthase reductase. Biochemistry 2002, 41, 13378–13385. [DOI] [PubMed] [Google Scholar]
- (60).Gherasim C; Rosenblatt DS; Banerjee R Polymorphic background of methionine synthase reductase modulates the phenotype of a disease-causing mutation. Hum Mutat 2007, 28, 1028–1033. [DOI] [PubMed] [Google Scholar]
- (61).Gulati S; Chen Z; Brody LC; Rosenblatt DS; Banerjee R Defects in auxiliary redox proteins lead to functional methionine synthase deficiency. J Biol Chem 1997, 272, 19171–19175. [DOI] [PubMed] [Google Scholar]
- (62).Campanello GC; Ruetz M; Dodge GJ; Gouda H; Gupta A; Twahir UT; Killian MM; Watkins D; Rosenblatt DS; Brunold TC; Warncke K; Smith JL; Banerjee R Sacrificial Cobalt-Carbon Bond Homolysis in Coenzyme B12 as a Cofactor Conservation Strategy. J Am Chem Soc 2018, 140, 13205–13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Leal NA; Olteanu H; Banerjee R; Bobik TA Human ATP: Cob(I)alamin adenosyltransferase and its interaction with methionine synthase reductase. J Biol Chem 2004, 279, 47536–47542. [DOI] [PubMed] [Google Scholar]
- (64).Yamanishi M; Labunska T; Banerjee R Mirror “base-off” conformation of coenzyme B12 in human adenosyltransferase and its downstream target, methylmalonyl-CoA mutase. J Am Chem Soc 2005, 127, 526–527. [DOI] [PubMed] [Google Scholar]
- (65).Stich TA; Yamanishi M; Banerjee R; Brunold TC Spectroscopic evidence for the formation of a four-coordinate Co2+ cobalamin species upon binding to the human ATP:cobalamin adenosyltransferase. J Am Chem Soc 2005, 127, 7660–7661. [DOI] [PubMed] [Google Scholar]
- (66).Padmakumar R; Taoka S; Padmakumar R; Banerjee R Coenzyme B12 is coordinated by histidine and not dimethylbenzimidazole on methylmalnyl-CoA mutase. J. Am. Chem. Soc. 1995, 117, 7033–7034. [Google Scholar]
- (67).Yamanishi M; Vlasie M; Banerjee R Adenosyltransferase: an enzyme and an escort for coenzyme B12? Trends Biochem Sci 2005, 30, 304–308. [DOI] [PubMed] [Google Scholar]
- (68).Padovani D; Labunska T; Palfey BA; Ballou DP; Banerjee R Adenosyltransferase tailors and delivers coenzyme B12. Nat Chem Biol 2008, 4, 194–196. [DOI] [PubMed] [Google Scholar]
- (69).Padovani D; Banerjee R A G-protein editor gates coenzyme B12 loading and is corrupted in methylmalonic aciduria. Proc Natl Acad Sci U S A 2009, 106, 21567–21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Padovani D; Banerjee R Assembly and protection of the radical enzyme, methylmalonyl-CoA mutase, by its chaperone. Biochemistry 2006, 45, 9300–9306. [DOI] [PubMed] [Google Scholar]
- (71).Padovani D; Labunska T; Banerjee R Energetics of interaction between the G-protein chaperone, MeaB and B12-dependent methylmalonyl-CoA mutase. J. Biol. Chem. 2006, 281, 17838–17844. [DOI] [PubMed] [Google Scholar]
- (72).Jost M; Cracan V; Hubbard PA; Banerjee R; Drennan CL Visualization of a radical B12 enzyme with its G-protein chaperone. Proc Natl Acad Sci U S A 2015, 112, 2419–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Cracan V; Padovani D; Banerjee R IcmF is a fusion between the radical B12 enzyme isobutyryl-CoA mutase and its G-protein chaperone. J Biol Chem 2010, 285, 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Campanello GC; Lofgren M; Yokom AL; Southworth DR; Banerjee R Switch I-dependent allosteric signaling in a G-protein chaperone-B12 enzyme complex. J Biol Chem 2017, 292, 17617–17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Lofgren M; Koutmos M; Banerjee R Autoinhibition and signaling by the switch II motif in the G-protein chaperone of a radical B12 enzyme. J Biol Chem 2013, 288, 30980–30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Lofgren M; Padovani D; Koutmos M; Banerjee R A switch III motif relays signaling between a B12 enzyme and its G-protein chaperone. Nat Chem Biol 2013, 9, 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Jost M; Born DA; Cracan V; Banerjee R; Drennan CL Structural Basis for Substrate Specificity in Adenosylcobalamin-dependent Isobutyryl-CoA Mutase and Related Acyl-CoA Mutases. J Biol Chem 2015, 290, 26882–26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Ruetz M; Campanello GC; McDevitt L; Yokom AL; Yadav PK; Watkins D; Rosenblatt DS; Ohi MD; Southworth DR; Banerjee R Allosteric Regulation of Oligomerization by a B12 Trafficking G-Protein Is Corrupted in Methylmalonic Aciduria. Cell Chem Biol 2019, 26, 960–969 e964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Chowdhury S; Banerjee R Evidence for quantum mechanical tunneling in the coupled cobalt carbon bond homolysis-substrate radical generation reaction catalyzed by methylmalonyl-CoA mutase. J. Am. Chem. Soc. 2000, 122, 5417–5418. [Google Scholar]
- (80).Shen H; Campanello GC; Flicker D; Grabarek Z; Hu J; Luo C; Banerjee R; Mootha VK The Human Knockout Gene CLYBL Connects Itaconate to Vitamin B12. Cell 2017, 171, 771–782 e711. [DOI] [PMC free article] [PubMed] [Google Scholar]


