Abstract
Purpose
Outcomes after the treatment for unresectable or advanced-stage hepatocellular carcinoma (HCC) are unsatisfied. We evaluated the therapeutic benefits of a combination therapy strategy for these patients through transarterial chemoembolization (TACE) plus sorafenib.
Patients and Methods
In total, 85 patients with HCC classified as intermediate and advanced stage from June 2012 to November 2017 were retrospectively investigated. We divided patients into the monotherapy (n=43; TACE alone) and combined therapy (n=42; TACE plus sorafenib) groups.
Results
Compared with the TACE alone group, the TACE plus sorafenib experienced significantly prolonged progression-free survival (PFS) (mean 21 months vs 12 months; P = 0.0005) and overall survival (OS) (mean 32 months vs 21 months; P = 0.0157). The disease control rate (DCR) of TACE plus sorafenib group was 80.95%, which was significantly increased than the TACE alone group (55.81%) (P<0.05), as well as objective response rate (ORR) (23.81% vs 16.28%). Besides, the rates of liver-related AEs and liver failure in the TACE plus sorafenib group were not increased in contrast to TACE alone group, and there were no new safety concerns. To sum up, the superiority of combination therapy with significantly prolonging progression-free and overall survival was observed, meanwhile finding a significant increase in tumor response rate and manageable safety in the combined therapy in contrast to the monotherapy group.
Conclusion
Based on unTACEble progression, the superiority of the combination therapy is that TACE plus sorafenib has been bringing about significantly better outcomes compared with TACE alone for HCC patients.
Keywords: hepatocellular carcinoma, sorafenib, chemoembolization, therapeutic, survival
Introduction
Hepatocellular carcinoma (HCC) is considered as one of the most common malignancy and the reason for death in patients with the second leading cancer-associated in China.1 Its incidence will continue to increase in the future. Most cases of HCC in China associated with hepatitis B virus (HBV) infection are already in the intermediate and advanced stages at the first diagnosis, and traditional radical surgery is not effective for the treatment of unresectable or advanced stage HCC due to the low rate of surgical resection and the high rate of postoperative recurrence.2 The treatment approach for HCC is associated with the stage and extent of disease, the degree of the underlying liver disease, and the overall performance status of the patient. Currently, for unresectable or advanced HCC, treatment methods include locoregional treatment3 (eg, transarterial chemoembolization), systemic therapy4 (eg, sorafenib, lenvatinib, regorafenib, PD1/PD-L1 immunotherapy), and locoregional ablation5 (eg, cryoablation, thermal ablation).
Hepatology associations worldwide6–9 recommend that transarterial chemoembolization (TACE) is currently adapted to the first-line therapy for HCC patients with intermediate stage (or Barcelona Clinic Liver Cancer [BCLC] stage B), that is multiple nodules, Child–Pugh A function, Eastern Cooperative Oncology Group Performance Status (ECOG-PS) grade 0 score and without macrovascular invasion (MVI) or extrahepatic metastasis. This is in spite of the fact that the characteristics of the extension of liver function degree, variable tumor numbers, and tumor sizes occur.8,10 The anti-tumor mechanism of TACE involves reducing the blood supply of the tumor feeding arteries by embolization, to reduce the tumor size and improve the prognosis.11 Nevertheless, it is usually necessary to repeat TACE to attain maximum tumor regression only because more than half of patients with BCLC-B profit from TACE. The liver function (such as the degree of blood supply of the hepatic artery to tumor thrombus12) and recurrence rate, as the main factors, affected the effectiveness of TACE.13 The latter is based on the premise that TACE-induced hypoxia induces vascular endothelial growth factor (VEGF) and hypoxia-inducible factor upregulation, which is essential for residual tumor growth, invasion, and metastasis.14,15 Therefore, TACE-based combination therapy has greatly improved the effectiveness of TACE treatment and expanded the scope of TACE treatment, which has put forward the strategy that the combination of TACE with systematic treatment (eg, sorafenib) will effectively control tumor growth.16
The multikinase inhibitor of sorafenib, regarded as the standard first-line therapy for advanced HCC, was capable to significantly prolong patients’ survival time in the SHARP trial.17 Given that patients with advanced HCC do not tolerate this treatment well in view of the emergence of sorafenib resistance and side effects,18 more efficient and safe treatments for advanced HCC are urgently required. Pinter and his colleagues19 found that the median survival time in the TACE alone was 9.2 months versus 7.4 months in the sorafenib alone for HCC patients with BCLC-C, without significant differences (P = 0.377), including the increase in the incidence rate of adverse events in patients receiving TACE (30.0% vs 17.0%). To enhance or improve the efficacy and tolerability of sorafenib, some trials20–23 (eg, post-TACE, SPACE, TACE-2, and TACTICS) have studied the efficacy of sorafenib by combining it with other locoregional treatments such as TACE, and explored the application value of TACE combined with sorafenib in the treatment of unresectable HCC. Park and his colleagues24 demonstrated that therapeutic outcomes, including progression-free survival (PFS; 5.2 months vs 3.6 months), overall survival (OS; 12.8 months vs 10.8 months), and disease control rate (DCR; 60.6% vs 47.3%, P = 0.005) were superior in the TACE plus sorafenib group than in the sorafenib alone group. Therefore, TACE or sorafenib alone does not have the optimal therapeutic effects, while TACE combined with sorafenib can achieve complementary effects. TACE induces the upregulation of angiogenic factors by ischemic liver injury to stimulate the growth of residual tumors,14,15 and an antiangiogenic agent (sorafenib) may complementarily inhibit VEGF receptors and platelet-derived growth factor (PDGF) to achieve anti-angiogenesis and control tumor growth.25,26
It is particularly important to objectively evaluate the efficacy of tumor therapy while seeking more effective drugs or programs to further improve the management of patients with tumors, especially given the evaluation criteria for new anti-tumor drugs in clinical trials. Currently, the RECIST/mRECIST standards have been commonly applied in the evaluation of tumor response. However, RECIST is not fully applicable to irregular tumors, tumors regressing unevenly after the treatment process, and for evaluating the efficacy of molecularly targeted drugs.27 In terms of evaluating tumor response, the modified solid tumor efficacy evaluation standard (mRECIST) was proposed, so that the solid tumor efficacy evaluation standard (RECIST) could be applicable to HCC.28 This aims to overcome some of the limitations of RECIST, such as measuring tumor reduction after locoregional and systemic treatments. It has also improved the evaluation criteria that may have led to the inaccurate assessment of disease progression, owing to the utilization of the traditional RECIST 1.1 criteria, because the clinical events were related to the natural progression of chronic liver disease. Similarly, Gillmore and his colleagues29 compared the effects between RECIST and mRECIST in the assessment of the efficacy of TACE in advanced-stage HCC, and found that the utilization of mRECIST criteria to evaluate the efficacy of TACE was more accurate in evaluating the ORR. Unfortunately, it was reported that there was still not paying attention to the modes of HCC progression in the most commonly applied for radiology criteria for the assessment of TACE response related to Response Evaluation Criteria in Solid Tumors or modified RECIST.30
The appropriate way to evaluate the results of embolization plus systemic therapy is currently unclear. Based on Response Evaluation Criteria in Solid Tumors (RECIST) or modified RECIST (mRECIST), the combination strategy of locoregional therapy and systemic therapy has been failing to show the superiority compared to monotherapy via several prospective studies and meta-study.20–22 In contrast, in the TACTICS trial, the novel TACE progression criteria defined as the liver cancer response assessment criterion (RECICL) is that assessing the efficacy of TACE plus sorafenib with unTACEable progression as the endpoint of PFS, without including new intrahepatic lesions.23 According to unTACEable progression, the trial showed that median PFS in TACE and sorafenib was significantly longer than that of TACE alone (25.2 months vs 13.5 months; HR = 0.59; 95% confidence interval [CI]: 0.41 to 0.87; P = 0.006), which illustrated that the combination of TACE and sorafenib has therapeutic benefits in patients with unresectable HCC. Therefore, it is particularly important to objectively evaluate the efficacy of tumor treatment, which is required for a more comprehensive and rigorous evaluation of embolization combined with systemic therapy.
In this study, we retrospectively analyzed the clinical data of TACE combined with sorafenib for the treatment of HCC patients at intermediate and advanced stages in our hospital between June 2012 and November 2017. We compared the efficacy and safety of TACE plus sorafenib with those of TACE alone for HCC, with unTACEable progression as the endpoint of PFS evaluation. Our findings suggested promising outcomes with TACE plus sorafenib.
Materials and Methods
Patients
The data of 85 patients with HCC at intermediate and advanced stages (BCLC-B and BCLC-C) treated between June 2012 and November 2017 were collected from the First Affiliated Hospital of Sun Yat-sen University. We conducted this study in August 2020. Patients (n=43) who were administered TACE alone were classified as the monotherapies group, whereas taking TACE plus sorafenib (n=42) were classified as the combination treatment group. A diagnosis of HCC was based on the clinical results of puncture biopsy and histological examination or early radiological examination, followed by subsequent flushing on dynamic liver imaging (such as computed tomography [CT] and/or magnetic resonance imaging [MRI]), and levels of alpha-fetoprotein (AFP). The patients were confirmed to have serum alpha-fetoprotein (AFP) levels ≥400 μg/L (tumor lesions examined by CT scans or MRI) or AFP levels <400 μg/L (tumor lesions examined by CT scans and MRI).
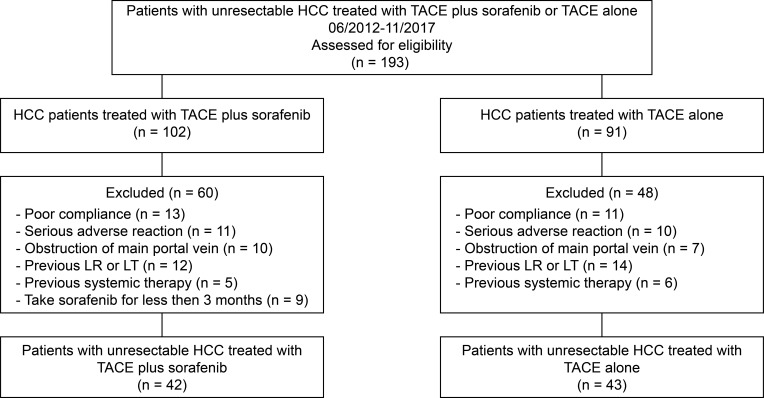
The relevant inclusion criteria included age of ≥30 years, at least one assessable lesion based on mRECIST criteria, Child–Pugh A or B function, the score of 0–2 according to ECOG-PS, and a life expectancy of ≥12 weeks. Patients were excluded if they experienced disease progression and death, or if they had been taking sorafenib for less than 3 months. Patients were discontinued from the study if they had poor compliance, serious adverse reactions, or had received any previous systemic therapy. These patients were not suitable candidates for curative therapy (eg, resection, liver transplantation) or had obstruction of main portal vein (Figure 1).
Figure 1.
Flow diagram showed selection criteria.
Abbreviation: TACE, transarterial chemoembolization.
All patients provided written informed consent, and the trial was approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University [2012–038]. This study was conducted in accordance with the Declaration of Helsinki. The access to personal information for Authors can be identified at any time of data capture.
Procedure and Treatment
The TACE procedure was performed rigorously according to the TACE protocol.31 Briefly, we inserted arterial catheter in the femoral artery through the Seldinger method and then into the hepatic artery. Under digital subtraction angiography (DSA), the tumor stain and arterial blood supply of the tumor were identified, and the catheter was plugged into the artery that supplies blood of tumor. This was followed by the injection of a lipiodol-based chemotherapeutic emulsion and blank microspheres with absorbable gelatin sponge particles to embolize the target blood vessel. Doctors selected the embolic materials according to the DSA findings. Nevertheless, it was essential to demand standardization starting from the choice of anticancer agents until the embolization endpoint.32 Many factors, including tumor size, vessel collateral and liver function, defined the dosages of the anticancer agents and the lipiodol injection.31,32 To minimize impairment of non-cancerous liver tissues, small microspheres were recommended owing to running distally in the vessels to release the chemotherapeutic agent very tightly into the tumor, with the superiority in tumor response and toxic effects.33 Moreover, it was reported that the preparation of the lipiodol chemotherapeutic drug emulsion was required to be mixed multiple times to avoid aggregation into larger droplets, which was not conducive to terminal embolization or catheter blockage.32 Enhanced CT was carried 4–6 weeks after TACE to evaluate the therapeutic effectiveness of tumor embolism. Antiemetic and liver protection treatments were routinely administered after surgery.
Patients were given 800 mg/day sorafenib 2 days after TACE in the combination treatment group. According to the adverse reactions (AEs), half the dosage is given or it can be continuously taken until the patient was relieved of symptoms of discontinuation. Eighty patients who were HBV DNA-positive received entecavir antiviral treatment first. Treatment was continued until severe deterioration of liver function or intolerance of TACE, etc.
Outcomes and Assessments
On the basis of the mRECIST criteria, tumor response was estimated every 4–6 weeks through enhanced dynamic CT or MRI,28 with tumor marker tests, liver function, and routine blood tests performed at the same time. After the follow-up, according to the mRECIST criteria, the patients were classified into complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). The co-primary endpoints contained PFS regarded as the progression from the timing of beginning to treat until untreatable (unTACEable) progression, and OS considered as randomization to death from any reason in this trial. The progression of disease was considered as untreatable (unTACEable) progression that the patients were not capable to further experiencing or profit from TACE. It was worth noting that the untreatable (unTACEable) progression as TACE refractoriness included extrahepatic spread, vascular invasion, Child–Pugh C, severe heart and lung disease, and deterioration of kidney function. Other secondary endpoints included ORR (equal to CR + PR) and DCR (equal to CR + PR + SD) both in TACE plus sorafenib and in TACE alone. Adverse events were evaluated through the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 and were divided into grades I–V.
Statistical Analysis
Pearson’s χ2 test and independent t-test were applied for comparing and analyzing the relationship between different types of variables. The Kaplan–Meier method for evaluating PFS and OS was performed, and the median time of the event was calculated as two-sided 95% confidence intervals (CIs). Statistical significance was set at P < 0.05. Statistical analyses were carried out using SPSS, version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
A total of 193 patients were gathered, of whom 85 fulfilled the eligibility criteria consisting of 43 patients (50.6%) in the TACE alone group and 42 (49.4%) in the TACE plus sorafenib group (Figure 1). Patient characteristics are summarized in Table 1. The two groups were similar in terms of age (mean 58.53 years vs 58.31 years, P = 0.901), sex distribution (proportion of males 72.09% vs 76.19%, P = 0.666), HBV and HCV infection (95.24% vs 97.67%, P=0.830), liver disease severity, and biochemical data (eg, AFP levels). Viral hepatitis accounted for almost all of the underlying causes of HCC. The majority of patients were classified as Child–Pugh A but nearly 30% of patients as the Child–Pugh B function. The ECOG-PS scores of most patients were 0 or 1. Of the 85 subjects enrolled, 48 (56.5%) were classified as BCLC stage B, and 37 (43.5%) as BCLC stage C. The patients’ HCC status is shown in Table 2. The sizes and numbers of HCC between these two groups were similar, and large HCC tumors (diameter over 5 cm) were found frequently in two groups. The occurrence of MVI and EHS tumors in 20 (47.62%) and 4 (9.52%) patients in the TACE plus sorafenib group, and in 22 (51.16%) and 3 (6.97%) patients in the TACE alone, respectively.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients Enrolled in This Study
| Characteristic | Group | χ2 | t | P | ||
|---|---|---|---|---|---|---|
| TACE Plus Sorafenib (n=42) | TACE Alone (n=43) | |||||
| Sex (n/%) | Male | 32 (76.19%) | 31 (72.09%) | 0.186 | – | 0.666 |
| Female | 10 (23.81%) | 12 (27.91%) | ||||
| Age (median ±SD) | Years | 58.31±7.83 | 58.53±8.11 | – | −0.125 | 0.901 |
| ECOG-PS(n/%) | 0 | 9 (21.43%) | 10 (23.26%) | 0.201 | - | 0.905 |
| 1 | 29 (69.05%) | 30 (69.77%) | ||||
| 2 | 4 (9.52%) | 3 (6.97%) | ||||
| Child-Pugh(n/%) | A | 29 (69.05%) | 29 (67.44%) | 0.488 | - | 0.783 |
| B | 11 (26.19%) | 13 (30.23%) | ||||
| C | 2 (4.76%) | 1 (2.33%) | ||||
| BCLC(n/%) | B | 23 (54.76%) | 25 (58.14%) | 0.099 | - | 0.754 |
| C | 19 (45.24%) | 18 (41.86%) | ||||
| Cirrhosis(n/%) | With | 39 (92.86%) | 38 (88.37%) | 0.501 | - | 0.479 |
| Without | 3 (7.14%) | 5 (11.63%) | ||||
| Viral hepatitis(n/%) | HBV | 39 (92.86%) | 41 (95.34%) | 0.372 | - | 0.830 |
| HCV | 1 (2.38%) | 1 (2.33%) | ||||
| None | 2 (4.76%) | 1 (2.33%) | ||||
| AFP(n/%) | AFP<400 ng/dl | 13 (30.95%) | 15 (34.88%) | 0.149 | - | 0.700 |
| AFP>400 ng/dl | 29 (69.05%) | 28 (65.12%) | ||||
Note: Results reported as N (%) unless otherwise indicated.
Abbreviations: AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; HBV, hepatitis B virus; HCV, hepatitis C virus; SD, standard deviation.
Table 2.
The Hepatocellular Carcinoma (HCC) Status of the Transarterial Chemoembolization Plus Sorafenib and Transarterial Chemoembolization Alone Groups
| HCC Status (n/%) | TACE Plus Sorafenib (n=42) | TACE Alone(n=43) | χ2 | P |
|---|---|---|---|---|
| HCC size | 0.190 | 0.909 | ||
| >5cm | 27 (64.29%) | 26 (60.47%) | ||
| 3–5cm | 12 (28.57%) | 13 (30.23%) | ||
| <3cm | 3 (7.14%) | 4 (9.30%) | ||
| Number of tumors | 0.165 | 0.921 | ||
| >5 | 8 (19.05%) | 8 (18.60%) | ||
| 3–5 | 22 (52.38%) | 21 (48.84%) | ||
| <3 | 12 (28.57%) | 14 (32.56%) | ||
| MVI | 20 (47.62%) | 22 (51.16%) | 0.107 | 0.744 |
| EHS | 4 (9.52%) | 3 (6.97%) | 1.556 | 0.459 |
| Lung | 2 (4.76%) | 1 (2.33%) | ||
| Lymph nodes | 1 (2.38%) | 2 (4.66%) | ||
| Bone | 1 (2.38%) | 0 (0.00%) |
Abbreviations: EHS, extrahepatic spread; HCC, hepatocellular carcinoma; MVI, macroscopic vascular invasion; TACE, transarterial chemoembolization.
Efficacy Outcomes
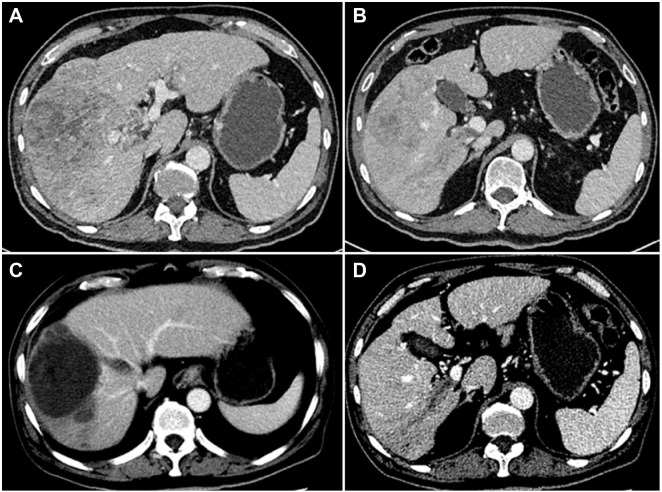
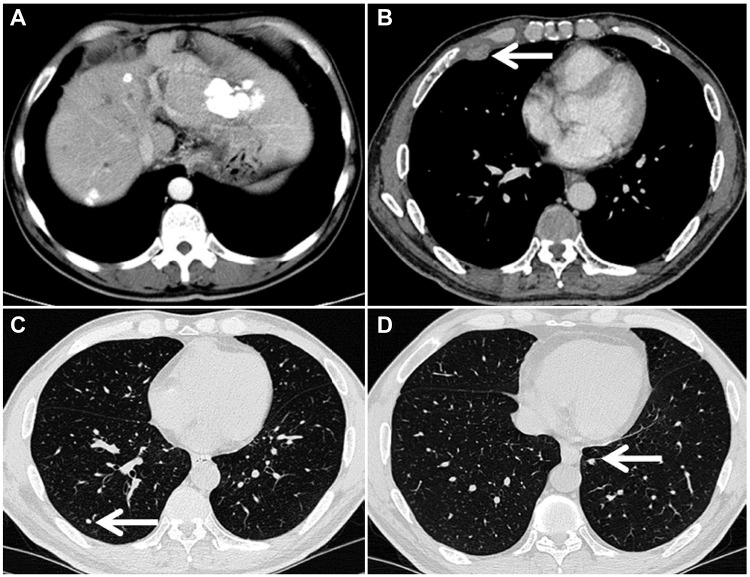
Tumor response was evaluated with CT and MRI on the basis of the mRECIST evaluation criteria every 4–6 weeks. A total of 85 patients in the TACE plus sorafenib group and the TACE alone group were followed up to assess the best tumor response. Based on the evaluation of the mRECIST criteria in the TACE plus sorafenib group (n = 42), there were 2 CR (4.76%), 8 PR (19.05%), 24 patients with SD (57.14%), and 8 patients with PD (19.05%). Meanwhile, the DCR (CR + PR + SD) was 80.95% and the ORR (CR + PR) was 23.81%. For the TACE alone group (n = 43), CR were seen in 0%, PR in 16.28%, SD in 39.53%, and PD in 44.19% of patients according to the mRECIST criteria, whereas DCR was seen in 55.81% and ORR in 16.28%. Interestingly, the DCR of the TACE plus sorafenib group was 80.95%, which was significantly increased than that of the TACE alone group (55.81%) as well as the ORR (23.81% vs 16.28%), and the difference was significant (χ2=9.633, P = 0.02, Table 3). In the TACE plus sorafenib group, there were 2 HCC patients with portal vein tumor thrombus (PVTT) who were not suitable for surgical resection. As shown in Figure 2, TACE combined with sorafenib resulted in good tumor control and PVTT necrosis. Although the intrahepatic tumor was controlled by TACE alone, the patient developed pleural and pulmonary metastases (Figure 3).
Table 3.
Outcomes in Patients Randomized to the TACE Plus Sorafenib and TACE Alone Groups
| Group (n/%) | N | Best Response | DCR | ORR | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | ||||||
| TACE plus sorafenib | 42 | 2 (4.76%) | 8 (19.05%) | 24 (57.14%) | 8 (19.05%) | 34 (80.95%) | 10 (23.81%) | 9.633 | 0.022 |
| TACE alone | 43 | 0 (0.00%) | 7 (16.28%) | 17 (39.53%) | 19 (44.19%) | 24 (55.81%) | 7 (16.28%) | ||
Note: Results reported as N (%) unless otherwise indicated.
Abbreviations: CR, complete response; DCR (CR+PR+SD), disease control rate; ORR (CR+PR), objective response rate; PD, progressive disease; PR, partial response; SD, stable disease; TACE, transarterial chemoembolization.
Figure 2.
Computed tomography image of the liver obtained from a 55-year-old male patient with a history of hepatitis B for 30 years. Contrast-enhanced CT imaging showed the presence of hepatocellular carcinoma with tumor thrombus in the right branch of the hepatic and portal veins (A and B). After 6 months of oral sorafenib combined with TACE, contrast-enhanced CT imaging showed tumor necrosis in the liver, and no blood supply was seen in the hepatic vein and PVTT (C and D).
Figure 3.
Computed tomography images of the chest and liver obtained from a 51-year-old male patient who had been treated with transarterial chemoembolization alone for 7 months. Although the intrahepatic lesions were controlled (A), there were right-sided pleural metastases (B, arrow shown) and bilateral lung metastases (C and D, arrow shown).
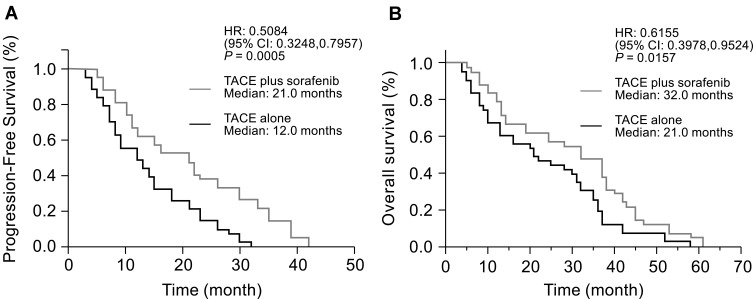
Furthermore, according to the RECICL criteria, median PFS, on the basis of unTACEable progression, was significantly prolonged in the TACE plus sorafenib group than in the TACE only group (21 months vs 12 months; HR= 0.5084; 95% CI: 0.3248 to 0.7957; P = 0.0005; Figure 4A). The median OS was 32 months for the TACE plus sorafenib group and 21 months for the TACE alone group (HR: 0.6155; 95% CI: 0.3978–0.9524; P = 0.0157; Figure 4B).
Figure 4.
Kaplan–Meier plots of median (A) progression-free survival and (B) OS in the TACE plus sorafenib and TACE alone groups.
Abbreviations: HR, hazard ratio; TACE, transarterial chemoembolization; CI, confidence interval.
These two groups were similar in tumor marker testing, liver function, and routine blood test results before treatment (P > 0.05), as shown in Table 4. In contrast, after treatment, the levels of total bilirubin (TBIL), aspartate aminotransferase (ALT), alanine aminotransferase (AST), cholinesterase (CHE), and albumin (ALB) in the TACE plus sorafenib group were higher than those in the previous treatment (P < 0.05), while the levels of AFP were lower than those before treatment (Table 5). Similarly, in the TACE alone group, the TBIL, ALT, and AST were also increased compared to those before treatment, whereas AFP and CHE were decreased compared to those before treatment (P < 0.05). Levels of CHE (4013.95±727.36 µ/L vs 4888.09±693.62 µ/L) and ALB (31.81±3.07 g/L vs 31.95±3.18 g/L) after treatment in the TACE alone group were lower than those before treatment, indicating that treatment with TACE alone caused liver damage. More importantly, in the TACE plus sorafenib group, the levels of both CHE and ALB were increased, indicating that the liver function of patients after TACE can be stabilized and the incidence of liver failure can be reduced.23 In addition, the level of AFP in the TACE plus sorafenib group was lower than that in the TACE alone group (229.34±91.25 µg/L vs 326.55±101.12 µg/L, P <0.05), as shown in Table 4, which shows that TACE combined with sorafenib is superior in the treatment of intermediate and advanced HCC compared to TACE alone.
Table 4.
Comparison of Biochemical Indices Before and After the First Transarterial Chemoembolization Procedure
| Time | Group | TBIL (µmol/L) | ALT (µ/L) | AST (µ/L) | CHE (µ/L) | ALB (g/L) | AFP (µg/L) |
|---|---|---|---|---|---|---|---|
| Before | TACE plus sorafenib (n=42) | 19.51± 3.94 | 55.11± 13.24 | 50.74± 11.69 | 4849.04± 701.91 | 32.13± 3.10 | 647.45± 268.52 |
| TACE alone (n=43) | 19.29± 4.23 | 55.45± 14.01 | 50.65± 11.88 | 4888.09± 693.62 | 31.95± 3.18 | 612.32± 272.14 | |
| t | 0.25 | −0.113 | 0.037 | −0.258 | 0.275 | 0.599 | |
| p | 0.803 | 0.910 | 0.971 | 0.797 | 0.784 | 0.551 | |
| After | TACE plus sorafenib (n=42) | 19.88± 3.47 | 57.90± 14.00 | 53.56± 11.79 | 5023.99± 835.30 | 33.17± 2.99 | 229.34± 91.25 |
| TACE alone (n=43) | 25.97± 3.77 | 67.35± 14.46 | 60.97± 11.96 | 4013.95± 727.36 | 31.81± 3.07 | 326.55± 101.12 | |
| t | −7.727 | −3.058 | −2.876 | 5.949 | 2.062 | −4.649 | |
| p | 0.000 | 0.003 | 0.005 | 0.000 | 0.042 | 0.000 |
Note: Results are presented as mean ± SD.
Abbreviations: AFP, alpha-fetoprotein; ALB, albumin; ALT, alanine transaminase; AST, aspartate transaminase; CHE, cholinesterase; TACE, transarterial chemoembolization; TBIL, total bilirubin.
Table 5.
Comparison of Biochemical Indices Before and After the First TACE Treatment Between the TACE Plus Sorafenib and TACE Alone Treatment Groups
| Time | Group | TBIL (µmol/L) | ALT (µ/L) | AST (µ/L) | CHE (µ/L) | ALB (g/L) | AFP (µg/L) |
|---|---|---|---|---|---|---|---|
| TACE plus sorafenib (n=42) |
Before | 19.51± 3.94 | 55.11± 13.24 | 50.74± 11.69 | 4849.04± 701.91 | 32.13± 3.10 | 647.45± 268.52 |
| After | 19.88± 3.47 | 57.90± 14.00 | 53.56± 11.79 | 5023.99± 835.30 | 33.17± 2.99 | 229.34± 91.25 | |
| t | −5.013 | −23.261 | −69.888 | −7.962 | −46.706 | 15.247 | |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| TACE alone (n=43) | Before | 19.29± 4.23 | 55.45± 14.01 | 50.65± 11.88 | 4888.09± 693.62 | 31.95± 3.18 | 612.32± 272.14 |
| After | 25.97± 3.77 | 67.35± 14.46 | 60.97± 11.96 | 4013.95± 727.36 | 31.81± 3.07 | 326.55± 101.12 | |
| t | −95.2720 | −148.439 | −632.856 | 152.356 | 6.850 | 10.944 | |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Results are presented as mean ± SD.
Abbreviations: AFP, alpha-fetoprotein; ALB, albumin; ALT, alanine transaminase; AST, aspartate transaminase; CHE, cholinesterase; TACE, transarterial chemoembolization; TBIL, total bilirubin.
Safety Outcomes
Patients restored taking 800 mg/day sorafenib 2 days after TACE in the TACE plus sorafenib group. At least 28 of the 42 patients who took sorafenib orally needed to reduce the dose of sorafenib (66.7%), mainly due to AEs. The most common AEs were hand-foot syndrome (66.67%), diarrhea (28.57%), rash (14.28%), and nausea (4.76%; Table 6). The most important grade 3 AEs were HFSR and diarrhea, whereas the incidence of fatigue, nausea, anorexia, and oral ulcers was relatively low. Patients in the TACE plus sorafenib group were relieved after the dose reduction of sorafenib and symptomatic treatment, and no other serious adverse reactions occurred.
Table 6.
Adverse Events Related to Sorafenib
| Adverse Event (n/%) | All Events | Grade | χ2 | P | |||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||
| Diarrhea | 12 (28.57%) | 8 (19.05%) | 3 (7.14%) | 1 (2.38%) | 0 (0.00%) | 5.869 | 0.970 |
| Hand-foot skin reaction | 28 (66.67%) | 19 (45.24%) | 6 (14.29%) | 3 (7.14%) | 0 (0.00%) | ||
| Rash | 6 (14.28%) | 4 (9.52%) | 2 (4.76%) | 0 (0.00%) | 0 (0.00%) | ||
| Fatigue | 7 (16.67%) | 5 (11.91%) | 2 (4.76%) | 0 (0.00%) | 0 (0.00%) | ||
| Anorexia and nausea | 2 (4.76%) | 2 (4.76%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Hypertension | 8 (19.04%) | 6 (14.28%) | 2 (4.76%) | 0 (0.00%) | 0 (0.00%) | ||
| Mouth ulcer | 3 (7.14%) | 3 (7.14%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Hoarseness | 2 (4.76%) | 1 (2.38%) | 1 (2.38%) | 0 (0.00%) | 0 (0.00%) | ||
Table 7 shows the adverse reactions of the TACE plus sorafenib group and the TACE alone group. After treatment, there was no significant difference in the incidence of new ascites (3 cases vs 4 cases), liver injury (6 cases vs 7 cases), hepatorenal syndrome (1 case vs 1 case), pleural effusion (2 cases vs 3 cases), spontaneous bacterial peritonitis (1 case vs 0 case), gastrointestinal bleeding (2 cases vs 1 case), ischemic cholecystitis (2 cases vs 2 cases), liver abscess (1 case vs 1 case), or inguinal hematoma (2 cases vs 3 cases) between the TACE plus sorafenib group and the TACE alone group (P > 0.05; Table 7). Although the most common is that it often exists underlying liver damage in patients with HCC, the rates of liver-related AEs and liver failure in the TACE plus sorafenib group were not increased in this study than in the TACE alone group, and there were no new safety concerns. Moreover, the recurrence rate of HCC in the TACE plus sorafenib group was significantly lower than that in the TACE alone group (8/42 vs 19/43, P < 0.05). This demonstrates the clinical feasibility and safety of sorafenib combined with TACE therapy for patients with intermediate and advanced stage HCC.
Table 7.
Comparison of TACE-Related Adverse Reactions Between the TACE Plus Sorafenib and TACE Alone Treatment Groups
| Adverse Event (n/%) | Group | χ2 | P | ||
|---|---|---|---|---|---|
| TACE Plus Sorafenib (n=42) | TACE Alone (n=43) | ||||
| New ascites | With | 3 (7.14%) | 4 (9.30%) | 0.131 | 0.513 |
| Without | 39 (92.86%) | 39 (90.70%) | |||
| Liver injury | With | 6 (14.29%) | 7 (16.28%) | 0.065 | 0.519 |
| Without | 36 (85.71%) | 36 (83.72%) | |||
| Hepatorenal syndrome | With | 1 (2.38%) | 1 (2.33%) | 0.000 | 0.747 |
| Without | 41 (97.62%) | 42 (97.67%) | |||
| Pleural effusion | With | 2 (4.76%) | 3 (6.98%) | 0.188 | 0.511 |
| Without | 40 (95.24%) | 40 (93.02%) | |||
| Spontaneous bacterial peritonitis | With | 1 (2.38%) | 0 (0.00%) | 1.036 | 0.494 |
| Without | 41 (97.62%) | 43 (100.00%) | |||
| Gastrointestinal bleeding | With | 2 (4.76%) | 1 (2.33%) | 0.370 | 0.491 |
| Without | 40 (95.24%) | 42 (97.67%) | |||
| Ischemic cholecystitis | With | 2 (4.76%) | 2 (4.65%) | 0.001 | 0.683 |
| Without | 40 (95.24%) | 41 (95.35%) | |||
| Liver abscess | With | 1 (2.38%) | 1 (2.33%) | 0.000 | 0.747 |
| Without | 41 (97.62%) | 42 (97.67%) | |||
| Inguinal hematoma | With | 2 (4.76%) | 3 (6.98%) | 0.188 | 0.511 |
| Without | 40 (95.24%) | 40 (93.02%) | |||
Abbreviation: TACE, transarterial chemoembolization.
Discussion
Primary carcinoma of the liver is closely related to liver cirrhosis and viral hepatitis, with the characteristic of subtle onset but high degree of malignancy. Furthermore, it is prone to metastasis and usually in the intermediate and advanced stage at the first time to diagnosis, which represents a lost opportunity for surgical intervention.2 The treatment approach for HCC has entered the era of individualized molecular targeted “precision” therapy for relevant genes, with remarkable curative effects and safety, meaning that it is quickly becoming the standard treatment for advanced HCC, with the continuous development of targeted drugs.
Based on the general classification and histopathological classification of HCC, as well as the stage of HCC, it plays a reference value for the determination of clinical treatment plan. However, clinicians have found that for the same type and stage of liver cancer, no matter how the clinical treatment is optimized, the prognosis is still very different. The development of molecular targeted therapy for cancer has made people start to think about the diagnosis and treatment of cancer. This phenomenon of molecular targeted therapy shows that the treatment of HCC cannot be uniform. Combined with the molecular phenotype of HCC through being divided to different subgroups, based on the traditional clinicopathological classification and staging of HCC, it may also improve the pertinence of different treatment methods for HCC, thus further improving the efficacy.34,35 It was reported that with regard to molecular features and histopathologic data, it may show a different response to these treatments as for example demonstrated by the case of OATP 1B1/1B3 mutation that determines a different imaging appearance of some HCCs.34 Molecular typing is followed by molecular targeted therapy of HCC and its subtypes can provide more targets for targeted therapy. Currently, the clinical application of sorafenib has brought dawn to the treatment of HCC, and it is still urgent to be further developed new and more effective molecular targeted therapeutic drugs. Because there are still limitations that the benefits of clinical survival time were observed after taking sorafenib treatment, emphasizing the desire for better treatment strategies.
Indeed, nearly 35–50% of patients with HCC also have portal vein tumor thrombus, with most of them classified as BCLC-C. Currently, sorafenib has been recommending as the first-line treatment in the Barcelona guidelines,36 but it is only partially effective for the treatment of HCC with portal vein tumor thrombus, leading to poor prognosis. It is worth noting that there have been many alternative treatments in the clinic, including surgical resection, liver transplantation, and TACE, which can improve the survival rate of advanced-stage HCC. TACE, considered as the first-line therapy for HCC patients at the intermediate stage, or BCLC-B tumors,6–9 can reduce the blood supply of the tumor by embolization of the supplying artery to taking tumor tissue necrosis and shrinkage or even disappearance of the tumor, resulting in better clinical outcomes.11 The disadvantage of TACE is that it is necessary to repeat TACE several times because of tumor recurrence and metastasis early after surgery, which may cause to the aggravation of liver function and the unsatisfactory of prognosis.37 Moreover, portal vein tumor thrombus can affect the blood supply to the liver to a certain extent, and TACE treatment further blocks the blood supply of the hepatic artery, which often leads to ischemia-related liver failure.38 Therefore, the survival after TACE remains relatively poor so that there is required for a treatment refinement able to improve the safety and effectiveness of the technology. One is to standardize the procedure of TACE, which could help interventional radiologists to achieve better results from TACE and contribute to a more reproducibility of the study results. The two principal TACE techniques are conventional TACE (cTACE) and drug-eluting bead TACE (DEB-TACE). It had been shown that in HCC patients, TACE with drug-eluting bead had advantages in controlling tumor progression and toxicity, thus reducing the risk of serious adverse events, compared with conventional TACE (cTACE).39,40 It cannot be ignored that when evaluating the effectiveness of a new treatment, its clinical benefits must be weighed according to its cost.41 Indeed, the direct incremental costs of DEB-TACE could be acceptable in respect to cTACE, although the price was slightly more expensive, it might represent a cost-effective alternative to cTACE with fewer complications.41 In addition, regarding to a correct standardization of the procedures, it is also possible to reduce the number of rays absorbed for both patients and radiologists and reduce possible stochastic effects.42 Increasing evidence is that the standard treatment of TACE is not suitable for all patients with intermediate and advanced stage HCC; hence, the second improvement is to widely advocate to refine the group of patients and implement individualized comprehensive treatment.43,44 A Phase II START trial44 showed that TACE plus sorafenib significantly improved the prognosis of HCC patients with portal vein tumor thrombus, with a 3-year OS rate of 86.1% and a low incidence of AEs. Interestingly, our study shows that in the TACE plus sorafenib group, there were two cases of HCC with portal vein tumor thrombus that could not be treated by surgery but could instead be well-controlled and achieve long-term survival without metastasis using TACE plus sorafenib. As shown in Figure 4, TACE combined with sorafenib has important clinical value for the treatment of HCC patients at intermediate and advanced stage. TACE alone is not effective in most cases; thus, combination therapy based on TACE has brought about a greater clinical benefit for HCC patients at intermediate and advanced stage.26,45,46 This is because even complete embolization with TACE cannot guarantee that the tumor is completely treated, especially in patients with portal vein, hepatic vein, and inferior vena cava cancer thrombi, and VEGF triggered by TACE-induced hypoxia promotes tumor angiogenesis and causes tumor recurrence.14,47,48 Sorafenib targets the VEGF receptor, RAF, and PDGF receptor, which explains its role in anti-angiogenesis and anti-tumor activity that can effectively inhibit tumor recurrence in HCC patients with tumor thrombi in the hepatic and portal veins. In order to enhance or improve the moderate efficacy of sorafenib, some trials have studied the efficacy of combining it with other therapies. Noticeably, a meta-analysis had proved the non-superiority in clinical outcome (OS, PFS, ORR and security etc.) of TACE with respect to TAE,49 which was require to be confirmed in broad non-inferiority trials with a large number of cases. Similarly, the hypoxia caused by TAE cannot be ignored; thus, it is reasonable and clinically significant to combine with antiangiogenic drugs, with the necessity to carry out large-scale clinical research and evaluation in the future. Besides, it was reported that the application of TACE chemotherapy drugs might further increase liver injury.50 But Y90 transarterial radioembolization, as a novel form of liver-directed with no significant vessel occlusion, and showed the similarity in survival outcome associated with OS, response rate and safety profile in addition to PFS as compared to TACE,51 which was benefited for HCC patient with PVT. It is worth mentioning that the comparison of efficacy and safety in these two procedures plus sorafenib to better define the treatment strategy in intermediate/advanced HCC patients.
The intention of combined therapy is to make use of the synergistic effect of two or more existing treatment methods to increase a patient’s survival benefit. A single-center retrospective study of 104 HCC patients with BCLC stage B/C (with or without PVTT) found that TACE plus sorafenib improved OS compared with sorafenib alone (HR 0.498; 95% CI: 0.278 to 0.892; P < 0.05).52 Similarly, it was demonstrated that in another retrospective study, TACE combined with sorafenib can significantly prolong OS compared to TACE alone.53 However, owing to the recurrence of new lesions after TACE, based on the evaluation of mRECIST or RECIST 1.1 criteria with regard to TTP or PFS, TACE plus sorafenib failed to exhibit therapeutic benefits compared with TACE alone in several previous randomized controlled trials (eg, TACE-2, and post-TACE).20,22 It is particularly important to objectively evaluate the efficacy of tumor treatment, which is required for a more comprehensive and rigorous evaluation of TACE interventional therapy and molecular targeted therapy. The assessment of locoregional treatment response was initially based on the European Society of Liver Diseases standards, but more recently, the RECIST/mRECIST criteria have been used.22 It has been suggested by Kudo that the limitation of RECIST and mRECIST lied in the rigor of evaluating intermediate stage HCC progression.23 In addition, the traditional efficacy evaluation standards are limited by the fact that they cannot identify the tumor necrosis caused by targeted drugs. Bruix and his colleagues54 first proposed that the appearance of new intrahepatic progression may not amply treatment failure after TACE, and that it is possible to continue to maintain treatment until the patient is incapable of being cured (ie, unTACEable progression), and this approach was applied in the SPACE trial.21 It was believed by Kudo and his colleagues that a new specific progression endpoint after TACE was supposed to be examined and proved in future TACE combination trials. They23 showed that repeated TACE was still effective for HCC, further illustrating that the measurement of the “progression” of intermediate-stage HCC according to RECIST and mRECIST criteria did not imply a failure of treatment, nor did it indicate the requirement of moving to next-line therapy. This is because regeneration of the original tumor or the occurrence of new intrahepatic lesions are the natural tumor biological characteristics of HCC, which may be necessary to extend the different endpoints of treatment to illustrate the clinical profits of the addition of sorafenib. Despite the application of “unTACEable progression” in the SPACE trial, there was no significant difference between the primary endpoint of TTP (HR = 0.797, P = 0.072) and the secondary endpoint of OS (HR = 0.898, P = 0.295) in the TACE plus sorafenib and TACE alone groups. It was suggested that the possible reason for this result was that the standard of disease progression, including the presence of new intrahepatic lesions, was so rigorous that it led to the early termination of sorafenib administration, resulting in a shorter course of sorafenib use and failure to improve the TTP.23 Not surprisingly, according to the TACE-2 trial,22 the combination therapy of TACE and sorafenib was not superior to the treatment with TACE alone in terms of PFS (mean 7.8 months vs 7.7 months, hazard ratio [HR] = 1.03, P = 0.850) and OS (18.8 months vs 19.6 months, HR = 1.03, P = 0.870) for advanced-stage HCC. It is speculated that the TACE-2 trial, based on RECIST 1.1, may force the early termination of TACE combined with sorafenib, resulting in failure to prolong the PFS and OS. In contrast, the therapeutic benefits of TACE plus sorafenib have been evaluated based on the RECICL criteria by Kudo, not involving treating new intrahepatic lesions as PD in the TACTICS trial.23 PFS was considered as the progression from the timing of beginning to treat until untreatable (unTACEable) progression that it was not capable to the patient to further suffer or profit from TACE. The TACTICS trial found that it can significantly prolong the median PFS of patients with unresectable HCC through TACE plus sorafenib treatment, compared with TACE treatment alone (25.2 months vs 13.5 months; HR = 0.59; 95% CI, 0.41–0.87; P = 0.006). Surprisingly, our study found that TACE plus sorafenib caused significantly longer median PFS than TACE alone (21 months vs 12 months; HR: 0.5084; 95% CI, 0.3248–0.7957; P = 0.0005). Similarly, the median OS increased significantly from 21 to 32 months (HR: 0.6155; 95% CI, 0.3978–0.9524; P = 0.0157). But in the TACTICS trial, median OS as the second co-primary endpoint was unable to be assessed. Moreover, in our study, ORR, as a secondary endpoint of the trial, was significantly increased in the TACE plus sorafenib (23.81%) than TACE alone (16.28%) groups. Other secondary endpoints of the TACE plus sorafenib group, such as DCR, were significantly higher than those in the TACE alone group (80.95% vs 55.81%). Compared with TACE alone, many AEs were more common in the TACE plus sorafenib group, such as hand foot syndrome (66.7%), which may be caused by sorafenib treatment. Compared with the TACE alone group, the rates of AEs in the TACE plus sorafenib group resulting from treatment with TACE were not higher in this study, with no new safety concerns. As mentioned above, the benefits of TACE plus sorafenib in comparison with TACE alone in patients with unresectable HCC, the findings suggested that TACE plus sorafenib for the treatment of unresectable HCC illustrated a controllable security and potential efficacy.
Nevertheless, the various treatments and prognostic options available for patients with HCC are affected by locating at the tumor stage and the damage degree of liver function.2 It is important for the treatment of patients with HCC to be accurately assessed and classified. On the one hand, the BCLC staging system is claimed by the size and number of tumors, whether there is macrovascular invasion (MVI) or extrahepatic spread (EHS), the stage of liver cirrhosis, and the patient’s performance status.55 According to the BCLC staging system, TACE is the first recommended as therapeutic strategy for HCC patients at the intermediate stage,36 and advanced HCC, or BCLC stage C, or progression resulting from TACE treatment is cured with systematic treatments such as sorafenib.48 Although included in the same stage, patients have different degrees of liver dysfunction and different tumor burdens, and their prognosis varies greatly, such as BCLC B stage involving heterogeneity.56,57 Regarding patients with intermediate-stage HCC, based on tumor burden, liver function, and PS, BCLC-B patients identified subgroups including stage B1, B2, B3 and B4, with clinically relevant different prognosis, and were assessed whether inclusion of the MELD score was benefit to prognostic tuning.57 Thus, a single therapeutic option may not be suitable for all intermediate-stage patients and strict prognostic stratification is required to provide appropriate treatment options. There are many factors that affect the prognosis, not only the stage of the tumor. In addition, it has also been reported that type-2 diabetes mellitus (DM) brings a three-fold risk of HCC, which seems to have a negative impact on the prognosis and clinical course of HCC patients independently of the underlying cause of cirrhosis.58 Advances in molecular typing have had a significant impact on the diagnosis and treatment of HCC, especially on the prognosis of patients with HCC.34 It is currently uncertain which patients will benefit from TACE again and which need to be switched to systemic therapy if the disease progresses. The TACTICS trial was the first randomized controlled trial with positive results from TACE plus sorafenib for unresectable HCC patients without vascular invasion or EHS, in contrast with the post-TACE, SPACE, and TACE-2 trials. The proportion of BCLC-A and B patients included in the TACTICS study was close to 89%, which may be one of the reasons for the longer PFS. The findings imply that the combination emerges promising, especially for advanced HCC (BCLC-C, 45.2%) that are generally eliminated from other trials.
With regard to advanced HCC, TACE plus sorafenib in contrast to sorafenib alone or TACE alone for advanced stage HCC may further raise our awareness of the real clinical profit of placing sorafenib into TACE in future studies. On the other hand, there is evidence that repeated TACE treatment can increase the incidence of adverse reactions, whose anti-tumor effect was offset by the negative impact on liver function in clinical practice.59 The liver function was associated with prognosis that affect clinical outcome. Interestingly, most patients have Child–Pugh A liver function in several trials, including the post-TACE, SPACE, TACE-2, and TACTICS trials (Child–Pugh ≤7), which may explain why TACE plus sorafenib attains promising outcomes for HCC patients at the intermediate and advanced stages. In addition, the negative results obtained in the post-TACE, SPACE, and TACE-2 trials may be due to inadequate definition of disease progression. “UnTACEable progression” was only defined by Bruix relatively recently, and includes major events such as extensive liver involvement, MVI/EHS, as well as intrahepatic progression related to impaired liver function and poor functional status, which is a contraindication for TACE treatment, and thus, it should not be used repeatedly in these settings. The TACTICS trial clearly showed that when TACE was considered effective for patients, TACE plus sorafenib should be continued in the intrahepatic progression. According to the RECICL criteria, patients undergoing Child–Pugh C liver function, MVI/EHS, excluding intrahepatic progression after TACE treatment, means that the patient needs to stop TACE treatment, which is the criterion as unTACEable progression or TACE refractory. It was found that the TACE plus sorafenib obtained a superior outcome by significantly prolonging times to vascular invasion, EHS, and stage progression, suggesting that TACE plus sorafenib significantly prevented the progression of intermediate toward advanced-stage HCC23 and improving prognosis. Therefore, the effective strategy is that TACE combined with an antiangiogenic agent (sorafenib) improves the prognosis of HCC patients, prolonging not only PFS but also OS (Figure 4). Collectively, the indications for TACE might further be expanded for advanced-stage HCC, especially with extrahepatic diseases in the combination of TACE and sorafenib due to the extensive intrahepatic tumor necrosis by TACE and the targeting extrahepatic disease of sorafenib. Patients with a poor prognosis tend to have higher HCC invasiveness, and it may be beneficial for these patients to start systemic therapy as soon as possible.
It is worth noting the timing of placing sorafenib to TACE and dosage of sorafenib, which is captured attention in tumor therapy studies. Three approaches with regard to the timing of sorafenib administration were proposed by Strebel.60 The means have been examined for the timing of placing sorafenib to TACE, as follows: (1) sequential administration, that is, anti-angiogenesis therapy (eg, sorafenib) after the completion of TACE treatment;20,61 (2) interrupted administration, patients treated with sorafenib to avoid possible AEs during TACE;21,22 and (3) continuous administration of anti-angiogenesis therapy (eg, sorafenib) throughout the embolization process without interruption before, during, or after TACE.23 The first two means have superiority in decreasing the risk of bleeding and complications, whereas the benefit of the third approach of continuous administration is to inhibit the upregulation of VEGF after TACE, relieving TACE-induced hypoxia and preventing tumor growth after TACE. Surprisingly, the results of the TACTICS trial indicate that continuous administration in TACE plus sorafenib prolongs PFS, extending the interval time between two TACE treatments, thus preventing the deterioration of liver function usually caused by repeated TACE. In our study, the median PFS was slightly lower than that of the TACTICS trial (21 months vs 25.2 months), which may be associated with the inhibition of VEGF by not taking sorafenib preoperatively. Thus, the expression levels of VEGF after TACE and the sensitivity of patients to sorafenib should also be recognized in order to make a more effective treatment plan. In addition, in the post-TACE trial sorafenib was prescribed at a low dose, with an average dose of 386 mg, which is significantly lower than that of 797 mg recommended by the SHARP trial17 and, along with a short duration of treatment, may be one of the reasons why TTP was not improved in that trial. Previous studies have reported that patients with a Child–Pugh score of 6 points and an ECOG-PS score of 1 before sorafenib treatment were prone to liver failure after the termination of sorafenib treatment.62 Therefore, systemic therapy should be commenced at an earlier time before a Child–Pugh score of 5 (Child–Pugh A) is identified and before refractory TACE. However, the SPACE trial was designed to conduct TACE at regular intervals, and unnecessary TACE may impair liver function or increase the adverse reactions to sorafenib. In contrast, in the TACTICS trial, TACE was carried out on-demand, and repeated TACE treatment was required when the lesion volume increased by more than 50% of the baseline volume, which extended the interval time between two TACE treatments; this trial suggested that TACE combined with sorafenib reduced the degree of liver function damage. As previously mentioned, it is worth noting the timing of placing sorafenib to TACE and the dosage of sorafenib as well as the standard of repeated TACE, in order to better understand the value of TACE combined with sorafenib in the treatment of HCC.
The main risk factor for HCC in China is HBV infection, with most affected patients experiencing underlying liver fibrosis or cirrhosis.2 A lack of antiviral therapy or TACE might cause HBV reactivation or liver decompensation.63,64 Our study found that all HCC patients had different degrees of liver function damage after TACE. Antiviral therapy with entecavir was performed in 82 patients with HBV/HCV before TACE treatment. The results of multi-factor analysis showed that antiviral therapy was an independent predictor of the efficacy of TACE in the treatment of HCC.65 It was reported that antiviral therapy reduced the risk of HBV reactivation and significantly stabilized the liver function of patients after TACE, thereby reducing the incidence of liver failure and prolonging the survival rate of patients with advanced HCC.65,66 It is worth noting that HBV combined with HCV infection will increase the risk of HCC. The burden and mortality of HCV-related cirrhosis combined with HCC in most countries, including Western countries, have increased in the past decade owing to ageing HCV population, and limited HCV therapeutic impact.67 This study benefited from its use of a large cohort of HCC patients with HBV (85 HCC patients from 80 HBV treatment recipients). But this particular study population (almost all HBV patients) does not represent a real limit because there is NOT a higher rate of de novo HCC occurrence or recurrence after DAA therapy in patients with previous HCV infection.68 Similarly, it has showed a beneficial effect and improved survival in HCC patients with HCV who were offered TACE, compared with whom were not offered TACE (14 months versus 7 months).69 The therapy strategy was inspired by the relationship between early HCC staging and early liver cirrhosis staging and survival improvement, that is, combining enhanced HCC screening in patients with HCV-related cirrhosis and DAA treatment of HCC patients could significantly improve survival.68,69 More importantly, it was required for the assessment of patient general status, such as the presence of sarcopenia or Child-Pugh classification and simple staging by Okuda as valuable tools in assessing the prognosis of patients.69,70 Therefore, antiviral therapy combined with TACE and sorafenib is an important treatment approach for HBV/HCV-related HCC, and its focus should be on the selection of a suitable population according to clinical classification, HBV/HCV infection, the patients’ own economic situation, and molecular prognostic markers.
There are several limitations to our study. First, the study design was retrospective and there may be selection or reporting bias. Second, this was a single-center study, with a limited sample size, and multi-center, large sample clinical data are required in the future. Moreover, most patients enrolled in our study had been diagnosed with Child–Pugh class A/B, BCLC stage B/C, and HBV infection. The treatment seems to be more clinically effective in patients with HCC (BCLC-B) and Child–Pugh class A disease. Further prospective research that it is necessary to analyze more cases and variables. Finally, the combination of TACE plus sorafenib therapy could potentially have a higher efficacy than therapy with TACE alone, but the efficacy for advanced HCC (BCLC-C), especially MVI/EHS, should be further determined by increasing the sample size and performing multi-factor analysis.
Conclusion
It is particularly important to objectively evaluate the efficacy of tumor treatment, which is required for a more comprehensive and rigorous evaluation of combination therapy of TACE and molecular targeted drugs. We used the end point of unTACEable progression to evaluate PFS to compare the therapeutic benefits between the combination of TACE plus sorafenib and treatment with TACE alone in patients with intermediate- and advanced-stage HCC. Based on unTACEable progression criteria, the combination of TACE with sorafenib brings about significantly better outcomes than TACE alone in patients with HCC, which are associated with prolonged progression-free survival, overall survival, and significantly increased tumor response rate, illustrating a controllable security and potential efficacy, with the advantage of extending the TACE interval to protect the patients’ liver function in order to stabilize the tumor cells. Most importantly, it is worth noting the timing of placing sorafenib to TACE and the degree of liver function damage as well as the standard of repeated TACE, to better understand the value of TACE combined with sorafenib in the treatment of HCC.
Acknowledgments
We would like to thank Editage (www.editage.cn) for their English language editing services.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Gilabert M, Bruix J, et al. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11(9):525–535. doi: 10.1038/nrclinonc.2014.122 [DOI] [PubMed] [Google Scholar]
- 3.Han G, Berhane S, Toyoda H, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response-based approach. Hepatology. 2020;72(1):198–212. doi: 10.1002/hep.31022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanoff HK, Chang Y, Lund JL, et al. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist. 2016;21:1113–1120. doi: 10.1634/theoncologist.2015-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590. doi: 10.1002/hep.27548 [DOI] [PubMed] [Google Scholar]
- 6.Loustaud-Ratti V, Debette-Gratien M, Carrier P. European association for the study of the liver and French hepatitis C recent guidelines: the paradigm shift. World J Hepatol. 2018;10:639–644. doi: 10.4254/wjh.v10.i10.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 8.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudo M, Trevisani F, Abou-Alfa GK, et al. Hepatocellular carcinoma: therapeutic guidelines and medical treatment. Liver Cancer. 2016;6:16–26. doi: 10.1159/000449343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047 [DOI] [PubMed] [Google Scholar]
- 12.Sun J, Shi J, Huang B, et al. The degree of hepatic arterial blood supply of portal vein tumor thrombus in patients with hepatocellular carcinoma and its impact on overall survival after transarterial chemoembolization. Oncotarget. 2017;8:79816–79824. doi: 10.18632/oncotarget.19767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725 [DOI] [PubMed] [Google Scholar]
- 14.Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878–2882. doi: 10.3748/wjg.v10.i19.2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x [DOI] [PubMed] [Google Scholar]
- 16.Dufour JF, Johnson P. Liver cancer: from molecular pathogenesis to new therapies: summary of the EASL single topic conference. J Hepatol. 2010;52:296–304. doi: 10.1016/j.jhep.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 18.Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized Phase III trials. J Clin Oncol. 2017;35:622–628. doi: 10.1200/JCO.2016.69.5197 [DOI] [PubMed] [Google Scholar]
- 19.Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590–599. doi: 10.1148/radiol.12111550 [DOI] [PubMed] [Google Scholar]
- 20.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 21.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64:1090–1098. doi: 10.1016/j.jhep.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 22.Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo controlled, double-blind, Phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–575. doi: 10.1016/S2468-1253(17)30156-5 [DOI] [PubMed] [Google Scholar]
- 23.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2019;69(8):1492–1501. doi: 10.1136/gutjnl-2019-318934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JW, Kim YJ, Kim DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70(4):684–691. doi: 10.1016/j.jhep.2018.11.029 [DOI] [PubMed] [Google Scholar]
- 25.Han K, Kim JH, Ko GY, et al. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: a comprehensive review. World J Gastroenterol. 2016;22:407–416. doi: 10.3748/wjg.v22.i1.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013 [DOI] [PubMed] [Google Scholar]
- 27.Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37:1–3. doi: 10.1002/mpo.1154 [DOI] [PubMed] [Google Scholar]
- 28.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 29.Gillmore R, Stuart S, Kirkwood A, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309–1316. doi: 10.1016/j.jhep.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 30.Tovoli F, Renzulli M, Granito A, et al. Radiologic criteria of response to systemic treatments for hepatocellular carcinoma. Hepat Oncol. 2017;4:129–137. doi: 10.2217/hep-2017-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JW, Park KW, Cho SH, et al. Risk of hepatitis B exacerbation is low after transcatheter arterial chemoembolization therapy for patients with HBV-related hepatocellular carcinoma: report of a prospective study. Am J Gastroenterol. 2005;100:2194–2200. doi: 10.1111/j.1572-0241.2005.00232.x [DOI] [PubMed] [Google Scholar]
- 32.Renzulli M, Peta G, Vasuri F, et al. Standardization of conventional chemoembolization for hepatocellular carcinoma. Ann Hepatol. 2020;22:100278. doi: 10.1016/j.aohep.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 33.Prajapati HJ, Xing M, Spivey JR, et al. Survival, efficacy, and safety of small versus large doxorubicin drug-eluting beads TACE chemoembolization in patients with unresectable HCC. AJR Am J Roentgenol. 2014;203(6):W706–W714. doi: 10.2214/AJR.13.12308 [DOI] [PubMed] [Google Scholar]
- 34.Vasuri F, Golfieri R, Fiorentino M, et al. OATP 1B1/1B3 expression in hepatocellular carcinomas treated with orthotopic liver transplantation. Virchows Archiv. 2011;459(2):141–146. doi: 10.1007/s00428-011-1099-5 [DOI] [PubMed] [Google Scholar]
- 35.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 36.Forner A, Reig ME, Bruix J, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133 [DOI] [PubMed] [Google Scholar]
- 37.Hiraoka A, Kumada T, Kudo M, et al. Hepatic function during repeated TACE procedures and prognosis after introducing sorafenib in patients with unresectable hepatocellular carcinoma: multicenter analysis. Dig Dis Sci. 2017;35:602–610. doi: 10.1159/000480256 [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Zhang C, Zhao Y, et al. Transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis: prognostic factors in a single-center study of 188 patients. Biomed Res Int. 2014;2014:194278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474–481. doi: 10.1016/j.jhep.2006.10.020 [DOI] [PubMed] [Google Scholar]
- 40.Lencioni R, Petruzzi P, Crocetti L. Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol. 2013;30(1):3–11. doi: 10.1055/s-0033-1333648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cucchetti A, Trevisani F, Cappelli A, et al. Cost-effectiveness of doxorubicin-eluting beads versus conventional trans-arterial chemo-embolization for hepatocellular carcinoma. Dig Liver Dis. 2016;48:798–805. doi: 10.1016/j.dld.2016.03.031 [DOI] [PubMed] [Google Scholar]
- 42.Compagnone G, Giampalma E, Domenichelli S, et al. Calculation of conversion factors for effective dose for various interventional radiology procedures. Med Phys. 2012;39(5):2491–2498. doi: 10.1118/1.3702457 [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Wang WJ, Guan S, et al. Sorafenib combined with transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patients. Ann Oncol. 2013;24:1786–1792. doi: 10.1093/annonc/mdt072 [DOI] [PubMed] [Google Scholar]
- 44.Chao Y, Chung YH, Han G, et al. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136:1458–1467. doi: 10.1002/ijc.29126 [DOI] [PubMed] [Google Scholar]
- 45.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443 [DOI] [PubMed] [Google Scholar]
- 46.Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today. 2005;41:773–784. doi: 10.1358/dot.2005.41.12.937959 [DOI] [PubMed] [Google Scholar]
- 47.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220 [DOI] [PubMed] [Google Scholar]
- 48.Wang B, Xu H, Gao ZQ, et al. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523–529. doi: 10.1080/02841850801958890 [DOI] [PubMed] [Google Scholar]
- 49.Facciorusso A, Bellanti F, Villani R, et al. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: a meta-analysis of randomized trials. United European Gastroenterol J. 2017;5(4):511–518. doi: 10.1177/2050640616673516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Facciorusso A, Licinio R, Muscatiello N, et al. Transarterial chemoembolization: evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol. 2015;7:2009–2019. doi: 10.4254/wjh.v7.i16.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Facciorusso A, Serviddio G, Muscatiello N. Transarterial radioembolization vs chemoembolization for hepatocarcinoma patients: a systematic review and meta-analysis. World J Hepatol. 2016;8(18):770–778. doi: 10.4254/wjh.v8.i18.770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu FX, Chen J, Bai T, et al. The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono-therapy in patients with BCLC stage B/C hepatocellular carcinoma. BMC Cancer. 2017;17(1):645. doi: 10.1186/s12885-017-3545-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren B, Wang W, Shen J, et al. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE alone for unresectable hepatocellular carcinoma: a propensity score matching study. J Cancer. 2019;10(5):1189–1196. doi: 10.7150/jca.28994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruix J, Reig M, Rimola J, et al. Clinical decision making and research in hepatocellular carcinoma: pivotal role of imaging techniques. Hepatology. 2011;54:2238–2244. doi: 10.1002/hep.24670 [DOI] [PubMed] [Google Scholar]
- 55.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122 [DOI] [PubMed] [Google Scholar]
- 56.Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906 [DOI] [PubMed] [Google Scholar]
- 57.Giannini EG, Moscatelli A, Pellegatta G, et al. Application of the intermediate-stage subclassification to patients with untreated hepatocellular carcinoma. Am J Gastroenterol. 2016;111(1):70–77. doi: 10.1038/ajg.2015.389 [DOI] [PubMed] [Google Scholar]
- 58.Facciorusso A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: recent findings and new perspectives. Curr Diabetes Rev. 2013;9(5):382–386. doi: 10.2174/15733998113099990068 [DOI] [PubMed] [Google Scholar]
- 59.Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 60.Strebel BM, Dufour JF. Combined approach to hepatocellular carcinoma: a new treatment concept for nonresectable disease. Expert Rev Anticancer Ther. 2008;8:1743–1749. doi: 10.1586/14737140.8.11.1743 [DOI] [PubMed] [Google Scholar]
- 61.Park JW, Koh YH, Kim HB, et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56:1336–1342. doi: 10.1016/j.jhep.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 62.Ogasawara S, Chiba T, Ooka Y, et al. Characteristics of patients with sorafenib-treated advanced hepatocellular carcinoma eligible for second-line treatment. Invest New Drugs. 2018;36:332–339. doi: 10.1007/s10637-017-0507-3 [DOI] [PubMed] [Google Scholar]
- 63.Jun BG, Kim YD, Kim SG, et al. Hepatitis B virus reactivation after radiotherapy for hepatocellular carcinoma and efficacy of antiviral treatment: a multicenter study. PLoS One. 2018;13:e0201316. doi: 10.1371/journal.pone.0201316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin XJ, Lao XM, Shi M, et al. Changes of HBV DNA after chemoembolization for hepatocellular carcinoma and the efficacy of antiviral treatment. Dig Dis Sci. 2016;61:2465–2476. doi: 10.1007/s10620-016-4167-5 [DOI] [PubMed] [Google Scholar]
- 65.Wang K, Jiang G, Jia Z, et al. Effects of transarterial chemoembolization combined with antiviral therapy on HBV reactivation and liver function in HBV-related hepatocellular carcinoma patients with HBV-DNA negative. Medicine. 2018;97:e10940. doi: 10.1097/MD.0000000000010940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan J, Yin X, Tang B, et al. Transarterial chemoembolization (TACE) combined with sorafenib in treatment of HBV background hepatocellular carcinoma with portal vein tumor thrombus: a propensity score matching study. Biomed Res Int. 2019;2019:2141859. doi: 10.1155/2019/2141859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yip B, Wantuck JM, Kim LH, et al. Clinical presentation and survival of Asian and non-Asian patients with HCV-related hepatocellular carcinoma. Dig Dis Sci. 2014;59:192–200. doi: 10.1007/s10620-013-2948-7 [DOI] [PubMed] [Google Scholar]
- 68.Guarino M, Sessa A, Cossiga V, et al. Direct-acting antivirals and hepatocellular carcinoma in chronic hepatitis C: a few lights and many shadows. World J Gastroenterol. 2018;24(24):2582–2595. doi: 10.3748/wjg.v24.i24.2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abbas Z, Siddiqui AU, Luck NH, et al. Prognostic factors of survival in patients with non-resectable hepatocellular carcinoma: hepatitis C versus miscellaneous etiology. J Pak Med Assoc. 2008;58(11):602–607. [PubMed] [Google Scholar]
- 70.Marasco G, Serenari M, Renzulli M, et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J Gastroenterol. 2020;55(10):927–943. doi: 10.1007/s00535-020-01711-w [DOI] [PMC free article] [PubMed] [Google Scholar]