Abstract
Brain tumors, a group of heterogeneous diseases, are the second most common cancer and the leading cause of cancer-related deaths in children. Insight into the prognosis of pediatric brain tumor survival has led to improved outcomes and could be further advanced through precision in prognosis. We analyzed the United States SEER population-based dataset of 15,723 pediatric brain tumor patients diagnosed and followed between 1975 and 2016 using a stratified Cox proportional hazards model. Mortality risk declined with increased age at diagnosis, the adjusted hazard ratio (aHR) (95% confidence interval) was 0.60 (0.55, 0.67) and 0.47 (0.42, 0.52) for ages at diagnosis 1-10 years and 10-19 years, respectively, when compared with infants. Non-Hispanic Caucasian patients showed a lower risk of mortality than non-Hispanic African Americans (1.21 (1.11, 1.32)) and Hispanics (1.21 (1.11, 1.32)). Primary tumor sites, grades, and histology showed substantial heterogeneity in mortality risk. Brainstem (2.62 (2.41, 2.85)) and Cerebrum (1.63 (1.46, 1.81)) had an elevated risk of mortality than lobes. Similarly, Grade II (1.32 (1.07, 1.62)), Grade III (3.39 (2.74, 4.19)), and Grade IV (2.18 (1.80, 2.64)) showed an inflated risk of mortality than Grade I. Compared to low-grade glioma, high-grade glioma (7.92 (7.09, 8.85)), Primitive neuroectodermal tumors (4.72 (4.15, 5.37)), Medulloblastoma (3.11 (2.79, 3.47)), and Ependymal-tumors (2.20 (1.95, 2.48)) had increased risk of mortality. County-level poverty and geographic region showed substantial variation in survival. This large population-based comprehensive study confirmed identified prognostic factors of pediatric brain tumor survival and provided estimates as epidemiologic evidence with greater generalization.
Keywords: Pediatric brain tumors, Survival, Population-based data, Heterogeneity, Prognostic factors
1. Introduction
Brain tumors account for more than 15% of children with cancer in the United States and are the second most common type of cancer in children [1]. More than 150 different brain tumors have been documented with several histological subtypes, and the diagnosis, treatment, and prognosis of childhood brain tumors heavily depend on the type, grade, size, and location of the tumor. Advances in diagnosis and treatment, as well as an understanding of the disease etiology in the last 2-3 decades, have resulted in substantial improvement in survival outcomes, but this has not happened equally in patients with all tumor types. The per-100,000 incidence rate of pediatric brain tumors has risen from 2.13 in 1975 to 2.99 in 2017, and the estimated 5-year survival rate has increased from 55% in 1975 to 76% in 2017 [2]. Age at diagnosis, race, sex, tumor grade, and tumor histologic subtypes and anatomic sites are the major identified prognostic factors. Studies have demonstrated that children diagnosed at an older age typically have better survival compared with those diagnosed at a younger age [3–6]. For children with medulloblastoma, the impact of age as a prognostic factor is hard to assess because different treatments are assigned according to patients’ age [7]. Racial variations in pediatric brain cancer survival have also been observed in previous studies. Non-Hispanic African American (NH-AA) children are at greater risk of death compared with their non-Hispanic Caucasian (NH-Caucasian) counterparts [3, 4]. Another frequently assessed factor is the tumor anatomic site, where the location at the brain stem and cerebrum are identified as having the worst survival [4, 6]. Sex is a risk factor for histologies such as low-grade gliomas and medulloblastomas, in which males are more vulnerable than females [3]. Tumor histology types such as glioblastomas, astrocytomas, medulloblastomas, ependymomas, and supratentorial primitive neuro-ectodermal tumors (PNET) greatly vary by prevalence, grades at diagnosis, and survival [8–11].
Socioeconomic and geographic factors have been reported to impact the incidence and survival outcomes of pediatric brain tumors [4, 12–17]. In the United States, the incidence rate of pediatric brain tumors is the highest in the Northeast region [17]. Survival also varied between Northeast and Midwest regions [4].
Published epidemiological studies on pediatric brain tumors are rare, relatively old, and mainly carried out with a focus on the disease occurrence [18–21]. Reports focusing on pediatric brain cancer survival and its prognostic factors are mainly based upon relatively small datasets of restricted histological types with limited comparisons [3–11]. A systemic and comprehensive epidemiologic study utilizing a population-based large dataset with long-term follow-up could better elucidate the variation in the survival patterns across prognostic factors and changes in the prognosis of these heterogeneous pediatric brain cancers due to the recent progress in the molecular understanding of tumor progression, diagnosis, and therapeutic responses. Such evaluation could lead to further refinement of risk groups that would enhance risk-based tailored therapy for additional improvement in the survival outcomes among the study population. In this study, we have utilized the population-based large dataset of the Surveillance Epidemiology and End Results (SEER) collected from patients diagnosed and followed-up between 1975 and 2016 to quantify the extent of associations of pediatric brain tumor survival with its prognostic factors, as the first study with over four decades of follow-up. The SEER dataset is large and well representative of the study population in the United States; thereby, an ideal to determine the epidemiological evidence of the multifaceted associations between pediatric brain cancer survival and its prognostic features.
2. Methods
2.1. Data Source
This study utilized 15,723 cases of pediatric (0–19 years) brain tumor patients, diagnosed between 1975 and 2016, from 18 SEER registries. The SEER program of the National Cancer Institute is the most comprehensive and reliable source of population-based information on cancer incidence and survival in the US [21]. The SEER registries are geographically diverse and cover approximately 27.8% of the US population with similar distribution to that of the general US population in regards to sex, race-ethnicity, and measures of poverty and education.
2.2. Study Variables
Study variables included age at diagnosis, sex, race-ethnicity, primary site, tumor grade, histology, year of diagnosis, percentage of persons in county below poverty level, geographic regions, survival time and vital status.
Age at diagnosis was recorded as <1, 1-4, 5-9, 10-14, and 15-19 years in the SEER dataset. We recoded this variable as <1, 1-9, and 10-19 years. Sex, a biological construct classified by chromosomes, was used as a nominal variable. We integrated categories of SEER variables “Race recode” and “Origin recode NHIA” to construct the following race-ethnicity groups: NH-Caucasian, NH-AA, Hispanic, and Non-Hispanic others (Others). The year of diagnosis was recorded in single-year interval in the SEER database. We recoded this variable as follows: 1975-1979, 1980-1984, 1985-1989, 1990-1994, 1995-1999, 2000-2004, 2005-2009, 2010-2014, and 2015-2016.
The SEER dataset included the primary site for brain tumors, utilizing the International Classification of Diseases for Oncology, third edition (ICD-O-3). We recoded this variable as follows: lobes (ICD-O-3 codes: C71.1-C71.4), brainstem (C71.7), cerebellum; nos (C71.6), cerebrum (C71.0), ventricle; nos (C71.5), and other sites including overlapping lesion of brain and brain, nos (C71.8-C71.9). Tumors in the SEER database were graded using the World Health Organization (WHO) grading system (Grades I-IV) basing on abnormalities of tumor cells and tissue under the microscope [22], as well as the potential rapidness in the tumor growth and spread. The tumor grades are not available for a large number of patients in the SEER database. We coded them as Unknown. Histologic classifications of brain tumors were done in the SEER data by the ICD-O-3/WHO 2008 definitions and were accessed using the SEER variable “ICD-O-3Hist/behav”. Histology was recoded as sub-groups reported in previously published studies [4, 8, 23]: ependymal tumors (ICD-O-3 code: 9391, 9392, 9393), low-grade glioma (9400, 9410, 9411, 9420, 9421), high-grade glioma (9401, 9440, 9441, 9442), Medulloblastoma (9470, 9471, 9472, 9474), other glioma (9380, 9381, 9382, 9424, 9431, 9432, 9450, 9451, 9460), primitive neuroectodermal tumors (PNET) (9473), and Others.
The percentage of persons with income below the poverty level was used as a county-level socioeconomic (SES) attribute and was grouped into quartiles. Quartile 1 included approximately 25% of patients from counties with the lowest percentage of persons below poverty level, whereas patients in Quartile 4 were from counties with the top 25% of percentage of persons below poverty level. SEER registries were located in six purchased/referred care delivery area (PRCDA) in the US. PRCDA included East, Pacific Coast, Northern Plains, Southern Plains, Southwest, and Alaska. Southern Plain is a new region with no patients included yet. There were only 27 patients in Alaska. We collapsed data from these two regions with that from the Pacific Coast.
The survival time referred to the duration of the time between diagnosis and death or the last day of follow-up. The SEER variable ‘Vital Status Recode’ documented whether the patient was alive or dead on the last available visit during the follow-up. The variable “SEER cause-specific death classification” was used to identify deaths caused by pediatric brain tumors. For the sensitivity analysis, those who did not experience death within the ten years after diagnosis were censored.
2.3. Statistical analysis
Distribution of the overall and brain tumor-specific mortality was summarized by demographics and clinical variables. Kaplan–Meier survival curve were used for the initial examination of crude survival time across prognostic factors. A univariable Cox proportional hazards model, stratified by the five-year interval of diagnosis, was used to examine the association between individual study variables and the risk of mortality of brain tumors. A multivariable stratified Cox proportional hazards model was used to determine the risk of mortality associated with prognostic factors in the US after simultaneous adjustment for known prognostic factors. Models were stratified by the year of diagnosis to account for the time-varying survival pattern. Hazard ratios (HR) and adjusted HR (aHR) for univariable and multivariable models, respectively, were reported along with the 95% confidence intervals (95% CI) and p -values. Additionally, we have repeated the analyses using only the first ten-year follow-up data to evaluate the sensitivity of the estimates using unequal follow-up time. All analyses were two-tailed with a level of significance of 0.05. The statistical software R (version 3.6.2) was used for the data analysis.
3. Results
Of the 15,723 patients included in the study, there were 56% female, 60% NH-Caucasian, 21% Hispanic, and 11% NH-AA children. More than half of the patients were diagnosed between ages 1 and 10 years. Low-grade glioma accounted for 37% of the patients, followed by medulloblastomas (15%). Of the 5,097 patients whose tumor grades were available in the SEER database, about 42% were identified with grade IV tumors. The overall mortality was 5,458 (35%), and the cancer-specific mortality was 4,739 (31%).
Table 1 demonstrated the distribution of overall and brain tumor-specific mortality over study variables of known prognostic factors of pediatric brain tumor survival. Figure 1 displayed the estimated Kaplan–Meier probability of surviving at any given time since the diagnosis by age group at diagnosis, race-ethnicity, sex, tumor histology, grade, and primary location. Table 2 presented the risk of mortality associated with each study factor with and without mutual adjustment for other factors. No substantial difference was observed in unadjusted risk between overall and brain tumor-specific mortality. Hence, results using overall mortality were presented for adjusted analysis as well as throughout the manuscript. Also, all estimation of mortality risk was accounted for survival variation due to differences in diagnosis years as a steady decline in the unadjusted risk of mortality was observed in patients diagnosed in later years. Unadjusted results from univariable Cox Proportional hazards models, in Table 2, revealed that age at diagnosis, race-ethnicity, histology type, tumor grade, and tumor primary site, county-level poverty, and geographic regions played a significant role in the mortality risk of pediatric brain tumors. Consistent with results from univariable model, all study factors but sex remained significant in the mutually adjusted multivariable stratified Cox proportional hazards regression model. Compared with patients diagnosed during infancy, patients diagnosed at an older age were associated with a lower risk of mortality, aHR (95% CI) was 0.60 (0.55, 0.67) and 0.47 (0.42, 0.52) for the age at diagnosis 1-9 years and 10-19 years, respectively. No substantial difference of sex effect was observed. Compared with NH-Caucasians, patients of other race-ethnic groups showed a substantially higher risk of mortality, aHR (95% CI): 1.25 (1.16, 1.35), 1.21 (1.11, 1.32), and 1.26 (1.14, 1.40) in Hispanics, NH-AA, and others, respectively. There were marked variations in the risk of mortality between tumor primary sites. When compared with lobes, the aHR (95% CI) was 2.62 (2.41, 2.85), 1.01 (0.91, 1.13), 1.63 (1.46, 1.81), and 1.30 (1.15, 1.48) in the brainstem, cerebellum; nos, cerebrum, and ventricle; nos, respectively. In contrast to the results in the univariable model, there was no difference in risk of mortality between lobes and cerebellum; nos after mutual adjustment for other factors. The risk of mortality increased with increasing levels of grades in both adjusted and unadjusted models. However, the extent of the difference in risk among grades reduced after controlling for other factors. The risk of mortality by histology subtypes also varied substantially. Ependymal tumors (aHR (95% CI): 2.20 (1.95, 2.48), high-grade glioma (7.92 (7.09, 8.85)), medulloblastoma (3.11 (2.79, 3.47)), other gliomas (3.38 (3.08, 3.71)), and PNET (4.72 (4.15, 5.37)) showed worse likelihood of survival compared to the low-grade glioma. The risk of death was comparable between ependymomas, medulloblastomas, and other gliomas. A steady increase in unadjusted risk of mortality was observed with a higher county-level poverty. The 4th Quartile of the county-level poverty measures remained significantly different from other three Quarters after adjustment for the remaining factors. In terms of PRCDA regions, the Northern plains emerged as the significantly worse with respect to survival when compared with the Pacific coast in the adjusted model. No substantial difference was observed in the hazard risk of mortality between the first ten years and the total follow-up time (Supplementary Table S1).
Table 1:
Distribution of pediatric brain tumor mortality, SEER data, 1975-2016.
| Variables | Overall | Brain tumor specific | ||||
|---|---|---|---|---|---|---|
| Total | Alive (%) | Dead (%) | Total | Alive (%) | Dead (%) | |
| Age Group at Diagnosis | ||||||
| <1 year | 912 | 433 (47.48) | 479 (52.52) | 883 | 483 (54.70) | 400 (45.30) |
| 01-09 years | 8354 | 5335 (63.86) | 3019 (36.14) | 8211 | 5488 (66.84) | 2723 (33.16) |
| 10-19 years | 6457 | 4497 (69.65) | 1960 (30.35) | 6221 | 4605 (74.02) | 1616 (25.98) |
| Sex | ||||||
| Female | 7134 | 4704 (65.94) | 2430 (34.06) | 6957 | 4827 (69.38) | 2130 (30.62) |
| Male | 8589 | 5561 (64.75) | 3028 (35.25) | 8358 | 5749 (68.78) | 2609 (31.22) |
| Race-ethnicity | ||||||
| NH-Caucasian | 9450 | 6322 (66.90) | 3128 (33.10) | 9222 | 6520 (70.70) | 2702 (29.30) |
| Hispanic | 3247 | 2094 (64.49) | 1153 (35.51) | 3145 | 2139 (68.01) | 1006 (31.99) |
| NH-AA | 1727 | 1019 (59.00) | 708 (41.00) | 1688 | 1065 (63.09) | 623 (36.91) |
| Others | 1299 | 830 (63.90) | 469 (36.10) | 1260 | 852 (67.62) | 408 (32.38) |
| Primary Tumor Site | ||||||
| Lobes | 3172 | 2200 (69.36) | 972 (30.64) | 3035 | 2235 (73.64) | 800 (26.36) |
| Brainstem | 3087 | 1473 (47.72) | 1614 (52.28) | 3024 | 1524 (50.40) | 1500 (49.60) |
| Cerebellum; nos | 4332 | 3251 (75.05) | 1081 (24.95) | 4271 | 3336 (78.11) | 935 (21.89) |
| Cerebrum | 1343 | 826 (61.50) | 517 (38.50) | 1300 | 859 (66.08) | 441 (33.92) |
| Ventricle; nos | 1031 | 681 (66.05) | 350 (33.95) | 1007 | 705 (70.01) | 302 (29.99) |
| Other brain sites | 2758 | 1834 (66.50) | 924 (33.50) | 2678 | 1917 (71.58) | 761 (28.42) |
| Tumor Grade | ||||||
| Grade I | 1076 | 943 (87.64) | 133 (12.36) | 1066 | 964 (90.43) | 102 (9.57) |
| Grade II | 1435 | 1133 (78.95) | 302 (21.05) | 1414 | 1164 (82.32) | 250 (17.68) |
| Grade III | 446 | 190 (42.60) | 256 (57.40) | 430 | 213 (49.53) | 217 (50.47) |
| Grade IV | 2140 | 963 (45.00) | 1177 (55.00) | 2051 | 1011 (49.29) | 1040 (50.71) |
| Unknown | 10626 | 7036 (66.21) | 3590 (33.79) | 10354 | 7224 (69.77) | 3130 (30.23) |
| Histology | ||||||
| Low-grade glioma | 5761 | 4932 (85.61) | 829 (14.39) | 5666 | 5020 (88.60) | 646 (11.40) |
| Ependymal tumors | 1236 | 781 (63.19) | 455 (36.81) | 1217 | 818 (67.21) | 399 (32.79) |
| High-grade glioma | 1346 | 322 (23.92) | 1024 (76.08) | 1245 | 344 (27.63) | 901 (72.37) |
| Medulloblastoma | 2294 | 1417 (61.77) | 877 (38.23) | 2270 | 1486 (65.46) | 784 (34.54) |
| Other | 1390 | 802 (57.70) | 588 (42.30) | 1341 | 847 (63.16) | 494 (36.84) |
| Other glioma | 3002 | 1700 (56.63) | 1302 (43.37) | 2909 | 1724 (59.26) | 1185 (40.74) |
| PNET | 694 | 311 (44.81) | 383 (55.19) | 667 | 337 (50.52) | 330 (49.48) |
| Percentage of Persons Below Poverty Level | ||||||
| Quartile 1 | 3950 | 2580 (65.32) | 1370 (34.68) | 3843 | 2687 (69.92) | 1156 (30.08) |
| Quartile 2 | 3848 | 2482 (64.50) | 1366 (35.50) | 3740 | 2560 (68.45) | 1180 (31.55) |
| Quartile 3 | 4391 | 2944 (67.05) | 1447 (32.95) | 4274 | 3002 (70.24) | 1272 (29.76) |
| Quartile 4 | 3528 | 2255 (63.92) | 1273 (36.08) | 3453 | 2323 (67.27) | 1130 (32.73) |
| PRCDA Regions | ||||||
| Pacific Coast | 7169 | 4715 (65.77) | 2454 (34.23) | 6963 | 4813 (69.12) | 2150 (30.88) |
| Northern Plains | 2215 | 1266 (57.16) | 949 (42.84) | 2153 | 1344 (62.42) | 809 (37.58) |
| East | 4994 | 3441 (68.90) | 1553 (31.10) | 4875 | 3533 (72.47) | 1342 (27.53) |
| Southwest | 1345 | 843 (62.68) | 502 (37.32) | 1324 | 886 (66.92) | 438 (33.08) |
| Year Group at Diagnosis | ||||||
| 1975-1979 | 748 | 262 (35.03) | 486 (64.97) | 724 | 338 (46.69) | 386 (53.31) |
| 1980-1984 | 706 | 289 (40.93) | 417 (59.07) | 689 | 334 (48.48) | 355 (51.52) |
| 1985-1989 | 905 | 472 (52.15) | 433 (47.85) | 874 | 516 (59.04) | 358 (40.96) |
| 1990-1994 | 1232 | 696 (56.49) | 536 (43.51) | 1212 | 765 (63.12) | 447 (36.88) |
| 1995-1999 | 1393 | 897 (64.39) | 496 (35.61) | 1369 | 924 (67.49) | 445 (32.51) |
| 2000-2004 | 3164 | 2060 (65.11) | 1104 (34.89) | 3098 | 2123 (68.53) | 975 (31.47) |
| 2005-2009 | 3157 | 2162 (68.48) | 995 (31.52) | 3056 | 2178 (71.27) | 878 (28.73) |
| 2010-2014 | 3214 | 2384 (74.18) | 830 (25.82) | 3122 | 2368 (75.85) | 754 (24.15) |
| 2015-2016 | 1204 | 1043 (86.63) | 161 (13.37) | 1171 | 1030 (87.96) | 141 (12.04) |
Note:
PRCDA: purchased/referred care delivery area
Figure 1. Survival of Pediatric Brain Tumor Patients by Prognostic Factors, SEER Data, 1975-2016.
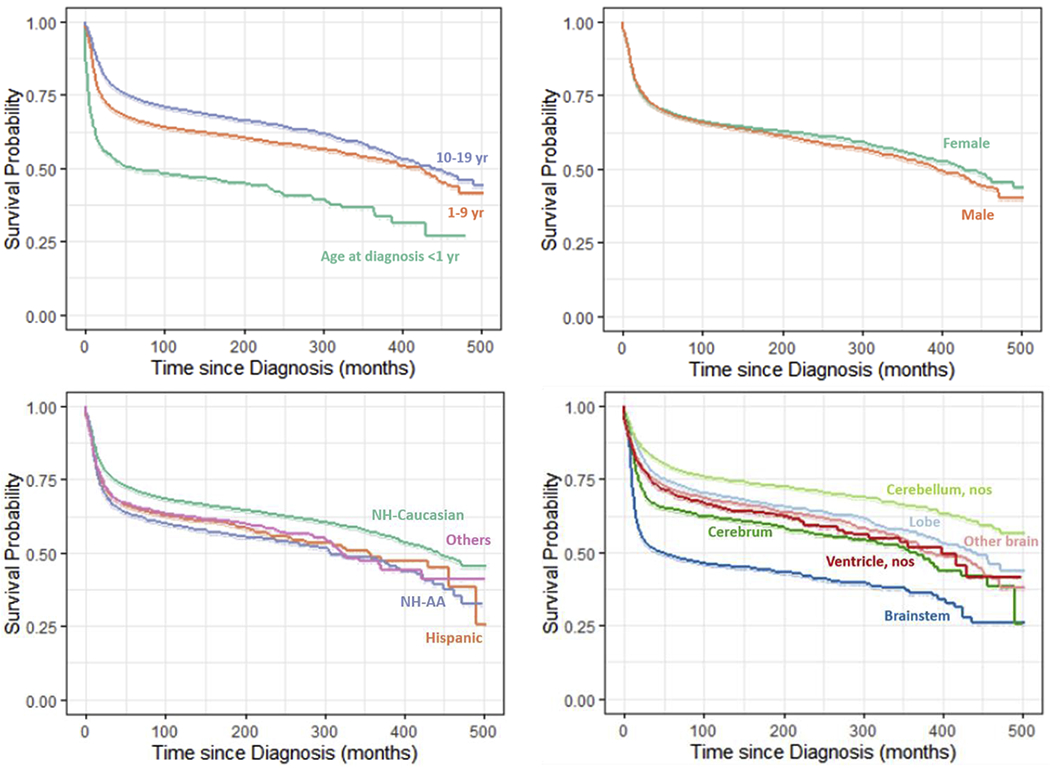
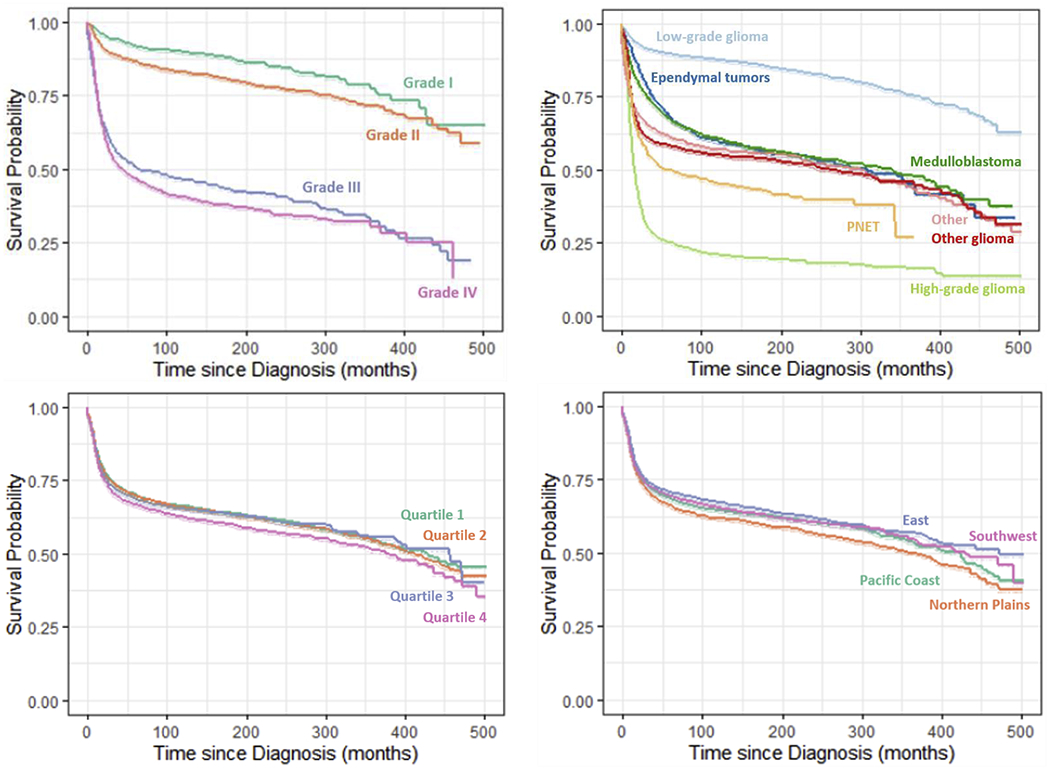
Prognostic factors included in the figures: age at diagnosis, sex, race-ethnicity, primary tumor site, tumor grade, histology, percentage of persons below poverty level, and US PRCDA regions.
Table 2:
Association between prognostic factors and survival of pediatric brain tumor patients in the univariable and multivariable Cox proportional hazards models stratified by year of diagnosis, SEER data, 1975-2016.
| Variables | Unadjusted Hazard Ratios (HR) | Adjusted Hazard Ratios (aHR) | ||||
|---|---|---|---|---|---|---|
| Overall | Brain tumor specific | Overall | ||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | aHR (95% CI) | p-value | |
| Age Group at Diagnosis | ||||||
| <1 year | ref | -- | ref | -- | ref | -- |
| 01-09 years | 0.53 (0.48, 0.58) | <0.01 | 0.58 (0.52, 0.64) | <0.01 | 0.60 (0.55, 0.67) | <0.01 |
| 10-19 years | 0.41 (0.37, 0.45) | <0.01 | 0.41 (0.37, 0.45) | <0.01 | 0.47 (0.42, 0.52) | <0.01 |
| Sex | ||||||
| Female | ref | -- | ref | -- | ref | -- |
| Male | 1.03 (0.98, 1.09) | 0.3 | 1.01 (0.96, 1.07) | 0.65 | 0.99 (0.94, 1.05) | 0.8 |
| Race-ethnicity | ||||||
| NH-Caucasian | ref | -- | ref | -- | ref | -- |
| Hispanic | 1.37 (1.28, 1.47) | <0.01 | 1.36 (1.26, 1.46) | <0.01 | 1.25 (1.16, 1.35) | <0.01 |
| NH-AA | 1.40 (1.29, 1.52) | <0.01 | 1.42 (1.30, 1.54) | <0.01 | 1.21 (1.11, 1.32) | <0.01 |
| Others | 1.29 (1.17, 1.42) | <0.01 | 1.28 (1.15, 1.42) | <0.01 | 1.26 (1.14, 1.40) | <0.01 |
| Primary Tumor Site | ||||||
| Lobes | ref | -- | ref | -- | ref | -- |
| Brainstem | 2.32 (2.14, 2.51) | <0.01 | 2.54 (2.33, 2.77) | <0.01 | 2.62 (2.41, 2.85) | <0.01 |
| Cerebellum; nos | 0.77 (0.70, 0.84) | <0.01 | 0.79 (0.72, 0.87) | <0.01 | 1.01 (0.91, 1.13) | 0.84 |
| Cerebrum | 1.38 (1.24, 1.54) | <0.01 | 1.43 (1.27, 1.60) | <0.01 | 1.63 (1.46, 1.81) | <0.01 |
| Ventricle; nos | 1.21 (1.07, 1.37) | <0.01 | 1.24 (1.09, 1.42) | <0.01 | 1.30 (1.15, 1.48) | <0.01 |
| Other brain sites | 1.11 (1.01, 1.21) | 0.03 | 1.10 (0.99, 1.21) | 0.07 | 1.30 (1.18, 1.42) | <0.01 |
| Tumor Grade | ||||||
| Grade I | ref | -- | ref | -- | ref | -- |
| Grade II | 1.51 (1.23, 1.85) | <0.01 | 1.66 (1.32, 2.10) | <0.01 | 1.32 (1.07, 1.62) | 0.01 |
| Grade III | 5.53 (4.49, 6.83) | <0.01 | 6.17 (4.88, 7.82) | <0.01 | 3.39 (2.74, 4.19) | <0.01 |
| Grade IV | 7.05 (5.89, 8.44) | <0.01 | 8.08 (6.59, 9.90) | <0.01 | 2.18 (1.80, 2.64) | <0.01 |
| Unknown | 3.39 (2.85, 4.03) | <0.01 | 3.86 (3.17, 4.70) | <0.01 | 1.73 (1.45, 2.07) | <0.01 |
| Histology | ||||||
| Low-grade glioma | ref | -- | ref | -- | ref | -- |
| Ependymal tumors | 3.13 (2.80, 3.52) | <0.01 | 3.44 (3.04, 3.90) | <0.01 | 2.20 (1.95, 2.48) | <0.01 |
| High-grade glioma | 9.64 (8.79, 10.58) | <0.01 | 11.06 (9.99, 12.24) | <0.01 | 7.92 (7.09, 8.85) | <0.01 |
| Medulloblastoma | 3.18 (2.89, 3.49) | <0.01 | 3.57 (3.21, 3.96) | <0.01 | 3.11 (2.79, 3.47) | <0.01 |
| Other | 4.06 (3.65, 4.51) | <0.01 | 4.39 (3.91, 4.94) | <0.01 | 3.31 (2.95, 3.70) | <0.01 |
| Other glioma | 4.20 (3.85, 4.59) | <0.01 | 4.88 (4.43, 5.37) | <0.01 | 3.38 (3.08, 3.71) | <0.01 |
| PNET | 5.60 (4.95, 6.33) | <0.01 | 6.11 (5.34, 6.99) | <0.01 | 4.72 (4.15, 5.37) | <0.01 |
| Percentage of Persons Below Poverty Level | ||||||
| Quartile 1 | ref | -- | ref | -- | ref | -- |
| Quartile 2 | 1.01 (0.94, 1.09) | 0.82 | 1.04 (0.96, 1.13) | 0.36 | 0.98 (0.91, 1.05) | 0.55 |
| Quartile 3 | 1.11 (1.03, 1.19) | <0.01 | 1.14 (1.05, 1.23) | <0.01 | 1.04 (0.97, 1.13) | 0.28 |
| Quartile 4 | 1.20 (1.11, 1.30) | <0.01 | 1.25 (1.15, 1.36) | <0.01 | 1.13 (1.04, 1.23) | <0.01 |
| PRCDA Regions | ||||||
| Pacific Coast | ref | -- | ref | -- | ref | -- |
| Northern Plains | 1.01 (0.93, 1.09) | 0.89 | 1.01 (0.93, 1.09) | 0.88 | 1.22 (1.12, 1.32) | <0.01 |
| East | 0.93 (0.87, 0.99) | 0.02 | 0.91 (0.85, 0.97) | 0.01 | 1.03 (0.96, 1.11) | 0.37 |
| Southwest | 0.91 (0.83, 1.01) | 0.07 | 0.92 (0.83, 1.02) | 0.11 | 1.09 (0.98, 1.21) | 0.1 |
| Year Group at Diagnosis | ||||||
| 1975-1979 | ref | -- | ref | -- | ||
| 1980-1984 | 0.98 (0.86, 1.12) | 0.78 | 1.01 (0.87, 1.17) | 0.9 | ||
| 1985-1989 | 0.79 (0.69, 0.90) | <0.01 | 0.77 (0.67, 0.89) | <0.01 | ||
| 1990-1994 | 0.76 (0.67, 0.86) | <0.01 | 0.72 (0.63, 0.83) | <0.01 | ||
| 1995-1999 | 0.62 (0.54, 0.70) | <0.01 | 0.62 (0.54, 0.71) | <0.01 | ||
| 2000-2004 | 0.64 (0.57, 0.72) | <0.01 | 0.63 (0.55, 0.71) | <0.01 | ||
| 2005-2009 | 0.62 (0.55, 0.69) | <0.01 | 0.60 (0.53, 0.68) | <0.01 | ||
| 2010-2014 | 0.59 (0.53, 0.67) | <0.01 | 0.59 (0.52, 0.67) | <0.01 | ||
| 2015-2016 | 0.64 (0.53, 0.77) | <0.01 | 0.61 (0.50, 0.75) | <0.01 | ||
Note:
CI: confidence interval;
All models were stratified by ‘Year Group at Diagnosis’ to estimate HR and aHR;
Adjusted Hazard Ratios (aHR): Hazard ratios of a factor estimated after mutually adjustment for other factors in the model;
aHR was estimated only overall mortality as there was no substantial difference in risk between overall and brain - specific mortality;
4. Discussion
Pediatric brain tumors are a group of deadly heterogeneous diseases with marked variation in patient characteristics, disease etiology, response to therapy, and survival. Patient’s demographic and clinical features that have been commonly identified with notable survival differences in previous studies include age at diagnosis, race-ethnicity, tumor location, tumor grade, and histologic subtypes [3–11]. In addition, SES and geographic variations in pediatric brain tumor survival have been identified in some studies [24]. Our study built on findings of previous studies, but provided estimates with greater accuracy, utilizing a large population-based dataset with long-term follow-up. Previous findings of prognostic factors were sometimes limited to patients with particular tumor locations or histologic subtypes. Our study utilized patients over four decades of all brain tumor types to compressively evaluate prognostic values of these factors.
Consistent with previous studies [3, 25–27], age at diagnosis remained a significant prognostic factor. Mortality risk decreased as age at diagnosis increased. When compared with infants, the hazard risk of mortality reduced by 40% and 53% in patients diagnosed at ages 1-9 years and 10-19 years, respectively. Age-related biological variation including distributional differences of tumor-histology, -grade, and -location, age-dependent treatment modality and response may be the main contributors of the survival disadvantage among younger patients [4, 6, 25–31]. The prevalence of relatively more aggressive histological lesions and tumor locations with a higher risk of mortality tended to be higher in younger age groups [25, 26]. This disparity was consistent in our SEER dataset. Also, patients with higher grades were more prevalent in infants in the SEER dataset. The worse outcomes in infants suggested the need for further innovation to treat and care for this group of patients.
Our study demonstrated highly prevalent survival disparities by racial and ethnic minorities. When compared with NH-Caucasians, the estimated adjusted hazards risk of mortality in NH-AA and Hispanic patients was 1.25 and 1.23, respectively. The findings of worse survival among NH-AA in our study mirrors the reports in previous studies [4, 32–34]. However, our findings of worse survival in Hispanics are consistent with some but not all as previous studies reported mixed results on the effect of this race-ethnic group [32, 33, 35]. In contrast to the findings reported by Siegel et al., the extent of survival disadvantage was alike among Hispanics and NH-AA patients in our study [4]. The racial differences in pediatric brain tumor survival could be attributed to the histology, differential access to the medical facilities, and response to therapies [4]. Enhanced measures to address modifiable race-ethnic survival disparities could further improve the prognosis of pediatric brain tumors.
Tumor grade is unknown for more than two-thirds of patients in the SEER data. Among the patients with reported tumor grade, mortality risk increased steeply with increased levels of tumor grades. This is consistent with current knowledge [4, 11].
Tumor anatomic site is commonly recognized as presenting wide-ranging differential survival outcomes in pediatric brain cancers [4, 11]. After controlling for known prognostic factors, the findings of our study revealed the following hierarchical order in the mortality risk of primary sites: cerebellum; nos, lobes, other brain, ventricle; nos, cerebrum, and brainstem. cerebellum; nos and brainstem exhibited significantly lower and higher risk of mortality when compared with other sites, consistent with previous reports [4, 28]. This prognosis of the brainstem tumor likely reflected a clinical reality. The brainstem tumor is difficult to treat and operate because either the tumor is inaccessible for treatment or surgical removal of the tumor may damage critical parts of the developing brain.
Like tumor sites, histological subtypes also consistently demonstrated diverse survival outcomes in previous studies [3, 4, 8, 11]. Low-grade glioma and high-grade glioma appeared as the most survival favorable and survival disadvantaged histologic subgroups, respectively, in our study which was consistent with previous reports [4, 28, 35]. PNET emerged as the second most survival disadvantaged subgroup, while ependymal tumors and medulloblastoma presented moderate risk of mortality following mild risk of low-grade glioma. The large change in magnitude of histology- and grade-attributed risk, after mutual adjustment for other factors, might reflect the complicated relation of these two factors with tumor site or other study factors in the prognosis of pediatric brain cancer.
Our study confirmed the role of SES and geographic region on the prognosis of pediatric brain tumor survival. Patients from counties with upper quartiles of percentages of person below poverty level showed a worse prognosis. The findings were consistent with that reported by previous studies [4, 17, 36]. While individually-based measures of SES are gold-standard, findings of our study suggested that community-level SES measures were also useful predictors of mortality risk and could point toward communities in need of resources to reduce SES-related survival disparities. Also, a possible explanation of the worst outcomes among children residing in Northern Plains might be the lack of adequate pediatric healthcare facilities in this region.
The greatest strength of our study was the utilization of a SEER population-based large dataset to describe survival of pediatric brain cancers by known prognostic factors with greater population representation than most studies previously published. This dataset allowed better categorization and estimation with better precision of known prognostic factors of pediatric brain survival, and finally, helped elucidate clinical realities. However, like most epidemiological studies, this study was not without weaknesses. First, findings of this study might be driven in part by the effect of unmeasured confounders including treatment. Secondly, SEER data might contain misclassification of patient demographics such as race, ethnicity, and socioeconomic status [37]. Thirdly, early diagnosis of tumors might be related to a favorable prognosis of survival. Lack of information on tumor stage at the time of diagnosis is a limitation of this study. Fourthly, inclusion of patients with only malignant brain tumors is also a limitation of this study. Fifthly, patients diagnosed in the recent past had shorter follow-up time, and had a lower risk of mortality as survival improved over time. Analytical challenges of these two limitations of the dataset were addressed through stratification of analyses by year of diagnosis. In addition, findings of the sensitivity analysis verified that varying follow-up time did not affect our results. Lastly, the proportion of Hispanic patients increased over time in SEER data. The stratified analysis handled this constraint of the population-based data successfully.
In conclusion, this study identified age at diagnosis, race-ethnicity, tumor grade, primary site, histologic subtypes, county-poverty level, and geographic regions as significant prognostic factors of pediatric brain tumor survival in the United States. This comprehensive study of well representative population-based SEER dataset with long-term follow-up enhanced estimates as epidemiologic evidence of the association of pediatric brain cancer with its prognostic factors in the United States. Improvement toward existing survival variation in demographic and biological host factors, disease etiology, SES as well as environmental barriers to accessing treatment may further reduce the risk of mortality. Epidemiological evidence of this study could suggest further elucidation of the underlying mechanism of complex interacting associations of prognostic factors with mortality risk, that eventually lead to identifying and implementing potential effective interventions to improve pediatric brain cancer survival in the United States.
Supplementary Material
Highlights:
Brain tumors are the 2nd leading cause of pediatric cancer death in the U.S.
Survival varies by tumor location, histology, age, race-ethnicity, and poverty.
Children diagnosed at older ages experience better survival outcomes.
Older age at diagnosis is associated with favorable tumor location and histology.
African American and Hispanic children are associated with higher mortality.
Acknowledgement:
The study was partially supported by the National Institutes of Health (NIH) COBRE Grant P30GM114736 (PI: Shaffer), and the National Institute of General Medical Sciences of the NIH IDeA Grant U54-GM104941 (PI: Hicks).
Footnotes
Conflict of Interest Statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address to both the Corresponding Author and the Cancer Epidemiology Journal.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Siegel RL, Miller KD and Jemal A, “Cancer Statistics, 2018,” CA: a cancer journal for clinicians, vol. 68, no. 1, pp. 7–30, January 2018. [DOI] [PubMed] [Google Scholar]
- [2].Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ and Cronin KA, “SEER Cancer Statistics Review, 1975-2017,” National Cancer Institute, Bethesda, MD, based on Novermber 2019 SEER data submission, posted to the SEER website, April 2020. [Google Scholar]
- [3].Claus EB and Black PM, “Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001,” Cancer, vol. 106, no. 6, pp. 1358–63, 15 Mar 2006. [DOI] [PubMed] [Google Scholar]
- [4].Siegel DA, Li J, Ding H, Singh SD, King JB and Pollack LA, “Racial and ethnic differences in survival of pediatric patients with brain and central nervous system cancer in the United States,” Pediatric Blood & Cancer, vol. 66, no. 2, p. e27501, Feb 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McGuire CS, Sainani KL and Fisher PG, “Both location and age predict survival in epnedymoma: a SEER study,” Pediatric Blood & Cancer, vol. 52, no. 1, pp. 65–9, Jan 2009. [DOI] [PubMed] [Google Scholar]
- [6].Qaddoumi I, Sultan I and Gajjar A, “Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results Database,” Cancer, vol. 115, no. 24, pp. 5761–70, 15 Dec 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mueller S and Chang S, “Pediatric brain tumors: current treatment strategies and future therapeutic approaches,” Neurotherapeutics, vol. 6, no. 3, pp. 570–86, Jul 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C and Barnholtz-Sloan JS, “CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015,” Neuro-oncology, vol. 20, no. suppl_4, pp. iv1–iv86, 1 Oct 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kilday J-P, Rahman R, Dyer S, Ridley L, Lowe J, Coyle B and Grundy R, “Pediatric ependymoma: biological perspective,” Molecular Cancer Research, vol. 7, no. 6, pp. 765–86, Jun 2009. [DOI] [PubMed] [Google Scholar]
- [10].Reddy AT, Janss AJ, Phillips PC, Weiss HL and Packer RJ, “Outcome for children with supratentorial primitive neuroectodermal tumors treated with surgery, radiation, and chemotherapy,” Cancer, vol. 88, no. 9, pp. 2189–93, 1 May 2000. [DOI] [PubMed] [Google Scholar]
- [11].de Araujo OL, da Trindade KM, Trompieri NM, Fontenele JB and Felix FHC, “Analysis of survival and prognostic factors of pediatric patients with brain tumor,” Journal de Pediatria, vol. 87, no. 5, pp. 425–32, 2011. [DOI] [PubMed] [Google Scholar]
- [12].Plascak JJ and Fisher JL, “Area-based socioeconomic position and adult glioma: a hierarchical analysis of surveillance epidemiology and end results data,” PLoS One, vol. 4, no. e60910, p. 8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Khanolkar AR, Ljung R, Talback M, Brooke HL, Carlsson S, Mathiesen T and Faychting M, “Socioeconomic position and the risk of brain tumour: a Swedish national population-based cohort study,” Journal of Epidemiology & Community Health, vol. 70, no. 12, pp. 1222–1228, 2016. [DOI] [PubMed] [Google Scholar]
- [14].Porter AB, Lachance DH and Johnson DR, “Socioeconomic status and glioblastoma risk: a population-based analysis,” Cancer Causes & Control, vol. 26, no. 2, pp. 179–185, 2015. [DOI] [PubMed] [Google Scholar]
- [15].Muquit S, Parks R and Basu S, “Socio-economic characteristics of patients with glioblastoma multiforme,” Journal of neuro-oncology, vol. 125, no. 2, pp. 325–329, 2015. [DOI] [PubMed] [Google Scholar]
- [16].Cote DJ, Ostrom QT, Gittleman H, Duncan KR, CreveCoeur TS, Kruchko C, Smith TR, Stampfer MJ and Barnholtz-Sloan JS, “Glioma incidence and survival variations by county-level socioeconomic measures,” Cancer, vol. 125, no. 19, pp. 3390–3400, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Siegel DA, Li J, Henley SJ, Wilson RJ, Lunsford NB, Tai E and Van Dyne EA, “ Geographic variation in pediatric cancer incidence-United States, 2003-2014,” Morbidity and Mortality Weekly Report, vol. 67, no. 25, pp. 707–713, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Johnson KJ, Cullen J, Barnholtz-Sloan JS, Ostrom QT, Langer CE, Turner MC, McKean-Cowdin R, Fisher JL, Lupo PJ, Partap S and Schwartzbaum JA, “Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review,” Cancer Epidemiology and Prevention Biomarkers, vol. 23, no. 12, pp. 2716–2736, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Miltenburg D, Louw DF and Sutherland GR, “Epidemiology of childhood brain tumors,” Canadian Journal of Neurological Sciences, vol. 23, no. 2, pp. 118–122, 1996. [DOI] [PubMed] [Google Scholar]
- [20].Baldwin RT and Preston-Martin S, “Epidemiology of brain tumors in childhood--a review,” Tooxicology and applied pharmacology, vol. 199, no. 2, pp. 118–131, 2004. [DOI] [PubMed] [Google Scholar]
- [21].Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on November 2017. submission. [Google Scholar]
- [22].Gupta A and Dwivedi T, “A simplified overview of World Health Organization classification update of central nervous system tumors 2016,” Journal of Neurosciences in Rural Practice, vol. 8, no. 4, pp. 629–641, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Packer RJ, “Childhood brain tumors: accomplishments and ongoing challenges,” Journal of child neurology, vol. 23, no. 10, pp. 1122–1127, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fang Z, Kulldorff M and Gregorio DI, “Brain cancer mortality in the United States, 1986 to 1995: A geographic analysis,” Neuro-Oncology, vol. 6, no. 3, pp. 179–187, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hankinson TC, Dudley RW, Torok MR, Patibandla MR, Dorris K, Poonia S, Wilkinson CC, Bruny JL, Handler MH and Liu AK, “Short-term mortality following surgical procedures for the diagnosis of pediatric brain tumors: outcome analysis in 5533 children from SEER, 2004-2011,” Journal of Neurosurgery: Pediatrics, vol. 17, no. 3, pp. 289–297, 2016. [DOI] [PubMed] [Google Scholar]
- [26].O’Kane R, Mathew R, Kenny T, Stiller C and Chumas P, “United Kingdom 30-day mortality rates after surgery for pediatric central nervous system tumors,” Journal of Neurosurgery: Pediatrics, vol. 12, no. 3, pp. 227–234, 2013. [DOI] [PubMed] [Google Scholar]
- [27].Bandopadhayay P, Bergthold G, London WB, Goumnerova LC, Morales La Madrid A, Marcus KJ, Guo D, Ullrich NJ, Chi SN and Beroukhim R, “Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database,” Pediatric blood & cancer, vol. 61, no. 7, pp. 1173–1179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, Stearns DS, Wolff JE, Wolinsky Y, Letterio JJ and Barnholtz-Sloan JS, “Alex’s Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007-2011,” Neuro-oncology, vol. 16, no. suppl_10, pp. x1–x36, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Samaan MC and Akhtar-Danesh N, “The impact of age and race on longevity in pediatric astrocytic tumors: A population-based study,” Pediatric blood & cancer, vol. 62, no. 9, pp. 1567–1571, 2015. [DOI] [PubMed] [Google Scholar]
- [30].Karalexi MA, Papathoma P, Thomopoulos TP, Ryzhov A, Zborovskaya A, Dimitrova N, Zivkovic S, Eser S, Antunes L, Sekerija M and Zagar T, “Childhood central nervous system tumour mortality and survival in Southern and Eastern Europe (1983-2014): gaps persist across 14 cancer registries,” European Journal of Cancer, vol. 51, no. 17, pp. 2665–2677, 2015. [DOI] [PubMed] [Google Scholar]
- [31].Smoll NR, “Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs),” Cancer, vol. 118, no. 5, pp. 1313–1322, 2012. [DOI] [PubMed] [Google Scholar]
- [32].Linabery AM and Ross JA, “Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975-1999,” Cancer, vol. 113, no. 9, pp. 2575–2596, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Austin MT, Hamilton E, Zebda D, Nguyen H, Eberth JM, Chang Y, Elting LS and Sandberg DI, “Health disparities and impact on outcomes in children with primary central nervous system solid tumors,” Journal of Neurosurgery: Pediatrics, vol. 18, no. 5, pp. 585–593, 2016. [DOI] [PubMed] [Google Scholar]
- [34].Holmes LJ, Chavan P, Blake T and Dabney K, “Unequal cumulative incidence and mortality outcome in childhood brain and central nervous system malignancy in the USA,” Journal of racial and ethnic health disparities, vol. 5, no. 5, pp. 1131–1141, 2018. [DOI] [PubMed] [Google Scholar]
- [35].Barnholtz-Sloan JS, Severson RK, Stanton B, Hamre M and Sloan AE, “Pediatric brain tumors in non-Hispanics, Hispanics, African American and Asians: differences in survival after diagnosis,” Cancer Causes & Control, vol. 16, no. 5, pp. 587–592, 2005. [DOI] [PubMed] [Google Scholar]
- [36].Mogensen H, Modig K, Tettamanti G, Talback M and Feychting M, “Socioeconomic differences in cancer survival among Swedish children,” British journal of cancer, vol. 114, no. 1, pp. 118–124, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gomez SL and Glaser SL, “Missclassification of race/ethnicity in a population-based cancer registry (United States),” Cancer causes & control, vol. 17, no. 6, pp. 771–781, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


