Abstract
This case report demonstrates the possibility of sarcoma biopsy needle track seeding from FNA/Core Needle Biopsy during the workup of a pediatric head and neck mass. Though not currently widely practiced in head and neck tumors, surgeons may consider placing biopsy tracks in the area of planned resection for suspected head and neck malignancies as is more common in approaches to extremity sarcoma.
Keywords: core needle biopsy, fine needle, head and neck cancer, needle biopsy, needle tract seeding, pediatric sarcoma
This case report demonstrates the possibility of sarcoma biopsy needle track seeding from FNA/Core Needle Biopsy during the workup of a pediatric head and neck mass. Though not currently widely practiced in head and neck tumors, surgeons may consider placing biopsy tracks in the area of planned resection for suspected head and neck malignancies as is more common in approaches to extremity sarcoma.
1. INTRODUCTION
Ultrasound‐guided fine‐needle aspiration (FNA) and core needle biopsies (CNB) are commonly used to diagnose head and neck malignancies. Needle tract seeding (NTS) from percutaneous biopsies, though uncommon, has been previously reported in various anatomic subsites. 1 Herein, we describe a case of NTS in a pediatric patient with high‐grade head and neck sarcoma. This case highlights important considerations in prebiopsy planning in the treatment of pediatric head and neck sarcomas.
2. CASE PRESENTATION
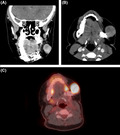
A 16‐year‐old girl presented with a left mandible tumor and a medical history significant for infant acute lymphocytic leukemia and anthracycline‐induced cardiomyopathy. (Figure 1) Risk factors for malignancy included cardiac transplant at age 9 and low‐dose external beam radiation for chemotherapy‐refractory retropharyngeal post‐transplant lymphoproliferative disorder at age 10. The diagnosis of a high‐grade spindle cell sarcoma of the mandible was made with ultrasound‐guided FNA and CNB at age 16. The biopsy was performed with multiple needle passes (5x 22‐gauge Chiba needles (Cook Medical Bloomington, IN, USA), 4x 18‐gauge BioPince (Argon Medical Devices Frisco, TX, USA) core biopsy) and a 17‐gauge introducer needle was used. Cytopathology was present to ensure specimen adequacy. A single skin nick was performed, and no freehand passes were obtained. Metastatic workup was negative, and resection was recommended after multidisciplinary discussion. The patient underwent radical segmental mandibulectomy, left selective neck dissection, and fibula free flap. The time span between the biopsy and the definitive resection was six weeks. During this time, the patient was closely followed by oncology and received one cycle of ifosfamide and doxorubicin. Surgical margins were negative, and there was no nodal involvement.
FIGURE 1.
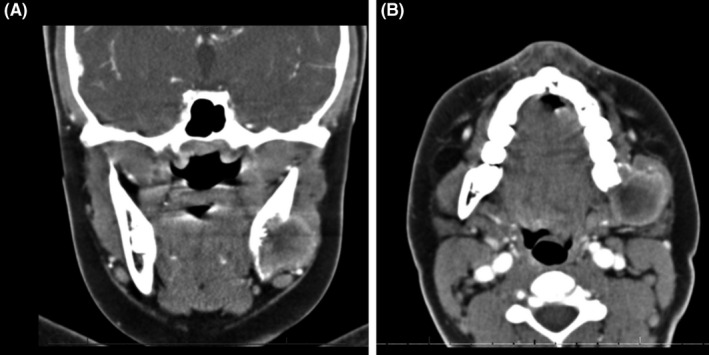
Maxillofacial CT scan with contrast (Philips Medical; Cambridge, MA, USA) in coronal (A) and axial (B) plane showed soft tissue mass centered at the left mandibular angle with cortical disruption. No cutaneous or subcutaneous involvement was evident on imaging
Surveillance PET/CT at three months following surgery suggested widespread bone metastases and a linear FDG‐avid lesion in the subcutaneous tissue connected to the skin corresponding to the original biopsy tract. (Figure 2) The patient underwent additional cycles of ifosfamide and doxorubicin given metastatic disease. Repeat imaging three months later showed interval growth of the soft tissue mass. (Figure 3) While the skin appeared uninvolved, the perifacial mass was conspicuous and caused the patient localized discomfort. After discussion with the family and patient, palliative wide local excision of the subcutaneous tissue mass was performed and revealed identical histology to that of the original sarcoma.
FIGURE 2.
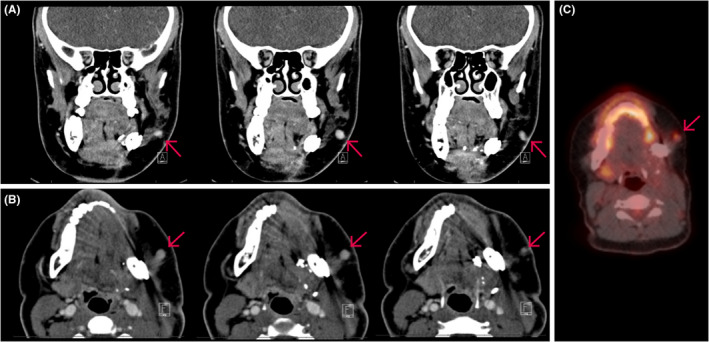
Three months postoperative PET/CT scan (Philips Medical; Cambridge, MA, USA). Representative image series of coronal (A) and axial (B) CT maxillofacial with contrast following left mandibulectomy and fibular graft reconstruction. Red arrow indicating tubular soft tissue lesion in the region of the previous needle biopsy tract. (C) Positron emission tomographic showed corresponding hypermetabolic soft tissue focus within the subcutaneous tissues of the left cheek
FIGURE 3.
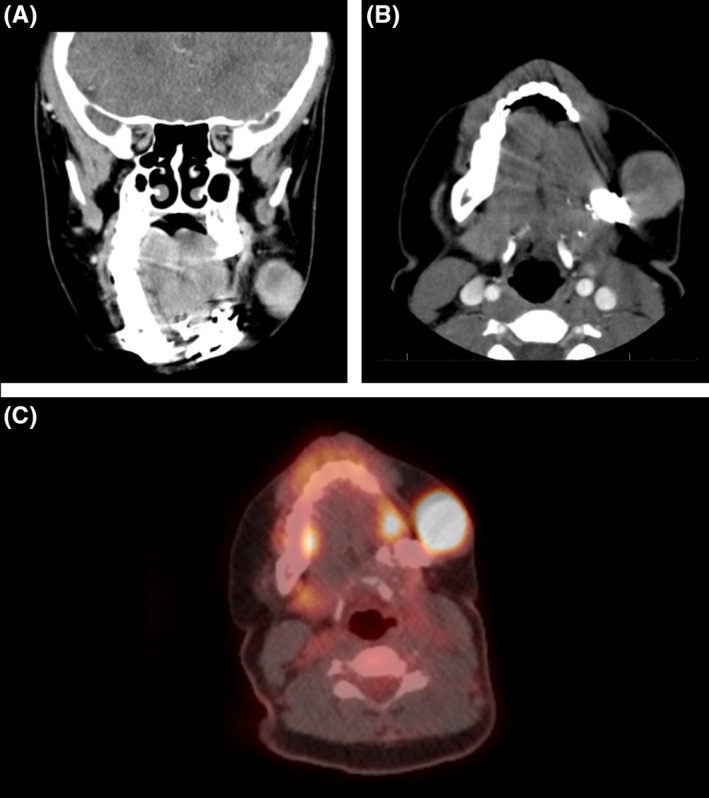
Six months postoperative repeat PET/CT scan (Philips Medical; Cambridge, MA, USA). Coronal (A) and axial (B) CT demonstrated significant interval enlargement of the left soft tissue mass. (C) Positron emission tomographic showed hypermetabolic uptake of the enlarged left cheek soft tissue mass. Red arrow indicating soft tissue lesion in the region of previous needle biopsy tract
3. DISCUSSION
NTS is a known potential complication of needle biopsy, particularly core biopsies. 1 The subcutaneous mass seen in our patient overlapped with the trajectory of the original needle biopsy. Moreover, the timeline of presentation and congruent histopathology strongly suggests this to be a case of NTS. This case is instructive as it documents a rare but important potential complication when treating pediatric head and neck sarcomas.
Overall, NTS is uncommon and reported predominately through case reports. 1 The patient presented had medical history notable for previous history of lymphoma, immunosuppression with organ transplant, and prior radiation treatment. Within the head and neck literature, a systematic review excluding thyroid and parathyroid lesions found NTS incidence following salivary and cutaneous malignancy to be 0.00012% for FNA and 0.0011% for CNB. 2 Another systematic review reported 19 cases of NTS following thyroid FNA between 1970 and 2014. 3
More aggressive histologies, such as the high‐grade spindle cell sarcoma case illustrated here, may portend a higher risk of NTS. For instance, in extremity sarcoma, excised needle tracts have been reported to harbor malignant cells in 13% of the samples. 1 As such, some surgeons have advocated for pretreatment biopsy to be performed in such a way that the biopsy tract will be included in the resected specimen. As opposed to patients with extremity sarcomas, this practice has not been widespread in managing pediatric head and neck sarcomas.
The excision of the biopsy tract would require careful planning for head and neck resections, as skin is often free from tumor involvement and, therefore, not typically resected. An additional consideration relates to the more judicious use of adjuvant therapy among pediatric head and neck patients. Binitie et al found that adjuvant radiation therapy with adult sarcoma patients did not have a local recurrence or metastatic rates with unresected CNB tracts. 4 Adjuvant radiation therapy carries elevated risks among pediatric patients, such as growth disruption and potential for the development of radiation‐associated neoplasms. 5 Therefore, surgical resection alone is the treatment of choice when possible in pediatric sarcoma patients.
Thus, a surgeon may want to opine on the cutaneous location and orientation of the biopsy needle's pathway into the tumor, particularly in suspected sarcoma cases. This gives the surgeon the option for routine excision of the needle biopsy tract should the diagnosis prove to be a sarcoma. Without prebiopsy planning, identification of the track to allow excision may be challenging. The paucity of evidence precludes assumptions on what may increase the risk of NTS, such as the size of the needle, number of passes, or histology of the tumor. For instance, in the case presented herein, NTS occurred despite using an introducer needle to act as a sheath. Instead, knowing that NTS is a distinct possibility should prompt a discussion between the surgeon and the interventional radiologist regarding the proposed biopsy location as it relates to the tumor, the anatomic muscular compartments, and the orientation of the future surgical resection incision. Furthermore, NTS is a rare occurrence, and additional research may help better elucidate the associated risk factors.
4. CONCLUSION
To our knowledge, this is the first reported case of pediatric head and neck sarcoma NTS following percutaneous biopsy. Pediatric head and neck sarcomas are uncommon with important anatomic considerations when planning the resection. Given the possibility of NTS among this patient population, surgeons should consider preplanning of the biopsy tract and removing the biopsy tract at the time of resection.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
PY and DH: drafted the manuscript and reviewed the literature. SD: performed the biopsy and provided expertise in interpreting the radiographic images. AD and DC: performed the procedure, conceptualized the study, and reviewed the literature. All authors were involved in patient care and gathered all clinical materials and images. All authors reviewed and approved the final version of the manuscript.
ETHICAL APPROVAL
The nature of this case report was reviewed and deemed exempt by the Baylor College of Medicine Institutional Review Board.
ACKNOWLEDGMENTS
Published with written consent of the patient.
You P, Haynes DA, Desai S, Dimachkieh A, Chelius D Jr. Needle tract seeding following percutaneous biopsy of pediatric head and neck sarcoma: A case report. Clin Case Rep. 2021;9:e04074. 10.1002/ccr3.4074
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Berger‐Richardson D, Swallow CJ. Needle tract seeding after percutaneous biopsy of sarcoma: Risk/benefit considerations. Cancer. 2017;123(4):560‐567. 10.1002/cncr.30370 [DOI] [PubMed] [Google Scholar]
- 2. Shah KSV, Ethunandan M. Tumour seeding after fine‐needle aspiration and core biopsy of the head and neck ‐ A systematic review. Br J Oral Maxillofac Surg. 2016;54(3):260‐265. 10.1016/j.bjoms.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 3. Polyzos SA, Anastasilakis AD. A systematic review of cases reporting Needle tract seeding following thyroid fine Needle biopsy. World J Surg. 2010;34(4):844‐851. 10.1007/s00268-009-0362-2 [DOI] [PubMed] [Google Scholar]
- 4. Binitie O, Tejiram S, Conway S, Cheong D, Temple HT, Letson GD. Adult soft tissue sarcoma local recurrence after adjuvant treatment without resection of core needle biopsy tract tumor. Clin Orthop Relat Res. 2013;471(3):891‐898. 10.1007/s11999-012-2569-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huh WW, Fitzgerald N, Mahajan A, Sturgis EM, Beverly Raney R, Anderson PM. Pediatric sarcomas and related tumors of the head and neck. Cancer Treat Rev. 2011;37(6):431‐439. 10.1016/j.ctrv.2011.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


