Abstract
Background
Cerebrovascular disorders have occurred more frequently in some Central Nervous System (CNS) disorders, such as epilepsy. Some CNS drugs have been associated with increased stroke risk. Our aim was to estimate the risk of ischaemic stroke in patients exposed to antiepileptic drugs (AED).
Methods
Population-based matched case-control study using SIDIAP database, based in electronic health records from primary healthcare from Catalonia, Spain. Cases were those patients with a registered diagnosis of first stroke during 2009–2014. Up to 10 controls were selected for each case and matched by sex, age, and geographic area and without a prior diagnosis of stroke. We considered global drug exposure to AED, past and current exposure and exposure in monotherapy to each AED.
Results
2,865 cases and 19,406 controls were exposed to AED during the study period. Global exposure to levetiracetam [(ORadj3.3, CI95 % 2.8-4.0)], phenytoin [ORadj1.5, CI95 % 1.2–41.9)], and valproic acid [(ORadj 1.3, CI95 % 1.1–1.6)], showed significantly association to ischaemic stroke that was also maintained with current exposure of levetiracetam [ORadj4.1, CI95 % 3.3–5.2)] and valproic acid [ORadj1.4, CI95 % 1.1–1.9)]. Current levetiracetam monotherapy showed a very high risk of ischaemic stroke [(ORadj 5.1, CI95 % 3.7–6.9)].
Conclusions
Drugs used for other conditions than epilepsy (pregabalin, gabapentin) were the most used AED and both did not show a risk. Levetiracetam shows a risk for stroke even when assessed in current monotherapy. The lack of data regarding the link with diagnosis and severity in our study makes it necessary to conduct further studies to confirm or dismiss our results, focussing on levetiracetam.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-021-02237-1.
Keywords: Antiepileptic drugs, Stroke, Drug exposure, Electronic health records, Primary healthcare
Background
Stroke is the second leading cause of death worldwide [1]. There are four types: ischaemic stroke, primary intracerebral haemorrhage, subarachnoid haemorrhage and undefined, being the ischaemic type the most frequent one (80–85 %) in Caucasian population [2, 3]. Stroke patients are at highest risk of death in the first weeks after the event, and between 20 and 50 % die within the first month depending on type, severity, age, comorbidity and effectiveness of treatment of complications. Those who survive can remain with or without disabilities, such as loss of speech and movement, making strokes the third cause of disability [1]. Over the last decades the total number of age-standardized rates of stroke mortality have decreased meanwhile stroke survivors have made the burden of stroke Disability-Adjusted Life Year (DALY lost) increase [2].
The pooled proportional frequency of ischaemic stroke in 2000–2008 was lower in low to middle income countries than in high-income countries (67 and 82 %, respectively) [2]. In high income countries in 2000–2008, early (21 days to 1 month) case fatality was between 13 and 23 %, [2] being in Catalonia mortality rate standardized by age 29.2 deaths/100,000 inhabitants in 2016 [4].
The most important modifiable risk factors for ischaemic stroke are hypercholesterolemia, hypertension, diabetes and smoking [5, 6].
Cerebrovascular disorders have occurred more frequently in some Central Nervous System (CNS) disorders, such as epilepsy [7–10] and also some CNS drugs have been reported to increase the risk for stroke, such as antidepressants, [11] anti-dementia drugs, [12] antipsychotics [13, 14] or antiepileptic drugs (AED) [15–17]. The mechanism suggested for AED is that they would increase risk of vascular diseases by accelerating atherosclerosis; for example cytochrome P450 enzyme-inducing AED as carbamazepine increase serum levels of total cholesterol and LDL-cholesterol and lipoprotein [16]. Concurrently, AED are often administered after a stroke to treat seizures or other conditions [18–20].
As there has been an increase in the chronic use of AED, on account of its use for the treatment of diseases different from epilepsy, like chronic neuropathic pain, migraine, anxiety and as mood stabilizers, [21] we think it is relevant to confirm if the risks detected in other studies are confirmed in our population. The aim of this study was to estimate the risk of ischaemic stroke in patients exposed to AED.
Methods
Study design
Population-based matched case-control study using data from primary healthcare (PHC) electronic records.
Study source
Information System for Research in Primary Care (SIDIAP) database; which contains data from 279 PHC centres from the Catalan Health Institute (ICS), covering 5.8 million people (80 % of the Catalan population) [22]. SIDIAP contains anonymized clinical information originated from different data sources: (1) ECAP (electronic health records in PHC of the ICS), including information since 2006 on sociodemographic characteristics, health conditions registered as International Classification Diseases Tenth Revision (ICD-10) [23] code, general practitioners’ (GP) prescriptions, clinical parameters and toxic habits, (2) laboratory data, (3) pharmacy invoice data corresponding to GP’s prescriptions (available since 2005) including information on all pharmaceutical products dispensed by communities pharmacies with Catalan Health System prescriptions by Anatomic Therapeutic and Chemical (ATC) [24] codes, and 4), Minimum Data Set at Hospital Discharge (CMBD-HA) [25] database for ICS hospitals, including diagnoses at hospital discharge registered as International Classification Diseases, Ninth Revision (ICD-9) codes [26].
Selection of cases and controls
Cases were those patients ≥ 18 years old suffering a first ischaemic stroke (CMBD-HA hospital admission ICD 9th codes: 433, 434, 435, 436, 437, 438) during 2009–2014 attended in hospitals from the ICS in Catalonia, Spain. Index date was the day of hospital admission for a stroke from January 2009 to December 2014.
A total of 10 controls were selected for each case, and matched by sex, age (+/- 1 year), geographic area and without previous diagnosis of stroke; any historic record of stroke before the selection year caused the exclusion as a control. The index date for controls was the same than for their respective case.
Variables
Age, sex, socioeconomic index (MEDEA) [27], body mass index (BMI), smoking status, alcohol intake, comorbidities, cardiovascular risk (classified as high, moderate, low or no risk), laboratory data including glomerular filtration rate (GFR) calculated per MDRD (Modification of Diet in Renal Disease), drug exposure and number of visits in PHC during the previous year to the index date.
Patients diagnosed with ischaemic heart disease and/or peripheral artery disease and/or diabetes with complications were classified with a high cardiovascular risk. Among the unclassified patients in the high category, those with uncomplicated diabetes, treatment with antidiabetic drugs or two or three of the following diagnoses: dyslipidaemia, hypertension, and smoking (active or ex-smoking) were classified as moderate cardiovascular risk. Patients who were not classified in any category were considered to be at low cardiovascular risk (divided into two groups: very low cardiovascular risk if no risk factors registered and low cardiovascular risk if one risk factor registered).
Exposure definition
The drugs of interest were antiepileptics, N03A from the ATC classification. Information of exposure was obtained from GP’s prescriptions and pharmacy invoice data. Current comedications before index date were also collected: anticoagulants, antiplatelets, diabetes and cardiac therapy, lipid modifying agents, hormonal contraceptives, non-steroidal anti-inflammatory drugs (NSAID), analgesics, and psychotropic drugs.
As first SIDIAP registers begin in 2005, a minimum period of four years was selected in order to assess the AED exposure.
We considered drug exposure when there was a dispensation of at least three packages of an active substance of any of the study drugs (AED) during the study period. Past exposure was considered if the dispensation had taken place more than one year before the index date. Current exposure was considered if patients had at least one dispensation within the three months before the index date. Trying to avoid indication bias, patients with one dispensation within the same month of the index date were excluded for the current exposure analysis. Also, current exposure to only one AED was also ascertained as monotherapy exposure.
Population size
For the study period SIDIAP had around 40,000 patients with a new stroke diagnosis registered. As CMBD-HA represents about a third of the SIDIAP total population (ICS hospitals and their primary care referral areas), we aimed to include approximately 12,000 cases. Assuming that the prevalence of exposure to the studied factors would be 1 % higher in cases, the available sample size would allow for detecting significant associations with OR ≥ 1.3 with a type I error of 5 % and an 80 % power.
Statistical analysis
We conducted multivariate models of conditional logistic regression, and we calculated crude and adjusted odds ratios (OR, ORadj) and their 95 % confidence interval (CI 95 %). The crude model adjusted by age, sex, year of the index date and cardiovascular risk. The variables used in the adjusted model were: age, sex, year of the index date, MEDEA index, BMI, smoking status, number of visits to PHC, GFR, and diagnoses of: arthrosis, dementia, depression, diabetes, dyslipidaemia, epilepsy, fibromyalgia, hypertension, ischaemic heart disease, neuropathy, peripheral arterial disease and gastrointestinal ulcer. Co-treatments (insulin, antihypertensive drugs, NSAID…) and all study drugs included were added in this model.
We estimated the crude and adjusted effects of the current, past and global exposure to AED, and the effect of each AED for patients exposed in monotherapy.
Results
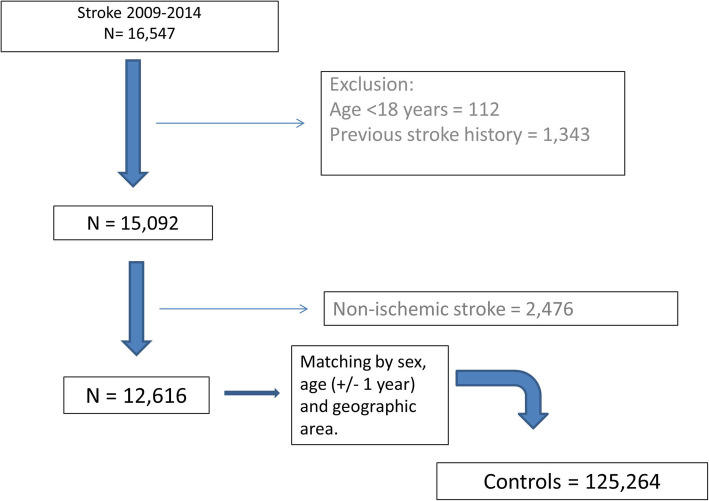
A total of 137,880 patients were included in the study; they were 12,616 cases with a first ischaemic stroke during 2009–2014 matched by sex, age and geographical region with 125,264 controls with no stroke history (Fig. 1). There were 56.3 % of men and their mean age was 72.6 (SD 13.2). Their baseline sociodemographic and clinical characteristics are described in Table 1 and their comedications at baseline, in Table 2. Cases and controls were different in most baseline characteristics highlighting the neuromental illness such as dementia (cases n = 828, 6.6 % and controls n = 6075, 4.8 %,), depression (853, 6.8 6870, 5.5) and epilepsy (cases n = 207, 1.6 % and controls n = 843, 0.7 %) and also in their ischaemic heart disease history (cases n = 1745, 13.8 % and controls n = 9656, 7.7 %), all statistically significant (p < 0,001). Also, the comedications at baseline showed differences (cardiac therapy for cases n = 2448, 19.4 % and for controls n = 15,565, 12.4 % and insulins cases n = 1276, 10.1 % and controls n = 4796, 3.8 %) but not for antidementia drugs (cases n = 2020, 16.0 % and controls n = 20,451, 16.3 %) and coxibs and oxicams (cases n = 2020, 16.0 % and controls n = 20,451, 16.3 %).
Fig. 1.
Study flowchart
Table 1.
Sociodemographic and clinical characteristics at baseline of patients exposed to Central Nervous System drugs
| N, % | Total N = 137,880 | Cases N = 12,616 | Controls N = 125,264 | p-value |
|---|---|---|---|---|
| Mean age, SD | 72.6, 13.2 | 72.9, 13.2 | 72.6, 13.2 | 0.009 |
| Sex, female | 60216, 43.7 | 5486, 43.5 | 54730, 43.7 | 0.658 |
| N visits in PHC | ||||
| Missing values | 15165, 11.0 | 682, 5.4 | 14483, 11.6 | <0.001 |
| <5 | 27055, 22.0 | 1860, 15.6 | 25195, 22.7 | <0.001 |
| 5-9 | 33155, 27.0 | 2460, 20.6 | 30695, 27.7 | |
| 10-24 | 46191, 37.6 | 4837, 40.5 | 41354, 37.3 | |
| ≥25 | 16314, 13.3 | 2777, 23.3 | 13537, 12.2 | |
| Smoking status | ||||
| Missing values | 18895, 13.7 | 1294, 10.3 | 17601, 14.1 | <0.001 |
| Current smokers | 16172, 13.6 | 2020, 17.8 | 14152, 13.1 | <0.001 |
| BMI mean, SD | 28.9, 4.7 | 28.8, 4.9 | 28.9, 4.7 | 0.273 |
| Missing values | 78443, 56.9 | 6610, 52.4 | 71833, 57.3 | <0.001 |
| Obesity | 21708, 36.5 | 2180, 36.3 | 19528, 36.5 | <0.001 |
| MEDEA | <0.001 | |||
| U | 8431, 6.1 | 1216, 9.6 | 7215, 5.8 | |
| R | 25909, 18.8 | 2343, 18.6 | 23566, 18.8 | |
| U1-U3 | 58111, 42.1 | 4955, 39.3 | 53156, 42.4 | |
| U4-U5 | 45429, 32.9 | 4102, 32.5 | 41327, 33.0 | |
| GFR | ||||
| Missing values | 56401, 40.9 | 4186, 33.2 | 52215, 41.7 | <0.001 |
| <30 mL/min/1.73m2 | 1599, 2.0 | 311, 3.7 | 1288, 1.8 | <0.001 |
| 30-44 | 5357, 6.6 | 802, 9.5 | 4555, 6.2 | |
| 45-59 | 12751, 15.6 | 1568, 18.6 | 11183, 15.3 | |
| ≥60 | 61772, 75.8 | 5749, 68.2 | 56023, 76.7 | |
| Cardiovascular risk, high | 14978, 10.9 | 2511, 19.9 | 12467, 10.0 | <0.001 |
| Comorbidities | ||||
| Anxiety | 12792, 9.3 | 1177, 9.3 | 11615, 9.3 | 0.836 |
| Arthrosis | 31921, 23.2 | 2823, 22.4 | 29098, 23.2 | 0.031 |
| Cancer | 24479, 17.8 | 2306, 18.3 | 22173, 17.7 | 0.106 |
| COPD | 16594, 12.0 | 1822, 14.4 | 14772, 11.8 | <0.001 |
| Dementia | 6903, 5.0 | 828, 6.6 | 6075, 4.8 | <0.001 |
| Depression | 7723, 5.6 | 853, 6.8 | 6870, 5.5 | <0.001 |
| Diabetes | 29158, 21.1 | 3890, 30.8 | 25268, 20.2 | <0.001 |
| Dyslipidaemia | 52057, 37.8 | 5007, 39.7 | 47050, 37.6 | <0.001 |
| Epilepsy | 1050, 0.8 | 207, 1.6 | 843, 0.7 | <0.001 |
| Fibromyalgia | 1266, 0.9 | 124, 1.0 | 1142, 0.9 | <0.001 |
| Hypertension | 73250, 53.1 | 7626, 60.4 | 65624, 52.4 | <0.001 |
| Ischaemic heart disease | 11401, 8.3 | 1745, 13.8 | 9656, 7.7 | <0.001 |
| Neuropathies | 5067, 3.7 | 575, 4.6 | 4492, 3.6 | <0.001 |
| Peripheral artery disease | 3888, 2.8 | 916, 7.3 | 2972, 2.4 | <0.001 |
| Ulcers | 4274, 3.1 | 444, 3.5 | 3830, 3.1 | 0.005 |
SD standard deviation, PHC primary healthcare, BMI body mass index, MEDEA socioeconomic index, (U unknown urban area, U# urban areas), R rural area, GFR glomerular filtration rate, COPD chronic obstructive pulmonary disease
Table 2.
Comedications at baseline of patients exposed to Nervous System drugs
| N, % | Total N = 137,880 |
Cases N = 12,616 |
Controls N = 125,264 |
p-value |
|---|---|---|---|---|
| Acetic acid derivatives | 58,208, 42.2 | 5652, 44.8 | 52,556, 42.0 | < 0.001 |
| Analgesics (metamizol and paracetamol) | 109,801, 79.6 | 10,771, 85.4 | 99,030, 79.1 | < 0.001 |
| Anti-dementia agents | 8907, 6.5 | 861, 6.8 | 8046, 6.4 | 0.080 |
| Antidepressants | 39,851, 28.9 | 4465, 35.4 | 35,386, 28.2 | < 0.001 |
| Antihypertensive agents | 3774, 2.7 | 576, 4.6 | 3198, 2.6 | < 0.001 |
| Antithrombotic drugs | 39,951, 29.0 | 8613, 68.3 | 31,338, 25.0 | < 0.001 |
| Antipsychotics | 20,024, 14.5 | 2361, 18.7 | 17,663, 14.1 | < 0.001 |
| Anxiolytics | 62,234, 45.1 | 6536, 51.8 | 55,698, 44.5 | < 0.001 |
| Beta-blockers | 18,022, 13.1 | 2864, 22.7 | 15,158, 12.1 | < 0.001 |
| Blood glucose-lowering drugs | 20,733, 15.0 | 2896, 23.0 | 17,837, 14.2 | < 0.001 |
| Calcium channel-blockers | 18,013, 13.1 | 2448, 19.4 | 15,565, 12.4 | < 0.001 |
| Cardiac therapy | 13,233, 9.6 | 2251, 17.8 | 10,982, 8.8 | < 0.001 |
| Coxibs and oxicams | 22,471, 16.3 | 2020, 16.0 | 20,451, 16.3 | 0.369 |
| Diuretics | 28,656, 20.8 | 3794, 30.1 | 24,862, 19.8 | < 0.001 |
| Gastrointestinal tract | 2505, 1.8 | 491, 3.9 | 2014, 1.6 | < 0.001 |
| Hypnotics and sedatives | 23,208, 16.8 | 2607, 20.7 | 20,601, 16.4 | < 0.001 |
| Insulins | 6072, 4.4 | 1276, 10.1 | 4796, 3.8 | < 0.001 |
| Lipid-modifying agents | 42,897, 31.1 | 6119, 48.5 | 36,778, 29.4 | < 0.001 |
| Opioids (phentanil, buprenorphine and tramadol) | 33,489, 24.3 | 3672, 29.1 | 29,817, 23.8 | < 0.001 |
| Propionic acid derivatives | 93,012, 67.5 | 8839, 70.1 | 84,173, 67.2 | < 0.001 |
| Psychostimulants, ADHD-agents and nootropics | 11,948, 8.7 | 1390, 11.0 | 10,558, 8.4 | < 0.001 |
| Renin-angiotensin agents | 54,778, 39.7 | 6657, 52.8 | 48,121, 38.4 | < 0.001 |
| Other anti-inflammatories (glucosamine and chondroitin sulphate) | 17,342, 12.6 | 1433, 11.4 | 15,909, 12.7 | < 0.001 |
ADHD attention deficit hyperactivity disorder
During the study period 2,865 (22.7 %) cases were exposed to AED (ATC classification N03) and 19,406 (15.5 %) were controls. Cases were more frequently exposed to all AED than controls (Table 3), except for pregabalin (38.4 % cases vs. 44.4 % controls exposed, p < 0.001). The drugs with the highest proportions of patients exposed were pregabalin (8,861 39.8 %) and gabapentin (9,720 43.6 %).
Table 3.
Exposure to antiepileptic drugs
| N, % | Total N = 22,271 | Cases N = 2,865 | Controls N = 19,406 | p-value |
|---|---|---|---|---|
| Carbamazepine exposure | 1238, 5.6 | 169, 5.9 | 1069, 5.5 | 0.407 |
| Current exposure | 321, 1.4 | 52, 1.8 | 269, 1.4 | |
| Past exposure | 202, 0.9 | 32, 1.1 | 170, 0.9 | |
| Monotherapy | 140, 0.6 | 15, 0.5 | 125, 0.6 | |
| Clonazepam | 4675, 21.0 | 570, 19.9 | 4105, 21.2 | 0.128 |
| Current | 1089, 4.9 | 135, 4.7 | 954, 4.9 | |
| Past | 1019, 4.6 | 124, 4.3 | 895, 4.6 | |
| Monotherapy | 525, 2.4 | 52, 1.8 | 473, 2.4 | |
| Gabapentin | 8861, 39.8 | 1138, 39.7 | 7723, 39.8 | 0.951 |
| Current | 1814, 8.1 | 296, 10.3 | 1518, 7.8 | |
| Past | 1994, 9.0 | 244, 8.5 | 1750, 9,0 | |
| Monotherapy | 1060, 4.8 | 177, 6.2 | 883, 4.6 | |
| Lamotrigine | 575, 2.6 | 94, 3.3 | 481, 2.5 | 0.012 |
| Current | 191, 0.9 | 38, 1.3 | 153, 0.8 | |
| Past | 146, 0.7 | 21, 0.7 | 125, 0.6 | |
| Monotherapy | 72, 0.3 | 14, 0.5 | 58, 0.3 | |
| Levetiracetam | 654, 2.9 | 231, 8.1 | 423, 2.2 | <0.001 |
| Current | 383, 1.7 | 166, 5.8 | 217, 1.1 | |
| Past | 62, 0.3 | 10, 0.3 | 52, 0.3 | |
| Monotherapy | 187, 0.8 | 90, 3.1 | 97, 0.5 | |
| Oxcarbazepine | 556, 2.5 | 83, 2.9 | 473, 2.5 | 0.137 |
| Current | 149, 0.7 | 27, 0.9 | 122, 0.6 | |
| Past | 131, 0.6 | 20, 0.7 | 111, 0.6 | |
| Monotherapy | 66, 0.3 | 11, 0.4 | 55, 0.3 | |
| Phenobarbital | 520, 2.3 | 85, 3.0 | 435, 2.2 | 0.018 |
| Current | 205, 0.9 | 39, 1.4 | 166, 0.9 | |
| Past | 124, 0.6 | 28, 1.0 | 96, 0.5 | |
| Monotherapy | 58, 0.3 | 10, 0.3 | 48, 0.2 | |
| Phenytoin | 610, 2.7 | 128, 4.5 | 482, 2.5 | <0.001 |
| Current | 232, 1.0 | 49, 1.7 | 183, 0.9 | |
| Past | 121, 0.5 | 25, 0.9 | 96, 0.5 | |
| Monotherapy | 96, 0.4 | 17, 0.6 | 79, 0.4 | |
| Pregabalin | 9720, 43.6 | 1100, 38.4 | 8620, 44.4 | <0.001 |
| Current | 1829, 8.2 | 239, 8.3 | 1590, 8.2 | |
| Past | 2586, 11.6 | 285, 9.9 | 2301, 11.9 | |
| Monotherapy | 1058, 4.8 | 119, 4.2 | 939, 4.8 | |
| Topiramate | 1088, 4.9 | 168, 5.9 | 920, 4.7 | 0.010 |
| Current | 176, 0.8 | 36, 1.3 | 140, 0.7 | |
| Past | 285, 1.3 | 45, 1.6 | 240, 1.2 | |
| Monotherapy | 77, 0.3 | 9, 0.3 | 68, 0.4 | |
| Valproic acid | 1037, 4.7 | 192, 6.7 | 845, 4.4 | <0.001 |
| Current | 367, 1.6 | 92, 3.2 | 275, 1.4 | |
| Past | 214, 1.0 | 28, 1.0 | 186, 1.0 | |
| Monotherapy | 175, 0.8 | 39, 1.4 | 136, 0.7 |
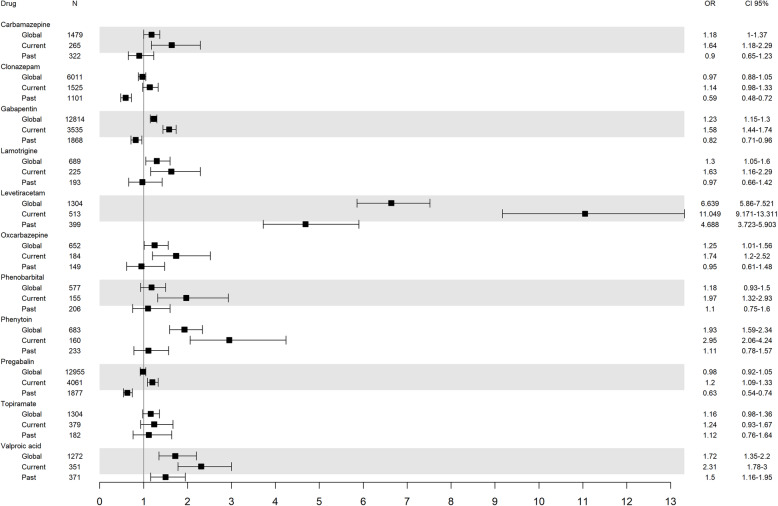
The effect of the AED exposure was adjusted for different baseline variables for the regression models (Fig. 2). Global exposure to AED showed a risk of 1.06 (95 % CI 0.99–1.13, p = 0.057) of ischaemic stroke and above all, the exposure to levetiracetam [(ORadj 3.3 CI95 % 2.8-4)], phenytoin [(ORadj 1.5 CI95 % 1.2–1.9)] and valproic acid [(ORadj 1.3 CI95 % 1.1–1.6)] did. Past exposure to lamotrigine [(ORadj 1.6 CI95 % 1.5–1.7)], phenobarbital [(ORadj 2.1 CI95 % 1.3–3.2)] and phenytoin [(ORadj 1.6 CI95 % 1-2.5)] showed a risk but only levetiracetam [(ORadj 4.1 CI95 % 3.3–5.2)] and valproic acid [(ORadj 1.4 CI95 % 1.1–1.9)] showed it when assessing current use. When the effect of AED in monotherapy was analysed, only levetiracetam (ORadj 5.1 CI95 % 3.7–6.9) was associated with a high risk of ischaemic stroke.
Fig. 2.
Risk of ischaemic stroke according to different exposures to antiepileptic drugs
Discussion
Most studies assessing risk of stroke with use of AED were cohort studies which did not analyse different times of exposure as in our study, our research analyse global, past, current and current monotherapy exposures through a case-control design. We found that global exposure to AED was not significantly associated to ischaemic stroke (OR 1.06, CI95 % 0.998–1.128) [8–17].
Among the classic AED (phenobarbital, phenytoin, valproic acid, carbamazepine and clonazepam) only the exposure to phenytoin and valproic acid showed a risk for ischaemic stroke that was even higher for current exposure in the case of valproic acid. The highest risk for these classics was for the old phenobarbital past exposure showing an adjusted risk of 2.0 (CI95 % 1.3–3.2). Regarding phenytoin and valproic acid, they demonstrated higher risk of stroke when compared to carbamazepine in the study of Hsieh et al. [16]. A pharmacokinetic explanation, regarding the role as potent inducers of cytochrome P450 enzymes (involved in cholesterol synthesis) of carbamazepine or phenytoin could substantially increase the risk of cardiovascular and cerebrovascular disease [28]. When compared to the new AED, only past exposure to lamotrigine (ORadj 1.6 CI95 % 1.5–1.7) showed a risk for stroke. Renoux et al. analysing the past exposure to AED did not find an increased risk of ischaemic stroke (RR 0.89, CI95 % 0.78–1.01) [15]. In the case of those AED which were associated to an increased risk of stroke for the past exposure but not for the current, we are not able to conclude if this higher risk was caused by the epilepsy itself or by the drug exposure [7, 18, 19, 29].
Nevertheless, measuring current exposure to AED may be more accurate in order to assess the real increase of risk of ischaemic stroke caused by these drugs. In our study, levetiracetam exposure was associated with the highest risk of stroke [OR adj 3.3 (CI95 % 2.8- 4.0)] even higher when assessing monotherapy exposure [OR adj 5.1 (CI95 % 3.7–6.9] was assessed. Levetiracetam shows a risk for stroke when handle the indication bias taken only first incident stroke cases excluding prescriptions in the same month of the index date. To be considered that levetiracetam is used for the most severe and for refractory epilepsies when other AED fail, information not available in our database, what could address a risk of the epilepsy itself. We have not found any studies with similar results for levetiracetam. Deeper studies have to be conducted, analysing the AED indications what could be relevant to support our results.
Clonazepam is a benzodiazepine usually used as an “add-on” medication for people who continue to have seizures while taking other seizure medicines. Not having diagnosis linked to its prescription can refer to its use for psychiatric disorders and not really to prevent seizures. It can also be prescribed as “if needed” which wouldn’t reflect its use.
The AED studied with higher prevalence of use in our population were gabapentin and pregabalin, drugs which have other indications with a more frequent use than epilepsy; both are authorized for neuropathic pain and pregabalin is also authorized to treat anxiety disorders, which are more prevalent disorders than epilepsy. None of these drugs show a risk for stroke for the different exposures estimated.
Among AED those with a mainly use in our country for epilepsy, phenytoin and valproic acid, showed a global risk [OR adj 1.5 (CI95 % 1.2–1.9)] [OR adj 1.34 (CI95 % 1.1–1.6)] which are not maintained in the past exposures meanwhile lamotrigine and phenobarbital showed a risk for the past exposure [OR adj 1.58 (CI95 % 1.5–1.7) and OR adj 2.08 (CI95 % 1.3–3.2) respectively], thus we cannot rule out that the epilepsy itself is increasing stroke risk.
One of the main limitations of our data is the lack of association between a drug prescription and a diagnosis registered, or the lack of registry of the severity of epilepsy. We cannot know the diagnosis which led to the AED prescription, not only seizures but also the possibility of other diagnosis that could lead to AED prescription such as tumour, hypoxia, and neuropathic pain, among others. The main cause of seizures in the elderly is stroke (52.3 %), [30] what can point to an indication bias in our study, we have even tried to handle it by excluding those patients a first stroke episode and excluding those AED prescriptions in the same month of the index date. There are no diagnostic images available in our database, so we were not able to examine a possible ischemic lesion by imaging study in either the controls or the cases, thus, this remains as a limitation inherent to our study.
Conclusions
Drugs used for other conditions than epilepsy were the most used AED (pregabalin, gabapentin). They didn’t show a risk for stroke.
An inherent risk of the epileptic condition for stroke cannot be dismissed with the present study results, but no link between drugs and diagnosis in our database made this a limitation in our research.
Levetiracetam shows a risk for stroke global exposure, current and also monotherapy.
Even our effort for handling the indication bias the lack of data regarding the diagnosis, severity and kind of epilepsy for it use should be address with further research focus on this active substance.
Supplementary Information
Table S1. Multivariate model.
Abbreviations
- AED
Antiepileptic drugs
- ATC
Anatomic Therapeutic and Chemical
- BMI
Body mass index
- CI
Confidence interval
- CMBD-HA
Minimum Data Set at Hospital Discharge
- CNS
Central Nervous System
- DALY
Disability-Adjusted Life Year
- ECAP
Electronic health records in Primary healthcare
- GFR
Glomerular filtration rate
- GP
General Practitioner
- ICD
International Classification of Diseases
- ICS
Catalan Health Institute
- MDRD
Modification of diet in renal disease
- MEDEA
Socioeconomic index
- NSAID
Non-steroidal anti-inflammatory drugs
- OR
Odds ratio
- PHC
Primary healthcare
- SD
Standard deviation
- SIDIAP
Information system for research in primary care
Authors’ contributions
Rosa Morros and Josep Ramon Marsal designed the study, Josep Ramon Marsal conducted the statistical analysis, all authors interpreted the results, Maria Giner-Soriano and Ainhoa Gomez-Lumbreras wrote the manuscript, all authors reviewed and approved the manuscript.
Funding
This study has been funded by Bioibérica S.A.
Availability of data and materials
All datasets are available at request to the corresponding author.
Ethics approval and consent to participate
The present study follows national and international regulations: Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects and Good Research Practice principles and guidelines. The study protocol has been approved by the Clinical Ethics Committee at IDIAPJGol, the reference institution for research in Primary Health Care of the ICS. Regarding the data contained in the databases and according to General Data Protection Regulation EU 2016/679, data included in SIDIAP are always anonymized. Thus, it is not necessary to ask for informed consent from the participants and so was waived by the Clinical Ethics Committee at IDIAPJGol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: a global response is needed. Bull World Health Organ. 2016;94:634-634A. doi: 10.2471/BLT.16.181636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–69. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 3.American Stroke Association. Types of Stroke and Treatment. 2017. https://www.stroke.org/en/about-stroke/types-of-stroke.
- 4.Magrinyà P., Garcia O., Masachs E., Medina A., Schiaffino A., Alava F. et al. Informe de Salut 2016. 2018.
- 5.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.CIR.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 6.Bejot Y, Caillier M, Ben Salem D, Couvreur G, Rouaud O, Osseby G-V, et al. Ischaemic stroke subtypes and associated risk factors: a French population based study. J Neurol Neurosurg Psychiatry. 2008;79:1344–8. doi: 10.1136/jnnp.2008.150318. [DOI] [PubMed] [Google Scholar]
- 7.Hsu SPC, Yeh CC, Chou YC, Shih CC, Hu CJ, Cherng YG, et al. Stroke risk and outcomes in epilepsy patients: Two retrospective cohort studies based on National Health Insurance in Taiwan. Atherosclerosis. 2019;280 2018:147–54. [DOI] [PubMed]
- 8.Jabareen A, Leker RR, Eyal S, Ekstein D. Treatment with antiepileptic drugs in patients with stroke. A change in clinical practice may be required. J Neurol Sci. 2018;395 September:4–7. [DOI] [PubMed]
- 9.Chang CS, Liao CH, Lin CC, Lane HY, Sung FC, Kao CH. Patients with epilepsy are at an increased risk of subsequent stroke: A population-based cohort study. Seizure. 2014;23:377–81. doi: 10.1016/j.seizure.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Gaitatzis A, Carroll K, Majeed A, Sander JW. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45:1613–22. doi: 10.1111/j.0013-9580.2004.17504.x. [DOI] [PubMed] [Google Scholar]
- 11.Biffi A, Scotti L, Corrao G. Use of antidepressants and the risk of cardiovascular and cerebrovascular disease: a meta-analysis of observational studies. Eur J Clin Pharmacol. 2017;73:487–97. doi: 10.1007/s00228-016-2187-x. [DOI] [PubMed] [Google Scholar]
- 12.Bohlken J, Weber S, Rapp MA, Kostev K. Continuous treatment with antidementia drugs in Germany 2003–2013: A retrospective database analysis. Int Psychogeriatrics. 2015;27:1335–42. doi: 10.1017/S1041610215000654. [DOI] [PubMed] [Google Scholar]
- 13.Gareri P, Segura-García C, Manfredi VG, Bruni A, Ciambrone P, Cerminara G, De Sarro G, De Fazio P. Use of atypical antipsychotics in the elderly: a clinical review. Clin Interv Aging. 2014;9:1363–73. [DOI] [PMC free article] [PubMed]
- 14.Zivkovic S, Koh CH, Kaza N, et al. Antipsychotic drug use and risk of stroke and myocardial infarction: a systematic review and meta-analysis. BMC Psychiatry 2019;19:189. [DOI] [PMC free article] [PubMed]
- 15.Renoux C, Dell’Aniello S, Saarela O, Filion KB, Boivin JF. Antiepileptic drugs and the risk of ischaemic stroke and myocardial infarction: A population-based cohort study. BMJ Open. 2015;5(8):e008365. [DOI] [PMC free article] [PubMed]
- 16.Hsieh C-Y, Lai EC-C, Yang Y-HK, Lin S-J. Comparative stroke risk of antiepileptic drugs in patients with epilepsy. Epilepsia. 2013;54:172–80. doi: 10.1111/j.1528-1167.2012.03693.x. [DOI] [PubMed] [Google Scholar]
- 17.Olesen JB, Abildstrøm SZ, Erdal J, Gislason GH, Weeke P, Andersson C, et al. Effects of epilepsy and selected antiepileptic drugs on risk of myocardial infarction, stroke, and death in patients with or without previous stroke: a nationwide cohort study. Pharmacoepidemiol Drug Saf. 2011;20:964–71. doi: 10.1002/pds.2186. [DOI] [PubMed] [Google Scholar]
- 18.Hassani M, Cooray G, Sveinsson O, Cooray C. Post-stroke epilepsy in an ischemic stroke cohort—Incidence and diagnosis. Acta Neurol Scand. 2020;141:141–7. doi: 10.1111/ane.13174. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Rajah G, Guo A, Wang Y, Wang Q. Pathogenesis of epileptic seizures and epilepsy after stroke. Neurol Res. 2018;40:426–32. doi: 10.1080/01616412.2018.1455014. [DOI] [PubMed] [Google Scholar]
- 20.Hanaya R, Arita K. The new antiepileptic drugs: Their neuropharmacology and clinical indications. Neurol Med Chir (Tokyo) 2016;56:205–20. doi: 10.2176/nmc.ra.2015-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agencia Española de Medicamentos y Productos Sanitarios. Utilización de medicamentos antiepilépticos en España durante el periodo 2008–2016. 2017.
- 22.SIDIAP. Information System for Research in Primary Care CatalanCASpanishESEnglish (UK).
- 23.WHO. ICD-10 Version: 2019. International Statistical Classification of diseases and Related Health Problems 10th Revision. 2019. https://icd.who.int/browse10/2019/en. Accessed 24 Apr 2020.
- 24.WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2019. 2019. https://www.whocc.no/atc_ddd_index/. Accessed 14 Aug 2020.
- 25.CatSalut. Servei Català de la Salut. Conjunt mínim bàsic de dades (CMBD). 2019. http://catsalut.gencat.cat/ca/proveidors-professionals/registres-catalegs/registres/cmbd/. Accessed 26 Nov 2020.
- 26.Ministerio de Sanidad PS e I. CIE9. https://eciemaps.mscbs.gob.es/ecieMaps/browser/index_9_mc.html.
- 27.Domínguez-Berjón M, Borrell C, Cano-Serral G, Esnaola S, Nolasco A, Pasarin M, et al. Construcción de un índice de privación a partir de datos censales en grandes ciudades españolas (Proyecto MEDEA) Gac Sanit. 2008;22:179–87. doi: 10.1157/13123961. [DOI] [PubMed] [Google Scholar]
- 28.Mintzer S, Skidmore CT, Abidin CJ, Morales MC, Chervoneva I, Capuzzi DM, et al. Effects of antiepileptic drugs on lipids, homocysteine, and C-reactive protein. Ann Neurol. 2009;65:448–56. doi: 10.1002/ana.21615. [DOI] [PubMed] [Google Scholar]
- 29.Feyissa AM, Hasan TF, Meschia JF. Stroke-related epilepsy. Eur J Neurol. 2019;26:18-e3. doi: 10.1111/ene.13813. [DOI] [PubMed] [Google Scholar]
- 30.Leppik IE. Status epilepticus in the elderly. Epilepsia. 2018;59:140–3. doi: 10.1111/epi.14497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multivariate model.
Data Availability Statement
All datasets are available at request to the corresponding author.