Abstract
Aim
The aim of this study was to determine the usefulness of COVID-GRAM and CURB-65 scores as predictors of the severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Caucasian patients.
Methods
This was a retrospective observational study including all adults with SARS-CoV-2 infection admitted to Hospital Universitario Marqués de Valdecilla from February to May 2020. Patients were stratified according to COVID-GRAM and CURB-65 scores as being at low–medium or high risk of critical illness. Univariate analysis, multivariate logistic regression models, receiver operating characteristic curve, and area under the curve (AUC) were calculated.
Results
A total of 523 patients were included (51.8% male, 48.2% female; mean age 65.63 years (standard deviation 17.89 years)), of whom 110 (21%) presented a critical illness (intensive care unit admission 10.3%, 30-day mortality 13.8%). According to the COVID-GRAM score, 122 (23.33%) patients were classified as high risk; 197 (37.7%) presented a CURB-65 score ≥2. A significantly greater proportion of patients with critical illness had a high COVID-GRAM score (64.5% vs 30.5%; P < 0.001). The COVID-GRAM score emerged as an independent predictor of critical illness (odds ratio 9.40, 95% confidence interval 5.51–16.04; P < 0.001), with an AUC of 0.779. A high COVID-GRAM score showed an AUC of 0.88 for the prediction of 30-day mortality, while a CURB-65 ≥2 showed an AUC of 0.83.
Conclusions
The COVID-GRAM score may be a useful tool for evaluating the risk of critical illness in Caucasian patients with SARS-CoV-2 infection. The CURB-65 score could be considered as an alternative.
Keywords: Coronavirus, COVID, CURB-65, COVID-GRAM, Severity score
Introduction
In December 2019, the novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in the city of Wuhan, China (Sohrabi et al., 2020). Spreading around the world in the early part of 2020, this disease outbreak is now considered a pandemic, with more than 45 million cases worldwide and more than 1 100 000 deaths by the end of October 2020, according to the World Health Organization (who.int/emergencies, 2020).
The clinical presentation of COVID-19 typically includes fever and pulmonary involvement (Shi et al., 2020, Lai et al., 2020), although almost any organ can be affected (Stokes et al., 2020). In some patients, the disease progresses quickly to respiratory failure and even death (Garcia-Alamino, 2020). The proportion of patients who become critically ill reaches almost 25% (Ye et al., 2020, Petrilli et al., 2020), and the case fatality rate has been shown to range between <0.1% and >25% (Li et al., 2020). Different risk factors have been associated with severe disease and mortality, including age and male sex (Hur et al., 2020, Michelozzi et al., 2020), various comorbidities (Alqahtani et al., 2020, Zhao et al., 2020), and some clinical laboratory and radiological findings (Zhang et al., 2020, Cappabianca et al., 2020).
Although only a small percentage of patients require admission to the intensive care unit (ICU) or mechanical ventilation, the COVID-19 population overwhelmed healthcare systems all over the world during the first wave of the pandemic and threatens to continue to do so. Some tools have been proposed to evaluate the risk of severe infection, in order to provide the most appropriate care and optimize limited resources (Yee et al., 2020, Zhou et al., 2020, Sprung et al., 2020, Ryan et al., 2020, Altschul et al., 2020). The COVID-GRAM score, validated to predict the risk of critical illness or death in the Chinese population, was one of the first published (Liang et al., 2020). The CURB-65 score (Lim et al., 2003) has been proposed for use in Spain as the reference prognostic tool for SARS-COV-2 pneumonia (mscbs.gob.es, 2020).
The aim of this study was to determine whether the COVID-GRAM score could also be used as a prognostic score at the time of hospital admission in Caucasian patients with SARS-CoV-2 infection, and to compare its accuracy with that of the CURB-65 score.
Methods
Design and inclusion
This retrospective observational cohort study was conducted from February 27 to May 25, 2020. All adults with a laboratory-confirmed SARS-CoV-2 infection admitted to a tertiary university hospital were included. The following exclusion criteria were applied: age <18 years; patients who had been included previously in the study. All patients received standard-of-care treatment according to the local protocol.
Data collection
The following patient characteristics at hospital admission were collected: age, sex, body temperature, respiratory rate, heart rate, arterial systolic and diastolic blood pressure, oxygen saturation (SaO2), and mental status. For the assessment of comorbidity, obesity and all other conditions included in the original study (Liang et al., 2020) were examined: number of comorbidities, chronic obstructive pulmonary disease (COPD), diabetes, hypertension, coronary artery disease, cerebrovascular disease, hepatitis B virus (HBV), cancer, chronic renal disease, and immunodeficiency disease. Laboratory parameters were recorded: haemoglobin, white blood cell, neutrophil, lymphocyte, and platelet counts, neutrophil to lymphocyte ratio, serum creatinine and urea, sodium, potassium, blood glucose, and levels of C-reactive protein (CRP), procalcitonin (PCT), lactate dehydrogenase (LDH), direct bilirubin, and total bilirubin. Chest radiography abnormalities were recorded. When direct bilirubin was unknown, total bilirubin was included if available. Unknown variables at admission were included as normal values. Admission to the ICU, length of hospital and ICU stay, and 30-day mortality were also included.
Altered mental status was defined as disorientation with respect to person, place, or time, stupor, or coma. Coronary artery disease was defined as the presence of a current or past history of angina or myocardial infarction. HBV infection was defined when serum surface antigen (HBsAg) and/or HBV viral load were detected, or when the patient had previously been diagnosed with HBV and hepatitis core antibody (anti-HBc) and hepatitis surface antibody (anti-HBs) were detected. Cancer history was defined as a current or past history of solid tumours or haematological malignancies.
The main outcome measure was ‘critical illness’, a composite endpoint that combines ICU admission and 30-day mortality. This endpoint has been adopted previously in other studies to assess the severity of infectious diseases (Liang et al., 2020).
The COVID-GRAM score was obtained after entering these variables into a calculation tool designed by Liang et al. (2020) (accessible at http://118.126.104.170/), which stratifies the patient according to the risk of critical illness as low, medium, or high. The CURB-65 score (Lim et al., 2003) was also calculated, with a CURB-65 score ≥2 being considered as a high risk of critical illness. Severe illness was defined as a qSOFA score ≥2 (Singer et al., 2016) and a World Health Organization (WHO) score ≥5 (who.int/blueprint, 2020).
Statistical analysis
All data were analysed and processed using SPSS software (IBM SPSS Statistics version 19.0 (IBM Corp., Armonk, NY, USA)). Qualitative variables were expressed as absolute frequencies and percentages, while quantitative variables were summarized as the mean and standard deviation (SD). Univariate methods were first used to test the differences between study subgroups, with the t-test for continuous variables and the Chi-square test for categorical variables. The independent variables significantly associated with critical illness or mortality in the univariate analysis and/or with clinical relevance according to the literature were entered into multivariate logistic regression models. The adjusted odds ratio (OR), relative risk (RR), and 95% confidence interval (CI) were calculated. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the COVID-GRAM score and CURB-65 score were calculated, and receiver operating characteristic (ROC) curves and the area under the curve (AUC) for predicting critical illness and 30-day mortality were calculated. Survival curves were generated by the Kaplan–Meier method, and the log-rank test was used to compare survival between groups. For all analyses, the significance level was set at 5%.
Results
Patient characteristics
A total of 523 patients (51.8% male, 48.2% female) were included during the study period. Mean age was 65.63 years (SD 17.89 years, range 19–105 years). Fifty-four (10.3%) patients required ICU admission, and 30-day mortality was 13.8%. Globally, 110 (21%) patients presented a critical illness. Twenty-eight (5.8%) patients had a WHO score ≥5 and 13 (2.8%) had a qSOFA score ≥2.
The main epidemiological and clinical characteristics of the patients, laboratory and chest radiography findings, and comparisons between patients with and without critical illness are shown in Table 1, Table 2, Table 3 .
Table 1.
Demographic characteristics of patients hospitalized with COVID-19 who did or did not develop critical illness.
| Total (n = 523) | Critical illness |
P-valuea | ||
|---|---|---|---|---|
| No (n = 413) | Yes (n = 110) | |||
| Age, mean (SD), years | 65.63 (17.89) | 63.58 (17.52) | 73.31 (17.25) | <0.001 |
| Sex, n (%) | 0.032 | |||
| Male | 271 (51.8) | 204 (49.4) | 67 (60.9) | |
| Female | 252 (48.2) | 209 (50.6) | 43 (39.1) | |
| Obesity, n (%) | 123 (23.5) | 91 (22) | 32 (29.1) | 0.121 |
| COPD, n (%) | 44 (8.4) | 28 (6.8) | 16 (14.5) | 0.009 |
| Diabetes, n (%) | 114 (21.8) | 83 (20.1) | 31 (28.2) | 0.068 |
| Hypertension, n (%) | 225 (43) | 157 (38) | 68 (61.8) | <0.001 |
| Coronary artery disease, n (%) | 53 (10.1) | 37 (9) | 16 (14.5) | 0.084 |
| Cerebrovascular disease, n (%) | 53 (10.1) | 33 (8) | 20 (18.2) | 0.002 |
| HBV infection, n (%) | 4 (0.8) | 3 (0.7) | 1 (0.9) | 0.180 |
| Unknown, n (%) | 162 (31) | |||
| Malignancy, n (%) | 56 (10.7) | 39 (9.4) | 17 (15.5) | 0.07 |
| Chronic kidney disease, n (%) | 51 (9.8) | 27 (6.5) | 24 (21.8) | <0.001 |
| Immunodeficiency, n (%) | 25 (4.8) | 16 (3.9) | 9 (8.2) | 0.06 |
| ≥1 comorbidities, n (%) | 312 (59.7) | 221 (53.5) | 91 (82.7) | 0.009 |
COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; SD, standard deviation.
T-test or Chi-square test (as appropriate in each case).
Table 2.
Clinical characteristics at admission and treatment of patients hospitalized with COVID-19 who did or did not develop critical illness.
| Total (n = 523) | Critical illness |
P-valuea | ||
|---|---|---|---|---|
| No (n = 413) | Yes (n = 110) | |||
| Unconsciousness, n (%) | 88 (16.8) | 53 (12.8) | 35 (31.8) | <0.001 |
| Dyspnoea, n (%) | 238 (45.5) | 163 (39.5) | 75 (68.2) | <0.001 |
| Haemoptysis, n (%) | 5 (1) | 3 (0.7) | 2 (1.8) | 0.296 |
| Chest pain, n (%) | 81 (15.5) | 68 (16.5) | 13 (11.8) | 0.231 |
| Fever, n (%) | 378 (72.3) | 289 (70) | 89 (80.9) | 0.23 |
| Dry cough, n (%) | 338 (64.6) | 267 (63.9) | 74 (67.3) | 0.514 |
| Headache, n (%) | 53 (10.1) | 48 (11.6) | 5 (4.5) | 0.029 |
| Rash, n (%) | 3 (0.6) | 3 (3.7) | 0 | 0.37 |
| Anosmia, n (%) | 31 (5.9) | 28 (6.8) | 3 (2.7) | 0.11 |
| Temperature >38 °C or <36 °C, n (%) | 138 (26.4) | 78 (18.9) | 60 (54.5) | <0.001 |
| Heart rate >90 beats/min, n (%) | 123 (23.5) | 80 (19.4) | 43 (39.1) | <0.001 |
| Respiratory rate, n (%) | <0.001 | |||
| <20 breaths/min | 271 (51.8) | 234 (56.7) | 37 (33.6) | |
| 20–30 breaths/min | 105 (20.1) | 60 (14.5) | 45 (40.9) | |
| >30 breaths/min | 22 (4.2) | 5 (1.2) | 17 (15.5) | |
| Unknown | 123 (23.5) | 112 (27.1) | 11 (10) | |
| Systolic blood pressure <100 mmHg, n (%) | 36 (6.9) | 27 (6.5) | 9 (8.2) | 0.645 |
| SaO2 <90%, n (%) | 105 (20.1) | 63 (15.3) | 42 (38.2) | <0.001 |
| High COVID-GRAM, n (%) | 122 (23.3) | 63 (15.3) | 59 (53.6) | <0.001 |
| CURB-65 score ≥ 2, n (%) | 197 (37.7) | 126 (30.5) | 71 (64.5) | <0.001 |
| qSOFA score ≥ 2, n (%) | 13 (2.48) | 7 (1.7) | 6 (5.5) | 0.002 |
SaO2, oxygen saturation.
Chi-square test.
Table 3.
Laboratory and chest radiography findings at admission among patients hospitalized with COVID-19 who did or did not develop critical illness.
| Total (n = 523) | Critical illness |
P-valuea | ||
|---|---|---|---|---|
| No (n = 413) | Yes (n = 110) | |||
| Neutrophil count, ×109/l, mean (SD) | 4.8 (2.7) | 4.5 (2.5) | 5.8 (3.2) | <0.001 |
| Lymphocyte count, ×109/l, mean (SD) | 1.2 (1.4) | 1.2 (1.1) | 1.2 (2.4) | 0.896 |
| Neutrophil to lymphocyte ratio, mean (SD) | 5.85 (5.91) | 5.00 (4.59) | 9.01 (8.63) | <0.001 |
| Platelet count, ×109/l, mean (SD) | 203.33 (92.28) | 207.71 (93.92) | 186.89 (84.22) | 0.035 |
| Haemoglobin, g/l, mean (SD) | 13.58 (1.69) | 13.67 (1.56) | 13.24 (2.10) | 0.049 |
| CRP, mg/l, mean (SD) | 8.35 (7.33) | 6.94 (6.07) | 12.94 (9.06) | <0.001 |
| Procalcitonin, ng/mL, mean (SD) | 0.3 (2.09) | 0.21 (1.85) | 0.61 (2.80) | 0.303 |
| Lactate dehydrogenase, U/l, mean (SD) | 223.98 (222.29) | 203.35 (140.70) | 301.16 (392.31) | 0.013 |
| Total bilirubin, mmol/l, mean (SD) | 0.43 (0.96) | 0.50 (1.06) | 0.17 (0.40) | 0.089 |
| Creatinine, μmol/l, mean (SD) | 1.32 (0.58) | 0.89 (0.47) | 1.34 (1.21) | <0.001 |
| Abnormal chest radiography, n (%) | 397 (75.9) | 299 (72.4) | 98 (89.1) | <0.001 |
CRP, C-reactive protein, SD, standard deviation.
T-test or Chi-square test (as appropriate in each case).
COVID-GRAM score and CURB-65 score for predicting critical illness
One hundred and twenty-two (23.33%) patients were classified as high risk according to their COVID-GRAM score, while 197 (37.7%) patients presented a CURB-65 score ≥2.
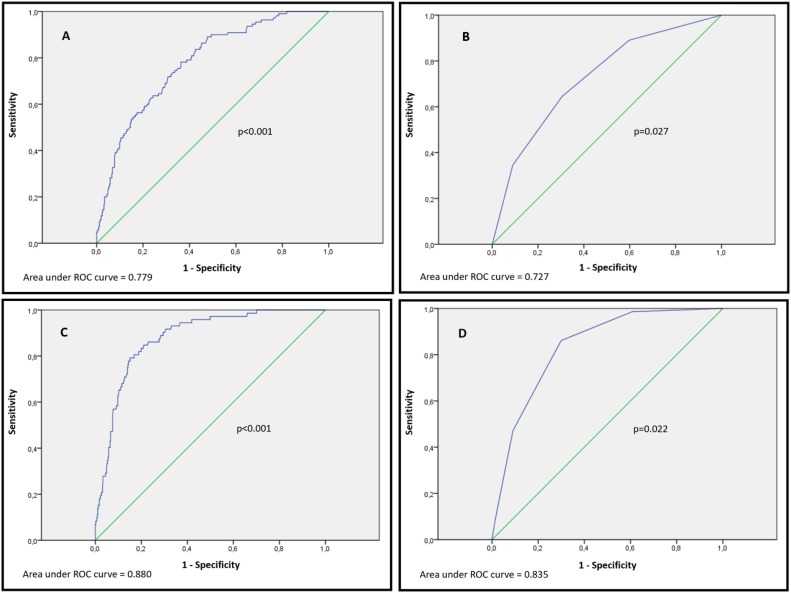
The proportion of patients with a high COVID-GRAM score was significantly greater in the group of patients with a critical illness compared to those without a critical illness (64.5% vs 30.5%, respectively; P < 0.001) (Table 1). A high COVID-GRAM score showed a sensitivity of 53%, specificity of 84%, PPV of 48%, and NPV of 87% for critical illness (Table 4 ). The ROC curve showed an AUC of 0.779 for predicting critical illness (Figure 1 A).
Table 4.
Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of high COVID-GRAM scores and CURB-65 scores ≥2 in predicting critical illness and 30-day mortality in 523 patients hospitalized with COVID-19.
| (TP/total positives) | (TN/total negatives) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||
| Critical illness | ||||||
| High COVID-GRAM score | 59/122 | 51/401 | 0.53 (0.44–0.62) | 0.84 (0.81–0.88) | 0.48 (0.39–0.57) | 0.87 (0.84–0.91) |
| CURB-65 score ≥2 | 71/197 | 39/326 | 0.64 (0.55–0.73) | 0.69 (0.65–0.73) | 0.36 (0.29–0.43) | 0.88 (0.85–0.92) |
| 30-day mortality | ||||||
| High COVID-GRAM score | 56/122 | 385/401 | 0.77 (0.68–0.87) | 0.85 (0.82–0.88) | 0.46 (0.37–0.55) | 0.96 (0.94–0.98) |
| CURB-65 score ≥2 | 62/197 | 10/316 | 0.86 (0.78– 0.94) | 0.70 (0.64–0.75) | 0.31 (0.25–0.38) | 0.97 (0.95–0.99) |
CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive.
Figure 1.
ROC curves to assess the accuracy of the COVID-GRAM score and CURB-65 score at admission for predicting critical illness and 30-day mortality in 523 patients hospitalized with COVID-19. (A) Accuracy of the COVID-GRAM score for predicting critical illness; (B) accuracy of the CURB-65 score for predicting critical illness; (C) accuracy of the COVID-GRAM score for predicting 30-day mortality; and (D) accuracy of the CURB-65 score for predicting 30-day mortality.
Logistic regression analysis was applied, adjusting by sex, obesity, and severity of illness at admission. A high COVID-GRAM score emerged as an independent predictor of critical illness (OR 9.40, 95% CI 5.51–16.04; P < 0.001) (Table 5 ).
Table 5.
Multivariable logistic regression model for predicting the development of critical illness in 523 patients hospitalized with COVID-19.
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Male sex | 1.65 (1.03–2.64) | 0.038 |
| Obesity | 2.25 (1.30–3.89) | 0.004 |
| qSOFA ≥2 | 0.658 (0.46–0.95) | 0.024 |
| High COVID-GRAM score | 9.40 (5.51–16.04) | <0.001 |
CI, confidence interval; LDH, lactate dehydrogenase; OR, odds ratio.
Since 162 patients (30.97%) had missing data, patients with complete data (n = 361, 69.03%) were analysed separately (n = 361). The proportion of patients with a high COVID-GRAM score was significantly greater in the group of patients with a critical illness than in the group without a critical illness (74.1% vs 14.7%; P < 0.001). When logistic regression analysis was applied in this group of patients, a high COVID-GRAM score was also shown to be an independent predictor of critical illness (OR 17.67, 95% CI 6.79–45.97; P < 0.001).
The sensitivity, specificity, PPV, and NPV of a CURB-65 score ≥2 for critical illness are shown in Table 4. The ROC curve showed an AUC of 0.727 for predicting critical illness (Figure 1B).
COVID-GRAM score and CURB-65 score for predicting 30-day mortality
The accuracy of the COVID-GRAM score for predicting 30-day mortality was evaluated. Among the patients with a high COVID-GRAM score, mortality was 10.7% (56 patients). A high COVID-GRAM score showed a sensitivity of 77%, specificity of 85%, PPV of 46%, and NPV of 96% (Table 4), and an AUC of 0.88 for 30-day mortality (Figure 1C). A high COVID-GRAM score emerged as an independent predictor of mortality when logistic regression analysis was applied (OR 20.42, 95% CI 10.72–38.90; P < 0.001).
When the group of patients with complete data was analysed separately, a high COVID-GRAM score also emerged as an independent predictor of mortality (OR 24.52, 95% CI 8.41–71.54; P < 0.001).
Among the patients with a high CURB-65 score, mortality was 11.85% (62 patients). A CURB-65 score ≥2 showed a sensitivity of 86%, specificity of 70%, PPV of 31%, and NPV of 97% (Table 4), and an AUC of 0.83 (Figure 1D) for 30-day mortality.
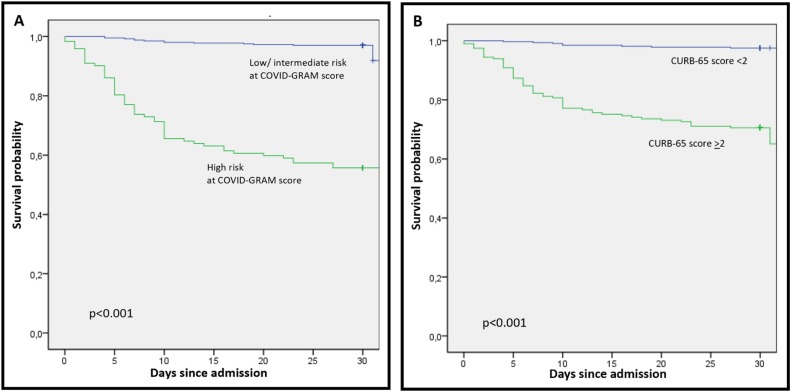
Kaplan–Meier curves for high COVID-GRAM score and CURB-65 score ≥2 for 30-day mortality are shown in Figure 2 .
Figure 2.
Kaplan–Meier curve for overall survival among 523 patients hospitalized with COVID-19 stratified by (A) COVID GRAM score and (B) CURB-65 score.
There was no significant difference in ICU admission according to the COVID-GRAM score (16.7% for high-risk patients vs 83.3% for low–intermediate-risk patients; P = 0.222) or the CURB-65 score (31.5% for CURB-65 score ≥2 vs 68.5% for CURB-65 score <2; P = 0.322).
Discussion
This appears to be the first study to analyse the usefulness of the COVID-GRAM score in a non-Chinese population. The strength of this study lies in the fact that it included all adults admitted with a diagnosis of COVID-19 to a tertiary-level European hospital during the beginning of the COVID-19 pandemic.
The mean age of the study patients was higher (65.63 vs 48.9 years) and the proportion of comorbidities was also higher (59.7% vs 25.1%) when compared to the study of Liang et al. (Liang et al., 2020). This could explain why critical illness and mortality were also higher among the present study patients compared to the original study (Liang et al., 2020) (21% vs 8.2%, and 13.8% vs 3.2%, respectively).
The results of this study are more similar in terms of mean age and comorbidities to those published in European and North American populations with SARS-CoV-2 infection (Richardson et al., 2020, Suleyman et al., 2020, Giacomelli et al., 2020, Guisado-Vasco et al., 2020), in which the reported mortality was even higher than among the present study patients (about 20% in most reports).
Univariate analysis identified different variables as predictors of critical illness, including age, male sex, the presence of comorbidities (especially hypertension, COPD, cerebrovascular disease, and chronic kidney disease), unconsciousness, dyspnoea, fever, tachycardia, tachypnoea, SaO2 <90%, high CRP levels, creatinine, LDH, neutrophil to lymphocyte ratio, and radiological confirmation of pneumonia. Likewise, the proportions of patients with a high COVID-GRAM score, WHO score ≥5, CURB65 score ≥2, and qSOFA score ≥2 were significantly higher in the group with a critical illness when compared to the group without a critical illness.
A high COVID-GRAM score at admission emerged as an independent predictor of critical illness, showing good sensitivity, specificity, and especially NPV. ROC curves showed good accuracy in predicting critical illness. Accuracy was even higher in the original study by Liang et al. (Liang et al., 2020); this difference in accuracy was perhaps influenced by the higher mean age and higher proportion with comorbidities among the present study patients. When the COVID-GRAM score was applied for predicting mortality, higher NPV and ROC curve values were obtained.
Interestingly, the accuracy of the CURB-65 score in predicting mortality was quite similar to that of the COVID-GRAM score. Although the AUC was slightly lower, the CURB-65 score showed a good NPV, and its simplicity of use makes it particularly attractive for routine clinical practice in COVID-19 patients, especially when quick decisions must be made, such as occurs in emergency departments. The results of this study on the accuracy of CURB-65 for mortality of patients with SARS-CoV-2 infection are similar to those reported in other publications (Guo et al., 2020, Ma et al., 2020, Fan et al., 2020, Satici et al., 2020, Wang et al., 2020, García Clemente et al., 2020).
This study has some limitations. First, the retrospective approach means that some variables were unknown from the COVID-GRAM and CURB-65 scores at admission. All unknown variables were assigned a value of normal, in order not to overestimate the results of the two scores. Second, all of the study participants were seen in the same hospital, and there may be differences among populations, even though similar patient characteristics as in other European and North American studies were observed (Richardson et al., 2020, Suleyman et al., 2020, Giacomelli et al., 2020, Guisado-Vasco et al., 2020). Finally, since all patients in this study were hospitalized, it is impossible to conclude if patients with a low risk of critical illness and mortality according to COVID-GRAM and CURB-65 could be safely discharged.
In summary, the COVID-GRAM score may be a useful tool for identifying Caucasian patients with SARS-CoV-2 infection who are at a low risk of critical illness and mortality. The CURB-65 score could be a good alternative, especially in situations of healthcare overload, where decisions must be made quickly. Further studies are needed to confirm whether these patients could be safely discharged and monitored on an outpatient basis.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethical approval was obtained from the Ethics Committee of Cantabria (internal code 2020.237).
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
HUMV-COVID group (in alphabetical order): Cristina Abad, Beatriz Abascal, Mario Agudo, Juan Alonso, Lucía Alonso, Inés Álvarez, Sofía Álvarez, Carlos Amado, Guido Andretta, Carlos Armiñanzas, Ana M. Arnaiz, Francisco Arnaiz, Laura Ayarza, Cristina Baldeón, María A. Ballesteros, Eva Barrero, María J. Bartolomé, Lara Belmar, Arancha Bermúdez, Ana Berrazueta, Carmen Blanco, Teresa Borderias, Marta Boya, Javier Burón, Alejandro Caballero, Marta Cabello, Vanesa Calvo-Rio, Sandra Campos, Violeta Cantero, Santiago Cantoya, Lucía Cañamero, Belén Caramelo, Juan M. Cerezo, Marina Cherchi, José M. Cifrián, Marina Cobreros, Alicia Cuesta, Sandra de la Roz, María del Barrio, Sara Delgado, Álvaro Díaz, Teresa Díaz de Terán, Juan J. Domínguez, Mathew Domínguez, María J. Domínguez, Carlos Durán, Patricia Escudero, Carmen Fariñas, Marina Fayos, Marlene Feo, Marta Fernández-Sampedro, Sonia Fernández-Jorde, Diego Ferrer, Patricia Fierro, Jimmy Flores, José Ignacio Fortea, María J. García, José D. García, Adrián García, Patricia García, Ana GarcíaMiguélez, Carmen García-Ibarbia, Luis Gibert, Aritz Gil, Alejandro J. Gil, Mónica González, Pablo González, Alejandro González, Paula González-Bores, Sofía Gonzalez-Lizarbe, Laura Gutiérrez, María C. Gutiérrez del Río, Marina Haro, Rosa Herreras, María S. Holanda, Andrés Insunza, David Iturbe, Sheila Izquierdo, José M. Lanza, Maite Latorre, Miguel Llano, Susana Llerena, Marta López, Miriam López, Carlos López, Ana López, Sara López-García, Laura López-Delgado, Iciar Lorda, José L. Lozano, Jorge Madera, Tamara Maestre, Adrián Magarida, Juan Martín, Marta Martín-Millán, Amaya Martínez, David Martínez, Gonzalo Martínez de las Cuevas, Joel Mazariegos, Iván Mazón, Jaime Mazón, Mireia Menéndez, Eduardo Miñambres, Víctor Mora, Pablo Munguía, José J. Napal, Iñigo Navarro, Sara Nieto, Tomás Obeso, Aitor Odriozola, Félix Ortiz, María Ortiz, Fernando Ortiz-Flores, Elsa Ots, Javier Pardo, Juan Parra, Raúl Parra, Ana C. Pascual, Yhivian Peñasco, José L. Pérez-Canga, Jesús Pérez del Molino, Nuria Puente, Leandra Reguero, Adriana Reyes, José A. Riancho, Eloy Rodríguez, Bryan Rodríguez, Juan C. Rodríguez-Borregán, Felix Romay, Cristina Ruiz, Luis J. Ruiz, Ana Ruiz, Jaime Salas, Zaida Salmón, Borja Sampedro, Juncal Sánchez-Arguiano, Laura Sánchez-Togneri, Juan Sánchez-Ceña, María J. Sanz-Aranguez, Patricio Seabrook, David Serrano, Marina Serrano, Nicolás Sierrasesumaga, Marta Sotelo, Borja Suberviola, Beatriz Tapia, Guillermo Tejón, Sandra Tello, Sonia Trabanco, Idoia Valduvieco, Carmen Valero, María T. Valiente, Lucrecia Yáñez, Zoilo Yusta, Miguel A. Zabaleta.
References
- Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul D.J., Unda S.R., Benton J., de la Garza Ramos R., Cezayirli P., Mehler M. A novel severity score to predict inpatient mortality in COVID-19 patients. Sci Rep. 2020;10:16726. doi: 10.1038/s41598-020-73962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappabianca S., Fusco R., de Lisio A., Paura C., Clemente A., Gagliardi G. Clinical and laboratory data, radiological structured report findings and quantitative evaluation of lung involvement on baseline chest CT in COVID-19 patients to predict prognosis. Radiol Med. 2020;12:1–11. doi: 10.1007/s11547-020-01322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G., Tu C., Zhou F., Liu Z., Wang Y., Song B. Comparison of severity scores for COVID-19 patients with pneumonia: a retrospective study. Eur Respir J. 2020;56 doi: 10.1183/13993003.02113-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alamino J.P. Epidemiological aspects, clinic and control mechanisms of Sars-Cov-2 pandemic: situation in Spain. Enferm Clin. 2020;(May) doi: 10.1016/j.enfcli.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Clemente M.M., Herrero Huertas J., Fernández Fernández A., De La Escosura Muñoz C., Enríquez Rodríguez A.I., Pérez Martínez L. Assessment of risk scores in covid-19. Int J Clin Pract. 2020 doi: 10.1111/ijcp.13705. [DOI] [PubMed] [Google Scholar]
- Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian Epidemic: a prospective cohort study. Pharmacol Res. 2020 doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisado-Vasco P., Valderas-Ortega S., Carralón-González M.M., Roda-Santacruz A., González-Cortijo L., Sotres-Fernández G. Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: a retrospective observational study (COQUIMA cohort) EClinicalMedicine. 2020;(October) doi: 10.1016/j.eclinm.2020.100591. 100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Zhou B., Zhu M., Yuan Y., Wang Q., Zhou H. CURB-65 may serve as a useful prognostic marker in COVID-19 patients within Wuhan, China: a retrospective cohort study. Epidemiol Infect. 2020;148:e241. doi: 10.1017/S0950268820002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur K., Price C.P.E., Gray E.L., Gulati R.K., Maksimoski M., Racette S.D. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163:170–178. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang S., Zhong F., Bao W., Li Y., Liu L. Age-dependent risks of incidence and mortality of COVID-19 in Hubei Province and other parts of China. Front Med (Lausanne) 2020;30(7):190. doi: 10.3389/fmed.2020.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Liang H., Ou L., Chen B., Chen A., Li C. China Medical Treatment Expert Group for COVID-19. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180:1–9. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Ng M., Xu S., Xu Z., Qiu H., Liu Y. Development and validation of prognosis model of mortality risk in patients with COVID-19. Epidemiol Infect. 2020;148:e168. doi: 10.1017/S0950268820001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelozzi P., de’Donato F., Scortichini M., De Sario M., Noccioli F., Rossi P. Mortality impacts of the coronavirus disease (COVID-19) outbreak by sex and age: rapid mortality surveillance system, Italy, 1 February to 18 April 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.19.2000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- mscbs.gob.es - Manejo clínico del COVID-19: atención hospitalaria, From https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos.htm. [Accessed 30 October 2020].
- Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C., Minc A., Caceres J., Balsalobre A., Dixit A., KaPik B. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.09.017. S0735-6757(20)30809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satici C., Demirkol M.A., Altunok E.S., Gursoy B., Alkan M., Kamat S. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int J Infect Dis. 2020;98:84–89. doi: 10.1016/j.ijid.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wang G., Cai X.P., Deng J.W., Zheng L., Zhu H.H. An overview of COVID-19. J Zhejiang Univ Sci B. 2020;21:343–360. doi: 10.1631/jzus.B2000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprung C.L., Joynt G.M., Christian M.D., Truog R.D., Rello J., Antes J.L. Adult ICU triage during the coronavirus disease 2019 pandemic: who will live and who will die? Recommendations to improve survival. Crit Care Med. 2020;48:1196–1202. doi: 10.1097/CCM.0000000000004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleyman G., Fadel R.A., Malette K.M., Hammond C., Abdulla H., Entz A. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hu Z.W., Hu Y., Cheng Y., Zhang H., Li H.C. Comparison of severity classification of Chinese protocol, pneumonia severity index and CURB-65 in risk stratification and prognostic assessment of coronavirus disease 2019] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:834–838. doi: 10.3760/cma.j.cn112147-20200226-00186. [DOI] [PubMed] [Google Scholar]
- who.int/blueprint - COVID-19 Therapeutic Trial Synopsis, from https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf. [Accesed 25 November 2020].
- who.int/emergencies - Coronavirus disease (COVID-19) pandemic, from https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Accessed 30 October 2020].
- Ye K., Tang F., Liao X., Shaw B.A., Deng M., Huang G. Does serum vitamin d level affect COVID-19 infection and its severity? A case-control study. J Am Coll Nutr. 2020;(October):1–8. doi: 10.1080/07315724.2020.1826005. [DOI] [PubMed] [Google Scholar]
- Yee J., Unger L., Zadravecz F., Cariello P., Seibert A., Johnson M.A. Novel coronavirus 2019 (COVID-19): emergence and implications for emergency care. J Am Coll Emerg Physicians Open. 2020;1:63–69. doi: 10.1002/emp2.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J.Y., Lee K.S., Ang L.W., Leo Y.S., Young B.E. Risk factors of severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis and meta-regression analysis. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Wang M., Zhang J., Ye J., Xu Y., Wang Z. Advances in the relationship between coronavirus infection and cardiovascular diseases. Biomed Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., He Y., Yang H., Yu H., Wang T., Chen Z. Development and validation a nomogram for predicting the risk of severe COVID-19: a multi-center study in Sichuan, China. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233328. [DOI] [PMC free article] [PubMed] [Google Scholar]