Abstract
Introduction:
The laparoscopic approach has become the gold standard for cholecystectomy. However, it could have some major complications. Among them, it can be considered postoperative pseudoaneurysms of the cystic or hepatic arteries. Haemobilia secondary to a cystic artery pseudoaneurysm is extremely rare.
Case Report:
Here we present a case from our centre of haemobilia in association with a cystic artery pseudoaneurysm, as a late complication of VLC. An 18-year-old girl underwent laparoscopic cholecystectomy; during surgery, due to viscero-perietal tight adhesions and due to the close proximity of the cystic duct to the biliary ducts, we suspected a bile duct injury. So, decision was taken to convert to open surgery: a suture was performed to repair the coledocic duct injury and an endoscopic papillotomy was performed with subsequent positioning of an endoscopic plastic biliary endoprothesis at the hepatocholedochus. One month after surgery, the patient showed clinical signs of hypovolemic shock. She underwent Computed Tomography Angiography, showing a possible arterial lesion, just adjacent to surgical clip. Therefore, patient underwent angiographic examination, which confirmed an 8 mm pseudoaneurysm arising from cystic artery, just adjacent to surgical clips. Superselective catheterization of vessel was performed, and two coils were released, until obtaining complete exclusion of the vascular lesion. The patient was discharged five days after procedure, with good general condition.
Conclusion:
Pseudoaneurysms of the cystic artery are uncommon entities, rarely reported in the literature, and often caused by cholecystitis or iatrogenic biliary injury. All conditions that are responsible for vessels’ injuries could also cause haemobilia. Even if pseudoaneurysm of cystic artery with haemobilia is a rare event, it has to be considered as a complication of VLC. Angiographic approach should be the treatment of choice.
Keywords: Haemobilia, Cystic artery pseudoaneurysm, Laparoscopic cholecystectomy
Introduction
The laparoscopic approach has become the gold standard for cholecystectomy (VLC). However, it could have some major complications, for example vascular ones (0.25%) (1-3). Among them, it can be considered postoperative pseudoaneurysms (PSAs) of the cystic or hepatic arteries, which is an uncommon complication, with fewer than 100 cases reported in the literature (4-10). The mechanism of PSA formation is unclear, but it likely involves vascular injury, for example thermal injury or erosion due to clips placement (11-14).
Patients who present postoperative PSA may develop bleeding in the form of hemoperitoneum, haemobilia, hematemesis, or melena. Haemobilia secondary to a cystic artery pseudoaneurysm is extremely rare (15,16).
Here we present a case from our centre of haemobilia in association with a cystic artery pseudoaneurysm, as a late complication of VLC. Our work is in line with the SCARE criteria (17).
Case Report
An 18-year-old girl was visited at our Surgery Clinic for a symptomatology characterized by vague and recurrent colic-type pain localized to the upper abdominal quadrants. Her GP, having seen the ultrasound and gastroscopy performed, directed the patient to our attention. In fact, the gastroscopy was negative for gastritis, while the ultrasound revealed the presence of cholelithiasis, without inflammatory features or biliary tract abnormalities. Her clinical history was silent for other pathologies or previous surgical interventions. She was scheduled for laparoscopic cholecystectomy, which was performed two months later in our Surgical Department, after that magnetic resonance cholangiography (MRC) excluded presence of biliary sludge in the extrahepatic biliary ducts. The standard technique with 4 ports was performed. Using monopolar L hook, Calot’s triangle was isolated, cystic duct and artery were identified and then clipped and dissected. Posterior dissection of the gallbladder was finally done, and it was removed using an endobag device. Due to viscero-perietal tight adhesions and due to the close proximity of the cystic duct to the biliary ducts, the Calot’s triangle exposure resulted complicated, and we suspected that a bile duct injury occurred in this surgical phase. So, decision was taken to convert to open surgery, thermal injury was confirmed, and a suture was performed to repair the choledochal duct injury. At the same time, after explorative intra-operative endoscopic retrograde cholangiopancreatography (ERCP), an endoscopic papillotomy was performed with subsequent positioning of an 11.5 Fr, 12 cm Cotton-Leung plastic stent (Cook Medical, USA) at the hepatocholedochus, to protect the injured section which had been surgically sutured. A week later, a new ERCP control examination showed a spread of the contrast out of the biliary tract, so another biliary prothesis of the same type was positioned.
One month after surgery, the patient had an upper gastrointestinal bleeding (hematemesis); she underwent urgent endoscopy and the biliary stent was removed. Blood residues were present in the stomach, but no signs of oesophageal or gastric bleeding were detected; laboratory studies revealed the following values: haemoglobin level, 8.8 g/L; white blood cells count, 11.9 × 103 /uL; haematocrit 25.2%; total bilirubin, 2.05 mg/dL. The patient showed clinical signs of hypovolemic shock, so she underwent urgent explorative laparotomy. An active bleeding was detected over the clipped cystic artery stump. To perform a correct haemostasis, the previous clip was removed and a new one was positioned.
Six days after this episode, the patient showed again signs of hypovolemic shock. Laboratory tests revealed: haemoglobin level, 8.2 g/L; white blood cells count, 11.3 × 103 /uL; haematocrit 23.7%. She underwent Computed Tomography Angiography (CTA), showing a possible arterial lesion, just adjacent to surgical clip, with choledocic luminal hyperdensity, as for haemobilia (Figure 1). Therefore, patient underwent digital subtraction angiography (DSA).
Figure 1.

Unenhanced Computed Tomography (CT) with coronal MIP (Maximum Intensity Projection) reconstructions showed a choledocic luminal hyperdensity, as for haemobilia (a). CT angiography showing an arterial lesion just adjacent to the surgical clip (b; red arrow).
After obtaining a femoral approach, selective catheterization of common hepatic artery and proper hepatic artery was performed. Diagnostic examination showed cystic artery originated from the very proximal left hepatic artery (Fig. 2), moreover, an 8 mm pseudoaneurysm arising from cystic artery, just adjacent to surgical clips, was confirmed (Fig. 2, 3). With a coaxial system, superselective catheterization of the vessel was performed (fig. 4a) and two coils (Interlock 3 mm x 6 cm; Boston Scientific) were released disto-proximally, according to “sandwich” technique, until obtaining complete exclusion of the vascular lesion (Fig. 4b). Finally, selective catheterization of superior mesenteric artery was performed to rule out possible retrograde flow into the pseudoaneurysm.
Figure 2.

DSA with injection from proper hepatic artery confirming a round-shaped pseudoaneurysm of the cystic artery, just laterally to the surgical clip (red arrow).
Figure 3.
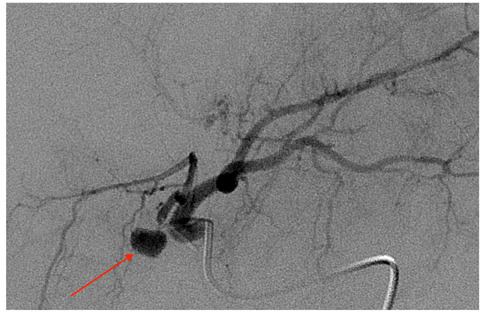
Selective catheterization of left hepatic artery showing the cystic artery presents an unusual origin from the very proximal left hepatic artery. The pseudoaneurysm is clearly detectable (red arrow).
Figure 4.

Super selective catheterization of cystic artery with clear evidence of the pseudoaneurysm (a); un - subtracted angiographic image showing release of two coils in distal-proximal fashion, according to “sandwich” technique, until obtaining complete exclusion of the vascular lesion (b).
Liver function tests were normal after the procedure and 3 days after that. The patient was discharged five days after procedure with good general condition.
An MRC was performed a month later, after endoscopic removal of the prosthesis. It reported “non-dilation of the intra and extra-hepatic biliary tracts: in particular, regular diameter of the hepatocoledocus; presence of minimal diameter reduction immediately after the confluence of the hepatic ducts. No free fluid effusion in the abdomen”. One year later, the patient again underwent control abdominal US and MRI, in the absence of symptoms. No dilatation of the biliary tract, neither abdominal effusion was detected.
Discussion
Pseudoaneurysms (PSAs) of the cystic arteries are uncommon entities, rarely reported in the literature, and often caused by cholecystitis and iatrogenic biliary injury. Other reported aetiologies are abdominal trauma, radiological procedures and systemic vasculitis (18). In case of iatrogenic complication, PSA formation results from a vessel wall injury, which could be mechanical or thermal, and which causes weakening of the artery and consequent periarterial hematoma (10,19). The thermal injury could result from the direct transfer of heat to the vessel, during the dissection of Calot’s triangle, or indirectly via a metal clip in contact with the artery. In fact, in many cases, the PSA has been found adjacent to the clips (2,20). The damage could also unintentionally occur in case of variations in the anatomic course of the right hepatic artery, and consequent misidentification of the cystic artery. Another factor contributing to pseudoaneurysm formation is the cytotoxic power of bile acids, in case of biliary leaks (10).
All these conditions that are responsible for vessels’ injuries could also cause hemobilia (12). This is a condition first described by Francis Glisson in 1654, which is defined as a communication between splanchnic and biliary circulation. Risk factors for haemobilia following cholecystectomy include variant bile duct anatomy, variant cystic artery anatomy, intraoperative adhesions, postoperative infection and bile leakage, which can cause erosion of the vascular wall (5,21,22). The incidence of haemobilia following an emergency VLC for acute cholecystitis has been reported to be 0.001%, while it has been observed to be 0.0003% for patients undergoing elective VLC (2).
Cystic artery pseudoaneurysms (CAP) are extremely rare vascular complications and are moreover uncommon causes of haemobilia: only 20 cases have been reported, of which only 9 following laparoscopic cholecystectomy (5,13,23-28).
The classic presentation of haemobilia is defined Quincke’s triad: right upper quadrant pain (biliary colic), upper gastrointestinal bleeding (melena and/or hematemesis), and obstructive jaundice. But this typical clinical presentation may be seen in less than 40% of haemobilia cases (29). Symptoms typically develop early in the postoperative period, but the average time between VLC and onset of bleeding seems to be 13–21 days (4,6-8,30), which is in line with what we reported in our paper. Presentations up to 120 days after surgery have also been reported (22,25).
The clinical evaluation of patients with haemobilia generally begins with upper-GI endoscopy or ERCP to exclude more common causes of bleeding and relieve the biliary obstruction. As in other cases of intra-abdominal bleeding, subsequent angiography (CT or conventional) is required to identify the source of bleeding (10).
In our patient, we decided to perform an endoscopic exam as first diagnostic approach at the first bleeding episode. No active bleeding was seen from the stomach, and we decided for urgent surgery because the patients showed clinical signs of hypovolemic shock. The CTA was performed after the second episode of anemization. Maybe an abdominal CTA before previous urgent surgical treatment could have shown the vascular complication and the haemobilia and could have solved the problem.
The reason of our choice to make a direct surgical indication was guided by the evidence that post-surgical bleeding is usually attributable to slippage of clips of the cystic artery or to liver bed bleeding (31). These are complications that can be easily surgically treated. Cystic artery pseudoaneurysms are extremely rare vascular complications, therefore this may explain the fact that we have not indicated to perform a CTA before surgery.
Transarterial or percutaneous embolization is the treatment of choice: coils can in fact allow complete embolization of a pseudoaneurysm without major complications. Our patient was treated successfully with this technique.
In the past, these vascular alterations were surgically managed with resection of the pseudoaneurysm and ligation of the cystic artery stump or right hepatic artery. But operative intervention in this setting are associated with high morbidity and mortality (10), so nowadays surgery is indicated in case of bile duct compression or injuries, failure of an embolization procedure or hemodynamic instability (2,18). In fact, with the advent of catheter-based therapies, pseudoaneurysms are now typically managed using endovascular transcatheter embolization, which can be performed using many embolic agents: coils, gel foam, N-butyl cyanoacrylate. Advantages of embolization include a less traumatic procedure, non-requirement of general anesthesia and a short recovery period (12). Failure may be due to the difficulty in approaching the PSA in the presence of a thrombosed proximal vessel. In patients where cannulation is feasible, embolisation failure could be related to an incomplete occlusion or to the presence of other smaller vessels supplying the pseudoaneurysm that were not seen on angiography (2).
Further treatment options included embolization of the right hepatic artery nonselectively (12).
Recently, some researchers reported the successful management of PSAs by injecting thrombin directly into the aneurysm (2,32). However, embolisation using this technique may be nonselective, resulting in potentially serious complications; injecting small amounts of matherial with Doppler guidance may reduce this risk.
Conclusion
In conclusion, even if pseudoaneurysm of cystic artery with haemobilia is a rare event, it has to be considered as a possible complication of VLC. Angiographic approach should be the treatment of choice. In our experience, this procedure seemed to ben feasible, safe, tolerable and effective.
Acknowledgments:
All authors disclose the absence of any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
Consent:
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Schietroma M, Pessia B, Mattei A, Romano L, Giuliani A, Carlei F. Temperature-Neutrophils-Multiple Organ Failure Grading for Complicated Intra-Abdominal Infections. Surg Infect (Larchmt) 2020 Feb;21(1):69–74. doi: 10.1089/sur.2019.092. doi: 10.1089/sur.2019.092. Epub 2019 Aug 28. [DOI] [PubMed] [Google Scholar]
- 2.Machado NO. Biliary complications post laparoscopic cholecystectomy: Mechanism, preventive measures, and approach to management - A review. Diagn Ther Endosc 2011. 2011:967017. doi: 10.1155/2011/967017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strasberg SM, Helton WS. An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB (Oxford) 2011;13:1–14. doi: 10.1111/j.1477-2574.2010.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bin Traiki TA, Madkhali AA, Hassanain MM. Hemobilia post laparoscopic cholecystectomy. J Surg Case Rep. 2015;(2):rju159. doi: 10.1093/jscr/rju159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrou A, Brennan N, Soonawalla Z, Silva MA. Hemobilia due to cystic artery stump pseudoaneurysm following laparoscopic cholecystectomy: Case presentation and literature review. Int Surg. 2012;97(2):140–4. doi: 10.9738/CC52.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schietroma M, Carlei F, Cappelli S, Pescosolido A, Lygidakis NJ, Amicucci G. Effects of cholecystectomy (laparoscopic versus open) on PMN-elastase. Hepatogastroenterology. 2007 Mar;54(74):342–5. [PubMed] [Google Scholar]
- 7.Feng W, Yue D, ZaiMing L, ZhaoYu L, Wei L, Qiyong G. Hemobilia following laparoscopic cholecystectomy: Computed tomography findings and clinical outcome of transcatheter arterial embolization. Acta Radiol. 2017;58(1):46–52. doi: 10.1177/0284185116638570. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson T, Travis S, Ettles D, et al. Hepatic artery angiography and embolization for hemobilia following laparoscopic cholecystectomy. Cardiovasc Intervent Radiol. 1999;22(1):20–4. doi: 10.1007/s002709900323. [DOI] [PubMed] [Google Scholar]
- 9.Rencuzogullari A, Okoh AK, Akcam TA, Roach EC, Dalci K, Ulku A. Hemobilia as a result of right hepatic artery pseudoaneurysm rupture: An unusual complication of laparoscopic cholecystectomy. Int J Surg Case Rep. 2014;5(3):142–4. doi: 10.1016/j.ijscr.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badillo R, Darcy MD, Kushnir VM. Hemobilia Due to Cystic Artery Pseudoaneurysm: A Rare Late Complication of Laparoscopic Cholecystectomy. ACG Case Rep J. 2017;4:e38. doi: 10.14309/crj.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madanur MA, Battula N, Sethi H, Deshpande R, Heaton N, Rela M. Pseudoaneurysm following laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int. 2007;6:294–8. [PubMed] [Google Scholar]
- 12.Kumar A, Sheikh A, Partyka L, Contractor S. Cystic artery pseudoaneurysm presenting as a complication of laparoscopic cholecystectomy treated with percutaneous thrombin injection. Clin Imaging. 2014;38:522–5. doi: 10.1016/j.clinimag.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Saldinger PF, Wang JY, Boyd C, Lang E. Cystic artery stump pseudoaneurysm following laparoscopic cholecystectomy. Surgery. 2002;131:585–6. doi: 10.1067/msy.2002.115353. [DOI] [PubMed] [Google Scholar]
- 14.Marchese M, Romano L, Giuliani A, Cianca G, Di Sibio A, Carlei F, Amicucci G, Schietroma M. A case of intrasplenic displacement of an endoscopic double-pigtail stent as a treatment for laparoscopic sleeve gastrectomy leak. Int J Surg Case Rep. 2018;53:367–369. doi: 10.1016/j.ijscr.2018.11.008. doi: 10.1016/j.ijscr.2018.11.008. Epub 2018 Nov 13. Erratum in: Int J Surg Case Rep. 2019;56:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergey E, Einstein DM, Herts BR. Cystic artery pseudoaneurysm as a complication of laparoscopic cholecystectomy. Abdom Imaging. 1995;20:75–7. doi: 10.1007/BF00199652. [DOI] [PubMed] [Google Scholar]
- 16.Bin Traiki TA, Madkhali AA, Hassanain MM. Hemobilia post laparoscopic cholecystectomy. J Surg Case Rep. 2015;(2):rju159. doi: 10.1093/jscr/rju159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler A, Orgill DP. For the SCARE Group. The SCARE 2018 Statement: Updating Consensus Surgical CAse REport (SCARE) Guidelines. International Journal of Surgery. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Butet Y, Bouras AF, Truant S, et al. Pseudoaneurysm of the cystic artery as a complication of laparoscopic cholecystectomy. Dig Liver Dis. 2012 May;44(5):449–50. doi: 10.1016/j.dld.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Poon R, Tuen H, Yeung C, et al. GI haemorrhage from fistula between right hepatic artery pseudoaneurysm and the duodenum secondary to acute cholecystitis. Gastrointest Endosc. 2000;51:491–3. doi: 10.1016/s0016-5107(00)70456-1. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava D, Chakravarti A, Gupta R, et al. Gastrointestinal bleeding from a false aneurysm of the hepatic artery after cholecystectomy. Am J Gastroenterol. 1996;91:395–6. [PubMed] [Google Scholar]
- 21.Wen F, Dong Y, Lu ZM, Liu ZY, Li W, Guo QY. Hemobilia after laparoscopic cholecystectomy: imaging features and management of an unusual complication. Surg Laparosc Endosc Percutan Tech. 2016;26:e18–e24. doi: 10.1097/SLE.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 22.Choudhary A, Barakat MT, Higgins LJ, et al. Choledochoscopic Identification of a Hepatic/Cystic Artery Pseudoaneurysm in a Patient with Hematemesis After Laparoscopic Cholecystectomy. Dig Dis Sci. 2017 Jun;62(6):1439–1442. doi: 10.1007/s10620-016-4243-x. [DOI] [PubMed] [Google Scholar]
- 23.Moses V, Keshava SN, Wann VC, et al. Cystic artery pseudoaneurysm after laparoscopic cholecystectomy presenting as haemobilia: a case report. Trop Gastroenterol. 2008 Apr-Jun;29(2):107–9. [PubMed] [Google Scholar]
- 24.Nakase Y, et al. Hemobilia and cystic artery stump pseudoaneurysm associated with liver abscess after a laparoscopic cholecystectomy: report of a case. Surg Today. 2008;38(6):567–71. doi: 10.1007/s00595-007-3663-9. [DOI] [PubMed] [Google Scholar]
- 25.England RE, Marsh PJ, Ashleigh R, Martin DF. Case report: pseudoaneurysm of the cystic artery: a rare cause of hemobilia. Clin Radiol. 1998;53(1):72–75. doi: 10.1016/s0009-9260(98)80041-x. [DOI] [PubMed] [Google Scholar]
- 26.Pessia B, Romano L, Giuliani A, et al. Rare case of upper gastrointestinal bleeding: Dieulafoy’ s lesion of duodenum. A case report. Ann Med Surg (Lond) 2019 Jul 13;45:19–21. doi: 10.1016/j.amsu.2019.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Molla Neto OL, Ribeiro AF. Saad WA Pseudoaneurysm of cystic artery after laparoscopic cholecystectomy. HPB. 2006;8:318–319. doi: 10.1080/13651820600869628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madanur MA, Battula N, Sethi H, et al. Pseudoaneurysm following laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int. 2007;6:294–298. [PubMed] [Google Scholar]
- 29.Schietroma M, Cappelli S, Carlei F, Pescosolido A, Lygidakis NJ, Amicucci G. “Acute abdomen”: early laparoscopy or active laparotomic-laparoscopic observation? Hepatogastroenterology. 2007 Jun;54(76):1137–41. [PubMed] [Google Scholar]
- 30.To K, Lai EC, Chung DT, et al. Cystic artery pseudoaneurysm with haemobilia after laparoscopic cholecystectomy. Hong Kong Med J. 2018 Apr;24(2):203–205. doi: 10.12809/hkmj176236. [DOI] [PubMed] [Google Scholar]
- 31.Kaushik R. Bleeding complications in laparoscopic cholecystectomy: Incidence, mechanisms, prevention and management. J Minim Access Surg. 2010;6(3):59–65. doi: 10.4103/0972-9941.68579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghoz A, Kheir E, Kotru A, Halazun K. Hemoperitoneum secondary rupture of cystic artery pseudoaneurysm. Hepatobiliary Pancreat Dis Int. 2007;6:321–3. [PubMed] [Google Scholar]


