Abstract
Background:
The giant haemorrhagic bursitis of the hip joint is a rare clinical condition that requires evidence-based guidelines for adequate diagnosis and management. Usually, this pathology requires conservative treatment; however, when abnormal size or clinical symptoms of compression of the surrounding noble structures are reported, an accurate differential diagnosis is required, in order to exclude other malignant conditions that can be included into differential diagnosis, and a surgical approach should be considered. The purpose of this work is to provide an appropriate description of the diagnostic and therapeutic path, providing an accurate analysis of the possible differential diagnoses.
Methods:
We report 2 cases of symptomatic haemorrhagic bursitis of the hip joint, confirmed by histological investigation. In both cases, the patients complained a peripheral nerve deficit of a single limb: one patient presented paresthesia of lateral femoral cutaneous nerve while the second peripheral edema due to compression of the proximal venous and lymphatic circulation.
Results:
Both cases were successfully managed by complete surgical excision of the mass, with no recurrence. There were no major complications, but in first case the nerve deficit was permanent.
Conclusions:
Giant hemorrhagic trochanteric bursitis is a rare condition, but it should be included in the differential diagnosis of soft tissue masses arising from the hip joint. Due to the rarity of this entity, a cautious exclusion process of all plausible differential diagnosis must be undertaken, in order to not miss the possibility of soft-tissue tumors, primarily malignant high-grade sarcomas. (www.actabiomedica.it)
Keywords: Trochanteric bursitis, giant bursitis, hip pain, differential diagnosis
Introduction
Trochanteric bursitis is a common disorder and frequent cause of lateral hip pain, affecting 8% to 15% of people of all ages (1), especially middle-aged women due to intrinsic anatomic patterns (2). Repetitive mechanical friction, blunt trauma, infection or inflammatory arthritis represent the most plausible factors related to this condition, although the underlying causes can be undetermined in a minority of cases (3). Surgical management for trochanteric bursitis is rarely performed, reserved for refractory cases to usual conservative therapy, including physical therapy and corticosteroid injections (4).
Unlike other anatomical locations (5), when trochanteric bursae dilate with fluid and synovial debris, they rarely reach such dimensions that enter into the differential diagnosis with soft-tissue tumors. In this context, several imaging modalities including ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) (6) could represent a precious help for correct diagnosis and may indicate proper treatment. On MRI, distended bursae are seen as hyperintense, near fluid-equivalent structures on T2-weighted images with acute bursitis demonstrating rim-enhancement on post-contrast imaging (7). Chronic bursitis complicated with hemorrhage and calcification have a more complex and varied appearance on MR imaging and makes it difficult to distinguish from other soft tissue masses (8, 9).
Hereinafter, we report two fairly rare cases of idiopathic giant trochanteric bursitis, both with concomitant neurological symptoms due to lateral femoro-cutaneous and sciatic nerve compression, respectively, and summarize the pathological conditions that can enter into the differential diagnosis with reference to the relevant literature.
Case report 1
A 58-year-old male presented with a 10-year history of painless right-sided trochanteric mass, which had become progressively uncomfortable and prominent over the past 12 months. There was no history of pelvic trauma or inflammatory disease of the pelvis.
On clinical examination, a painless tender mass measuring about 10 cm in its greatest diameter was noted, mobile relative to superficial cutaneous layers but fixed to underlying structures (Figure 1). The overlying skin was normal with no signs of infection. Paresthesia in the anterior region of the ipsilateral thigh was reported. The range of motion of the hip was not limited, although a limitation due to moderate pain with passive hip adduction was signaled. All laboratory tests, including red and white blood count with differential count, erythrocyte sedimentation rate, C-reactive protein and rheumatoid factor, were normal.
Figure 1.

Clinical examination of the painless tender mass, measuring about 10 cm in its greatest diameter, mobile relative to superficial cutaneous layers but fixed to underlying structures.
The patient was initially evaluated with pelvis radiographs that showed no significant patterns in both hip joints. MRI demonstrated a neo-formation of almost 11 centimetres long e 7 centimetres thick, isointense on fat-suppressed T2-weighted images, with hypointensity, relative to fat, on T1-weighted images (Figure 2). This formation was located in the lateral region of the proximal leg and involved the course of the tensor fascia lata muscle that appeared hypothrofic. The hip joint did not show significant alteration. In the inguinal region there were two lymph nodes of increased size but less than one centimetre.
Figure 2.
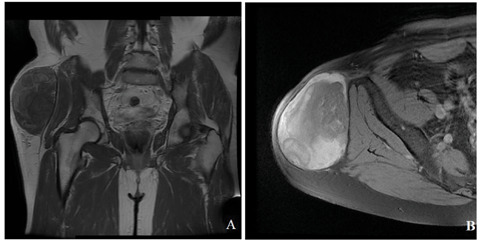
MRI demonstrated a mass of almost 11 centimetres long e 7 centimetres thick, with hypo-intense, relative to fat, on T1-weighted images (A) and hyper-intense on fat-suppressed T2-weighted images after administration of contrast medium (B).
An ultrasound-guided fine needle aspiration biopsy showed brown granulation-like tissue, with histologic presence of hemorrhagic and necrotic tissue associated to histiocytic infiltrate, suggestive of an old hemorrhagic mass, with no evidence of infection or neoplasia. At this point, we decided to proceed with a wide margin excision.
Case report 2
A 44-year-old caucasian man presented with a solid mass in the right gluteal region. The mass had slowly enlarged over the past 5 years, but recently progressively increased in size. Moreover, he complained of numbness in the right back thigh during the previous month. In medical history, he is not affected by major pathologies and does not take pharmacotherapy.
Physical examination revealed a palpable 10×5 cm hard elastic mass in the right gluteal region. The hip range of motion was no affected but marked hypoesthesia was observed in the sciatic nerve region from the posterior aspect of the thigh to the posterior aspect of the leg. A manual muscle test demonstrated that the strength of the biceps muscle was reduced to 3/5. All laboratory tests, including red and white blood count with differential count, erythrocyte sedimentation rate, C-reactive protein and rheumatoid factor, were normal.
X-ray examination revealed no hip of femoral alteration. MRI demonstrated a big mass of almost 9 centimetres long and 7 centimetres thick, extended from the iliac crest to the great trochanter. The gluteus maximum was laterally dislocated and the mass presented a course towards the piriformis muscle. The MRI signal was isointense to muscle on T1W images and hyperintense on T2W images (Figure 3). The heterogeneity of the content, of probable haemorrhagic origin, needed further investigation. The hip joint and the other adjacent structures showed no alteration of relief. The ultrasound biopsy shown hemorrhagic and necrotic tissue associated to histiocytic infiltrate that could no orientate towards a diagnosis of certainty.
Figure 3.

MRI demonstrated a big mass of almost 9 centimetres long and 7 centimetres thick, extended from the iliac crest to the great trochanter, with consequent dislocation of the gluteus maximum muscle The MRI signal was isointense to muscle on T1W images (A) and hyper-intense on T2W images (B).
Also, in this case, we decided to proceed with a wide margin excision, with complete resolution of patient’s symptoms in the postoperative stay (Figure 4).
Figure 4.

Intraoperative imagine during excision of the mass.
Discussion
Bursae are synovial-lined, potential spaces between bony prominences and surrounding soft tissue structures. Working as cushions, bursae provide a gliding interface between opposed moving components and reduce pressure and mechanical friction (10).
Approximately 20 different bursae have been identified around the pelvis (7, 11), but mostly 3 of these structures are widely well described. These include the trochanteric bursa, which separates the gluteus maximus from the greater trochanter and the short rotator muscles, the ischial bursa, situated over the tuberosity of the ischium, and the gluteo-femoral bursa, which separates the iliotibial tract from the vastus lateralis. Due to its superficial position and proximity to large tendons, bursa around hip can inflame inflamed and become a common reason for presentation to the orthopedic surgeon, more rarely for progressive increasing size mimicking soft-tissue tumors. According to our knowledge, these are the first two reported cases of hemorrhagic trochanteric bursitis with similar features, especially as regard dimensions and invasiveness to surrounding soft tissue structures. Furthermore, the singularity of these cases lies in the absence of associated systemic pathological conditions. In literature, several other cases of huge bursitis from different anatomical districts are reported.
Iliopsoas bursitis is a rare pathological condition, secondary to various hip diseases (such as rheumatoid arthritis, osteoarthritis, and femoral head osteonecrosis) that develop because of a communication with the hip joint present in approximately 14 % of the human population (12). In quite a lot of reported cases, it entered into differential diagnosis with soft-tissue tumors arising from the hip, since it can present as a massive inguinal mass with concomitant neurological and vascular symptoms due to direct pressure against adjacent structures such as the femoral nerve and veins (13 – 16).
Tayfur et al. (17) reported a case of a huge trochanteric bursa due to a rare manifestation of musculoskeletal tuberculosis, treated by drainage along with one-year antituberculosis therapy. Stahnke et al. (18) described two cases of calcific infrapatellar and prepatellar hemorrhagic bursitis, that deserve particular attention for the singular radiological and pathological findings. The presence of dystrophic calcification within the lesion, associated to the heterogeneous and varied radiological appearance, represent an unusual misleading entity that may suggest a mineralizing soft tissue sarcoma such as synovial sarcoma.
As a matter of fact, giant bursae may indicate an underlying neoplastic process that must not go unnoticed. Tuncer et al. (19) reported a singular case of a huge scapulothoracic bursitis with synovial osteochondromatosis, which was caused by osteochondroma arising from the inner surface of the scapula. Maheshwari et al. (20) presented two rare cases of pigmented villonodular bursitis arising from the pes anserinus bursa. The involvement of a true bursa without articular communication represents a rarity.
Synovial sarcomas are malignant soft tissue tumors that should be taken into consideration when excluding possible differential diagnosis of giant bursitis. Despite occurring near joints, rarely the synovial sarcoma may arise from intraarticular sources, such as synovium, bursa or tendon sheath (21, 22). The slowly and insidious growth together with the long duration of symptoms noted in some cases also after 10 years (21) could represent a further confounding pattern. However, the high risks of local recurrence and distant metastases (23, 24) prejudice the long-term survivorship and should alert physician when evaluating a bursitis with consistent features.
Conclusions
In conclusion, giant hemorrhagic trochanteric bursitis is a rare condition that should be included in the differential diagnosis of soft tissue masses arising from the hip joint. Due to the rarity of this entity, a cautious exclusion process of all plausible differential diagnosis must be undertaken, in order to not miss the possibility of soft-tissue tumors, primarily malignant high-grade sarcomas.
In this context, as with other lesions, clinical and radiographic features along with close histological correlation are essential for correct diagnosis and adequate treatment protocol.
Abbreviations:
- CT:
computed tomography
- MRI:
magnetic resonance imaging
Ethical approval and consent to participate:
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Due to the retrospective nature of the present manuscript, without experimental procedures or drugs, the approval of Institutional Ethics Committee was not required, as per regulation.
Consent for publication:
Written informed consent for publication of her clinical details and clinical images was obtained from the patients.
Funding source:
No external funding was received for the initiation or completion of this study.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Segal NA, Felson DT, Torner JC, Zhu Y, Curtis JR, Niu J, Nevitt MC. Multicenter Osteoarthritis Study Group. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil. 2007;88(8):988–92. doi: 10.1016/j.apmr.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearon AM, Cook JL, Scarvell JM, Neeman T, Cormick W, Smith PN. Greater trochanteric pain syndrome negatively affects work, physical activity and quality of life: a case control study. J Arthroplasty. 2014;29(2):383–6. doi: 10.1016/j.arth.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Seidman AJ, Varacallo M. StatPearls [Internet]. Treasure Island. FL: StatPearls Publishing; 2019 Jan-2019 Feb 15. Trochanteric Bursitis. [PubMed] [Google Scholar]
- 4.Lustenberger DP, Ng VY, Best TM, Ellis TJ. Efficacy of treatment of trochanteric bursitis: a systematic review. Clin J Sport Med. 2011;21(5):447–53. doi: 10.1097/JSM.0b013e318221299c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khodaee M. Common Superficial Bursitis. Am Fam Physician. 2017 Feb 15;95(4):224–231. [PubMed] [Google Scholar]
- 6.Hirji Z, Hanjun JS, Choudur HN. Imaging of Bursae. J Clin Imaging Sci. 2011;1:22. doi: 10.4103/2156-7514.80374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman MV, Stensby JD, Long JR, Currie SA, Hillen TJ. Beyond the greater trochanter: a pictorial review of the pelvic bursae. Clin Imaging. 2017;41:37–41. doi: 10.1016/j.clinimag.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Murphy MD, Kransdorf MJ, Smith SE. Imaging of soft tissue neoplasm in the adult: malignant tumours. Semin Musculoskeletal Radiol. 1999;1:39–58. doi: 10.1055/s-2008-1080050. [DOI] [PubMed] [Google Scholar]
- 9.Morton MJ, Berquist TH, McLeod RA, Unni KK, Sim FH. MR imaging of synovial sarcoma. AJR Am J Roentgenol. 1991;156:337–340. doi: 10.2214/ajr.156.2.1846054. [DOI] [PubMed] [Google Scholar]
- 10.Foisneau-Lottin A, Martinet N, Henrot P, Paysant J, Blum A, André J. Bursitis, adventitious bursa, localized soft-tissue inflammation, and bone marrow edema in tibial stumps: the contribution of magnetic resonance imaging to the diagnosis and management of mechanical stress complications. Arch Phys Med Rehabil. 2003;84:770–7. doi: 10.1016/s0003-9993(02)04808-6. [DOI] [PubMed] [Google Scholar]
- 11.Williams A, Newell RL. In: Gray’s Anatomy. 39th ed. Philadelphia, USA: Elsevier; 2005. Pelvic girdle, gluteal region and hip joint; pp. 1447–1448. [Google Scholar]
- 12.Chandler SB. The iliopsoas bursa in man. Anat Rec. 1934;58:235–240. [Google Scholar]
- 13.Iwata T, Nozawa S, Ohashi M, Sakai H, Shimizu K. Giant iliopectineal bursitis presenting as neuropathy and severe edema of the lower limb: case illustration and review of the literature. Clin Rheumatol. 2013;32(5):721–5. doi: 10.1007/s10067-013-2223-5. [DOI] [PubMed] [Google Scholar]
- 14.Monaghan N, Nwawka OK, Wyss JF. Giant iliopsoas bursa presenting as a large pulsatile groin mass. PMR. 2014;6(9):857–9. doi: 10.1016/j.pmrj.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Shao J, Qiu C, Chen Y, Liu B. Synovial cysts of the hip joint: a single-center experience. BMC Surg. 2018, 5;18(1):113. doi: 10.1186/s12893-018-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelini A, Zanotti G, Berizzi A, Staffa G, Piccinini E, Ruggieri P. Synovial cysts of the hip. Acta Biomed. 2018;88(4):483–490. doi: 10.23750/abm.v88i4.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tayfur Ö, Kılıç L, Karadağ Ö, Akdoğan A, Kerimoğlu Ü, Uzun Ö. Tuberculous bursitis of the greater trochanter mimicking ankylosing spondylitis. Eur J Rheumatol. 2015;2(1):31–32. doi: 10.5152/eurjrheumatol.2015.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahnke M, Mangham DC, Davies AM. Calcific haemorrhagic bursitis anterior to the knee mimicking a soft tissue sarcoma: report of two cases. Skeletal Radiol. 2004;33(6):363–6. doi: 10.1007/s00256-003-0743-9. [DOI] [PubMed] [Google Scholar]
- 19.Tuncer K, Izgi E, Cankaya B, Ogul H, Kantarci M. Huge Bursitis and Bursal Synovial Osteochondromatosis Associated With Scapular Osteochondroma Mimicking a Giant Calcific Mass of the Chest Wall. Am J Phys Med Rehabil. 2019;98(1):e1–e3. doi: 10.1097/PHM.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 20.Maheshwari AV, Muro-Cacho CA, Pitcher JD., Jr Pigmented villonodular bursitis/diffuse giant cell tumor of the pes anserine bursa: a report of two cases and review of literature. Knee. 2007;14(5):402–7. doi: 10.1016/j.knee.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Ly JQ, LaGatta LM, Beall DP, Packard J. Massive recurrent synovial sarcoma of the hip: radiologic-histologic correlation in a case of failed magnet therapy. Clin Imaging. 2005;29(4):291–3. doi: 10.1016/S0899-7071(03)00016-0. [DOI] [PubMed] [Google Scholar]
- 22.Akhanoba F, Zill-E-Huma R, Pollock R, Edwards SJE. Synovial sarcoma in an HIV-positive pregnant woman and review of literature. BMJ Case Rep. 2019;12(1) doi: 10.1136/bcr-2018-227646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieg AH, Hefti F, Speth BM, Jundt G, Guillou L, Exner UG, von Hochstetter AR, Cserhati MD, Fuchs B, Mouhsine E, Kaelin A, Klenke FM, Siebenrock KA. Synovial sarcomas usually metastasize after>5 years: a multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol. 2011;22:458–67. doi: 10.1093/annonc/mdq394. [DOI] [PubMed] [Google Scholar]
- 24.Palmerini E, Staals EL, Alberghini M, Zanella L, Ferrari C, Benassi MS, Picci P, Mercuri M, Bacci G, Ferrari S. Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution. Cancer. 2009;115(13):2988–98. doi: 10.1002/cncr.24370. [DOI] [PubMed] [Google Scholar]


