Abstract
This case of secondary sclerosing cholangitis (SSC‐CIP) emphasizes the need to provide follow‐up care for patients that have recovered from COVID‐19 in order to understand the complexity of SARS‐CoV‐2 associated sequela.
Keywords: biliary epithelium, cholangiopathy, COVID‐19, liver injury, SARS‐CoV‐2, secondary sclerosing cholangitis
This case of secondary sclerosing cholangitis (SSC‐CIP) emphasizes the need to provide follow‐up care for patients that have recovered from COVID‐19 in order to understand the complexity of SARS‐CoV‐2 associated sequela.

1. INTRODUCTION
In several patients infected with COVID‐19, liver injury has been observed; however, the pathogenesis is unclear. This article explores a case of secondary sclerosing cholangitis (SSC) following severe ARDS due to COVID‐19. Causes of biliary damage associated with SARS‐CoV‐2 were evaluated with an emphasis on SSC in the critically ill.
Infection with the novel severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) has developed into a pandemic with over 111 million‐infected individuals worldwide. 1 , 2 Despite being predominantly associated with respiratory symptoms, SARS‐CoV‐2 has been linked to abnormalities of liver function tests in up to 69% of hospitalized patients. 3 Whereas most liver enzyme alterations normalize together with clinical convalescence, abnormalities persist and aggravate in some patients. The exact causes of liver and bile duct injury in SARS‐CoV‐2 remain elusive. The differentiation of predominant pathological mechanisms in the individual affected patient is challenging but important for management and prognosis.
In this report, we describe a rare case of secondary sclerosing cholangitis (SSC) complicating the course of severe COVID‐19 and review potential mechanisms of liver and bile duct pathology caused by SARS‐CoV‐2. SSC is characterized by peribiliary inflammation and fibrosis leading to irreversible biliary damage. 4 , 5 Possible mechanisms precipitating SSC in this patient include drug‐related toxicity, direct virus‐ or immune‐mediated as well as hypoxia‐associated cell injury. 6 , 7 , 8 Several cases of SSC have been described in critically ill patients characterized by rapid progression to liver cirrhosis. 9 Recently, another case of SSC following a severe infection with multi‐organ failure due to COVID‐19 has been published stressing the importance of understanding the pathogenesis of COVID‐19‐associated biliary damage. 10
2. CASE REPORT
A 47‐year‐old male patient (BMI 24) was admitted to a district hospital because he suffered from shortness of breath. He had been suffering from fever and dry cough for about ten days prior to admission. His past medical history did not contain any relevant liver diseases. The patient did not take recreational drugs, reported moderate alcohol consumption and did not take medications on a regular basis.
The patient was intubated shortly after admission due to severe hypoxemia which was attributed to SARS‐CoV‐2‐mediated ARDS. He received lopinavir/ritonavir (800mg/200mg per day) for 3 days and subsequently remdesivir (200 mg loading dose on the first day and then 100mg/per day) for 10 days (d) within the compassionate use program. 11 Due to suspected bacterial superinfection, an antibiotic regimen with piperacillin/tazobactam and clarithromycin followed by a therapy with meropenem was initiated for 10d. The patient suffered from a severe form of ARDS and had to be ventilated with intermittent use of prone positioning and PEEP values up to 16mbar. Weaning was successful at day 25 after admission. Catecholamines were only required for 2d compensating circulatory depressive adverse effects of sedative drugs. The patient did not receive extracorporeal life support. Upon admission, surrogate markers for biliary injury, such as ALP and GGT, were only mildly elevated (ALP 1.56xULN and GGT 4.05xULN) (Table 1). Bilirubin levels were within normal limits. Markers for hepatocyte damage, such as AST and ALT, were slightly elevated (AST 2.08xULN, ALT 2.22xULN). In contrast, inflammatory markers such as crp (c‐reactive protein) were significantly increased upon admission (crp 66.80xULN). During the course of his treatment, the patient's inflammatory markers were steadily improving, so that crp levels were almost within normal range at follow‐up days 77 and 91 (Figure 1A). AST and ALT continuously increased after admission until they reached a maximum at day 20 after initial presentation (AST 13.43xULN, ALT 16.76xULN). Subsequently, AST and ALT levels improved and were only about 2‐2.5‐fold following day 91 postadmission (AST 2.23xULN, ALT 2.18xULN). Biliary damage‐associated markers GGT, ALP, and bilirubin continuously increased after the patient's admission (Figure 1). Following day 51, laboratory values of GGT and ALP reached an almost constant level (GGT 28.60xULN at day 51 and 27.80xULN at day 77; ALP 9.91xULN at day 51, and 8.95xULN at day 77), whereas serum bilirubin levels were continuously increasing to a maximum value of 21.63xULN (21.63 mg/dl) at the last follow‐up (day 144, Figure 1). Liver sonography (day 39) demonstrated multiple changes in the diameter of small‐ and medium‐sized intrahepatic bile ducts as well as increased sonographic reflexes of the biliary ducts as it can be seen in sclerosing cholangitis. 12 MRCP (day 38) as well as MRI of the liver were consistent with sonographic findings. Follow‐up MRCP at day 79 indicated an aggravated accentuation of intra‐ and extrahepatic biliary ducts (Figure 2). There were no signs of malignant liver tumors, hepatic metastasis, or mechanical obstruction of the biliary ducts on liver imaging. Liver biopsy was performed at day 50 revealing slight to moderately enlarged portal tracts with a mixed inflammatory infiltrate, degenerative changes of the bile duct epithelium, and ductular reaction, as well as focal biliary metaplasia of the periportal hepatocytes. In addition, perivenular canalicular cholestasis, beginning hepatocyte dropout, and a few bile infarcts could be seen. These changes were considered consistent with intensive care unit‐associated secondary sclerosing cholangitis without casts (Figure 3). Due to the continuous deterioration of his liver function, the patient has successfully undergone liver transplantation.
TABLE 1.
Table of the patient's laboratory values of bilirubin in mg/dL, AST in U/L, ALT in U/L, ALP in U/L, GGT in U/L and crp in mg/dL or relative to the upper normal limit (ULN) of the respective laboratory range

Laboratory results were taken at different time points after first presentation of the patient at the regional hospital (d = 0). Absolute values in red indicate an increase above normal level. Positive (+) or negative (‐) test result for SARS‐CoV‐2 PCR in nasal/throat swab/tracheal fluid at the respective time points is indicated in the last column of the table.
FIGURE 1.
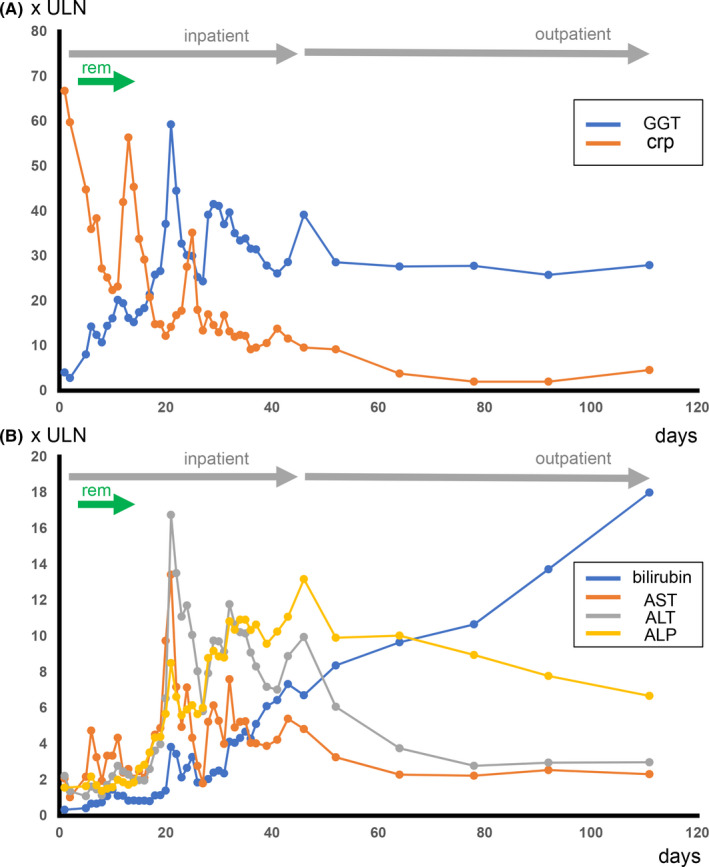
A. Serum levels of GGT (blue) and CRP (orange) at initial admission and at several time points up to 90 days follow‐up after initial presentation. Serum blood levels are depicted as amount relative to upper limit of normal of the respective laboratory. Time in hospital is indicated by a gray arrow. Time of remdesivir (rem) application is indicated by a green arrow. B. Serum levels of bilirubin (blue), alkaline phosphatase (ALP) (yellow), ALT (gray) and AST (orange) at initial admission and at several time points until 90 d follow‐up after initial presentation in hospital. Serum blood levels are depicted as time the upper normal limit of normal (x‐times ULN)
FIGURE 2.

MRCP of the patient at follow‐up at day 79 after initial presentation (anterior‐posterior) showing multiple changes in the diameter of small‐ and medium‐sized intrahepatic bile ducts and an accentuation of the biliary ducts as it can be seen in sclerosing cholangitis
FIGURE 3.

A. Overview of the liver biopsy showing intact architecture of the liver tissue (HE, 2×). B. Detail of an enlarged portal tract with mixed inflammatory infiltrate and degenerative changes of the bile duct epithelium (star) (HE, 10×). C. Periportal bile infarct (arrows) (HE, 10×). D. Bile infarct (arrow) of the zone 3 (*=central vein) (HE, 10×). E. Immunohistochemistry for CK7 highlights the distorted bile ducts (4×). F. Immunohistochemistry for Ki67 shows the high rate of proliferation of the bile duct epithelia (arrow) and the hepatocytes (4×)
3. DISCUSSION
We are reporting about a rare case of secondary sclerosing cholangitis (SSC) after severe infection with SARS‐CoV‐2. Most probable causes for development of SSC in our patient comprise endotheliitis/microthrombosis, direct viral damage to the biliary epithelium, drug‐induced‐, hypoxia‐, and/or inflammation‐mediated biliary injury.
3.1. Viral Damage
The angiotensin‐converting enzyme 2 (ACE2) and the serine protease TMPRSS2 were identified as receptors for viral host cell entry. 13 , 14 ACE2 and TMPRSS2 are expressed on several different cells of the human body, including cholangiocytes suggesting a possible direct interaction of SARS‐CoV‐2 with the biliary epithelium. 15 , 16 , 17 After inoculation of human liver ductal organoids with SARS‐CoV‐2 for 24 hours, the viral nucleocapsid protein could be detected in a small number of biliary epithelial cells indicating successful viral entry. Moreover, analysis of the viral genomic RNA confirmed viral replication in biliary epithelial cells. RNA sequencing analysis of SARS‐CoV‐2 infected biliary organoids demonstrated an increased expression of genes associated with cell death induction as well as a reduced expression of genes associated with tight junction integrity/epithelial cell barrier (eg, Claudin‐1) and biliary transport (eg, SLC10A2). 17 However, it remains unclear if cholangiocytes are infected by SARS‐CoV‐2 in a significant number of COVID‐19 patients. 16 , 17 Access of the virus to the biliary epithelium could be via the blood or through the intestine. While no SARS‐CoV‐2 RNA was detected in the blood of our patient at any time point, SARS‐CoV‐2 RNA was present in stool samples on at least one occasion. Our own analysis of gallbladder bile, gallbladder brush cytology, as well as gallbladder and liver histology from 5 deceased SARS‐CoV‐2 patients and one COVID‐19 patient, who underwent cholecystectomy, showed no evidence for the presence of viral RNA by PCR. This finding indicates that viral replication in biliary and gallbladder epithelium above the detection level is not generally observed in patients with moderate and severe COVID‐19 disease.
3.2. Drug‐induced SSC
Another possible reason for the development of SSC in our patient is drug‐related cytotoxic effects. Drug‐induced liver injury (DILI) can be caused by direct cytotoxicity of the substance or more often by an idiosyncratic reaction. Although it is rare, DILI can cause a sclerosing cholangitis‐like phenotype. 18 , 19 While admitted to ICU, our patient received a number of drugs with potential hepatotoxic effects such as meropenem, piperacillin/tazobactam, lopinavir/ritonavir, and remdesivir. Antibiotics have been described as causes of acute cholestasis, cholestatic hepatitis and, rarely, of vanishing bile duct syndrome. 19 , 20 A drug‐induced SSC due to meropenem or piperacillin has not been described to date. Combination therapy with lopinavir/ritonavir has previously been discussed as a risk factor for DILI and its use was associated with an increased likelihood of abnormalities in liver function tests. 21 , 22 Consequently, therapy with lopinavir/ritonavir could be a contributing factor in the development of SSC in our patient. Additionally, our patient received a treatment with remdesivir. For this relatively new drug, no severe DILI has been reported yet, although liver enzyme elevations are frequently reported also in patients treated with remdesivir. 22 Due to application of remdesivir in a limited number of patients, rare cases of cholestatic liver damage following remdesivir cannot be ruled out. Since DILI is a very common side effect of pharmacological therapy, it is possible that at least one of these drugs had an amplifying effect on the development of biliary damage in this patient. However, the development of SSC as a consequence of drug toxicity alone is highly unlikely in our patient.
3.3. SSC in critically ill patients
A very common cause for SSC is hypoxemia in critically ill patients (SSC‐CIP). 23 , 24 , 25 Although the exact mechanisms are unknown, it is speculated that focal hypoxia leads to biliary necrosis and peribiliary fibrosis development. SSC‐CIP is characterized by no prior history of liver disease, the absence of signs of mechanical biliary obstruction, and a prolonged admission to ICU with the need for mechanical ventilation. 23 , 24 An increased incidence of SSC‐CIP in severe viral pneumonia after infection with influenza has been published before. 26 , 27 Our patient had been admitted to the ICU for a duration of 31 days, was on mechanical ventilation for more than 25 days, and received high pressure mechanical ventilation with a PEEP up to 16 mbar, partially in prone position. These factors may have triggered the development of SSC‐CIP in this patient. The time period of mechanical ventilation reported for patients with SSC‐CIP varied between 26 and 41 days, with a peak PEEP of 12.8mbar. 23 , 24 , 25 , 28 , 29 Another factor closely associated with SSC‐CIP is prolonged hypotension with a mean arterial pressure of less than 65 mmHg and need for vasopressor drugs. Most reports hypothesize that systemic hypotension for a prolonged period represents the most important risk factor in SSC‐CIP. 29 , 30 We were not able to observe prolonged hypotension in our patient, though. Additionally, the development of microemboli and microthrombi in the context of hypercoagulability and endotheliitis may be another reason for local hypoxemia of the biliary tract in critically ill patients. 31 , 32 , 33
3.4. Inflammation‐mediated SSC
Development of SSC has also been reported in patients with auto‐inflammatory diseases and inflammatory syndromes with involvement of the liver such as eosinophilic cholangitis or mast cell cholangiopathy. 34 , 35 , 36 , 37 Moreover, congenital or acquired immunodeficiency syndromes and bacterial or viral infections have been associated with SSC. Underlying mechanisms are unknown, but it is suggestive that inflammatory infiltrates and mediators are contributing to focal damage of biliary ducts and fibrosis development. 38 In animal models, TNFα‐Tnfrsf1 signaling was important for development of sclerosing cholangitis in a chronic infection with cryptosporidium parvum. 39 Thus, it is fathomable that inflammatory infiltrates triggered by SARS‐CoV‐2 infection may contribute to SSC development in our patient.
Although a direct viral effect in the development of SSC in our patient with severe SARS‐CoV‐2 cannot be ruled out, the most probable explanation for his liver disease is multi‐factorial due to drug‐, hypoxia‐ and immune‐mediated injury to the biliary epithelium. Patients recovering from COVID‐19 may face a number of sequelae not only due to long‐term ICU treatment and chronic lung injury but also in response to the dysregulated immune response during and after SARS‐CoV‐2 infection. 40 Especially in children and young adults an increase in auto‐immune and auto‐inflammatory diseases such as multisystemic inflammatory syndrome have been reported. 41 , 42 , 43 It was hypothesized that the interaction of the virus with the host immune response may render predisposed individuals more susceptible toward an environmental insult, for example, drug toxicity, triggering auto‐immune/auto‐inflammatory diseases, which may apply to SSC development in our patient. 44 With the ongoing pandemic of SARS‐CoV‐2, more reports of SSC and liver damage are to be expected.
In conclusion, we were able to report a case of SSC in a patient without prior medical history of biliary duct disease after a severe ARDS due to infection with SARS‐CoV‐2. Although a virus‐induced biliary damage cannot be excluded, SSC‐CIP seems to be the most probable diagnosis in our patient. The case emphasizes the complexity of SARS‐CoV‐2 associated sequela and underscores the need to offer follow‐up to patients recovered from COVID‐19.
CONFLICT OF INTEREST
The authors do not have any conflicts of interest to declare regarding this work. Verena Keitel received speaker's reimbursement from Falk Foundation, Albireo and Abbvie. Torsten Feldt reports role as country principal investigator for Germany for the SIMPLE studies for remdesivir and membership in the national advisory board for remdesivir, both without personal fees. Caroline Klindt reports personal fees and book gifts from Elsevier Publishing. Björn‐Erik Jensen reports personal fees from Gilead, personal fees from ViiV, personal fees from Janssen‐Cilag, personal fees from Theratechnologies, all outside the submitted work. Tina Senff, Timo Brandenburger, Sandra Hauka, Maximilian Seidl, Lars Schimmöller, Jörg Timm, Johannes G. Bode, Tom Luedde, Gerald Antoch, Bahne H. Bahners, Irene Esposito and Alexander Killer have nothing to disclose.
AUTHOR CONTRIBUTIONS
Caroline Klindt, Björn‐Erik Jensen, Timo Brandenburger, Torsten Feldt, Verena Keitel, Johannes G. Bode, Alexander Killer and Bahne H. Bahners cared for the patient, Caroline Klindt and Verena Keitel wrote the first draft of the manuscript. Caroline Klindt, Björn‐Erik Jensen, Timo Brandenburger, Torsten Feldt, Verena Keitel, Johannes G. Bode, Alexander Killer performed literature search. Gerald Antoch and Lars Schimmöller performed, interpreted, and wrote the part on the MRI; Maximilian Seidl, Irene Esposito, Sandra Hauka, Tina Senff and Jörg Timm performed histopathological and viral analysis of liver, interpreted the data on bile, gallbladder samples as well as biological samples. Caroline Klindt, Bahne H. Bahners, Gerald Antoch, Irene Esposito, Maximilian Seidl and Lars Schimmöller designed figures. Caroline Klindt, Björn‐Erik Jensen, Timo Brandenburger, Torsten Feldt, Alexander Killer, Gerald Antoch, Tina Senff, Lars Schimmöller, Sandra Hauka, Jörg Timm, Maximilian Seidl, Bahne H. Bahners, Irene Esposito, Tom Luedde, Johannes G. Bode, Verena Keitel critically read and revised the manuscript.
ETHICAL STATEMENT
The patient mentioned in the case report gave written informed consent to have his case published, including laboratory reports, histopathological results, and radiological imaging. Examinations and treatment of the patient were made in accordance with current clinical guidances and recommendations for COVID‐19. The patient received off‐label/compassionate use treatment with antiviral therapies for COVID‐19 as described. The patient/patient's relative gave informed written consent for these treatments.
ACKNOWLEDGEMENTS
We thank Lisa Knopp for technical support. Published with written consent of the patient.
Klindt C, Jensen B‐E, Brandenburger T, et al. Secondary sclerosing cholangitis as a complication of severe COVID‐19: A case report and review of the literature. Clin Case Rep. 2021;9:e04068. 10.1002/ccr3.4068
Johannes G. Bode and Verena Keitel shared last.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO Coronavirus Disease (COVID‐19) Dashboard [Internet]. 2020. Available from: https://covid19.who.int/. Accessed on 21th Feb 2021.
- 3. Bloom PP, Meyerowitz EA, Reinus Z, et al. Liver Biochemistries in Hospitalized Patients With COVID‐19. Hepatology. 2020. 10.1002/hep.31326 [DOI] [PubMed] [Google Scholar]
- 4. Ruemmele P, Hofstaedter F, Gelbmann CM. Secondary sclerosing cholangitis. Nat Rev Gastroenterol Hepatol. 2009;6(5):287‐295. [DOI] [PubMed] [Google Scholar]
- 5. Lazaridis KN. Sclerosing cholangitis epidemiology and etiology. J Gastrointest Surg. 2008;12(3):417‐419. [DOI] [PubMed] [Google Scholar]
- 6. Gossard AA, Angulo P, Lindor KD. Secondary sclerosing cholangitis: a comparison to primary sclerosing cholangitis. Am J Gastroenterol. 2005;100(6):1330‐1333. [DOI] [PubMed] [Google Scholar]
- 7. Imam MH, Talwalkar JA, Lindor KD. Secondary sclerosing cholangitis: pathogenesis, diagnosis, and management. Clin Liver Dis. 2013;17(2):269‐277. [DOI] [PubMed] [Google Scholar]
- 8. Kirchner GI, Rümmele P. Update on sclerosing cholangitis in critically Ill patients. Viszeralmedizin. 2015;31(3):178‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hentschel F, Bornscheuer T, Lüth S. Secondary cholangitis of the critically ill. Z Gastroenterol. 2019;57(8):977‐982. [DOI] [PubMed] [Google Scholar]
- 10. Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS‐CoV‐2 infection. BMJ Case Rep. 2020;13(11):e237984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grein J, Ohmagari N, Shin D, et al. Compassionate use of Remdesivir for patients with severe covid‐19. N Engl J Med. 2020;382(24):2327‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Voigtländer T, Negm AA, Schneider AS, et al. Secondary sclerosing cholangitis in critically ill patients: model of end‐stage liver disease score and renal function predict outcome. Endoscopy. 2012;44(11):1055‐1058. [DOI] [PubMed] [Google Scholar]
- 13. Tan ND, Qiu Y, Xing X‐B, et al. Associations Between Angiotensin‐Converting Enzyme Inhibitors and Angiotensin II Receptor Blocker Use, Gastrointestinal Symptoms, and Mortality Among Patients With COVID‐19. Gastroenterology. 2020;159 (3):1170‐1172.e1. 10.1053/j.gastro.2020.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271‐80 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agarwal A, Chen A, Ravindran N, To C, Thuluvath PJ. Gastrointestinal and liver manifestations of COVID‐19. J Clin Exp Hepatol. 2020;10(3):263‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang L, Han Y, Nilsson‐Payant BE, et al. A human pluripotent stem cell‐based platform to study SARS‐CoV‐2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(1):125‐36.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao B, Ni C, Gao R, et al. Recapitulation of SARS‐CoV‐2 infection and cholangiocyte damage with human liver ductal organoids. Protein & Cell. 2020;11(10):771‐775. 10.1007/s13238-020-00718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI. Drug‐induced liver injury: Interactions between drug properties and host factors. J Hepatol. 2015;63(2):503‐514. [DOI] [PubMed] [Google Scholar]
- 19. Sundaram V, Björnsson ES. Drug‐induced cholestasis. Hepatol Commun. 2017;1(8):726‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diseases BMNIoDaDaK . LiverTox: Clinical and Research Information on Drug‐Induced Liver Injury: Meropenem 2012‐ [updated 17.01.2017].
- 21. Griffin LM, Watkins PB, Perry CH, St Claire RL, Brouwer KL. Combination lopinavir and ritonavir alter exogenous and endogenous bile acid disposition in sandwich‐cultured rat hepatocytes. Drug Metab Dispos. 2013;41(1):188‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug‐induced liver injury and COVID‐19 infection: the rules remain the same. Drug Saf. 2020;43(7):615‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gelbmann CM, Rümmele P, Wimmer M, et al. Ischemic‐like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007;102(6):1221‐1229. [DOI] [PubMed] [Google Scholar]
- 24. Gudnason HO, Björnsson ES. Secondary sclerosing cholangitis in critically ill patients: current perspectives. Clin Exp Gastroenterol. 2017;10:105‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leonhardt S, Veltzke‐Schlieker W, Adler A, et al. Secondary sclerosing cholangitis in critically ill patients: clinical presentation, cholangiographic features, natural history, and outcome: a series of 16 cases. Medicine (Baltimore). 2015;94(49):e2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pischke S, Fischer L, Lohse AW. Very severe secondary sclerosing cholangitis as a sequela of influenza. Dtsch Arztebl Int. 2017;114(25):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weig T, Schubert MI, Gruener N, et al. Abdominal obesity and prolonged prone positioning increase risk of developing sclerosing cholangitis in critically ill patients with influenza A‐associated ARDS. Eur J Med Res. 2012;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin T, Qu K, Xu X, et al. Sclerosing cholangitis in critically ill patients: an important and easily ignored problem based on a German experience. Front Med. 2014;8(1):118‐126. [DOI] [PubMed] [Google Scholar]
- 29. Leonhardt S, Veltzke‐Schlieker W, Adler A, et al. Trigger mechanisms of secondary sclerosing cholangitis in critically ill patients. Crit Care. 2015;19:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ben‐Ari Z, Levingston D, Weitzman E, et al. Secondary sclerosing cholangitis following major burn. Ann Hepatol. 2015;14(5):695‐701. [PubMed] [Google Scholar]
- 31. Ronco C, Reis T, Husain‐Syed F. Management of acute kidney injury in patients with COVID‐19. Lancet Respir Med. 2020;8(7):738‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Erkelens GW, Vleggaar FP, Lesterhuis W, van Buuren HR, van der Werf SD. Sclerosing pancreato‐cholangitis responsive to steroid therapy. Lancet. 1999;354(9172):43‐44. [DOI] [PubMed] [Google Scholar]
- 35. Miura F, Asano T, Amano H, et al. Resected case of eosinophilic cholangiopathy presenting with secondary sclerosing cholangitis. World J Gastroenterol. 2009;15(11):1394‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goode EC, Simpson BW, Rushbrook SM. A rare cause of cholangiopathy. Gastroenterology. 2013;144(7):e14‐e15. [DOI] [PubMed] [Google Scholar]
- 37. Baron TH, Koehler RE, Rodgers WH, Fallon MB, Ferguson SM. Mast cell cholangiopathy: another cause of sclerosing cholangitis. Gastroenterology. 1995;109(5):1677‐1681. [DOI] [PubMed] [Google Scholar]
- 38. Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006;44(5):1063‐1074. [DOI] [PubMed] [Google Scholar]
- 39. Ponnuraj EM, Hayward AR. Requirement for TNF‐Tnfrsf1 signalling for sclerosing cholangitis in mice chronically infected by Cryptosporidium parvum. Clin Exp Immunol. 2002;128(3):416‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hui DS, Joynt GM, Wong KT, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020;324(3):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID‐19. Nat Rev Rheumatol. 2020;16(8):413‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


