Abstract
Mixed medullary‐follicular‐derived carcinoma is a very rare event. It is extremely important to make the correct diagnosis, due to prognostic and treatment implications. A genetic study of these patients is advisable to exclude the presence of MEN 2.
Keywords: carcinoma, medullary, papillary, thyroid
Mixed medullary‐follicular‐derived carcinoma is a very rare event. It is extremely important to make the correct diagnosis, due to prognostic and treatment implications. A genetic study of these patients is advisable to exclude the presence of MEN 2.

1. INTRODUCTION
Medullary and papillary thyroid carcinoma (MTC and PTC) are two distinct neoplasms which, respectively, originate from follicular and parafollicular C cells. These conditions have always been considered different from each other in terms of their incidence, cell origin, and histopathological features. The simultaneous occurrence of MTC and PTC in the same patient is an uncommon event, and the occurrence of true mixed follicular medullary carcinomas is extremely rare. We present a 60‐year‐old man with a mixed medullary‐papillary carcinoma of the thyroid and papillary and medullary component metastases on the lymph nodes, who was referred to an endocrinology consultation after the diagnosis of thyroid nodules during a cervical ultrasound scan. Mixed medullary‐follicular‐derived carcinomas are a very rare event in clinical practice, and a constant debate exists regarding whether this event should be considered coincidental, rather than just bring the result of a common genetic alteration. It is of extreme importance to make the correct diagnosis as this influences the prognostic and has implications in terms of treatment. Radioactive iodine therapy and thyroid stimulating hormone (TSH) suppression therapy can be recommended for the treatment of a PTC, whereas the treatment of a MTC is essentially based on a radical surgical approach, which only requires levothyroxine replacement therapy. The genetic study of patients with this prognosis is also pertinent, in order to exclude the presence of multiple endocrine neoplasia type 2.
Papillary thyroid carcinoma is the most common (approximately 85%) thyroid carcinoma and is derived from thyroid follicular cells of the endoderm. 1 In contrast, MTC is an uncommon malignant epithelial neoplasm (5%‐10% of all thyroid cancers 2 ), which originates from the parafollicular C cell and is traditionally assumed to evolve from the ultimobranchial body of the neural crest. 3 The cell origin, histopathologic features, and prognosis of these tumors are different, and it is very rare to come across the simultaneous occurrence of these tumors. 4 , 5 , 6 , 7 , 8 , 9 , 10 Indeed, both type of carcinoma can occur in the same patient as a collision tumor, or as a mixed medullary‐follicular thyroid carcinoma (MMFTC) (a real mixed tumor, with the immunoreactivity and morphological features of both types of carcinoma). 11
We present a case report of this rare association and provide a short review of the literature of this rare condition.
2. CASE REPORT
The patient in question was a 60‐year‐old man with a history of atrial fibrillation (which was diagnosed in 2015), associated with amiodarone‐induced thyrotoxicosis (AIT). An evaluation was accordingly made of the thyroid gland. The thyroid was palpable during a neck examination, without the detection of either thyroid nodules or cervical lymph nodes. An ultrasound examination of the neck showed a 22 mm (the largest dimension) solid nodule on the left thyroid lobe (on a multinodular thyroid gland), with irregular margins and microcalcifications. Furthermore, a supraclavicular suspicious lymph node was also noticed.
The patient underwent fine‐needle aspiration (FNA) cytology in another hospital, which suggested the possibility of a MTC with supraclavicular lymph node metastasis. The patient was then referred to our hospital for additional investigations and surgical treatment. He presented no other physical abnormalities and had no history of prior radiation therapy. In addition, his family history was negative for thyroid cancer and other endocrinopathies.
Serum calcitonin was elevated (428.5 pg/mL; NL: <14.3 pg/mL), with normal urinary normetanephrines and metanephrines. Preoperative levels of TSH (1.90 mU/L) and free T4 (0.963 ng/dL) were normal, even without any medication for the thyroid function (after suspension of amiodarone). The patient also had a normal serum level of calcium, phosphorus, and parathyroid hormone.
A total thyroidectomy with bilateral cervical lymph node dissection (Levels II to VII) was performed. The thyroid gland weighed 35.3 g, with a bosselated surface. A poorly defined white/tan nodule was identified in the middle third of the left lobe, measuring 2.3 × 1.5 × 1.2 cm. A second nodule with similar characteristics was observed on the lower pole of the left lobe, measuring 1.9 × 1 cm. The dissected soft tissue from the neck was carefully examined for lymph nodes, with each region being sampled separately. 68 lymph nodes were isolated, the largest being 2.6 cm.
On microscopic examination, the tumor was determined to have the largest dimension of 23 mm and to be composed of two components which were intimately intertwined. The cells had PTC characteristics in one of these components, with strongly and uniformly positive staining for thyroglobulin, while being negative for calcitonin (Figures 1A and 2).
FIGURE 1.
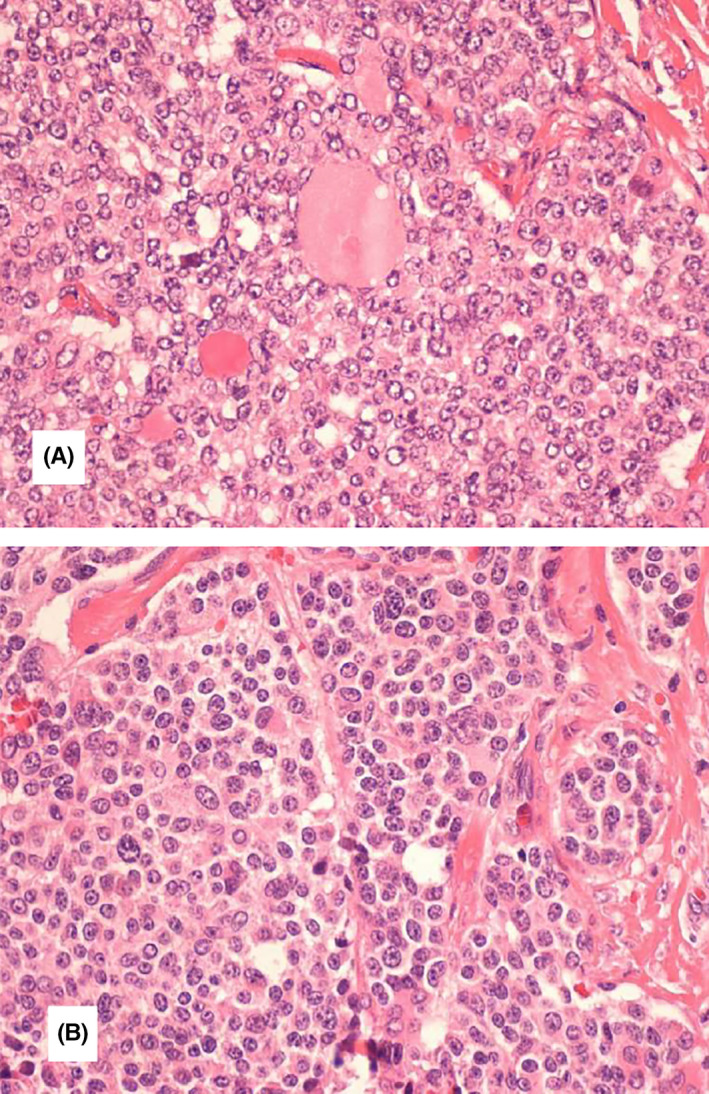
Thyroid section showing mixed medullary and papillary thyroid carcinoma. A, Cells with characteristics of papillary thyroid carcinoma (irregular nuclear membrane, nuclear overlap and clarified chromatin), sometimes with follicle formation. B, Interspersed with salt and pepper chromatin cells. Histological type (WHO): Mixed carcinoma (composed) by a component of medullary carcinoma and another of follicular cells with characteristics of papillary carcinoma in the left lobe; In the right lobe, a 1 mm papillary microcarcinoma is observed. Multifocality/intrathyroid spread: observed, with sizes from 1 to 23 mm. Vascular and lymphatic invasion: Observed. Infiltrative pattern. Invasion of the gland capsule: Observed Extra‐thyroid extension: Observed; Limited, however, without involvement of striated muscle tissue. Margins: Not involved by neoplasia. Lymph nodes: Metastases (from the medullary and papillary components) are observed in 12 of the 38 lymph nodes isolated on the left and 8 of the 10 in the central region; Size of the largest metastasis: 20 mm—T2N1BM0
FIGURE 2.
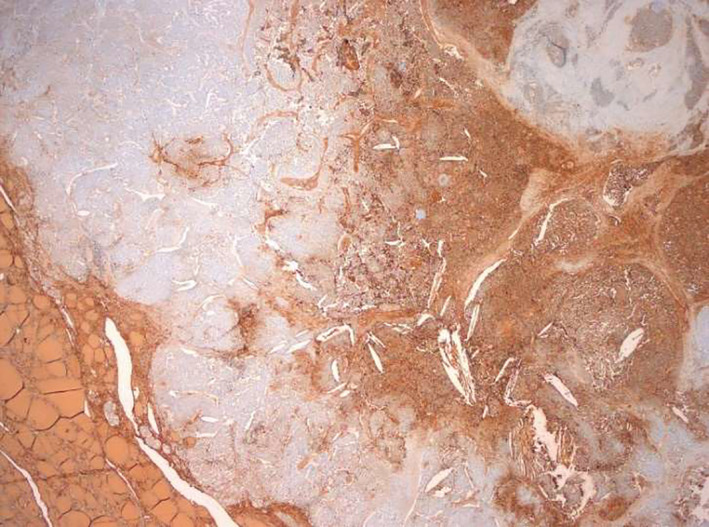
Thyroglobulin expression in the follicular component of the tumor. Thyroglobulin+ in the normal thyroid parenchyma (lower left corner) and in the papillary thyroid carcinoma component (right)
The other component showed characteristics which are consistent with MTC, namely a strong immunoreactivity to calcitonin, synaptophysin, chromogranin A, while being negative for thyroglobulin (Figures 1B and 3). Vascular invasion was present. Limited extrathyroid extension was also observed, but with no involvement of striated muscle tissue. A 1 mm papillary microcarcinoma was concurrently observed in the right lobe. Eight out of ten isolated central lymph nodes had metastases from both components (the largest being 14 mm wide), as well as 12 out of 38 of the left lymph nodes (the largest being 20 mm wide). A final diagnosis identified a mixed medullary‐papillary carcinoma of the thyroid and the patient accordingly underwent ablative treatment with I131. As a MTC can be associated with hereditary syndromes, such as MEN 2, a mutational analysis was performed to investigate the presence of RET germline mutations, which was negative.
FIGURE 3.
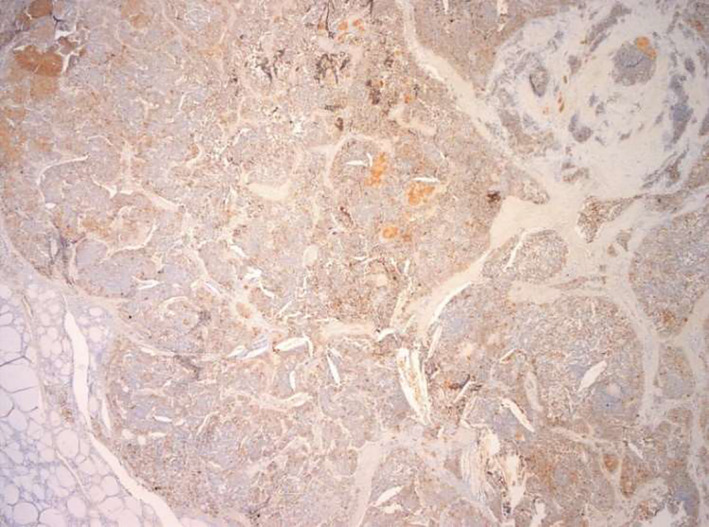
Calcitonin expression in the medullary component of the tumor. Calcitonin+ in the medullary carcinoma component
Serum levels of calcitonin returned back to normal after the surgical procedure and are currently (1 year and 2 months after) stable (calcitonin: 0.800 pg/mL; nondetectable levels of thyroglobulin and of antithyroglobulin antibodies, with normal thyroid function). During a subsequent ultrasound scan of the neck, signs of lateral cervical emptying were observed, with some cervical lymph nodes having no pathological morph‐dimensional criteria. The largest was 8 mm wide and was situated in the 1/3 lower‐right carotid jugular chain, with evidence of an eccentric adipose hilum with hilar Doppler flow, which was referred for ultrasound surveillance. The patient is currently undergoing substitution therapy and is clinically well 1 year after surgery.
3. DISCUSSION
The occurrence of a MTC and a PTC jointly forming a mixed tumor is very rare, although it has been reported in the past. 8
Medullary thyroid carcinoma was first described in 1959 by Hazard et al, in a patient with thyroid carcinoma which showed a solid, nonfollicular structure, with amyloid in the stroma. 12 Later, in 1979, follicular structures between cells with typical medullary features were described. 13 Subsequently detectable thyroglobulin in the foci with follicular appearance in such atypical MTCs were identified, and it was proposed that these tumors could represent a new disease, which was named "mixed medullary‐follicular carcinoma" (MMFC). 14 , 15 MMFC was defined as a distinct entity and was classified by the World Health Organisation (WHO) in 1988 16 as "tumors which show the morphological features of both a medullary carcinoma with immunoreactivity for calcitonin, and a follicular carcinoma with immunoreactivity for thyroglobulin”. This condition is different from the existence of MTCs with follicles 17 or papillae, 18 as well as from MTCs with normal follicles which are entrapped within the tumor. Later, in 2004, 19 high rates of simultaneous PTC (13.8%) in patients with MTC were reported, similar to the results of 2010, when Kim et al identified the presence of PTC in 19% of patients with MTC. 20 Nevertheless, the true mixed nature of these tumors remains a rare entity. 8
The etiology of this mixed tumor is not well understood, although several theories have been proposed. 21 One possibility is that the tumor could originate from a common progenitor cell, 22 maybe in the ultimobranchial cell rest, with subsequent divergent differentiation in parafollicular and follicular cell lines. Another explanation for the existence of this tumor is the presence of a common tumorigenic stimulus (such as exposure to radiation) which promotes the oncogenic transformation of both cells types. 23 Some authors suggest the potential role of RET germline mutations in the development of both tumor types, although the genetic analysis of RET oncogene in this type of tumor leads to conflicting results. 24 , 25 Another possible and more plausible theory is that of the “hostage hypothesis,” where the malignant transformation of C cells with entrapment of normal follicles leads to a microenvironment which subsequently stimulates the neoplastic development of the trapped follicular cells. 26
With regards our case, it is important to highlight the atypical presentation of this rare carcinoma, that is, the incidence of hyperthyroidism, which is not considered to be one of the primary symptoms of thyroid cancer. 27 The association between thyrotoxicosis and thyroid cancer is rare and little‐recognized, which can lead to a delay in the diagnosis of this tumor and, consequently, to a worse prognosis. Even so, an increasing number of studies have been carried out which demonstrate that this association is possible and that a careful assessment of the thyroid gland is essential whenever any thyroid dysfunction is detected. 27 The most commonly reported presentation of MMFTC is a slowly increasing swelling of the neck. 28 , 29 , 30
In most cases, lymph node metastases were reported at diagnosis, as a single tumor cell population, or, alternatively, as a mixture of both components within the same lymph node. 30 , 31 , 32 Foci consistent with mixed medullary and papillary carcinoma were observed in our case, and immune‐histo‐chemical findings which support both types of carcinoma were also detected in these foci.
Mixed medullary‐follicular thyroid carcinoma is more frequent in middle‐aged patients, who, in almost all cases have a high serum calcitonin level, 9 , 30 as in the case of our patient. Tumor size varies and is generally unifocal. 26 Multifocal tumors are more frequent in patients with MEN 2A. 5
In the cases of our patient, although the FNA cytology of the thyroid nodule suggested the possibility of a single MTC, subsequent microscopic evaluation identified the presence of follicular structures with nuclear papillary features. This finding highlights the importance of the need to carry out a complete histological tumor evaluation, as FNA is a sensitive initial examination, yet it may not be representative of the total tumor area.
Treatment of MMFTC is mainly driven by the medullary component. However, the treatment of this type of tumor remains controversial, 26 not only on account of the few reported cases, but also due to the use of different treatment strategies in the reported cases. Surgery (including of the tumor and areas of lymphatic drainage—Levels II to VII) is accepted as being the first‐choice option, 33 whereas the role of adjuvant therapies (radioiodine and chemotherapy) remains controversial, possibly due to the tumors being mainly of medullary origin. However, as these tumors are constituted of thyroglobulin immune‐reactive cells, the efficacy of these adjuvant therapies was put into questions, at least for the follicular component. 30 , 34 Our patient had abnormally high levels of calcitonin at the time of diagnosis and was treated initially with surgery and subsequently underwent ablative treatment with I131, due to the papillary component.
In the follow‐up of this type of tumor, it could be helpful to measure blood calcitonin and thyroglobulin levels to guide the therapeutic approach. Our patient is currently stable and is under surveillance. A recent study investigated 183 patients with simultaneous PTC‐MTC and it was found that 45% of these patients were disease‐free after >10 years from diagnosis, with their prognosis being mainly determined by the medullary component of the tumor. 4 Measurement of calcitonin in the presurgical work‐up of thyroid nodules with positive cytology for PTC has also been advocated to assist the early diagnosis of concomitant MTC. Although the guidelines suggest that there is no clear evidence in favor of measuring the serum calcitonin during the preoperatory period of patients with thyroid nodules, 35 should the FNA of a patient with MMFTC have only detected the papillary component of the tumor, then the measurement of this marker could lead to a correct diagnosis, which would otherwise have remained undetected up until the patient was submitted to surgical resection.
A precise and early diagnosis of this uncommon variety of thyroid carcinoma is essential to ensure both an adequate treatment of the patient and also the carrying out of genetic screening (with the objective to exclude MEN2 syndromes and familial MTC—FMTC). The role of FNA cytology in the diagnosis of this type of tumors is limited and can lead to misdiagnosis. Accordingly, it is fundamental to correlate the cytological diagnosis with serum calcitonin levels and also to confirm the diagnosis during the immune‐histochemical investigation of the surgical specimen.
With regard to treatment with amiodarone, AIT can be found in 3% to 12% of patients who take this drug 27 ; however, the presence of thyroid cancer in thyroidectomy specimens removed for AIT is rare, 28 , 29 In recent years, several case reports have raised the possibility of an association between amiodarone and cancer. 36 , 37 Thyroid dysfunction resulting from the administration of amiodarone can be induced in patients with previous thyroid carcinoma, 38 or it can be manifested after the introduction of this drug. 39 Even though our patient had been evaluated by another hospital for AIT and we were given access to a few details regarding his etiological assessment and approach, as far as we know, this is the first time that a case of mixed thyroid carcinoma has been reported in patients being treated with amiodarone.
The outcomes of several mechanisms may have been responsible for what happened to our patient, with examples including, but not being limited to the following: amiodarone inducing the neoplastic transformation of pre‐existing nodules; the onset of AIT due to the presence of an underlying previously unknown thyroid cancer; or as a consequence of the occurrence of an additional etiological mechanism that affects this particular type of mixed tumor. Although we cannot be certain, it is very probable that this case highlights the importance of the need to carry out a detailed assessment of the thyroid gland in patients who are undergoing treatment with amiodarone which is not only analytical, but also involves an ultrasound scan.
4. CONCLUSION
This paper describes an atypical presentation of an extremely rare type of tumor in clinical practice and it highlights the importance of carrying out an adequate assessment of the thyroid gland when any type of thyroid dysfunction is detected. This case report also highlights the importance of measuring preoperative calcitonin levels and of carrying out a complete histological examination (both morphological and immunohistochemically), together with the need to meticulously dissect a sample of the lymph node in search of metastatic disease.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
VG: wrote this case report. CC, JO, APS, MJ, PF, and DC: reviewed the draft. MF: helped in writing this case report. IT was involved in the patient care and also reviewed the draft.
ETHICAL APPROVAL
All procedures performed in the study were in accordance with the ethical standards of the national guidelines.
PATIENT CONSENT
Written informed consent was obtained from the patient.
ACKNOWLEDGMENTS
We would like to acknowledge all the endocrinologists, surgeons, geneticists, radiologists, and pathologists of IPO‐Porto.
Guerreiro V, Costa C, Oliveira J, et al. Mixed medullary—papillary thyroid carcinoma with mixed lymph node metastases: A case report. Clin Case Rep. 2021;9:04165. 10.1002/ccr3.4165
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Fahiminiya S, de Kock L, Foulkes WD. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375(23):2306‐2307. [DOI] [PubMed] [Google Scholar]
- 2. Fagin JA, Wells SA. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375(23):2307. [DOI] [PubMed] [Google Scholar]
- 3. Johansson E, Andersson L, Örnros J, et al. Revising the embryonic origin of thyroid C cells in mice and humans. Development. 2015;142(20):3519‐3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Appetecchia M, Lauretta R, Barnabei A, et al. Epidemiology of simultaneous medullary and papillary thyroid carcinomas (MTC/PTC): an Italian Multicenter Study. Cancers. 2019;11(10):1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rossi S, Fugazzola L, De Pasquale L, et al. Medullary and papillary carcinoma of the thyroid gland occurring as a collision tumour: report of three cases with molecular analysis and review of the literature. Endocr Relat Cancer. 2005;12(2):281‐289. [DOI] [PubMed] [Google Scholar]
- 6. Younes N, Shomaf M, Al HL. Simultaneous medullary and papillary thyroid carcinoma with lymph node metastasis in the same patient: case report and review of the literature. Asian J Surg. 2005;28(3):223‐226. [DOI] [PubMed] [Google Scholar]
- 7. Erhamamci S, Reyhan M, Kocer NE, Nursal GN, Torun N, Yapar AF. Simultaneous occurrence of medullary and differentiated thyroid carcinomas. Report of 4 cases and brief review of the literature. Hell J Nucl Med. 2014;17(2):148‐152. [DOI] [PubMed] [Google Scholar]
- 8. Ciampi R, Romei C, Pieruzzi L, et al. Classical point mutations of RET, BRAF and RAS oncogenes are not shared in papillary and medullary thyroid cancer occurring simultaneously in the same gland. J Endocrinol Invest. 2017;40(1):55‐62. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Yuan L, Yang D, Jin Y. Serum calcitonin negative mixed medullary‐follicular carcinoma initially diagnosed as medullary thyroid carcinoma by fine‐needle aspiration cytology: a case report and review of the literatures. Diagn Cytopathol. 2018;46(8):690‐693. [DOI] [PubMed] [Google Scholar]
- 10. Samarasinghe S, Yuksel S, Mehrotra S. Intermixed medullary and papillary thyroid cancer in a patient with renal cell carcinoma. Endocrinol Diabetes Metab Case Rep. 2020;2020: 10.1530/EDM-20-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: A commentary on the second edition. Cancer. 1989; 63(5):908‐911. [DOI] [PubMed] [Google Scholar]
- 12. Hazard JB, Hawk WA, Crile G. Medullary (solid) carcinoma of the thyroid; a clinicopathologic entity. J Clin Endocrinol Metab. 1959;19(1):152‐161. [DOI] [PubMed] [Google Scholar]
- 13. Bussolati G, Monga G. Medullary carcinoma of the thyroid with atypical patterns. Cancer. 1979;44(5):1769‐1777. [DOI] [PubMed] [Google Scholar]
- 14. Pfaltz M, Hedinger CE, Mühlethaler JP. Mixed medullary and follicular carcinoma of the thyroid. Virchows Arch A Pathol Anat Histopathol. 1983;400(1):53‐59. [DOI] [PubMed] [Google Scholar]
- 15. Hales M, Rosenau W, Okerlund MD, Galante M. Carcinoma of the thyroid with a mixed medullary and follicular pattern: morphologic, immunohistochemical, and clinical laboratory studies. Cancer. 1982;50(7):1352‐1359. [DOI] [PubMed] [Google Scholar]
- 16. Sobin LH. Histological typing of thyroid tumours. Histopathology. 1990;16(5):513. [DOI] [PubMed] [Google Scholar]
- 17. Harach HR, Williams ED. Glandular (tubular and follicular) variants of medullary carcinoma of the thyroid. Histopathology. 1983;7(1):83‐97. [DOI] [PubMed] [Google Scholar]
- 18. Kakudo K, Miyauchi A, Takai S, Katayama S, Kuma K, Kitamura H. C cell carcinoma of the thyroid—papillary type. Acta Pathol Jpn. 1979;29(4):653‐659. [DOI] [PubMed] [Google Scholar]
- 19. Biscolla RP, Ugolini C, Sculli M, et al. Medullary and papillary tumors are frequently associated in the same thyroid gland without evidence of reciprocal influence in their biologic behavior. Thyroid. 2004;14(11):946‐952. [DOI] [PubMed] [Google Scholar]
- 20. Kim WG, Gong G, Kim EY, et al. Concurrent occurrence of medullary thyroid carcinoma and papillary thyroid carcinoma in the same thyroid should be considered as coincidental. Clin Endocrinol. 2010;72(2):256‐263. [DOI] [PubMed] [Google Scholar]
- 21. Valeria P. Un raro caso di tumore misto midollare‐pappilare della tiroide. In: Porcu Giuseppe CP, Carlo C, Stefania M, Novella G, Angelo N eds. Cagliari, Italy: Chirurgia; 2000. [Google Scholar]
- 22. Ljungberg O, Ericsson UB, Bondeson L, Thorell J. A compound follicular‐parafollicular cell carcinoma of the thyroid: a new tumor entity? Cancer. 1983;52(6):1053‐1061. [DOI] [PubMed] [Google Scholar]
- 23. Triggs SM, Williams ED. Experimental carcinogenesis in the thyroid follicular and C cells. A comparison of the effect of variation in dietary calcium and of radiation. Acta Endocrinol. 1977;85(1):84‐92. [PubMed] [Google Scholar]
- 24. Papi G, Corrado S, Pomponi MG, Carapezzi C, Cesinaro A, LiVolsi VA. Concurrent lymph node metastases of medullary and papillary thyroid carcinoma in a case with RET oncogene germline mutation. Endocr Pathol. 2003;14(3):269‐276. [DOI] [PubMed] [Google Scholar]
- 25. Adnan Z, Arad E, Dana J, Shendler Y, Baron E. Simultaneous occurrence of medullary and papillary thyroid microcarcinomas: a case series and review of the literature. J Med Case Rep. 2013;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Volante M, Papotti M, Roth J, et al. Mixed medullary‐follicular thyroid carcinoma. Molecular evidence for a dual origin of tumor components. Am J Pathol. 1999;155(5):1499‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fu H, Cheng L, Jin Y, Chen L. Thyrotoxicosis with concomitant thyroid cancer. Endocr Relat Cancer. 2019;26(7):R395‐R413. [DOI] [PubMed] [Google Scholar]
- 28. Hanna AN, Michael CW, Jing X. Mixed medullary‐follicular carcinoma of the thyroid: diagnostic dilemmas in fine‐needle aspiration cytology. Diagn Cytopathol. 2011;39(11):862‐865. [DOI] [PubMed] [Google Scholar]
- 29. Dusková J, Janotová D, Svobodová E, Novák Z, Tretiník P. Fine needle aspiration biopsy of mixed medullary‐follicular thyroid carcinoma. A report of two cases. Acta Cytol. 2003;47(1):71‐77. [DOI] [PubMed] [Google Scholar]
- 30. Nangue C, Bron L, Portmann L, et al. Mixed medullary‐papillary carcinoma of the thyroid: report of a case and review of the literature. Head Neck. 2009;31(7):968‐974. [DOI] [PubMed] [Google Scholar]
- 31. Apel RL, Alpert LC, Rizzo A, LiVolsi VA, Asa SL. A metastasizing composite carcinoma of the thyroid with distinct medullary and papillary components. Arch Pathol Lab Med. 1994;118(11):1143‐1147. [PubMed] [Google Scholar]
- 32. Mizukami Y, Nonomura A, Michigishi T, Noguchi M, Ishizaki T. Mixed medullary‐follicular carcinoma of the thyroid gland: a clinicopathologic variant of medullary thyroid carcinoma. Mod Pathol. 1996;9(6):631‐635. [PubMed] [Google Scholar]
- 33. Sadat Alavi M, Azarpira N. Medullary and papillary carcinoma of the thyroid gland occurring as a collision tumor with lymph node metastasis: A case report. J Med Case Rep. 2011;5:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papotti M, Volante M, Komminoth P, Sobrinho‐Simões M, Bussolati G. Thyroid carcinomas with mixed follicular and C‐cell differentiation patterns. Semin Diagn Pathol. 2000;17(2):109‐119. [PubMed] [Google Scholar]
- 35. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inaba H, Suzuki S, Takeda T, Kobayashi S, Akamizu T, Komatsu M. Amiodarone‐induced thyrotoxicosis with thyroid papillary cancer in multinodular goiter: case report. Med Princ Pract. 2012;21(2):190‐192. [DOI] [PubMed] [Google Scholar]
- 37. Petrulea MS, Lencu C, Piciu D, Lisencu CI, Georgescu CE. Challenges of thyroid cancer management in amiodarone‐treated patients: a case report. Clujul Med. 2015;88(4):550‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Charles C, Dhatariya KK. Amiodarone‐induced thyrotoxicosis after total thyroidectomy for metastatic follicular thyroid cancer. AACE Clin Case Rep. 2020;6(2):e70‐e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saad A, Falciglia M, Steward DL, Nikiforov YE. Amiodarone‐induced thyrotoxicosis and thyroid cancer: clinical, immunohistochemical, and molecular genetic studies of a case and review of the literature. Arch Pathol Lab Med. 2004;128(7):807‐810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


