Abstract
Early detection and surveillance of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) virus are key pre-requisites for the effective control of coronavirus disease (COVID-19). So far, sewage testing has been increasingly employed as an alternative surveillance tool for this disease. However, sampling site characteristics impact the testing results and should be addressed in the early use stage of this emerging tool. In this study, we implemented the sewage testing for SARS-CoV-2 virus across sampling sites with different sewage system characteristics. We first validated a testing method using “positive” samples from a hospital treating COVID-19 patients. This method was used to test 107 sewage samples collected during the third wave of the COVID-19 outbreak in Hong Kong (from June 8 to September 29, 2020), covering sampling sites associated with a COVID-19 hospital, public housing estates, and conventional sewage treatment facilities. The highest viral titer of 1975 copy/mL in sewage was observed in a sample collected from the isolation ward of the COVID-19 hospital. Sewage sampling at individual buildings detected the virus 2 days before the first cases were identified. Sequencing of the detected viral fragment confirmed an identical nucleotide sequence to that of the SARS-CoV-2 isolated from human samples. The virus was also detected in sewage treatment facilities, which serve populations of approximately 40,000 to more than one million people.
Keywords: Sewage surveillance, SARS-CoV-2, COVID-19, Sewage system characteristics, Early warnings, Hong Kong
Graphical abstract
1. Introduction
Since the shedding of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2; the causative virus of coronavirus disease [COVID-19]) in stool has been reported (Xu et al., 2020), a large number of studies have detected the genetic signal of this virus in sewage across various regions and countries (Usman et al., 2020). Viral shedding in feces can occur in symptomatic, asymptomatic, or pre-symptomatic carriers (Xu et al., 2020), thus opening the possibility of using sewage surveillance of SARS-CoV-2 as an early warning system for monitoring the COVID-19 in the community. The infectiousness of COVID-19 prior to the onset of symptoms was estimated to account for approximately 44% of disease transmission (He et al., 2020). Therefore, detecting the early warning signals for the identification of mild and asymptomatic cases in communities is a key pre-requisite for developing effective strategies that may help to control such pre-symptomatic transmission of the disease.
While sewage testing for SARS-CoV-2 is a promising tool to characterize and supplement the COVID-19 epidemiology, this emerging method can be fraught with challenges and possible misinterpretations. Although multiple research teams have generated valuable data for the development of testing protocols for SARS-CoV-2 in sewage (Ahmed et al., 2020a; Jafferali et al., 2021; Pérez-Cataluña et al., 2021; Philo et al., 2021; Westhaus et al., 2021), no standard sewage testing methods are currently available. One major reason for this is that the complexity of sewage, which varies with sewage systems and could greatly affect the ability to detect SARS-CoV-2 in sewage. To date, most studies have investigated the use of sewage surveillance in conventional sewage treatment facilities to track the trends in COVID-19 infection, while the effects of sampling site characteristics on the detection of the virus require further elucidation.
A few studies (Nemudryi et al., 2020; Wu et al., 2020) have shown high-quality correlations between the strengths of the SARS-CoV-2 signals in sewage and the COVID-19 incidence rates in corresponding sewer sheds, suggesting the potential use of sewage testing for assessing the development trend of COVID-19. For example, a study (Wu et al., 2020) tested 116 sewage samples from the local sewage treatment facilities during the entire outbreak period from January to May 2020 and explored the correlations between the strengths of the SARS-CoV-2 signals in those samples to the infection trend in the community. This study demonstrated the high-quality correlations. They concluded that the dynamics of SARS-CoV-2 in sewage treatment works can be used to predict the trend of COVID-19 transmission, nearly 4–10 days ahead of the actual clinical reporting of cases. In addition to the early warning and infection trend analysis, the integration of current data sources to set actionable assessment criteria can be effective in the use of this emerging method. However, there are multiple uncertainties in estimating the community prevalence of this disease that pose a challenge in its effective evaluation, including the percentage of patients shedding the virus in their feces, variation of the viral load in stool (from 10 2.7 to 107.6 copy/mL in confirmed COVID-19 cases in Hong Kong (Cheung et al., 2020; Pan et al., 2020)), degradation and distribution of viruses in the sewage system, and the impact of the matrix components in sewage. These uncertainties affect the sensitivity and robustness of the method. The challenges of informative sewage surveillance, such as method validation using proof-of-concept sites (such as a COVID-19 hospital), should be addressed to make it a robust supplement method for clinical surveillance.
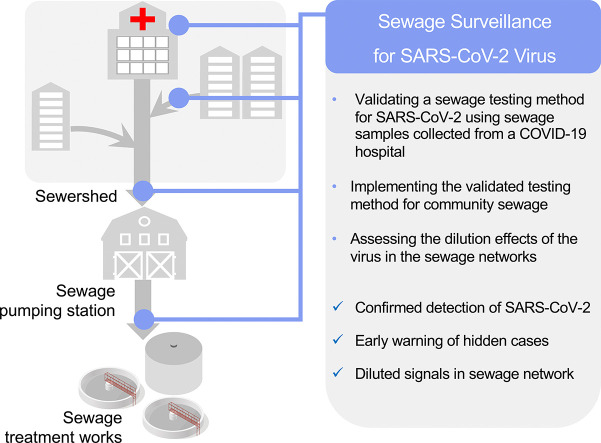
This study is the first case report of wastewater-epidemiology of COVID-19 in Hong Kong. Our objectives included: (1) Validating a sewage testing method for SARS-CoV-2 using sewage samples collected from a hospital treating COVID-19 patients; (2) Implementing the validated testing method for the sewage samples collected from community sewage of individual apartment blocks in Hong Kong to be used as an early warning tool; and (3) Assessing the dilution effects of the virus in the sewage networks. In this study, we included multiple process controls for different steps of the testing method to minimize contamination and ensure the reliability of the results (Fig. 1 ). A total number of 107 sewage samples collected during a COVID-19 outbreak in Hong Kong were tested, and we showed that the genetic signal of SARS-CoV-2 was detected in sewage of two apartment buildings 2 days prior to the report of the first clinical cases in the two buildings. Additionally, dilution of the viral signals was observed in samples associated with the sewage networks of the COVID-19 hospital. The sensitivity and limitations of this method with respect to large sewage treatment facilities were also discussed.
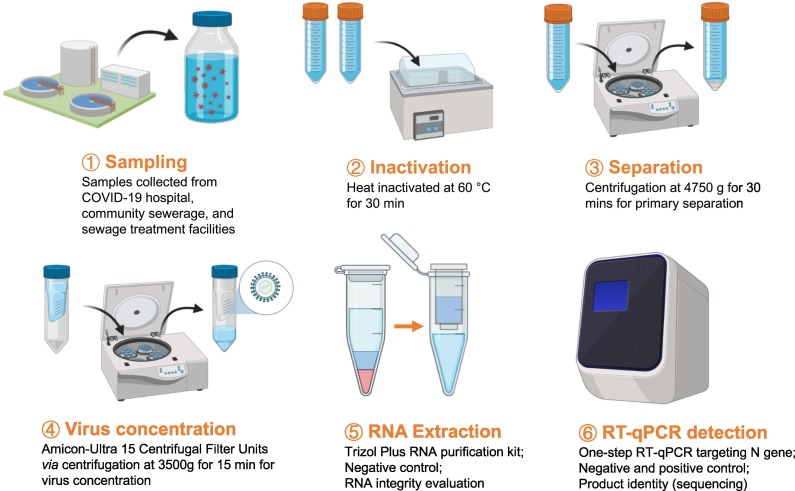
Fig. 1.
Analytical workflow for quantification of SARS-CoV-2 in sewage sample.
2. Materials and methods
2.1. Sewage sample collection
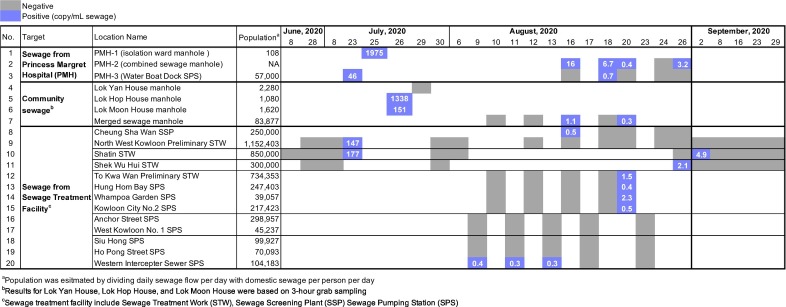
A total number of 107 sewage samples were collected from 20 locations in Hong Kong from June 8, 2020 to September 29, 2020 by the Drainage Services Department (DSD) of Hong Kong. The epidemiological data for the sampling period was summarized in Fig. S1. The surveyed locations included manholes of the isolation ward building of a hospital treating COVID-19 patients and the associated downstream sites, manholes of individual buildings from public housing estates in the Wong Tai Sin District Tsz Wan Shan area (a hotspot area during the third wave of COVID-19 in Hong Kong), as well as inlets of sewage treatment facilities, including Sewage Treatment Work (STW), Sewage Screening Plant (SSP), and Sewage Pumping Station (SPS). Except for the three samples collected at manholes of individual buildings were 3 h composite (Table 1 ), the other samples were all 24 h composite, with 15 min intervals. All sewage samples were preserved at 4 °C until processing. Samples were inactivated at 60 °C for 30 min prior to subsequent pre-treatment, viral RNA extraction and detection via reverse transcription - quantitative polymerase chain reaction (RT-qPCR).
Table 1.
Sewage testing data for 107 samples taken during a COVID-19 outbreak in Hong Kong.
2.2. Sewage sample pre-treatment
For the sample pre-treatment, the inactivated sewage sample was first separated into the supernatant and pellet by centrifugation at 4750g for 30 min, and then the supernatant was used for the concentration. For the concentration of the SARS-CoV-2 genetic material from sewage samples, we adopted a method based on the ultrafiltration principle (Medema et al., 2020) among other existing methods based on other principles, such as membrane filtration (Ahmed et al., 2020b), polyethylene glycol (PEG) precipitation (Wu et al., 2020; Zhang et al., 2020), aluminum chloride (AlCl3) precipitation (Randazzo et al., 2020a; Randazzo et al., 2020b), and ultracentrifugation (Ahmed et al., 2020b; Jafferali et al., 2021). The supernatant was collected and concentrated by centrifugation at 3500 g for 15 min using the Amicon-Ultra 15 Centrifugal Filter Units (a molecular weight cut-off of 10 kDa; Merck Millipore). The retentate from the filter was collected for RNA extraction.
2.3. Viral RNA extraction and RT-qPCR
RNA extraction for the 107 sewage samples was conducted using the Trizol plus RNA Purification Kit (Thermofisher) following the manufactures' protocols. After RNA extraction, a one-step N-gene-specific RT-qPCR (Chu et al., 2020; Pan et al., 2020; Vogels et al., 2020), was then performed for the detection of viral signals (Table S1). The one-step RT-qPCR was carried out for 45 cycles with a 20 μL reaction mixture using the TaqMan Fast Virus one-step Master Mix (Thermo Fisher, USA). In a typical 20 μL reaction, it contains 5 μL of 4 × TaqMan Fast Virus 1-Step Master Mix, 500 nM of forward primer, 500 nM of reverse primer, 250 nM of probe, and 4 μL of RNA template were used. The RT-qPCR condition was as follows: 50 °C for 5 min, 95 °C for 20 s, 45 cycles at 95 °C for 5 s and 58 °C for 30 s. If the cycle threshold (Ct) value of a sewage sample was ≤45, the sample was considered to have a SARS-CoV-2 signal. To quantify the copy number of the viral RNA, a standard curve was generated by using the serially diluted plasmid DNA containing the target gene (10 to 107 copy/reaction). The quantification limit of the assay was 10 copy/reaction. The primer-probe set used in the N-gene-specific RT-qPCR for the detection of the SARS-CoV-2 RNA enabled the detection of SARS-CoV-2 viral RNA. The standard curve has a dynamic range of 10–107 copy/reaction, and good correlation coefficient (R2) (0.99–1). For quality assurance and quality control (QA/QC), the reagents in the RNA extraction kit were used as the negative control (as the “reagent blank”) for RNA extraction and quantification steps. The no-template control (NTC) was included as a negative control for RT-qPCR. For one of the samples, the RT-qPCR product was sent out for Sanger sequencing at the Beijing Genomics Institute (BGI) to get the sequence of the product.
3. Results and discussion
3.1. Detection of the virus in the sewage network of a hospital treating COVID-19 patients
As shown in Table 1, among 107 sewage samples tested during the third wave of the COVID-19 outbreak in Hong Kong (Fig. S1), the signal of SARS-CoV-2 was detected in 23 out of 107 (21%) sewage samples. These included seven (out of 12, 58%) samples collected from sites associated with the sewage network of a hospital treating COVID-19 patients, four (out of 8, 50%) samples collected from the manholes of individual buildings, and 12 (out of 87, 14%) samples collected from the sewage treatment facilities, including STW, SSP, and SPS, that serve populations of approximately 40,000 to more than 1 million people. Overall, the used sewage testing method for SARS-CoV-2 is technically feasible for the samples of a sampling site, scaling from the manholes of individual buildings to the inlets of large sewage treatment facilities. The comparable detection rates of the virus in the sites associated with the COVID-19 hospital and sites of community sewage system suggested the effectiveness of the method to be used in the present study for the quantification of SARS-CoV-2 in the community sewage. Besides, since the sites associated with sewage network of the COVID-19 hospital served as the “positive” sites compared to the other sites being tested, the incidence of detection of positive signals (i.e., the detection rate) in these sites associated with the COVID-19 hospital may indicate the effects of sampling randomness in providing information about the presence or absence of the virus in the sewage system. Another study (Gonçalves et al., 2020) reported a detection rate at a similar level (10 out of 15, 66.7%) for the hospital sewage collected at downstream pumping stations.
A strikingly high level of SARS-CoV-2 concentration (1975 copy/mL sewage) was observed in the sewage directly collected from the manhole (PMH-1) of the isolation ward of the COVID-19 hospital (Fig. S2). Two other studies also reported the detection of SARS-CoV-2 in the untreated sewage and disinfected sewage (Wang et al., 2020; Zhang et al., 2020) of COVID-19 hospitals. The reported concentration range was from 4.7 copy/mL to 17 copy/mL in the treated sewage disinfected with sodium hypochlorite. Along the sewage network of the COVID-19 hospital, the samples taken from another manhole (PMH-2) at the downstream of the sewage system had a range of 0.4 copy/mL to 16 copy/mL, while the further downstream samples at the downstream pumping station (Water Boat Dock SPS, PMH-3) had a concentration range of 0.7 copy/mL to 46 copy/mL. These results showed the dilution of the viral signal along the sewage pipeline, from the sampling point to its downstream sites. Complete absence of signals was also observed for sites PMH-2 and PMH-3, which may due to the effect of sampling randomness or the virus dilution at downstream sewage treatment facilities.
3.2. Early detection of the virus at individual buildings
Since early July of 2020, Hong Kong has been experiencing its third wave of COVID-19 outbreak. The Wong Tai Sin district in particular, had emerged as a hotspot. Therefore, sewage samples were collected and analyzed from individual buildings in this hotspot area. SARS-CoV-2 was detected in sewage samples, 2 days before COVID-19 was first diagnosed in two individual buildings of the housing estates located in the Wong Tai Sin district (Fig. 2a). The two samples were collected from the manholes of Lok Hop House (1080 residents) and Lok Moon House (1620 residents) on July 26 and had a concentration levels of 1338 copy/mL and 151 copy/mL, respectively (Fig. S3). The results were obtained on July 26, 2020. Community testing by clinical diagnosis for these two buildings started on July 27, 2020, and the participation rates for community testing were 80% and 85% for Lok Hop House and Lok Moon House, respectively. On July 29, 2020, the first confirmed cases for each building (case #2903 for Lok Hop House, and case #2920 for Lok Moon House) were reported. Later on, there was another infected case (case #3916) for the Lok Hop House who was confirmed on August 7, 2020. The RT-qPCR product from the sewage sample of Lok Hop House was sequenced to confirm its identity. For the targeted region of 110 bp, it was 100% identical to the sequence of the SARS-CoV-2 virus isolated from a human sample (Fig. 2b). Implementation of suitable quality indicators for the process is considered to be essential for the performance of the method and also as a way to improve the reproducibility and reliability of the testing results (O'Reilly et al., 2020). The sequence-based identification of viruses is a useful quality indicator for the testing method. Overall, these results indicate that the sewage surveillance can give early signals for the individual buildings even if there are only one or two infected cases in a building with over 1000 residents. The use of sewage surveillance for SARS-CoV-2 to obtain early warning signals for potential community outbreaks has been mainly reported in retrospective studies (La Rosa et al., 2020; Nemudryi et al., 2020; Randazzo et al., 2020a), while only two studies (Medema et al., 2020; Randazzo et al., 2020b) have indicated the use of large sewage treatment works covering a population of approximately 28,000 to 101,000 people. In our study, sewage testing was used to provide information about the presence of hidden infected individuals shedding SARS-CoV-2 to a single-building sewage system, which served approximately 1000 residents, before the first cases of COVID-19 was identified.
Fig. 2.
(a) Correlation between epidemiological details and sewage positive sample taken from the sewage system of public housing estates and (b) Confirmation of the identify of RT-qPCR product from a sewage sample of Lok Hop House by Sanger sequencing. NCBI Accession Number for the reference SARS-CoV-2 is MT929054.1.
It is important to note that negative samples do not simply imply the absence of the virus in the sewage. Randomness in sampling can be a source of uncertainty, which was observed in the present study for sites associated with the COVID-19 hospital. Besides, samples taken from Lok Yan House on 29 July 2020 was tested negative for SARS-CoV-2, but there was a reported infection case of infection (#2881) still living in that building at the sampling time of sampling. The absence of this SARS-CoV-2 signal could also be due to the impact of the randomness of sewage sampling as well as the variability of viral shedding rate in COVID-19 patients. This implies that the applied testing method could miss the positive cases in a catchment area, which is an inevitable consequence of the randomness of sewage sampling, such as unevenness in virus distribution and sewage flow.
3.3. Detection of the virus at large sewage treatment facilities
Despite its promising results, monitoring at the individual building level for a large city is an extremely resource-demanding task. As monitoring the virus concentration at downstream sewage treatment facilities can be used to infer the virus infection trends (Schmidt, 2020), testing for sewage collected from sewage treatment facilities is more applicable for longitudinal sewage surveillance. However, this strategy is also fraught with challenges when the concentration of SARS-CoV-2 virus in sewage is low. In this study, we showed that the viral signal of SARS-CoV-2 can be detected in the sewage entering large sewage treatment facilities, which serve more than 1.1 million people. For a total of 87 samples collected from such large treatment facilities, 14 samples were tested positive for SARS-CoV-2 with concentrations ranging from 0.3 copy/mL to 177 copy/mL. For the other 73 samples, the measurements were below the method detection limit. Considering that all the samples were from large sewage treatment facilities, positive results were randomly observed. Such randomness is prone to dilution of the genetic signal in the sewer system, which may reach the marginal level of the detection method. To overcome the dilution effects at downstream sewage treatment facilities, a testing method with high sensitivity is required.
A study (Haramoto et al., 2020) reported the detection of SARS-CoV-2 RNA in effluent samples using a large sample volume of 5 L, while the influent samples with a small volume of 200 mL tested negative in the same study. This indicated that concentrating a large volume of sewage samples could increase the chances of detecting SARS-CoV-2. Other research opportunities to improve the sensitivity of our current testing method lie in aspects of lowering the quantification limits of the whole method, including optimizations of the sampling strategy to peak hours of the shedding of fecal matter, evaluations of virus concentration methods, extraction protocols for sewage samples, and assessments of primer-probe sets and kits used for the RT-qPCR. For the quantification of SARS-CoV-2 using RT-qPCR, a comparison of different primer-probe sets targeting various genetic loci of the virus is essential to discern their performance in sewage samples. In addition, the utilization of a larger reaction volume for one-step RT-qPCR with an increased sample template could also be considered to lower the detection limit per unit volume of sewage.
Although the association between sewage measurements of SARS-CoV-2 and clinically reported COVID-19 cases has been observed in sewage treatment facilities by studies using different testing methods and diverse sewage matrices (Hata et al., 2021; Medema et al., 2020; Weidhaas et al., 2021), a similar relationship was not observed in this study. Factors affecting the magnitude of such relationships include differences in the population sizes of sewer sheds, the sewage testing method, and the overall epidemiological context of the outbreak. In our study, such correlation analysis was limited by the low concentrations of SARS-CoV-2 in the tested sewage samples (generally a few copies per mL for sewage treatment facilities compared with up to 1975 copy/mL in the manhole of the COVID-19 hospital), potentially due to the dilution effects in the sewage networks as well as the relatively low prevalence of infection cases in local communities. The assessment of the correlation between the sewage surveillance data and the clinic testing data is critical. To address the limitations of this study, a longitudinal sewage analysis covering the majority of the COVID-19 outbreak periods is needed to avoid potential misinterpretation of the sewage testing results.
4. Conclusions
-
•
The employed method has been demonstrated to be applicable in quantifying SARS-CoV-2 in samples collected from manholes outside individual buildings as well as entrances for large sewage treatment facilities. In particular, we evaluated the performance of the sewage testing method by analyzing “positive” samples from the sewage network associated with a COVID-19 hospital.
-
•
The highest viral concentration of 1975 copy/mL was detected in a sewage sample collected from the isolation ward of the COVID-19 hospital. From the 12 samples collected from the sampling sites in the sewage network of the COVID-19 hospital, seven samples were tested positive for SARS-CoV-2.
-
•
Our results provided a 2-day early notice of hidden cases in two individual apartment buildings. Besides, the presence of SARS-CoV-2 was confirmed in the RT-qPCR product by Sanger sequencing. This promising results of this study prompt us to seek further solutions to apply the sewage surveillance method for wider applications to help with the control of COVID-19.
-
•
During the third outbreak of COVID-19 in Hong Kong, 12 out of 87 samples collected from large sewage treatment facilities, including Sewage Treatment Work, Sewage Screening Plant, and Sewage Pumping Station, were tested positive for SARS-CoV-2. The viral concentration ranged from 0.3 copy/mL to 177 copy/mL. Dilution of viral signal was observed in large sewage treatment facilities.
CRediT authorship contribution statement
Xiaoqing Xu: Methodology, Investigation, Formal analysis, Data curation. Xiawan Zheng: Methodology, Investigation, Formal analysis, Data curation. Shuxian Li: Investigation. Nga Sze Lam: Investigation. Yulin Wang: Investigation. Daniel K.W. Chu: Resources. Leo L.M. Poon: Resources, Writing – review & editing. Hein Min Tun: Resources, Writing – review & editing. Malik Peiris: Methodology, Resources, Writing – review & editing. Yu Deng: Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Gabriel M. Leung: Conceptualization, Methodology, Resources, Writing – review & editing. Tong Zhang: Conceptualization, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Hong Kong Drainage Services Department and the Environmental Protection Department under the Environmental Bureau of Hong Kong SAR, China. The authors would like to thank for the financial support of HKU Faculty of Engineering COVID-19 Action Seed Funding. Xiaoqing Xu and Xiawan Zheng would like to thank The University of Hong Kong for the Postgraduate Studentship.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.148000.
Appendix A. Supplementary data
Supplementary material
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F., Chan P.P., Lung K., Tso E., Liu R., Ng Y., Chu M.Y., Chung T.W., Tam A.R. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Pan Y., Cheng S.M., Hui K.P., Krishnan P., Liu Y., Ng D.Y., Wan C.K., Yang P., Wang Q. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66(4):549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J., Koritnik T., Mioč V., Trkov M., Bolješič M., Berginc N., Prosenc K., Kotar T., Paragi M. Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. Sci. Total Environ. 2020;755 doi: 10.1016/j.scitotenv.2020.143226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Hara-Yamamura H., Meuchi Y., Imai S., Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Jafferali M.H., Khatami K., Atasoy M., Birgersson M., Williams C., Cetecioglu Z. Benchmarking virus concentration methods for quantification of SARS-CoV-2 in raw wastewater. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Ferraro G.B., Veneri C., Iaconelli M., Bonadonna L., Lucentini L., Suffredini E. SARS-CoV-2 has been circulating in northern Italy since December 2019: evidence from environmental monitoring. Sci. Total Environ. 2020;750 doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1(6) doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly K.M., Allen D.J., Fine P., Asghar H. The challenges of informative wastewater sampling for SARS-CoV-2 must be met: lessons from polio eradication. Lancet Microbe. 2020;1(5):e189–e190. doi: 10.1016/S2666-5247(20)30100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cataluña A., Cuevas-Ferrando E., Randazzo W., Falcó I., Allende A., Sánchez G. Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo S.E., Keim E.K., Swanstrom R., Ong A.Q., Burnor E.A., Kossik A.L., Harrison J.C., Demeke B.A., Zhou N.A., Beck N.K. A comparison of SARS-CoV-2 wastewater concentration methods for environmental surveillance. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.144215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo, W., Cuevas-Ferrando, E., Sanjuan, R., Domingo-Calap, P. and Sanchez, G. 2020a. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Available at SSRN 3586696. [DOI] [PMC free article] [PubMed]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C. Watcher in the wastewater. Nat. Biotechnol. 2020;38(8):917–920. doi: 10.1038/s41587-020-0620-2. [DOI] [PubMed] [Google Scholar]
- Usman M., Farooq M., Hanna K. 13th. Vol. 54. Environ. Sci. Technol.; 2020. Existence of SARS-CoV-2 in Wastewater: Implications for its Environmental Transmission in Developing Communities; pp. 7758–7759. [DOI] [PubMed] [Google Scholar]
- Vogels C.B., Brito A.F., Wyllie A.L., Fauver J.R., Ott I.M., Kalinich C.C., Petrone M.E., Casanovas-Massana A., Muenker M.C., Moore A.J. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT–qPCR primer–probe sets. Nat. Microbiol. 2020;5(10):1299–1305. doi: 10.1038/s41564-020-0761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the coronavirus disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., Hoffman K., Jamal R., Heck P., Zhang Y. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S., Weber F.-A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T. Detection of SARS-CoV-2 in raw and treated wastewater in Germany–suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., Bonneau R., Brown M.A., Bushman M., Chai P.R., Duvallet C., Erickson T.B., Foppe K., Ghaeli N., Gu X., Hanage W.P., Huang K.H., Lee W.L., Matus M., McElroy K.A., Nagler J., Rhode S.F., Santillana M., Tucker J.A., Wuertz S., Zhao S., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. medRxiv. 2020 doi: 10.1016/j.scitotenv.2021.150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. Msystems. 2020;5(4) doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S. Potential spreading risks and disinfection challenges of medical wastewater by the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang hospital. Sci. Total Environ. 2020;741:140445. doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material