Abstract
Objectives
To determine if commercially available mouthwash with β-cyclodextrin and citrox (bioflavonoids) (CDCM) could decrease the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) salivary viral load.
Methods
In this randomized controlled trial, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR-positive patients aged 18–85 years with asymptomatic to mild coronavirus disease 2019 (COVID-19) symptoms for <8 days were recruited. A total of 176 eligible patients were randomly assigned (1:1) to CDCM or placebo. Three rinses daily were performed for 7 days. Saliva sampling was performed on day 1 at 09.00 (T1), 13.00 (T2) and 18.00 (T3). On the following 6 days, one sample was taken at 15.00. Quantitative RT-PCR was used to detect SARS-CoV-2.
Results
The intention-to-treat analysis demonstrated that, over the course of 1 day, CDCM was significantly more effective than placebo 4 hours after the first dose (p 0.036), with a median percentage (log10 copies/mL) decrease T1–T2 of –12.58% (IQR –29.55% to –0.16%). The second dose maintained the low median value for the CDCM (3.08 log10 copies/mL; IQR 0–4.19), compared with placebo (3.31 log10 copies/mL; IQR 1.18–4.75). At day 7, there was still a greater median percentage (log10 copies/mL) decrease in salivary viral load over time in the CDCM group (–58.62%; IQR –100% to –34.36%) compared with the placebo group (–50.62%; IQR –100% to –27.66%). These results were confirmed by the per-protocol analysis.
Conclusions
This trial supports the relevance of using CDCM on day 1 (4 hours after the initial dose) to reduce the SARS-CoV-2 viral load in saliva. For long-term effect (7 days), CDMC appears to provide a modest benefit compared with placebo in reducing viral load in saliva.
Keywords: β-cyclodextrin, Citrox, Coronavirus disease 2019, Mouthwash, Saliva, Severe acute respiratory syndrome coronavirus 2, Viral load
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may be transmitted via saliva, even in patients who do not cough or have other respiratory symptoms [1,2]. SARS-CoV-2 is identified in 91.7% of saliva samples from individuals with coronavirus disease 2019 (COVID-19), and the load can reach up to 1.2 × 108 copies/mL [[3], [4], [5]]. When a person sneezes, converses, breathes or coughs, saliva droplets containing microorganisms are produced [2]. The size of these droplets and the quantity produced depend on the individual. Consequently, the risk of transmission is also variable [2]. The contamination between people in close contact (1–3 m) occurs through saliva droplets (>60 μm) [6]. The transmission between two persons separated by a distance of up to 7–8 m is the result of virus-laden aerosols (droplets <60 μm) [7,8].
The use of mouthwashes is an ‘adjuvant’ treatment part of the usual treatment or individual prophylaxis, especially in oral health. Considering mouthwashes as agents that can decrease the viral load of SARS-CoV-2 is an extremely attractive concept [[9], [10], [11], [12]]. However, there is no in vivo evidence to recommend mouthwashes to control SARS-CoV-2 viral load. Among antiviral molecules contained in mouthwashes, hydrogen peroxide, β-cyclodextrin, flavonoids, essential oils, cetylpyridinium chloride or povidone-iodine could be of interest in the fight against SARS-CoV-2 [9,10,13]. The antiviral activity of β-cyclodextrin–citrox mouthwash (CDCM) in our trial is based on β-cyclodextrin and citrox (flavonoids). These molecules have demonstrated antiviral activity against several viruses [[14], [15], [16], [17], [18], [19], [20]], but evidence for their action against SARS-CoV-2 was based only on in silico studies [10].
The objective of our study was to describe the evolution of salivary SARS-CoV-2 viral load in COVID-19 outpatients receiving mouthwashes with or without antivirals.
Materials and methods
A protocol of this trial has been published [21] and the trial registered at ClinicalTrials.gov (NCT04352959).
Study design
The study was a multicentre, double-blind randomized controlled trial with two parallel arms (1:1). Participants were enrolled at four French hospital centres, and monitoring occurred at home. Written informed consent was obtained from each participant before enrolment.
The ‘Committee for the Protection of Persons South Mediterranean III’ (France) reviewed and approved the clinical trial protocol. The study was conducted in compliance with the principles of the Declaration of Helsinki.
Participants
The population was ambulatory adults with asymptomatic and mild clinical COVID-19 symptoms who had voluntarily presented at the hospital for a screening qualitative PCR test. Asymptomatic patients are defined as individuals without clinical signs whereas mild corresponds to outpatients and patients with clinical symptoms without pneumonia manifestations on image results [22].
Eligibility was restricted to adults aged 18–85 years old with a clinical diagnosis of COVID-19 infection, asymptomatic or mild clinical symptoms that had been present for <8 days, virological confirmation, an understanding and acceptance of the trial and written agreement to participate in the trial.
The exclusion criteria were pregnancy, breastfeeding, an inability to comply with the protocol, a lack of written agreement, mouthwash use on a regular basis (more than once a week), an inability to answer questions and a lack of cooperation.
Randomization and masking
Eligible patients were randomly assigned (1:1) to either the CDCM group or the placebo group. The randomization sequence with permutation blocks of size 4 was prepared using e-CRF Voozalyon 1.3 (Voozanoo, Caluire, France) (see Supplementary material, Appendix S1).
Once enrolled, participants each received three 200 mL medication vials. Each vial contained either a mouthwash containing the antiviral components (β-cyclodextrin (0.1%) and citrox (0.01%)) or placebo, which had similar appearance and content but without the above-mentioned antiviral components; the labels on the vials were identical. All participants, investigators, statisticians and laboratory staff were masked to medication vials and treatment allocation.
Procedures
Participants were instructed to use three mouthwashes per day (at 09.00, 14.00 and 19.00), with either 30 mL of CDCM or placebo, both provided by Curaden AG (Kriens, Switzerland) for 1 min (see Supplementary material, Fig. S1). Participants were instructed to collect their saliva by trained nurses using the ‘Saliva Collection System’ kit (Greiner Bio-one, Kremsmünster, Austria). Saliva sampling was performed on the first day at T1 (09.00, before the first mouthwash) and then at T2 (13.00) and T3 (18.00). On the following 6 days, only one sample was taken at 15.00. The rationale for performing the pure saliva sampling before and not after the mouthwash was to collect the amount of viral load that had accumulated in the previous hours following the previous rinsing.
SARS-CoV-2 RNA detection and quantification were performed at laboratories of the National Reference Centre for Respiratory Viruses (Lyon, France) (see Supplementary material, Appendix S1 and Table S1). The viral load in saliva was calculated as the number of RNA copies per mL of saliva.
Outcomes
Primary outcome measures included changes from baseline SARS-CoV-2 in salivary samples at two time-points, 4 and 9 hours, within 1 day after the first intake. Secondary outcome measures included changes from baseline SARS-CoV-2 in salivary samples at 6 days after the first dose.
Statistical analysis
Sample-size calculations were estimated using the freeware STPLAN (Version 4.5, Department of Biomathematics, University of Texas MD, USA). The sample size was based on a minimal viral load difference of 1 log10 copies/mL between placebo and CDCM groups, a common standard deviation of 2 log10 copies/mL, a power of 0.9 and a type I error of 5%. It was calculated that at least 70 individuals per group would be necessary. With an estimated drop-out rate of 25%, 88 individuals per group were required (unilateral test).
Intention-to-treat analyses were performed on the imputed data from all randomized patients using Multiple Imputation by Chained Equations, based on a Monte Carlo–Markov chain algorithm under missing at random data hypothesis. We performed a paired non-parametric Wilcoxon signed rank test with Bonferroni correction comparing the decrease of viral load over time: T1 versus T2, T1 versus T3 and T1 versus day 7 in both groups. Then the two groups were compared at each time thanks to a non-parametric Mann–Whitney U test. Finally, a mixed-effect linear model (viral load repeated data along time from day 1 T1 to day 7) was performed with group (CDCM/placebo) as fixed effect and individuals as random effect.
Additionally, per-protocol analysis using the same methodology as the intention-to-treat analyses was performed based on participants with a complete set of outcome data at day 1.
Post hoc subgroup analyses were performed on the data sets with Day 1 T1 values starting at the first quartile (Q1, patients' baseline viral load >2.94 log10 copies/mL (intention-to-treat) or 2.95 log10 copies/mL (per-protocol)), the second quartile (Q2, patients' baseline viral load >4.01 log10 copies/mL; 4.12 log10 copies/mL (per-protocol)) and the third quartile (Q3, patients' baseline viral load >5.03 log10 copies/mL; >5.16 log10 copies/mL (per-protocol)).
Except for the Mann–Whitney U test, the other tests were based on the unilateral hypothesis (H1: CDCM < placebo). All analyses other than sample-size calculations and graphic illustrations were performed using R (version 3.6.0, The R Foundation for Statistical Computing Platform).
Results
Study design and analysis set
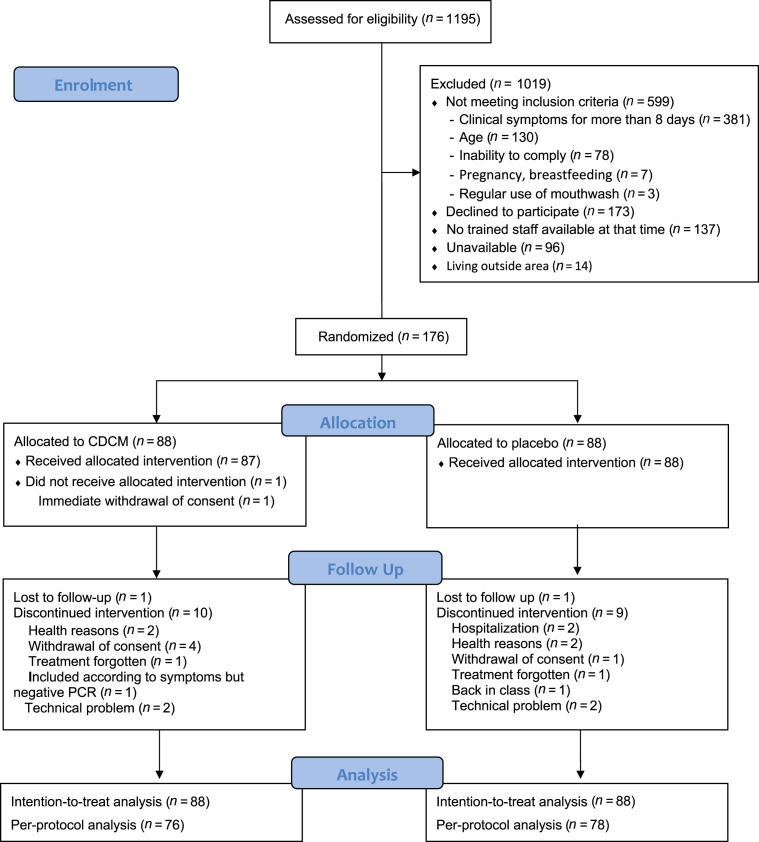
This trial [21] was conducted between 9 June and 11 December 2020. Out of 1195 selected patients, 176 met the inclusion criteria and were randomized. These patients constituted the intention-to-treat population (Fig. 1 ). The baseline characteristics of the two study groups were similar (Table 1 ). The mean age was 43.06 ± 5.56 years, ranging from 18 to 77 years; 80/176 (45.45%) of patients were male. A total of 157/169 (92.90%) were outpatients, and 130/167 (77.84%) of participants had no medical antecedents. Among the participants, 15/175 (8.57%) were asymptomatic and 160/175 (91.43%) had mild symptoms, with 3.58 ± 2.25 of the symptoms on the COVID-19 report forms. Participants were randomized within 4 days (interquartile range (IQR) 3–5 days) of symptom onset. The first saliva specimens were collected at a median time of 4 days (IQR 3–5 days) after nasopharyngeal PCR-positive results. Median initial viral load was 4.01 log10 copies/mL (IQR 2.94–5.03 log10 copies/mL, range 0–10.19 log10 copies/mL). The first quartile (Q1) corresponded to a viral load starting at 2.94 log10 copies/mL, whereas the second (Q2) corresponded to a viral load starting at 4.01 log10 copies/mL, and the third (Q3) corresponded to a viral load starting at 5.03 log10 copies/mL.
Fig. 1.
BBCovid trial profile.
Table 1.
Baseline characteristics of the study groups (intention-to-treat analysis)
| CDCM | Placebo | |
|---|---|---|
| Gender, n/N (%) | ||
| Male | 41/88 (46.59%) | 39/88 (44.32%) |
| Female | 47/88 (53.41%) | 49/88 (55.68%) |
| Age (years) | n = 88 | n = 88 |
| Mean ± SD | 42.06 ± 14.97 | 44.08 ± 16.16 |
| Median; range (IQR) | 41.5; 18–76 (30–52) | 42; 18–77 (30–56) |
| No co-morbidity, n/N (%) | 65/82 (79.27%) | 65/85 (76.47%) |
| Diagnosis to inclusion time (days) | n = 87 | n = 83 |
| Mean ± SD | 3.71 ± 4.11 | 2.84 ± 1.57 |
| Median; range (IQR) | 4; 0–38 (2–4.5) | 3; 0–8 (2–4) |
| Initial place of care, n/N (%) | ||
| Home | 80/85 (94.12%) | 77/84 (91.67%) |
| Hospital | 5/85 (5.88%) | 6/84 (7.14%) |
| Nursing care | 0/85 (0.00%) | 1/84 (1.19%) |
| Diagnostic delay to day 1 (days) | n = 87 | n = 84 |
| Mean ± SD | 4.3 ± 3.34 | 3.61 ± 1.48 |
| Median; range (IQR) | 4; 0–31 (3–5) | 4; 1–8 (3–5) |
| Delay clinical signs to day 1 (days) | n = 76 | n = 82 |
| Mean ± SD | 5.59 ± 1.54 | 5.46 ± 1.67 |
| Median; range (IQR) | 6; 2–9 (4.75–7) | 6; 1–9 (4.25–7) |
| Delay PCR to day 1 (days) | n = 85 | n = 84 |
| Mean ± SD | 4.11 ± 1.53 | 3.73 ± 1.45 |
| Median; range (IQR) | 4; 1–8 (3–5) | 4; 0–8 (3–5) |
Abbreviations: CDCM, β-cyclodextrin-citrox mouthwash; IQR, interquartile range; SD, standard deviation.
Change in SARS-CoV-2 salivary viral load during the first day for all the patients
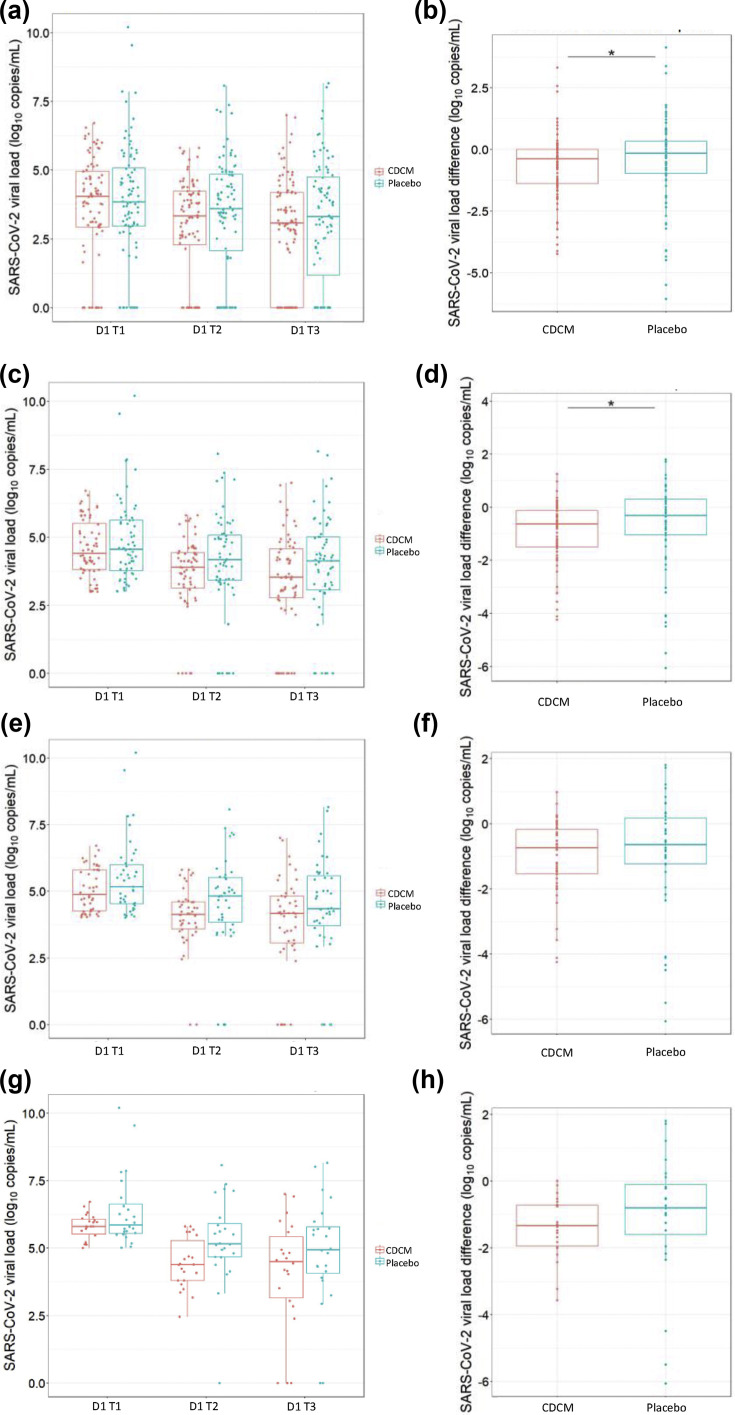
The SARS-CoV-2 salivary load continuously decreased between T1–T2 and T2–T3 for the CDCM group and the placebo group (Fig. 2 ). The median viral load was lower in the CDCM group compared with the placebo group at T2 and T3. A significant difference was observed in viral load reduction in the before–after comparison of the same patients receiving CDCM versus no difference for the placebo group from T1 to T2 (p 0.036) (Table 2 ). The percentage median decrease (log10 copies/mL) was –12.58% (IQR –29.55% to –0.16%) for CDCM versus –6.74% (IQR –21.16% to 10.44%) for placebo. At T3, the salivary viral load decreases were significant for both groups compared with T1 (CDCM: p < 0.001; placebo: p 0.002). However, no significant difference between the two groups was detected. Similar results were obtained with the per-protocol analysis (see Supplementary material, Tables S2, S3 and S4 and Fig. S2).
Fig. 2.
Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) salivary load within the mouthwash cohorts at day 1 (intention-to-treat analysis). (a) Evolution for all patients. (b) SARS-CoV-2 viral load difference between T1 and T2 for all patients. (c) Evolution for patients with a SARS-CoV-2 viral load >2.94 log10 copies/mL at day 1 T1. (d) SARS-CoV-2 viral load difference between T1 and T2 for patients with a SARS-CoV-2 viral load >2.94 log10 copies/mL at day 1 T1. (e) Evolution for patients with a SARS-CoV-2 viral load >4.01 log10 copies/mL at day 1 T1. (f) SARS-CoV-2 viral load difference between T1 and T2 for patients with a SARS-CoV-2 viral load >4.01 log10 copies/mL at day 1 T1. (g) Evolution for patients with a SARS-CoV-2 viral load >5.03 log10 copies/mL at day 1 T1. (h) SARS-CoV-2 viral load difference between T1 and T2 for patients with a SARS-CoV-2 viral load >5.03 log10 copies/mL at day 1 T1.
Table 2.
Salivary SARS-CoV-2 load evolution for the CDCM and placebo groups (intention-to-treat analysis)
| CDCM | Placebo | p valuea | |
|---|---|---|---|
| Global | n = 88 | n = 88 | |
| Day 1 T1 | |||
| Median (IQR) | 4.05 (2.94–4.96) | 3.85 (2.97–5.08) | |
| Day 1 T2 | n = 88 (ID = 7) | n = 88 (ID = 5) | |
| Median (IQR) | 3.33 (2.29–4.23) | 3.60 (2.07–4.83) | |
| Median difference T1–T2 (IQR) | –0.38 (–1.39 to 0.00) | –0.15 (–0.97 to 0.33) | 0.036 |
| p valueb | <0.001 | 0.039 | |
| % decrease T1–T2 median (IQR) | –12.58% (–29.55% to –0.16%) | –6.74% (–21.16% to 10.44%) | |
| Day 1 T3 | n = 88 (ID = 11) | n = 88 (ID = 7) | |
| Median (IQR) | 3.08 (0–4.19) | 3.31 (1.18–4.75) | |
| Median difference T1–T3 (IQR) | –0.24 (–1.55 to 0.06) | –0.30 (–1.23 to 0.22) | 0.270 |
| p valueb | <0.001 | 0.002 | |
| % decrease T1–T3 median (IQR) | –10.67% (–37.30% to 3.25%) | –9.79% (–28.53% to 9.21%) | |
| Day 7 | n = 88 (ID = 14) | n = 88 (ID = 19) | |
| Median (IQR) | 0 (0–1.34) | 1.62 (0–1.70) | |
| Median difference T1–day 7 (IQR) | –2.07 (–4.03 to –0.50) | –2.11 (–3.35 to –0.86) | 0.388 |
| p valueb | <0.001 | <0.001 | |
| % decrease T1–day 7 median (IQR) | –58.62% (–100% to –34.36%) | –50.62% (–100% to –27.66%) | |
| Mean difference | –0.17 (90% CI –0.39 to 0.06) | ||
| MLM p valuec | 0.112 | ||
Abbreviations: CDCM, β-cyclodextrin-citrox mouthwash; ID, imputation data; IQR, interquartile range; MLM, mixed linear model; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are expressed in log10 copies/mL of saliva or in % for the % of variation calculated with values expressed in log10 copies/mL.
Mann–Whitney U test of the differences with a unilateral alternative hypothesis (H1 CDCM < Placebo).
Wilcoxon rank signed test of the differences.
Mixed linear model of the concentrations over time with a unilateral alternative hypothesis (H1 CDCM < Placebo).
Change in the SARS-CoV-2 salivary viral load during the first day based on patients' baseline viral load
The time-by-time outcomes on day 1 according to the initial salivary load were analysed in three subgroups based on the quartiles. The descriptive results and the quantitative results are presented in Fig. 2 and the Supplementary material (Table S5).
For patients with an initially salivary SARS-CoV-2 load >2.94 log10 copies/mL, there was a significant difference in the reduction in the viral load between T1 and T2. CDCM had an effect, whereas the placebo did not (p 0.036). The salivary viral load significantly decreased over the T1–T3 period for both groups; however, there was a more positive impact (log10 copies/mL) for the CDCM group (–15.74%, IQR –38.53% to –1.48% for CDCM versus –10.84%, IQR –28.65% to 3.82% for placebo).
For patients with an initial salivary SARS-CoV-2 load >4.01 log10 copies/mL, the results did not show a significant difference between CDCM and placebo at T2 (p 0.182) or T3 (p 0.257). The median percentage decrease (log10 copies/mL) between T1 and T3 was –16.35% for CDCM (IQR –37.42% to –3.56%) and –12.30% for placebo (IQR –27.19% to 1.53%).
For patients with an initial SARS-CoV-2 saliva load >5.03 log10 copies/mL, the quantitative results showed no significant difference between the two groups for periods T1–T2 and T1–T3. The median percentage decrease (log10 copies/mL) at T1–T3 was –24.14% for CDCM (IQR –41.05% to –4.49%) and –14.15% for placebo (IQR –27.71% to –4.21%). Similar results were obtained with the per-protocol analysis (see Supplementary material, Table S6).
Change in SARS-CoV-2 salivary viral load at 7 days for all the patients
The changes from baseline in the amount of SARS-CoV-2 in salivary samples at 7 days were analysed as intention-to-treat.
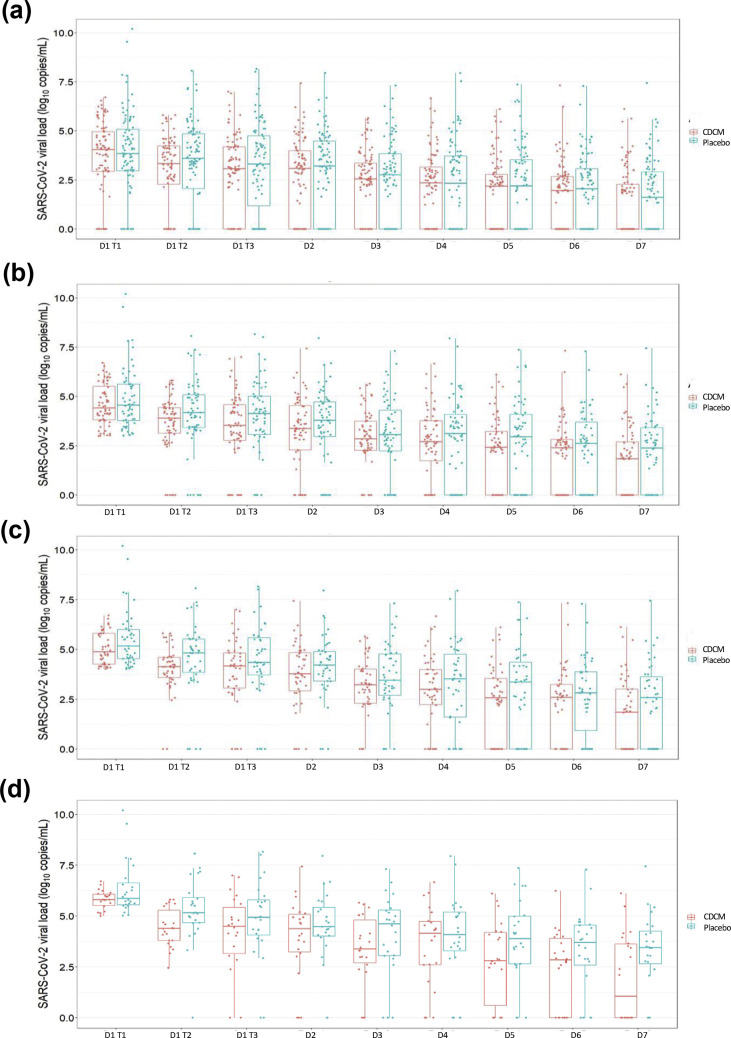
A continuous decrease over the 7 days for the CDCM group and the placebo group was observed (Fig. 3 ). At day 7, no significant difference between patients receiving CDCM and those receiving placebo was observed (p 0.388) (Table 2, and see Supplementary material, Table S4). In both groups, the viral load was significantly lower on day 7 than on day 1 T1 (p < 0.001). With the linear mixed model, a higher but non-significant (p 0.112) reduction in the viral load was observed in the CDCM group (mean difference –0.17 log10 copies/mL, 90% CI –0.39 to 0.06). Similar results were obtained with the per-protocol analysis (see Supplementary material, Table S4, Figs S3 and S4).
Fig. 3.
Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) salivary load within the mouthwash cohorts from day 1 to day 7 (intention-to-treat analysis). (a) Evolution for all patients. (b) Evolution for patients with a SARS-CoV-2 viral load >2.94 log10 copies/mL at day 1 T1. (c) Evolution for patients with a SARS-CoV-2 viral load >4.01 log10 copies/mL at day 1 T1. (d) Evolution for patients with a SARS-CoV-2 viral load >5.03 log10 copies/mL at day 1 T1.
Change in SARS-CoV-2 salivary viral load at 7 days based on patients' baseline viral load
At day 7, the median salivary viral load was always lower for the CDCM group than for the placebo group (Fig. 3). No significant differences between the two groups were observed (Table 2). For initial salivary loads >5.03 log10 copies/mL, the median percentage decrease (log10 copies/mL) at day 7 compared with that at T1 for the CDCM group versus the placebo group was –84.28% (IQR –100% to –36.52%) versus –44.20% (IQR –59.59% to 33.61%). With the linear mixed model, a more significant reduction in the viral load was observed for patients with an initial viral load >4.01 log10 copies/mL in the CDCM group; mean difference –0.25 log10 copies/mL (90% CI –0.47 to –0.04; p 0.028). Likewise, the analysis of patients with an initial viral load >5.03 log10 copies/mL indicated a mean difference in the CDCM group versus the placebo group of –0.36 log10 copies/mL (90% CI –0.64 to 0.08; p 0.019). Similar results were obtained with the per-protocol analysis (see Supplementary material, Table S5, Figs S3 and S4).
Discussion
The primary end-point demonstrated an effect of CDCM versus placebo. A single CDCM rinse significantly reduced the risk of SARS-CoV-2 contamination from saliva. Over the course of 1 day, the first CDCM rinse significantly reduced the viral load, and the second dose maintained this low value, compared with placebo.
The post-hoc analysis based on patients' baseline viral load led to several observations. First, among participants with an initial load >2.94 log10 copies/mL, a significant decrease in the CDCM group was observed in the 4 hours separating T2 from T1. Second, their salivary viral load significantly decreased over the T1 to T3 period in both groups; however, there was a more positive impact in the CDCM group than in the placebo group. The decrease observed in the placebo group could be explained by the natural decrease in salivary viral load over the course of a day, by the effect of oral rinsing or by the presence of excipients that can be considered as potential active ingredients [23]. Third, for patients with initial loads >4.01 log10 copies/mL, a higher but non-significant percentage of viral load reduction was observed for the CDCM group than for the placebo group. One hypothesis is that, for participants with the highest viral load, the frequency of mouthwash use could be insufficient to significantly impact the viral load within a short period of time.
Moreover, the post-hoc analysis based on patient age concluded that the age was not related to initial viral load at day 1 T1 (Spearman correlation test, p 0.07), nor to the evolution up to day 1 T1 (Spearman correlation test, p 0.74). No impact over time was observed (mixed linear model p 0.302).
Concerning the antiviral load responses to mouthwashes on day 7, our trial provided unclear evidence for the general population. By eliminating the fluctuation effect, there was a greater drop in salivary viral load over time in the CDCM group. For patients with an initial viral SARS-CoV-2 load >4.01 log10 copies/mL or >5.03 log10 copies/mL, CDCM significantly reduced the salivary viral load more quickly than placebo.
Our study had limitations related to the time elapsed from the first salivary collection to the time delay estimate for adults without clinical symptoms. Thirty per cent of individuals with infection never develop symptoms [24,25]. Since infectivity appears to peak at or before symptom onset, the initial viral load data underestimate the salivary concentration load of the general population during the incubation period [26]. Second, in our RCT, the proportion of missing data was 12% (13% in the Active group; 11% in the Control group). The reasons for missing data—completely random missing data, random missing data and non-random missing data—were not specified. Third, in both groups, some participants had no SARS-CoV-2 load at day 1 T1 (28/176, 15.9%). These data may have affected the power of the tests and/or affected the viral load reduction values. Moreover, due to the recruitment period (June–December 2020), the question of extrapolation of the results to variant strains that have emerged since that time arises.
Conclusion
CDCM had a significant beneficial effect on reducing SARS-CoV-2 salivary viral load in adults with asymptomatic or mild COVID-19, 4 hours after the initial dose. For long-term effect, the benefit to recommend CDMC appears limited, even if three daily rinses had a beneficial effect on reducing the SARS-CoV-2 salivary viral load 7 days after the initial intake in adults with high salivary viral loads at baseline. CDMC appears to provide a modest benefit compared with placebo in reducing viral load in saliva.
Transparency declaration
All authors have completed the ICMJE uniform disclosure form. DB reports non-financial support and other from Curaden AG Switzerland, outside the submitted work. All other authors declare that they have no conflicts of interest.
This work was partially supported by Curaden AG, Kriens, Switzerland and by Laboratory ‘Systemic Health Care’, EA4129, University of Lyon, France. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All the authors have full access to all the data in the study and had final responsibility for the decision to submit for publication.
Authors contributions
FC, DB and CD proposed the original study idea. FC was the coordinating officer of this trial. FC, PT, CD and DB designed the trial and study protocol. MV, PT, MR and FC contributed to the data interpretation and PT, FC, MV and HP verified the data. EGD, AE, MEL and GI were responsible for the site work including the recruitment, follow up and data collection. HP monitored the trial. MBD and MV were responsible for the laboratory analysis. PT and MR did the main analysis. FC and DB wrote the first draft the manuscript and CD, MBD, MR, MV and PT contributed to the revision of the manuscript. All authors reviewed and accepted the paper before submission.
Access to data
Florence Carrouel has full access to the data and is the guarantor for the data.
Acknowledgements
We acknowledge the contribution of all the patients and trial team members at each recruitment site. Most particularly, Sophie Lengagne, Eva Geraud and Anthea Loiez from the Emile Roux Hospital Centre (le Puy-en-Velay, France); Louis Gauthier from the Protestant Infirmary (Lyon, France), Séverine Poupblanc, Anne-Hélène Boivin and Jérome Dimet from the Intercommunal Hospital Centre of ‘Mont de Marsan et du Pays des Sources’ (Mont de Marsan, France); and Armand Sophie, Caroline Gagneux, Adrien Didelot, Matthieu Pecquet, Marie Paul Perraud and Josiane Thimonier (Cadres des services) from Saint Joseph Saint Luc Hospital (Lyon, France). We also thank all the technicians from the CNR for their work. We thank Stephane Morisset independent statistician and special adviser. We also extend our thanks to Eric Bomel, EZUS, University Lyon1 who provided central administrative support to the project, and Ursula Sutter from Greiner Bio-One GmbH (St Gallen, Switzerland) and Dr Eric Gonzalez Garcia from Greiner Bio-One GmbH (Kremsmuenster, Austria) who kindly provided technical support.
Editor: A. Huttner
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.05.028.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Xu R., Cui B., Duan X., Zhang P., Zhou X., Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12:1–6. doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baghizadeh Fini M. Oral saliva and COVID-19. Oral Oncol. 2020;108:104821. doi: 10.1016/j.oraloncology.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To K.K.W., Yip C.C.Y., Lai C.Y.W., Wong C.K.H., Ho D.T.Y., Pang P.K.P. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25:372–378. doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 4.To K.K.-W., Tsang O.T.-Y., Chik-Yan Yip C., Chan K.-H., Wu T.-C., Chan J.M.C. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon J.G., Yoon J., Song J.Y., Yoon S.Y., Lim C.S., Seong H. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Academies of Sciences E. National Academies Press (US); 2020. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic (April 1, 2020) [PubMed] [Google Scholar]
- 7.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrouel F., Conte M.P., Fisher J., Gonçalves L.S., Dussart C., Llodra J.C. COVID-19: a recommendation to examine the effect of mouthrinses with β-cyclodextrin combined with citrox in preventing infection and progression. J Clin Med. 2020;9 doi: 10.3390/jcm9041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrouel F., Gonçalves L.S., Conte M.P., Campus G., Fisher J., Fraticelli L. Antiviral activity of reagents in mouth rinses against SARS-CoV-2. J Dent Res. 2021;100:124–132. doi: 10.1177/0022034520967933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell V.B., Thomas D., Stanton R., Maillard J.-Y., Murphy R.C., Jones S.A. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function. 2020 doi: 10.1093/function/zqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyers C., Robison R., Milici J., Alam S., Quillen D., Goldenberg D. Lowering the transmission and spread of human coronavirus. J Med Virol. 2021;93:1605–1612. doi: 10.1002/jmv.26514. [DOI] [PubMed] [Google Scholar]
- 13.Herrera D., Serrano J., Roldán S., Sanz M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin Oral Investig. 2020 doi: 10.1007/s00784-020-03413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalani S., Poh C.L. Flavonoids as antiviral agents for enterovirus A71 (EV-A71) Viruses. 2020;12 doi: 10.3390/v12020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou M., Liu H., Li J., Yao X., Chen Y., Ke C. Structure-activity relationship of flavonoid bifunctional inhibitors against Zika virus infection. Biochem Pharmacol. 2020;177:113962. doi: 10.1016/j.bcp.2020.113962. [DOI] [PubMed] [Google Scholar]
- 16.Braga S.S. Cyclodextrins: emerging medicines of the new millennium. Biomolecules. 2019;9 doi: 10.3390/biom9120801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B.Q., Fu T., Dongyan Y., Mikovits J.A., Ruscetti F.W., Wang J.M. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem Biophys Res Commun. 2000;276:534–538. doi: 10.1006/bbrc.2000.3485. [DOI] [PubMed] [Google Scholar]
- 18.Jones S.T., Cagno V., Janeček M., Ortiz D., Gasilova N., Piret J. Modified cyclodextrins as broad-spectrum antivirals. Sci Adv. 2020;6 doi: 10.1126/sciadv.aax9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goncharova E.P., Kostyro Y.A., Ivanov A.V., Zenkova M.A. A Novel sulfonated derivative of β-cyclodextrin effectively inhibits influenza A virus infection in vitro and in vivo. Acta Naturae. 2019;11:20–30. doi: 10.32607/20758251-2019-11-3-20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braga S.S., Barbosa J.S., Santos N.E., El-Saleh F., Paz F.A.A. Cyclodextrins in antiviral therapeutics and vaccines. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13030409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrouel F., Viennot S., Valette M., Cohen J.-M., Dussart C., Bourgeois D. Salivary and nasal detection of the SARS-CoV-2 virus after antiviral mouthrinses (BBCovid): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:906. doi: 10.1186/s13063-020-04846-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gülsen A. Simple classification of COVID-19 patients. J Lung Pulm Respir Res. 2020;7:62–63. [Google Scholar]
- 23.Pottel J., Armstrong D., Zou L., Fekete A., Huang X.-P., Torosyan H. The activities of drug inactive ingredients on biological targets. Science. 2020;369:403–413. doi: 10.1126/science.aaz9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson M.A., Quandelacy T.M., Kada S., Prasad P.V., Steele M., Brooks J.T. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pullano G., Di Domenico L., Sabbatini C.E., Valdano E., Turbelin C., Debin M. Underdetection of cases of COVID-19 in France threatens epidemic control. Nature. 2020 doi: 10.1038/s41586-020-03095-6. [DOI] [PubMed] [Google Scholar]
- 26.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.