Abstract
Background & aims
Malnutrition following intensive care unit (ICU) stay is frequent and could be especially prominent in critically ill Coronavirus Disease 2019 (COVID-19) patients as they present prolonged inflammatory state and long length stay. We aimed to determine the prevalence of malnutrition in critically ill COVID-19 patients both at the acute and recovery phases of infection.
Methods
We conducted a prospective observational study including critically ill COVID-19 patients requiring invasive mechanical ventilation discharged alive from a medical ICU of a university hospital. We collected demographic, anthropometric and ICU stay data (SAPS2, recourse to organ support and daily energy intake). Nutritional status and nutritional support were collected at one month after ICU discharge (M1) by phone interview and at 3 months after ICU discharge (M3) during a specialized and dedicated consultation conducted by a dietitian. Malnutrition diagnosis was based on weight loss and body mass index (BMI) criteria following the Global Leadership Initiative on Malnutrition. Primary outcome was the prevalence of malnutrition at M3 and secondary outcomes were the evolution of nutritional status from ICU admission to M3 and factors associated with malnutrition at M3.
Results
From march 13th to may 15th, 2020, 38 patients were discharged alive from the ICU, median [IQR] age 66 [59–72] years, BMI 27.8 [25.5–30.7] kg/m2 and SAPS2 47 [35–55]. Thirty-three (86%) patients were followed up to M3. Prevalence of malnutrition increased during the ICU stay, from 18% at ICU admission to 79% at ICU discharge and then decreased to 71% at M1 and 53% at M3. Severe malnutrition prevailed at ICU discharge with a prevalence of 55% decreasing 32% at M3. At M3, the only factors associated with malnutrition in univariate analysis were the length of invasive mechanical ventilation and length of ICU stay (28 [18–44] vs. 13 [11–24] days, P = 0.011 and 32 [22–48] vs. 17 [11–21] days, P = 0.006, respectively), while no ICU preadmission and admission factors, nor energy and protein intakes distinguished the two groups. Only 35% of undernourished patients at M3 had benefited from a nutritional support.
Conclusion
Malnutrition is frequent, protracted and probably underrecognized among critically ill Covid-19 patients requiring invasive mechanical ventilation with more than half patients still being undernourished three months after ICU discharge. A particular attention should be paid to the nutritional status of these patients not only during their ICU stay but also following ICU discharge.
Keywords: COVID-19, Malnutrition, Pneumonia, SARS-Cov 2, Intensive care unit, Mechanical ventilation
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection reached pandemic proportions on March, 2020 [1]. Although obesity is a significant risk factor for severe forms and mortality [2], this infection seems to constitute a significant nutritional risk, as significant weight loss and clinical cachexia were reported during acute coronavirus disease 2019 (COVID-19) [3]. This could be even truer in severe COVID-19 patients hospitalized in intensive care unit (ICU), which represents approximately 5% of patients with SARS-CoV-2 infection [4]. Indeed, weight loss and acute skeletal muscle wasting following ICU is common [5,6] and the prominent inflammatory profile of severe COVID-19 [7,8] and long length of stay in ICU [9] could lead to especially high rates of malnutrition. Nevertheless, there are almost no data on the risk of malnutrition in critically ill COVID-19 patients and their short- and long-term nutritional status after ICU discharge remains to be specified. The main objective of this study was to assess and follow the nutritional status of severe COVID-19 patients who undergone invasive mechanical ventilation (IMV) in the ICU both at the acute phase of infection then at 1 and 3 months after ICU discharge.
2. Materials and methods
2.1. Study population
We included all patients admitted in the medical ICU of European Georges Pompidou Hospital in Paris (France) for laboratory confirmed SARS-CoV-2 infection (positive reverse transcription polymerase chain reaction), who required IMV and who were discharged alive from the ICU. All subjects included in this study were informed about the use of their data for research purposes and those who objected to the reuse of their data were excluded, in accordance to French legislation. The study protocol was approved by a local ethic committee (IRB: #00011928).
2.2. Data collected
We prospectively collected data during the entire ICU stay and at one (M1) and three months (M3) after ICU discharge. During the ICU stay, we collected anthropometric data with weight variations by Hillrom® TotalCare SpO2RT® ICU beds with weighing function, usual and ICU admission weight, body mass index (BMI) defined by weight (kg)/height (m2), simplified acute physiology score II (SAPS2), duration of IMV, recourse to organ support (vasopressors and kidney replacement therapy (KRT)), recourse to prone positioning, biological data with albumin, prealbumin and C-reactive protein (CRP) and daily energy intake administered during the stay. At M1, we collected anthropometric, biological and nutritional support data available by contacting the dietitian and/or physician in charge of the patient at that time. At M3, a dedicated consultation was conducted by both a medical nutritionist and a dietitian from the Nutrition Department of the same hospital, during which we collected anthropometric, weight using SECA® 635 weighting machine, clinical and biological data, as well as body composition by bio-impedancemetry (InBody S10®) and a quantification of ingesta based on the CIQUAL food nutritional composition table issued by the French Food Administration (Agence Nationale de Sécurité Sanitaire Alimentation, Environment, Travail (ANSES)): https://ciqual.anses.fr/). Importantly, the quantification was performed by the same dietitian for all patients at all timepoints. In case of incapacity to come to the visit, a phone interview with the patient was planned.
2.3. Outcomes
The primary outcome was the evolution of the nutritional status including prevalence and severity of malnutrition, during the acute phase of infection in the ICU and at M1 and M3. We also assessed the impact of demographic factors, severity at ICU admission, organ support (duration of IMV, use of vasopressor and KRT) and nutritional support on severe malnutrition and on persistence malnutrition at M1 and M3 after ICU discharge, respectively.
2.4. Nutritional assessment
The diagnosis of malnutrition was made using the Global Leadership Initiative on Malnutrition (GLIM) [10], who requires at least one etiologic criterion among reduced food intake or assimilation, inflammation or disease burden and at least one phenotypic criterion among non-volitional weight loss, low BMI and reduced muscle mass. We considered that an etiologic criterion of malnutrition was always present due to COVID-19 induced inflammation. Phenotypic criteria were assessed using BMI and % weight loss. Moderate malnutrition was defined by weight loss between 5 and 10% within the past 6 months or 10–20% beyond 6 months and/or BMI <20 kg/m2 (if age <70 years) or BMI <22 kg/m2 (if age ≥70). Severe malnutrition was defined by weight loss over 10% within the past 6 months or over 20% beyond 6 months and/or BMI <18.5 kg/m2 (if age <70 years) or BMI <20 kg/m2 (if age ≥70). Nutritional status was at ICU admission, ICU discharge, one month (M1) and three months (M3) following ICU discharge was determined by body mass index and (kg/m2) and weight loss (%) criteria. At M3, nutritional status was additionally determined by body composition and muscle function criteria for patients with on-site visit, we also considered a handgrip test <26 kg for men and <16 kg for women, and bio-impedancemetry fat-free index <17 kg/m2 for men and <15 kg/m2 for women and skeletal muscle mass index <7 kg/m2 for men and <5.7 kg/m2 for women in favor of malnutrition as recommended in good practice recommendation issued by the French National Authority for Health (Recommendation de Bonne Pratique Haute Autorité de Santé: Diagnostic de la dénutrition de l'enfant et de l'aldulte, https://www.has-sante.fr/jcms/p_3118872/fr/diagnostic-de-la-denutrition-de-l-enfant-et-de-l-adulte). Daily energy and protein intakes and the corresponding percentage of energy needs and protein coverage were computed using energy needs of 25 kcal/kg/day and 1.3 g/kg/day in ICU according to the ESPEN guidelines [11]and using energy needs of 30 kcal/kg/day and 1 g/kg/day after ICU's discharge, according to the ESPEN guidelines in context of Sars-Cov-2 infection [12].
2.5. Statistical analyses
Continuous variables were expressed as median and interquartile range. Categorical variables were expressed as absolute values and percentages. Owing to the lack of knowledge regarding the recently spreading SARS-CoV-2 pandemic when designing the study, no sample size calculation could be performed prior to the beginning of the study. In order to investigate factors associated with malnutrition, we compared different patients' characteristics according to their nutritional status at ICU discharge and at M3 after ICU discharge using Wilcoxon test for continuous variables and Fisher's exact test for categorial variables. Statistical tests were considered significant if P < 0.05. All statistical analyses were conducted using R studio software version 3.3.2 (http://www.r-project.org).
3. Results
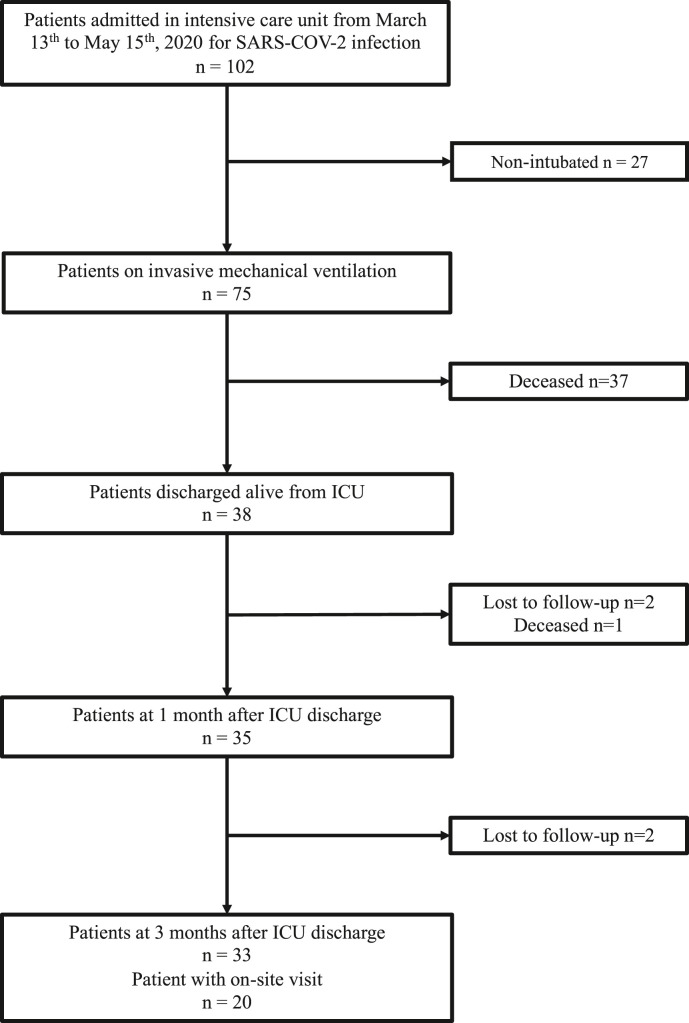
The study was conducted during the first wave of the pandemic in France, from March 13th to may 15th, 2020. During this period, we included 38 patients admitted in the medical ICU of European Georges Pompidou Hospital (France) for SARS-CoV-2 infections, who required IMV and who were discharged alive from the ICU. Accordingly, nutritional evaluations of patients at M3 were carried out from June 15th to September 15th, 2020. Among the 38 patients included, data were available in 35 patients at M1 (1 patient died within the month of ICU discharge and 2 were lost to follow-up) and in 33 patients at M3 (2 additional patients were lost to follow-up). Twenty patients had the on-site dedicated follow-up consultation and data of the remaining 13 patients were gathered through phone interview (see flowchart in Fig. 1 ).
Fig. 1.
Flowchart. Abbreviations: ICU: Intensive Care Unit; SARS-CoV-2: Severe Acute Respiratory Distress Syndrome – Coronavirus – 2.
At ICU admission, participants median [interquartile range] age and BMI were 66 [59, 72] years old and 27.8 [25.5, 30.7] kg/m2 respectively (Table 1 ). Only 7 patients (18%) had malnutrition with a prevalence of moderate and severe malnutrition of 5 (13%) and 2 (5%), respectively. More than half of undernourished patients were overweight (58%) (Table 1).
Table 1.
General and nutritional characteristics at ICU admission.
| Nutritional Status |
p-valueb | |||
|---|---|---|---|---|
| Characteristic | All patients N = 38a |
No malnutrition N = 31 (82%)a |
Malnutrition N = 7 (18%)a |
|
| Demographic characteristics | ||||
| Age (years) | 66 [59, 72] | 64 [58, 71] | 71 [65, 74] | 0.181 |
| Male sex | 29 (76%) | 22 (71%) | 7 (100%) | 0.164 |
| Hypertension, n (%) | 21 (55%) | 16 (52%) | 5 (71%) | 0.427 |
| CKD, n (%) | 10 (26%) | 8 (26%) | 2 (29%) | 1.0 |
| Dyslipidemia, n (%) | 8 (21%) | 8 (26%) | 0 (0%) | 0.307 |
| History of neoplasia, n (%) | 4 (11%) | 3 (9.7%) | 1 (14%) | 1.0 |
| Diabetes, n (%) | 15 (39%) | 13 (42%) | 2 (29%) | 0.681 |
| COPD, n (%) | 4 (11%) | 3 (9.7%) | 1 (14%) | 1.0 |
| Active smoker, n (%) | 5 (13%) | 3 (9.7%) | 2 (29%) | 0.223 |
| Anthropometric characteristics | ||||
| Usual weight (kg) | 84 [72, 95] | 80 [73, 93] | 95 [79, 96] | 0.336 |
| Weight (kg) | 82 [75, 91] | 83 [76, 92] | 81 [73, 88] | 0.510 |
| BMI (kg/m2) | 27.8 [25.5, 30.7] | 28.6 [25.6, 31.2] | 27.1 [22.0, 28.2] | 0.094 |
| Overweight, n (%) | 22 (58%) | 18 (58%) | 4 (57%) | 1.0 |
| Obesity, n (%) | 10 (26%) | 10 (32%) | 0 (0%) | 0.156 |
Results are expressed by median [interquartile range] for continuous data and n (%) for categorial data. P-values shown result from Wilcoxon test for continuous data and Fisher's exact test for categorial data between 2 categories of nutritional status.
Abbreviations: BMI: Body Mass Index; COPD: Chronic Obstructive Pulmonary Disease; CKD: Chronic Kidney Disease; Obesity: BMI≥30 kg/m2; Overweight: BMI≥25 kg/m2.
Median [IQR]; n (%).
Mann-Whithney test; Fisher's exact test.
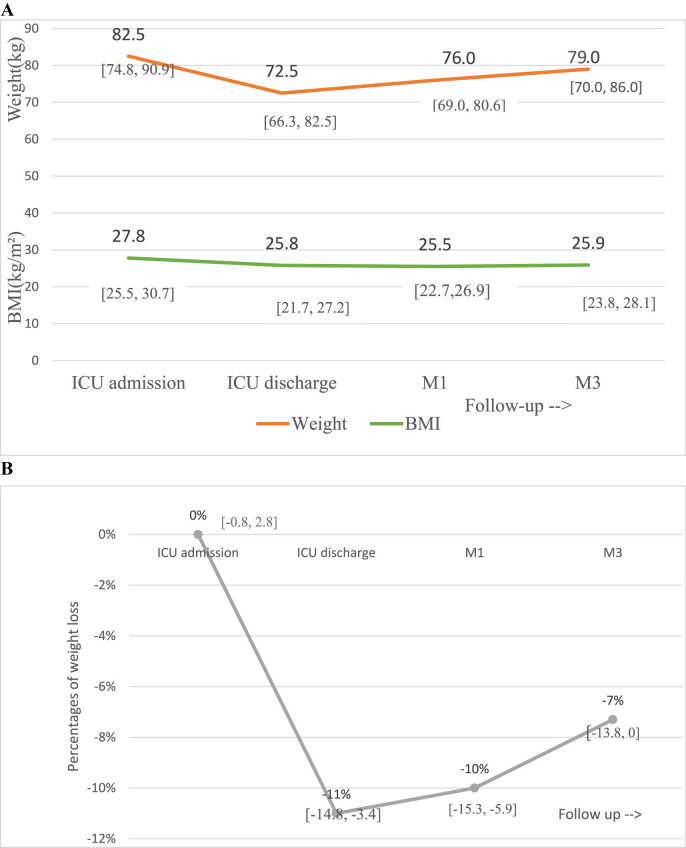
During the ICU stay, median weight loss was −11% [-3.4, −14.8]. After ICU discharge, patients presented a gradual but moderate increase in weight, with a median gain of 3 kg [-1, 7.4] in the 3 months following discharge, resulting in an overall median weight loss of −7% [0, −13.8] at M3 as compared to ICU admission (Fig. 2 ). The evolution of nutritional status at the different time points of the study is illustrated on Fig. 3 . Overall, according to the international GLIM criteria, the prevalence of malnutrition markedly increased during the ICU stay, from 18% at ICU admission to 79% at ICU discharge. It started to decrease at M1 with a remaining prevalence of 71%. At M3, the prevalence of malnutrition was 53%. Severe malnutrition prevailed at ICU discharge with a prevalence of 55% and decreased at 45% at M1 and at 32% at M3 at the benefit of moderate malnutrition. When taking into account the additional criteria of reduction of function and mass muscle to define malnutrition at M3 as proposed in the French guidelines (bio-impedancemetry and handgrip test), the prevalence of moderate malnutrition raised from 21% to 34%, increasing the overall rate of undernutrition from 53% to 66%.
Fig. 2.
Weight evolution of patients from ICU admission to 3 months post-ICU discharge. A Median weight and BMI variation with interquartile range at ICU admission, ICU discharge, one month (M1) and three months (M3) from ICU discharge. B Variations of weight expressed as percentage of usual weight at the different timepoints of the study with interquartile range.Abbreviations: BMI: Body Mass Index; ICU: Intensive Care Unit.
Fig. 3.
Nutritional status of patients from ICU admission to 3 months post-ICU discharge. Nutritional status was at ICU admission, ICU discharge, one month (M1) and three months (M3) following ICU discharge was determined by body mass index and (kg/m2) and weight loss (%) criteria. At M3, nutritional status was additionally determined by body composition and muscle function criteria for patients with on-site visit (M3∗).Abbreviations: ICU: Intensive Care Unit.
Factors associated with malnutrition (GLIM criteria) at M3 are presented in Table 2 . Length of IMV and length of stay in ICU were significantly longer in the malnutrition group as compared with patients without malnutrition at M3 (28 [18, 44] vs. 13 [11, 24] days, P = 0.011 and 32 [22, 48] vs. 17 [11, 21] days, P = 0.006, respectively). No preadmission or ICU admission factors, nor the energy and protein intake during the ICU stay distinguished the two groups.
Table 2.
Factors associated with the nutritional status at three months after ICU discharge according to GLIM criteria.
| Nutritional Status |
p-valueb | |||
|---|---|---|---|---|
| Characteristic | All patients N = 33a |
No malnutrition N = 13a |
Malnutrition N = 20a |
|
| Demographic characteristics at ICU admission | ||||
| Age (years) | 65 [59, 71] | 60 [56, 67] | 66 [62, 72] | 0.319 |
| Male sex | 26 (79%) | 8 (62%) | 18 (90%) | 0.084 |
| COPD | 4 (12%) | 1 (7.7%) | 3 (15%) | 1.0 |
| History of neoplasia | 2 (6.1%) | 0 (0%) | 2 (10%) | 0.508 |
| Active smoker | 4 (12%) | 1 (7.7%) | 3 (15%) | 1.0 |
| CKD | 10 (30%) | 4 (31%) | 6 (30%) | 1.0 |
| Obesity | 9 (27%) | 3 (23%) | 6 (30%) | 1.0 |
| BMI (kg/m2) | 28.6 [25.8, 30.9] | 27.1 [25.0, 29.1] | 28.6 [27.3, 31.0] | 0.136 |
| ICU stay characteristics | ||||
| SAPS2 | 47 [35, 55] | 49 [42, 54] | 46 [35, 56] | 0.825 |
| Duration of IMV (days) | 22 [12, 38] | 13 [11, 24] | 28 [18, 44] | 0.011 |
| Vasopressor | 26 (79%) | 11 (85%) | 15 (75%) | 1.0 |
| Duration of treatment of vasopressor (days) | 4.5 [1.8,8.2] | 4.0 [2.0, 6.0] | 7.0 [2.0, 11.0] | 0.154 |
| Prone positioning | 10 (30%) | 2 (15%) | 8 (40%) | 0.245 |
| KRT | 14 (42%) | 4 (31%) | 10 (50%) | 0.275 |
| Length of ICU stay (days) | 23 [17, 39] | 17 [11, 21] | 32 [22, 48] | 0.006 |
| Anthropometrics at M3 | ||||
| Weight (kg) | 79 [70, 86] | 72 [67, 86] | 80 [73, 86] | 0.357 |
| BMI (kg/m2) | 25.9 [23.8, 28.1] | 26.10 [23.18, 28.24] | 25.71 [24.37, 27.40] | 0.839 |
| Nutritional support | ||||
| Coverage of energy need during ICU (%) | 80 [73, 91] | 80 [74, 88] | 80 [71, 92] | 0.685 |
| Protein intakes during ICU (g/kg/day) | 1.03 [0.87, 1.17] | 1.00 [0.87, 1.06] | 1.10 [0.92, 1.21] | 0.204 |
| Nutritional support within 3 months of ICU discharge | 10 (30%) | 3 (23%) | 7 (35%) | 1.0 |
Results are expressed as median [interquartile range] for continuous data and n (%) for categorial data. P-values result from Wilcoxon test for continuous data and Fisher's exact test for categorial data between the two categories of nutritional status.
Abbreviations:COPD: Chronic Obstructive Pulmonary Disease; CKD: Chronic Kidney Disease; IMV: Invasive Mechanical Ventilation; KRT: Kidney Replacement Therapy; BMI: Body Mass Index; Obesity: BMI≥30 kg/m2; SAPS2: Simplified Acute Physiology Score II; Nutritional support: oral nutritional supplement or enteral nutrition.
Bold values are significant results.
Median [IQR]; n (%).
Mann-Whithney test; Fisher's exact test.
At M3, a complete nutritional assessment comprising body composition, muscle function and energy intake measurements could be performed in the 20 patients who could come to the on-site visit (Table 3 ). No significant differences were found in fat-free mass, skeletal muscle mass and energy intake also all these variables were lower in the malnutrition group as compared to the non-malnutrition group. No patient met the diagnostic criteria of malnutrition using body composition parameters, while almost all malnourished patients (85%) met the handgrip criteria for undernutrition.
Table 3.
Body composition, muscle function and energy intakes at 3 months after ICU discharge.
| Nutritional Status |
p-valueb | |||
|---|---|---|---|---|
| Characteristic | All patients, N = 20a | No malnutrition N = 5 (25%)a |
Malnutrition N = 15 (75%)a |
|
| Anthropometrics | ||||
| Weight (kg) | 80 [70, 86] | 86 [80, 86] | 74 [68, 84] | 0.190 |
| BMI (kg/m2) | 26.0 [24.3, 28.1] | 28.0 [26.1, 28.2] | 25.5 [24.1, 28.1] | 0.600 |
| Handgrip (n = 18) | ||||
| Absolute value (kg) | 19 [12, 30] | 38 [30, 40] | 15 [12, 23] | 0.014 |
| Abnormal | 11 (61%) | 0 (0%) | 11 (85%) | 0.002 |
| Bio-impedancemetry | ||||
| Fat mass (kg) | 21.5 [17.3, 25.2] | 23.4 [18.8, 26.8] | 20.1 [16.1, 24.0] | 0.432 |
| Free-fat mass | ||||
| Absolute value (kg) | 59 [47, 61] | 60 [54, 62] | 59 [46, 61] | 0.295 |
| Index (kg/m2) | 18.9 [16.9, 19.6] | 19.0 [16.9, 19.5] | 18.9 [17.2, 19.7] | 0.930 |
| Normal | 20 (100%) | 5 (100%) | 15 (100%) | 1.0 |
| Skeletal muscle mass | ||||
| Absolute value (kg) | 31.5 [25.3, 33.8] | 32.5 [28.7, 34.2] | 30.7 [24.9, 33.5] | 0.407 |
| Index (kg/m2) | 10.3 [9.2, 10.7] | 10.4 [9.1, 10.6] | 10.3 [9.3, 10.8] | 1.0 |
| Normal | 20 (100%) | 5 (100%) | 15 (100%) | 1.0 |
| Biological data | ||||
| Albumin (g/L) | 41.5 [38.3, 45.0] | 45.0 [39.0, 46.0] | 41.0 [38.2, 44.5] | 0.334 |
| Prealbumin (g/L) (n = 19) | 0.28 [0.24, 0.33] | 0.33 [0.28, 0.34] | 0.26 [0.24, 0.31] | 0.378 |
| CRP (mg/L) (n = 16) | 1 [1, 3] | 1 [1, 3] | 1 [1, 3] | 0.787 |
| Nutritional data | ||||
| Nutritional support | 5 (25%) | 1 (20%) | 4 (27%) | 1.0 |
| Energy intake (kcal/day) | 1800 [1438, 2142] | 2100 [1800, 2300] | 1550 [1375, 2015] | 0.116 |
| Protein intake (g/kg/day) | 1.05 [0.83, 1.18] | 1.00 [0.78, 1.10] | 1.06 [0.92, 1.18] | 0.793 |
| Coverage of energy needs (%) | 80 [72, 93] | 83 [80, 88] | 78 [71, 94] | 0.432 |
These data concerned the subgroup of 20 patients who had on-site visit at 3 months of ICU discharge. Results are expressed as median [interquartile range] for continuous data and n (%) for categorial data. P-values result from Wilcoxon test for continuous data and Fisher's exact test for categorial data between the nutritional status categories.
Abbreviations: BMI: Body Mass Index; CRP: C-Reactive Protein.
Bold values are significant results.
Median [IQR]; n (%).
Mann-Whithney test; Fisher's exact test.
4. Discussion
In this study, we show that the prevalence of malnutrition markedly increased during the ICU stay up to 79% at ICU discharge. This prevalence is much higher than reported in COVID-19 patients in previous studies, with a prevalence of malnutrition ranging from 37.5% to 52.7% [[11], [12], [13], [14]]. However these studies were not carried out exclusively in ICU. Bedock et al. [15] found a prevalence of 66.7% in critically ill patients, which remains below the one we found. Lew et al. [16] reported a prevalence of malnutrition ranging from 38% to 78% but outside of −19. Our results thus confirm that ICU patients with severe COVID-19 are at especially high risk of malnutrition. Importantly, we also showed that nutritional recovery after SARS-CoV-2 infection seems to be very slow, with a prevalence of malnutrition which remains high at 66% with all criteria available at M3. To our knowledge, there is no other study reporting the evolution of the nutritional status in the medium to long term after ICU discharge of serious COVID-19 patients. Indeed, the prevalence of malnutrition started to decrease at M1, but with more than half patients still being undernourished at that time. Yet the proportion of severe malnutrition, predominant at ICU discharge, decreased from M1 at the benefit of moderate malnutrition. Although the relatively small sample of patients precludes to draw definite conclusions, determinants of persistent malnutrition seemed to lie within the length of ICU stay and of organ support rather than related to preadmission characteristics or nutritional support during and after the ICU stay, as hypothesized by Virgens et al. [17] and others [16]. Interestingly, only 32% of patients in whom undernutrition persisted at M3 had benefited from a nutritional support, suggesting that malnutrition was either underrecognized or undertreated in this population. This reinforces the call for a specific nutritional management of COVID-19 patients, as recently emphasized by ESPEN [12] and others [[18], [19]].
In a subpopulation, we were able to gather a comprehensive assessment of nutritional status at M3, comprising body composition and energy intake. While most of the studies report the classical anthropometrics data, a substantial part of the diagnosis of undernutrition was made by a muscle strength deficit, which underlines the importance of testing grip strength in clinical practice [18]. This test could better correlate with sarcopenia and functional outcome. Indeed, ICU-acquired weakness is present in more than half of critically ill COVID-19 patients, with a clear association to functional outcome at hospital discharge [20]. On the contrary, fat-free mass and skeletal muscle mass did not differ between group. Lastly, the protein intake at M3 for undernourished patients was insufficient but the coverage of energy needs was relatively satisfactory with a mean of nearly 80% for all patients. These last results suggest that the part of physical activity in nutritional care remains to be strengthened and/or that the deterioration of muscle function was the predominant impairment. Paying particular attention to strengthening muscle function could have a favorable impact on nutritional status at M3 after ICU discharge.
The main limitations of our study are the monocentric design with relatively small sample size, together with a few patients lost to follow-up. On the other hand, the study population was composed of homogeneous patients in terms of severity of disease and ICU management, which is not always the case in other studies evaluating the prevalence of undernutrition. Indeed, patients were managed according to international guidelines of the Surviving Sepsis Campaign updated for the COVID-19 and our patients characteristics and outcomes were comparable to the ones of mechanically ventilated patients observed in large multicentric cohorts. To our knowledge, this study is the first to follow the prevalence and severity of malnutrition of patients hospitalized in ICU for a severe COVID-19 infection requiring IMV, during both the acute phase and until 3 months during the recovery phase of the infection. The high prevalence of malnutrition and its slow recovery after ICU discharge emphasize the need to evaluate the nutritional status of patients not only during the acute phase of the SARS-CoV-2 infection but also after discharge in order to provide adequate nutritional and physical support.
5. Conclusion
Severe malnutrition is frequent in critically-ill COVID-19 patients requiring mechanical ventilation and even increases up to one month after ICU discharge, regardless of nutritional support during and after ICU discharge. Three months after ICU discharge, more than half patients still suffer from malnutrition. It seems necessary to provide constant nutritional care to these patients on a multimodal basis and in a prolonged manner.
Author contributions
C. Rives-Lange: Conceptualization, Methodology, Formal analysis, Writing – original draft; A. Zimmer: Investigation, Formal analysis, Writing – original draft; A. Merazka: Investigation; C. Carette: Investigation, Writing – review & editing; A. Martins-Bexinga: Investigation; C. Hauw-Berlemont: Investigation, Writing – review & editing; Emmanuel Guerot: Investigation; AS. Jannot: Formal analysis, Writing – review & editing; JL. Diehl: Writing – review & editing; S. Czernichow: Conceptualization, Methodology, Writing – review & editing; B. Hermann: Methodology, Investigation, Formal analysis, Writing – original draft.
Funding
None.
Conflicts of interest
The authors declare no conflict of interest in relation to this work.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Medica Atenei Parm. 19 mars. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czernichow S., Beeker N., Rives-Lange C., Guerot E., Diehl J., Katsahian S., et al. Obesity doubles mortality in patients hospitalized for severe acute respiratory syndrome coronavirus 2 in Paris hospitals, France: a cohort study on 5,795 patients. Obesity. déc 2020;28(12):2282–2289. doi: 10.1002/oby.23014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anker M.S., Landmesser U., Haehling S., Butler J., Coats A.J.S., Anker S.D. Weight loss, malnutrition, and cachexia in COVID-19: facts and numbers. J Cachexia Sarcopenia Muscle. déc 2020;31 doi: 10.1002/jcsm.12674. jcsm.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 7 avr 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Valla F.V., Baudin F., Gaillard Le Roux B., Ford-Chessel C., Gervet E., Giraud C., et al. Nutritional status deterioration occurs frequently during children's ICU stay∗. Pediatr Crit Care Med. août 2019;20(8):714–721. doi: 10.1097/PCC.0000000000001979. [DOI] [PubMed] [Google Scholar]
- 6.Puthucheary Z.A., Rawal J., McPhail M., Connolly B., Ratnayake G., Chan P., et al. Acute skeletal muscle wasting in critical illness. J Am Med Assoc. 16 oct 2013;310(15):1591. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 7.Kox M., Waalders N.J.B., Kooistra E.J., Gerretsen J., Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. J Am Med Assoc. 20 oct 2020;324(15):1565. doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. déc 2020;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. janv 2021;47(1):60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cederholm T., Jensen G.L., Correia M.I.T.D., Gonzalez M.C., Fukushima R., Higashiguchi T., et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. févr 2019;10(1):207–217. doi: 10.1002/jcsm.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. févr 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. juin 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L., et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. juin 2020;74(6):871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouget A., Vardon-Bounes F., Lorber P., Vavasseur A., Marion O., Marcheix B., et al. Prevalence OF malnutrition IN COVID-19 inpatients: the NUTRICOV study. Br J Nutr. 21 déc 2020:1–24. doi: 10.1017/S0007114520005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedock D., Bel Lassen P., Mathian A., Moreau P., Couffignal J., Ciangura C., et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN. déc 2020;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lew C.C.H., Yandell R., Fraser R.J.L., Chua A.P., Chong M.F.F., Miller M. Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review. J Parenter Enter Nutr. juill 2017;41(5):744–758. doi: 10.1177/0148607115625638. [DOI] [PubMed] [Google Scholar]
- 17.Virgens I.P.A., Santana N.M., Lima S.C.V.C., Fayh A.P.T. Can COVID-19 be a risk for cachexia for patients during intensive care? Narrative review and nutritional recommendations. Br J Nutr. 5 nov 2020:1–9. doi: 10.1017/S0007114520004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caccialanza R., Laviano A., Lobascio F., Montagna E., Bruno R., Ludovisi S., et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. juin 2020;74:110835. doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeejeebhoy K.N., Keller H., Gramlich L., Allard J.P., Laporte M., Duerksen D.R., et al. Nutritional assessment: comparison of clinical assessment and objective variables for the prediction of length of hospital stay and readmission. Am J Clin Nutr. 1 mai 2015;101(5):956–965. doi: 10.3945/ajcn.114.098665. [DOI] [PubMed] [Google Scholar]
- 20.COVID -19 Consortium. Van Aerde N., Van den Berghe G., Wilmer A., Gosselink R., Hermans G. Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med. nov 2020;46(11):2083–2085. doi: 10.1007/s00134-020-06244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]