Abstract
Adult dolphins are capable of sleeping with one eye open and exhibiting slow wave activity in the electroencephalogram (EEG) of one hemisphere at a time. The aim of this study was to examine the postpartum sleep behavior of bottlenose dolphin calves and their mothers. The behavior of three dolphin mother-calf pairs was monitored from birth to 13 months postpartum. Dolphin mothers and their calves exhibited a complete disappearance of rest at the surface for a minimum of 2 months postpartum, swimming in echelon formation on average 97–100% of the observation time. Calves surfaced to breathe more often than their mothers between the postpartum age of 2 and 8 weeks. During the first postpartum month two dolphin mothers surfaced with both eyes open on average in 93 and 98% of the time while in their calves both eyes were open in 90 and 60% of the cases. In calves, the eye directed toward the mother was open more often (on average 95% of all observations in calf 1 and 99% in calf 2) than the eye directed to the opposite side (82% in calf 1 and 60% in calf 2). Our data indicate that dolphin mothers and calves are highly active and vigilant during the initial period of the calf’s life, continuously monitoring their position relative to each other by sight during wakefulness and sleep. We hypothesize that episodes of EEG slow wave activity at this time are likely to be brief, fragmenting EEG defined sleep into short episodes.
Keywords: Bottlenose dolphin, postpartum behavior, calf, rest, sleep, EEG, cetaceans
1. Introduction
All terrestrial mammals studied to date exhibit minimal activity and maximal total sleep amounts at birth, with sleep gradually decreasing, and activity gradually increasing to adult levels as the animal grows to maturity [1]. In contrast to terrestrial mammals, killer whale (Orcinus orca) and bottlenose dolphin (Tursiops truncatus) neonates are highly active after birth, with their mothers not resting at the surface during the first 3 weeks postpartum [2,3]. This surface behavior is a common rest behavior in captive killer whales and dolphins [2–7]. Previous studies have noted a high level of activity in dolphin calves when compared to their mothers but did not examine this behavior in detail [8,9].
Terrestrial mammals have been shown to remain immobile during sleep with both eyes closed at all times. However, dolphins are capable of sleeping during swimming and exhibiting slow wave activity in one hemisphere while the other hemisphere displays low voltage activity [10]. The eye which is opposite to the sleeping hemisphere is predominantly closed or in an intermediate state while the eye opposite to the waking hemisphere is more frequently open [11–13]. To date, an association between rest behavior and eye state, in freely swimming dolphins, has been studied in only one species – the pacific white-sided dolphin (Lagenorhynchus obliquidens, [14]). During slow echelon swimming, when dolphins switched their positions, they also switched eye state in such a way, that the open eye was always directed toward their schoolmates. Similar, but limited, observations were also obtained from bottlenose dolphins [8,9]. Lilly [15] speculated that dolphins need to keep one eye open to scan their environment to avoid predation, while Goley [14] suggested that the open eye allows them to maintain visual contact with group members. Sekiguchi and Kohshima [9] suggested that captive bottlenose dolphins keep one eye open to avoid collisions with the pool wall. Although these hypotheses differ in minor details, they clearly emphasize the sentinel function of the asymmetrical eye state in sleeping dolphins and the ability of dolphins to be asleep and vigilant simultaneously.
The maintenance of visual contact should be of even greater importance to cetacean mothers and their newborn calves. However, the behavioral aspects of sleep in dolphin neonates and their mothers have not been examined in detail. In the present study we paid particular attention to the eye state of bottlenose dolphin mothers and their calves, as this parameter has been shown to be a useful indicator of behavioral state [12].
2. Materials and methods
2.1. Subjects and experimental conditions
The behavior of three bottlenose dolphin calves and their mothers was videotaped and visually monitored from birth to 13 months of age. The behavior of 2 non-pregnant females, one pregnant female, and one adult male was also examined for comparison. The observations were conducted in accordance with the National Institute of Health Guide for the Care and Use of Experimental Animals and were approved by VA GLAHS Sepulveda and UCLA Animal Research Committees. The characteristics of the dolphins and the timing of the observations are presented in Table 1.
Table 1.
Time and duration of video-recording and visual observations.
| Dolphins | Status at the time of observations | Time resident in the facility and age | Video-recording: postpartum age (for pairs) and duration | Eye state observations: postpartum age and duration |
|---|---|---|---|---|
| Female 1 | Mother | 1/10 years | 1–3 days: 58h incl. 3 nights (22–07h) 14 days: 1 night (22–07h) 28 days: 2 nights (22–07h) 57 days: 48h incl. 2 nights (22–07h) |
3–10 weeks: for 3–5h continuously between 05–21h. |
| Calf 1 | Born to female 1 | ≤8 weeks | ||
| Female 2 | Mother | 5/9 years | 2–4 days: 40h incl. 3 nights (22–07h) 8–9 days: 2 nights (22–07h) 17 days: 36h incl. 2 nights (22–07h) 28 days: 2 nights (22–07h) |
2–4 days: 2 nights (00–06h) 8–9 days: 2 nights (00–06h) 28–29 days: 2 nights (00–06h) |
| Calf 2 | Born to female 2 | ≤5 weeks | ||
| Female 3 | Mother | 8/13 years | 3–6 days: 24h incl. 3 nights (21–06h) 20 days: 3 nights (22–07h) 15 weeks: 3 nights (22–07h) 54 weeks: 2 nights (22–07) |
- |
| Calf 3 | Born to female 3 | ≤54 weeks | ||
| Female 2 | Pregnant | 5/9 years | 36h, incl. 2 nights (22–07h) | - |
| Female 4 | Nonpegnant | 4/8 years | 36h, incl. 2 nights (22–07h) | - |
| Female 5 | Nonpregnan | 4/6 years | 36h, incl. 2 nights (22–07h) | - |
| Male | Father of calves 2,3 | 9/18 years | 36h, incl. 2 nights (22–07h) | - |
Pair 1 was housed at the Utrish Marine Station of the Russian Academy of Sciences (Novorossiysk, Russia) in a circular tank 13 m in diameter and 4.5 m deep. This pair was alone in the pool during the first two months postpartum and together with two adult females during most of the 3rd month after that. Pairs 2 and 3 were housed at the Gelendgick Dolphinarium (Gelendgick, Russia) in an indoor rectangular pool, 25 m × 14 m, with depth ranging from 1.5 to 3.5 m. Calves 2 and 3 were born in the dolphinarium, 15 months apart. Each pair was kept alone in the pool during the first 3 days postpartum. Afterwards, both pairs were housed together with 2–3 adult dolphins.
Over the entire observation period, the illumination was natural during the daytime at both sites. In order to observe the dolphins at night, additional illumination was turned on above the pools (<200 Lux at the water surface in Pair 1 and approximately 300 Lux in pairs 2 and 3) during the night (20:00–08:00). Dolphins were adapted to this level of illumination because it was set by the dolphinarium at least 1 month prior to each calf’s birth to continuously monitor the dolphin female during the last portion of her pregnancy. The lights continued to be on at night during the first month postpartum for continuous observation of the neonate performed by the personnel of the dolphinarium and 2 days prior to each subsequent series of observations after that, at our request.
The pool at Utrish (pair 1) was cleaned and refilled with fresh seawater 2–3 times per week. The dolphins were fed 3–4 times per day between 08:00 and 20:00. This pair was not disturbed at any other time. Dolphins housed in the Gelendgick Dolphinarium (pairs 2 and 3) were trained and fed 3–4 times per day between 9:00 and 19:00 and were not disturbed between 20:00 and 07:00. During the daytime, all dolphins were generally active at this site because of the presence of personnel and visitors in the vicinity of the pool. Females 2 and 3 participated in 1-h shows, 1–5 times per day depending on the season, starting at the postpartum age of 2 weeks. The temperature of water in the pool varied between 20 and 24 °C in Utrish (pair 1) and between 18 and 22 °C in Gelendgick (pairs 2 and 3). However, the temperature did not change more than 2 °C within one night.
2.3. Video recording and observation of behavior
Behavior of dolphin mothers and their calves was observed (videotaped and visually monitored) both during the day and nighttime at different postpartum ages beginning from several hours after birth until calves were 13 months of age (Table 1). The behavior of female 2 (mother of calf 2) was also videotaped for 2 consecutive nights when she was pregnant (2 months prior to the calf’s birth). We also monitored the behavior of 2 non-pregnant females and one adult male when they were housed in the same pool for 2 consecutive nights.
2.3. Observation of eye state
In addition to videotaping the dolphin behavior, the state of both eyes was documented in two mother-calf pairs (1 and 2) from 1 week to 3 months postpartum during slow resting circular swimming. To accomplish this, two observers took positions at opposite ends of the pool. On average, in 74% of all observations, the state of eyes was noted when dolphins surfaced to breathe and in the remaining cases when the dolphins swam near the observers close to the water surface. Observation of eye state in pair 1 was conducted between 05:00 and 22:00 for 3–5 hours per day. The time of observations in this pair was changed randomly from day to day, so that the entire observation period (05:00–22:00) was covered two times at the postpartum age of 3–4 weeks and two times at the age of 7–10 weeks. Recording of eye state in pair 2 was performed during most of the nighttime (00:00–06:00). The detailed timing of the observations is given in Table 1. During analysis we considered only those cases in which two eyes were observed within an interval of less than 15 sec. The eye states in pair 1 were analyzed separately for the postpartum ages of 3–4 and 7–10 weeks. The eye state data for pair 2 were calculated separately for 3 postpartum ages (1, 2 and 4 weeks) based on the data collected during two consecutive nights in each case. The total eye state observation time was 53 hours in pair 1 and 48 hours in pair 2.
2.4. Scoring of behavior
The behavior of all dolphins was scored from videotapes in real time as active swimming, circular swimming, and floating (or resting) at the surface if an uninterrupted episode continued for at least 10 sec, as described in other publications [5–7,9,16]. Active swimming was marked by variable speed, irregular trajectory, and evident features of activity, such as eating, association with conspecifics, emission of sounds, etc. Circular swimming was scored when dolphins swam at least one complete circle without evidence of active behavior as described above. Dolphins were considered floating and resting at the surface if they stopped at the surface with their dorsal fin above the water or slowly drifted without evident paddle movements. We also continuously noted respiration acts of the mothers and their calves.
2.5. Data analysis
The high level of daytime activity (approximately between 08:00 and 21:00) in dolphins housed in the Gelendgick Dolphinarium was confirmed with 24-h video recordings. Therefore, only the nighttime data collected between 22:00 and 07:00 was used for comparison. All data for the dolphin mothers and their calves were grouped by weeks of postpartum age studied (e.g., animals of 1–7 days of age were grouped into the 1 week category). Average numbers are reported as mean ± standard error (SE). Analyses of several thousands of individual breaths in dolphin mothers and their calves were averaged to create the means, comprised ten 20-minute intervals (5 per each of two consecutive nights in the series of observations), each started at the beginning of every hour between 01:00 and 05:00. This averaging technique was used to avoid the inflated degrees of freedom resulting from very large sample sizes. Differences in the mean breathing pauses between the calves and their mothers were evaluated by paired T-test. We also calculated the percentage of apneas (breathing pauses longer than 40 sec). Dolphin pairs 2 and 3 rarely exhibited breathing pauses longer than 60 sec (<3% of the total number of all pauses in females 2 and 3). Therefore, the 40-sec criteria was chosen because this apnea duration was found to be approximately twice as long as the average breathing pause in pairs 2 and 3 and 1.5 times longer in pair 1 during most observation periods. One and two way ANOVA was performed to assess the difference between the state of two eyes in dolphin mothers and their calves. Because of the small number of subjects and the fact that the observations were conducted at different experimental conditions we were limited in the usage of statistical tests.
3. Results
3.1. Continuous swimming
Although captive adult dolphins spend a significant portion of time resting at the surface, this behavior was not observed in dolphin mothers during the initial postpartum period. Calves swam in continuous formation with their mothers at all times. Each pair often changed the trajectory and speed of swimming (especially during the first 2 weeks postpartum), and displayed an immediate orienting response to changes in the behavior of other animals and to all events in the vicinity of pool (e.g., presence of personnel). The calves also frequently changed positions next to their mothers.
Mother 1 was not observed floating at the surface throughout the entire period of observation except for a few stops at the surface at 8 weeks postpartum (Table 2). Two other mothers resumed resting at the surface (floating time was greater than 1% of the nighttime) when their calves were 3 weeks of age (mother 2) or older (mother 3). In mother 2, rest at the surface occupied less than 3% of the nighttime between 3–4 weeks postpartum. The duration of rest at the surface was greatest in mother 3 at 15 weeks postpartum. However, the greatest measured amount of rest at the surface in mother 3 (22.4% of the nighttime) was significantly less than that observed for females without calves and in one adult male (52–73% of the nighttime). There was a considerable difference between the time spent resting at the surface in dolphin females without calves (females 2,4 and 5, on average 64.6±6.5% of the observation time) and in mothers 1–3 at the postpartum age of 1 week (on average 0.2±0.2%, n=3), 2–4 weeks (0.9±0.5%). Calves 1 and 2 swam continuously during the entire observation period. The duration of time they briefly stopped at the surface was less than 0.1% of the nighttime. Calf 3 rested at the surface 3–4% of the observation time at the postpartum ages of 15 weeks and 13 months. The total duration of floating time in this calf was only 18% of that of its mother. Because of the small number of subjects we did not have enough power to evaluate the significance of the developmental changes of the behavioral pattern in the studied calves and their mothers.
Table 2.
Total duration of the behaviors documented in adult bottlenose dolphins (females without calves, male, females with calves) and calves at different postpartum ages as percent of the nighttime.
| Dolphins | Age (weeks) / Status | Active swimming | Circular swimming | Rest at the surface |
|---|---|---|---|---|
| Pair 1 | ||||
| Mother | 1 | 6.4±3.2 | 93.6±3.2 | 0.0 |
| Calf | 6.9±3.2 | 93.1±3.2 | 0.0 | |
| Mother | 2 | 4.9±0.6 | 95.1±0.6 | 0.0 |
| Calf | 5.1±0.6 | 93.9±0.6 | 0.0 | |
| Mother | 4 | 4.4±3.1 | 95.6±3.1 | 0.0 |
| Calf | 4.7±3.1 | 95.3±3.1 | 0.0 | |
| Mother | 8 | 14.1±3.5 | 85.6±3.9 | 0.3±0.3 |
| Calf | 16.5±4.7 | 83.5±4.9 | 0.0 | |
| Pair 2 | ||||
| Mother | 1 | 22.8±2.4 | 76.7±2.7 | 0.5±0.3 |
| Calf | 31.7±0.7 | 68.2±0.8 | 0.1±0.1 | |
| Mother | 2 | 9.4±4.5 | 89.7±4.3 | 0.9±0.1 |
| Calf | 10.4±4.4 | 89.5±4.3 | 0.1±0.1 | |
| Mother | 3 | 14.8±5.9 | 82.6±6.0 | 2.6±0.3 |
| Calf | 27.3±3.6 | 72.7±3.6 | 0.0 | |
| Mother | 4 | 23.5±3.6 | 73.9±4.3 | 2.6±0.4 |
| Calf | 32.6±6.0 | 67.4±6.1 | 0.0 | |
| Pair 3 | ||||
| Mother | 1 | 2.6±1.2 | 97.4±1.2 | 0.0 |
| Calf | 9.0±2.0 | 91.0±2.0 | 0.0 | |
| Mother | 3 | 3.3±0.7 | 95.7±1.5 | 1.0±0.5 |
| Calf | 16.0±1.5 | 83.5±2.6 | 0.5±0.3 | |
| Mother | 15 | 12.9±3.5 | 64.7±10.0 | 22.4±2.0 |
| Calf | 41.8±7.8 | 54.1±8.9 | 4.1±0.7 | |
| Mother | 54 | 18.3±1.3 | 67.7±2.1 | 14.0±0.3 |
| Calf | 39.4±1.9 | 58.0±2.8 | 2.6±0.6 | |
| Female 2 | Pregnant | 27.0±2.6 | 0.0 | 73.0±2.6 |
| Female 4 | Nonpregnant | 31.9±2.9 | 16.4±1.2 | 51.7±1.8 |
| Female 5 | Nonpregnant | 28.8±2.2 | 2.2±0.6 | 69.0±2.8 |
| Male | - | 27.4±4.8 | 14.1±1.1 | 58.5±5.9 |
Each number is average of 2 consecutive nights (22.00–07.00). The values are presented as mean ± standard error (SE).
3.2. Breathing pattern
During the first two weeks postpartum, mothers and calves surfaced to breathe and submerged synchronously, breathing within 5 sec of each other on average in 90, 95 and 96 % of cases (pairs 1–3, respectively). In most of the remaining cases dolphin calves surfaced alone breaking the synchrony while the mother took a longer pause and continued swimming. Over at least the first 4 weeks postpartum, the mothers exhibited a greater number of apneas (pauses longer than 40 sec) compared to their calves, except at the age of 1 week in pair 1 (Fig. 1). The mean duration of breathing pauses in each calf was shorter than that in its mother at the same postpartum age, except at 1 week postpartum (Fig. 1). When the calves were between 2 and 15 week old the difference between the average breathing pause in the mother and her calf was statistically significant with T-test for 7 out of 8 comparisons. The mean breathing pause and percentage of apneas did not change substantially in the mothers and their calves during the first 8 weeks postpartum. Mother 3 noticeably increased the duration of intervals between surfacing for breathing and the percentage of apneas at the postpartum age of 15 weeks and older compared to 1–3 weeks postpartum.
Figure 1.
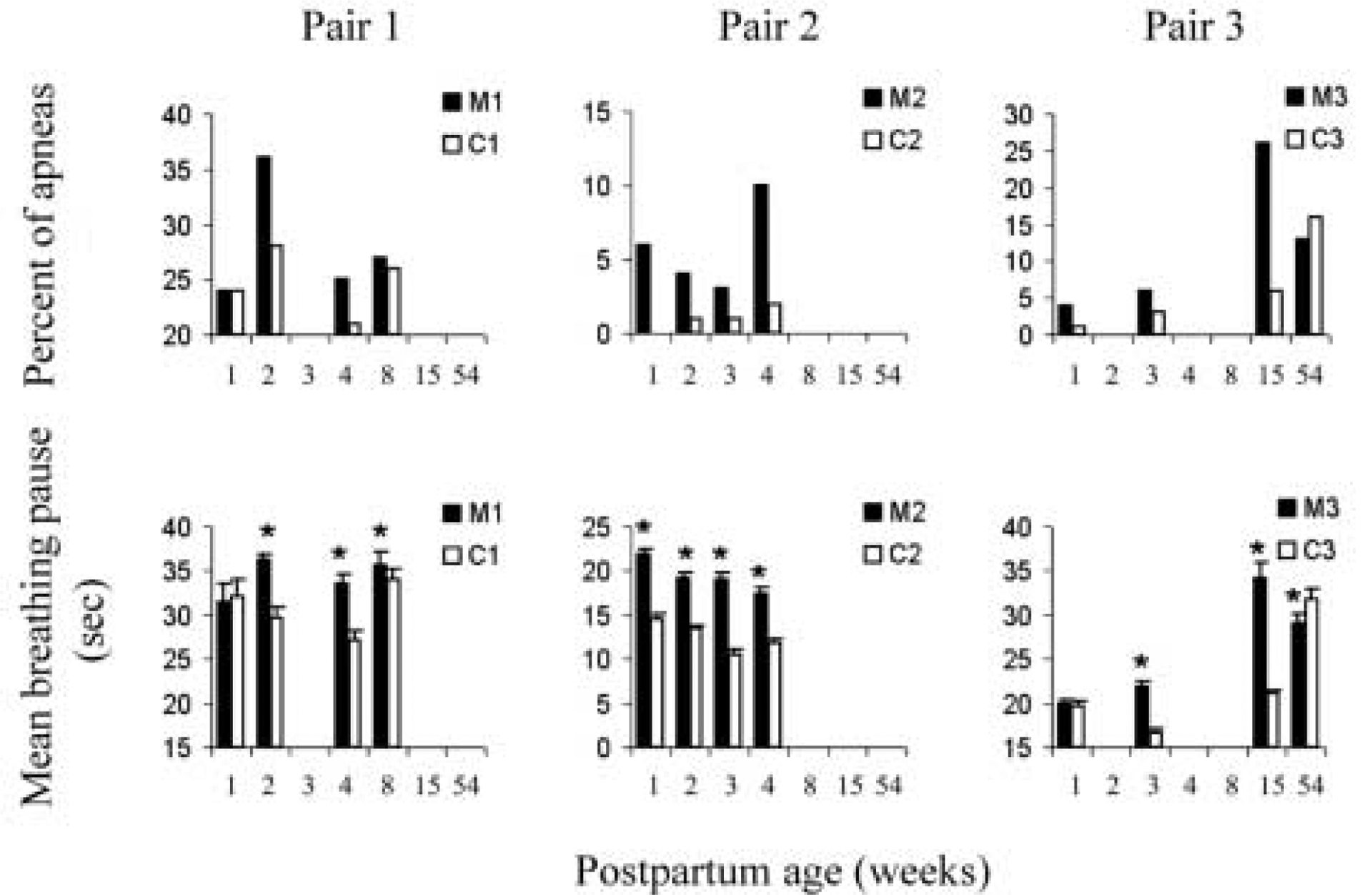
Characteristics of the breathing pattern in bottlenose dolphin mothers and their calves. Top diagrams show proportion of apneas (pauses longer than 40 sec) in mothers (M1-M3) and in calves (C1-C3) as percent of all breathing pauses during the observation period. The bottom diagrams are mean breathing pauses ± standard error (SE). The means are the average of 10 measurements as described in the methods. Asterisks indicate a significant difference (p<0.05) between mothers and their calves.
3.3. State of two eyes in dolphins mothers and calves
In pair 1 the state of both eyes was documented 870 times in the calf (on average 35 times per hour) and 600 times in the mother (44 times per hour). In pair 2 the state of two eyes was noted during 1140 times in the calf (on average 72 times per hour) and 1570 times in the mother (50 times per hour, 200–550 times / night) over the entire period. The average interval between observing both eyes was 11 sec in pair 1 (56% of all intervals in the calf and 86% of all intervals in the mother were shorter than 10 sec) and 7 sec in pair 2 (87% and 81% of the intervals were shorter than 10 sec in the calf and the mother, respectively).
During the entire period of observation, dolphin mothers were observed surfacing or swimming mostly with both eyes open (91–100% of the time during different observation periods, Fig. 2). During the first postpartum month, female 1 was observed with both eyes open in 93% of the cases (percentage of all observations at 3–4 weeks postpartum) and female 2 on average in 97.8±0.2% of the cases (3 periods of observations at 1–4 weeks postpartum). Over the entire period of observation, for both females, one eye was rarely seen in an intermediate state (on average 8% of the time in mother 1 and 2% in mother 2), while the other eye was open. Only on a few occasions (<0.4%) was one eye noted to be closed in mother 1 while the other eye was open.
Figure 2.
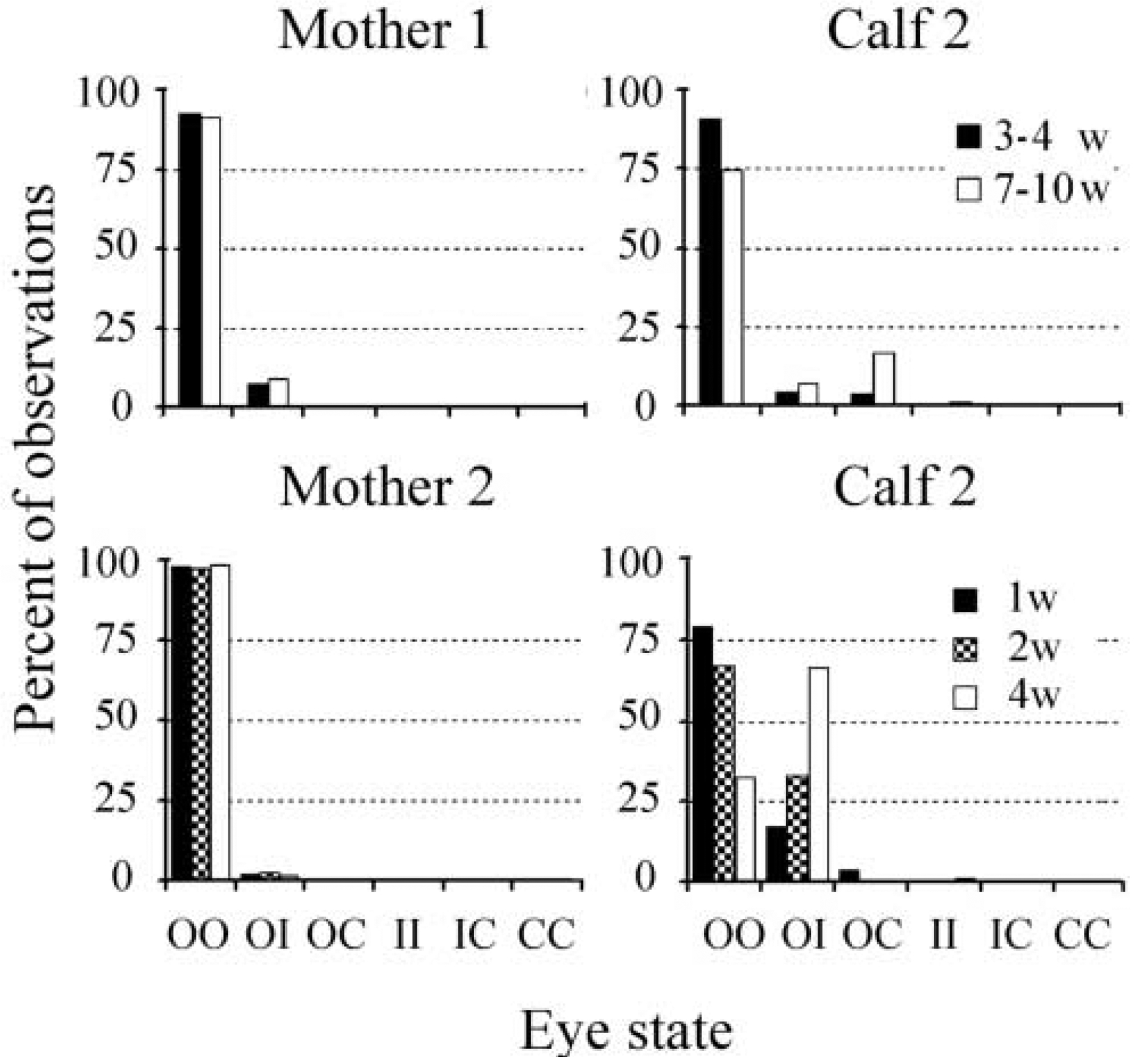
State of two eyes in two bottlenose dolphin mother-calf pairs (pair 1 and 2) at different postpartum age. The first letter signifies the state of one eye (O-open, I-intermediate, C-closed) and the second letter marks the state of the other eye. W is age in weeks. For each period of observation the data is presented as percent of all cases in which both eyes were seen simultaneously (in most cases within intervals less than 10 sec). There was no difference between the states of the right and left eye (see the text). Note that the amount of eye closure (complete or intermediate) increased during the first three months postpartum in both calves.
Calves also surfaced most often with both eyes open, particularly at younger ages (Fig. 2). In calf 1, which was observed mostly during the light hours, the percentage of instances of surfacing with both eyes open, at the postpartum age of 3–4 weeks, was similar to that of its mother (90% of the observation time in the calf and 93% in the mother). In the remaining cases, the calf was seen with only one eye in a closed or intermediate position while the other eye was open (4 and 5% of the observations, respectively). During several surfacings (approximately 1% of all cases) one eye was closed while the other eye was in an intermediate position. When the calf was 7–10 weeks old, the percentage of surfacings with both eyes open decreased by approximately 15%. At this time, the calf surfaced with one eye closed four times more often than when it was younger (4% at the age of 3–4 weeks and 17% at the age of 7–10 weeks). At the same time, the percentage of surfacings with both eyes open in the mother did not change compared to the period when the calf was 3–4 week old.
In calf 2, which was observed during the nighttime, the percentage of surfacing with both eyes open varied between 32 and 79% of the observation cases (on average 59±14% for 3 age periods) with the greatest value recorded at the calf’s youngest age (Fig 2). During other time calf 2 was seen with one eye open while the other eye was in an intermediate state (on average 39±14%, n=3), or occasionally closed (1.4±1.1%). Rarely (<2% of the observations on different nights) was calf 2 seen with both eyes in an intermediate position or with one eye in an intermediate state and the other eye closed. At all time points this calf was observed with two eyes open less frequently than its mother.
We found no difference between the percentage of cases when the right and left eyes were seen open in both mothers (one way ANOVA, mother 1: F1,2=1.81, p=.31, mother 2: F1,2=3.45, p=.14) and their calves (calf 1: F1,2=5.75, p=.14, calf 2: F1,2=7.71, p=.54) during different periods of observations. This indicates an absence of asymmetry in the state of two eyes in the calves and their mothers at the time of surfacing. Over the entire observation period, bilateral eye closure was not observed in mothers or their calves when they were engaged in slow circular swimming.
3.4. State of eyes and position of the dolphin mother and calf
Both mother-calf pairs swam predominantly in a counterclockwise direction (>97% of the observation time). During periods of slow circular swimming calves took position next to their mothers, swimming along either the inner or outer circle in bouts and tended to resume position on the same side, even when they surfaced to breathe alone. The asymmetry in eye state in the calf occurred in bouts. The eye state of calves changed depending on their position next to their mothers, in such a way, that the eye directed to the mother was open more often than the eye directed to the opposite side (Fig. 3). As shown in Table 3, in both calves the eye directed to the mother was predominantly open (91–97% of the observations in calf 1 and 99–100% in calf 2). The opposite eye (directed to the center of the pool or the pool wall) was observed open in a smaller proportion of cases (68%−93% in calf 1 and 35–90% in calf 2). In the remaining cases, the eye not directed to the mother was in an intermediate (on average 12±4% in calf 1, n=4, 2 eyes and 2 observation periods; 38±9% in calf 2, 2 eyes and 3 observation periods) or closed state (on average 5±1% and 1±1%, respectively in calf 1 and 2). When analyzed for the entire periods of observation, the difference in the state of two eyes in the calves reached significance for the factor “direction” (toward the mother or opposite side; two way ANOVA, F1,4=7.72, p=.05) but no difference was found between the state of the right and left eyes (F1,4=0.02, p=.89). The eyes in the dolphin mothers were open most of the time (Figure 2–3, Table 3). There was no difference between the states of the right and the left eye in both mothers regardless of whether the eye was directed to her calf or to the opposite side (Factor “direction”: F1,4=0.74, p=.43; Factor “eye”: F1,4=1.07, p=.35).
Figure 3.
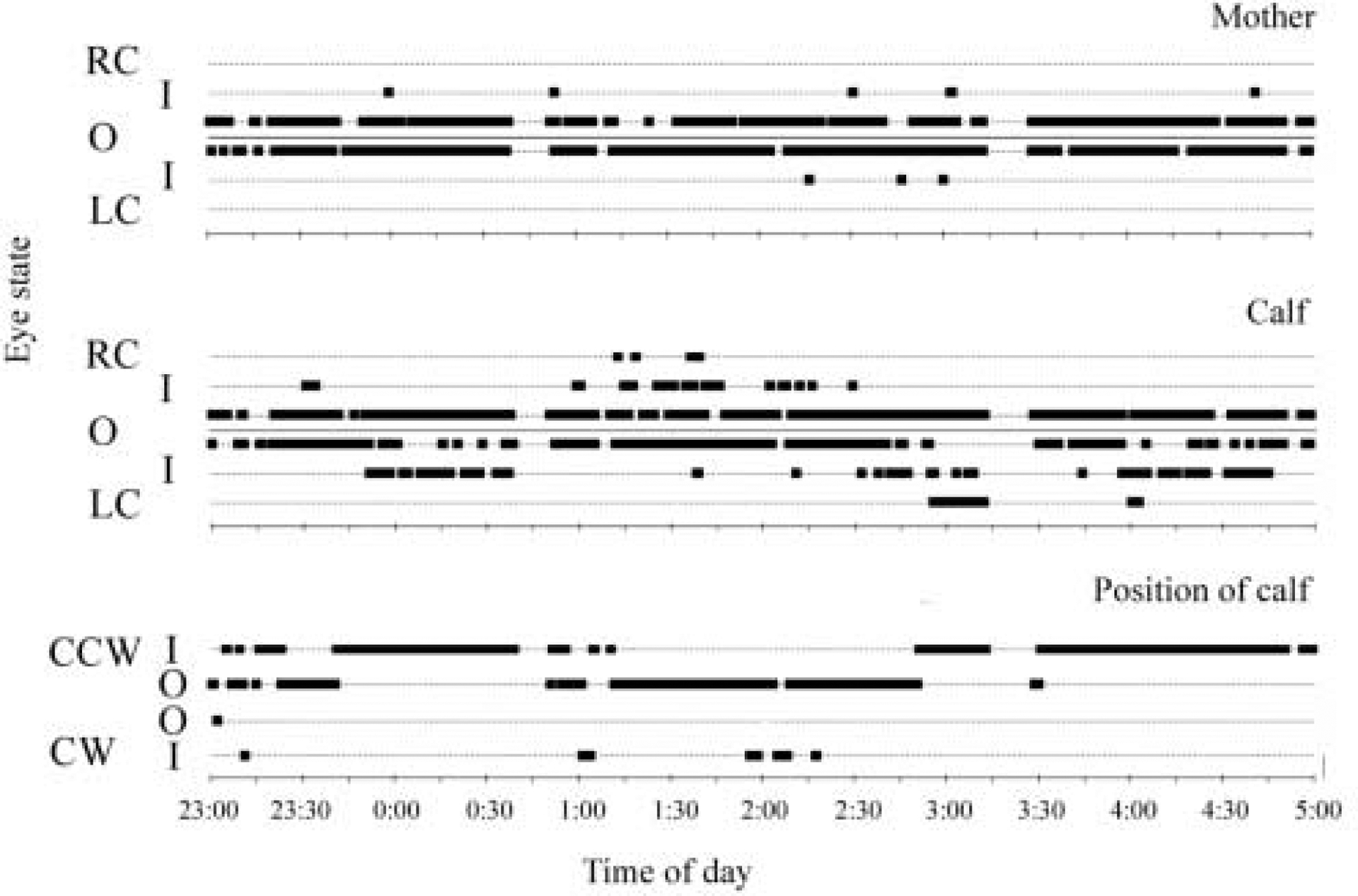
Simultaneous recording of eye state in a bottlenose dolphin mother and her calf (pair 2) during slow circular swimming. The state of each eye (R -right, L -left) is marked as open (O), closed (C) or intermediate (I). The position of the calf relative to its mother is shown as inner (I) or outer (O). The direction of swimming in the dolphin pair is marked as counterclockwise (CCW) or clockwise (CW). Interruptions in the record of eye state and calf position (at 0:45 and 3:15) represented gross periods of activity during which time dolphin eyes could not be continuously monitored. However, when visible, both eyes were open at these times.
Table 3.
State of eye in dolphin mothers and their calves as function of their relative positions and the age of calves.
| Dolphin pair / Age in weeks | Calves | Mothers | ||||||
|---|---|---|---|---|---|---|---|---|
| Eye directed to the mother | Eye directed to the opposite side | Eye directed to the calf | Eye directed to the opposite side | |||||
| Right | Left | Right | Left | Right | Left | Right | Left | |
| Pair 1 | ||||||||
| 3–4 w | 95% (137) | 97% (219) | 88% (206) | 93% (235) | 98% (221) | 96% (165) | 94% (164) | 90% (222) |
| 7–10 w | 91% (227) | 97% (256) | 68% (227) | 81% (311) | 97% (323) | 95% (312) | 96% (329) | 92% (321) |
| Mean (n=2) | 93±2 | 97±0 | 78±10 | 87±6 | 98±1 | 96±1 | 95±1 | 91±1 |
| Pair 2 | ||||||||
| 1w | 100% (412) | 99% (753) | 90% (605) | 60% (321) | 98% (548) | 99% (371) | 96% (348) | 100% (883) |
| 2w | 100% (450) | 100% (490) | 79% (424) | 55% (429) | 100% (321) | 100% (477) | 95% (307) | 100% (610) |
| 4w | 99% (399) | 99% (265) | 42% (404) | 35% (253) | 100% (148) | 100% (179) | 99% (163) | 95% (65) |
| Mean (n=3) | 100±0 | 99±0 | 70±15 | 50±8 | 99±1 | 100±0 | 97±1 | 98±2 |
| Mean for both eyes | ||||||||
| Pair 1 (n=4) | 95.0±1.4 | 82.5±5.4 | 96.5±0.6 | 93.0±1.3 | ||||
| Pair 2 (n=6) | 99.5±0.2 | 60.2±9.5 | 99.5±0.4 | 97.5±1.1 | ||||
The data are presented as percentage of the cases in which the eye was seen open. The total number of observations is given in parenthesis. The average values are mean ± standard error (SE).
4. Discussion
Continuous swimming is a remarkable feature of the dolphin neonate and their mother behavior as shown in this study. High level of activity of bottlenose dolphin calves and almost complete absence of rest at the surface for several postpartum months were mentioned in several publications (e.g, [8,9,17,18,19]) but have not been documented in detail prior to this study. We [2,3] and others [20] noted that similar changes occur in killer whales. All these data have important implications for our understanding the functions of sleep in cetaceans and of sleep in general.
Sleep as defined by EEG slow waves can occur in cetaceans during floating at the surface, slow stereotypic swimming, and also while lying on the bottom [10, 21]. Near continuous swimming appears to be characteristic of all small adult cetaceans and their calves (e.g., the Commerson’s dolphin, Cephalorhynchus commersonii [22]; harbor porpoise, Phocoena phocoena [23,24]; white side dolphin [14]; Amazonian river dolphin, Inia geoffrensis [25]). All these species weigh less than 150 kg [26]. Almost continuous swimming is also a behavioral feature of calves of larger size cetacean species and their mothers (e.g., the bottlenose dolphin and killer whale [2,3]; 1 month old calves weight 50–80 kg and 150–200 kg, respectively, with lengths less than 1 and 2 m [26]), indicating that sleep in all these animals must occur during swimming. In contrast, long periods of immobility while floating at the surface or lying on the bottom of pools are characteristic of all large cetaceans studied to date (e.g. beluga, Delphinapterus leucas [27], killer whale [2,3], gray whale, Eschrichtius robustus [28]; adult belugas weigh 500–1400 kg, killer whales weigh 2600–9000 kg, and gray whales reach 35000 kg [26]). Rest at the surface has also been reported to be among the most typical sleep behavior in middle size cetaceans such as adult bottlenose dolphins in captivity [4,5,7,9,16]. As we showed in this study it may occupy between 50 and 70% of the nighttime in adult dolphins. The available data suggests that the length and weight of the animal, rather than the developmental status of dolphins and whales, determine whether continuous swimming occurs or not. Therefore, according to behavioral criteria, large dolphins and whales more closely resemble terrestrial mammals, indicating that motion is not a necessary feature of sleep for all cetacean species.
One obvious danger for infant dolphins born in captivity is collision with the walls of the pool. This happened to one of the calves in this study, several hours after its birth, when the mother made a sharp turn too close to the corner of the pool. However, we did not see any evidence that dolphin (this study) or killer whale [3] mothers tried to prevent their calves from swimming outside of the circle followed by the mother, where the calves could potentially hit the wall. As we mentioned in the results section, all 3 dolphin mothers swam predominantly in a counterclockwise direction. At the same time the calves swam an approximately similar amount of time both along the inner and outer circles (Fig 3). It is also important to emphasize that both dolphin and killer whale mothers resumed resting at the surface when their calves still remained active almost 24/h/day, swimming near their mothers or joining other dolphins swimming at that time. There is no question that calves followed their mother but the data available suggest that dolphin calves also have an “internal drive” for continuous swimming during the initial period of their lives.
Although several studies have reported continuous swimming in captive cetaceans, we are aware of only one study by Mann and Smuts [29], which attempted to quantify rest behavior in dolphin infants in the wild. This was done in Shark Bay, Australia. In this study, rest behavior was defined as “slow travel, < 2mph, frequent floating at the surface, frequent direction changes” which is a more general definition than the criteria we used in this study. It has been reported that neonatal bottlenose dolphins did not stop moving for more than a few seconds during the first postpartum week, however older calves floated on the surface for up to several minutes. These data are in full agreement with ours, indicating that continuous swimming is characteristic of infant dolphins born both in captivity and in the wild. On the other hand, when the data collected in Shark Bay were averaged for the whole period of observation (postpartum age of 1–10 weeks) the rest time in the calves occupied on average 21 and 50% of the time in two areas of observations (non-provisioning and provisioning). These data indicate that calves in the wild may spend a larger portion of time resting at the surface for reasons unknown to us. On the other hand, the estimate of the total duration of rest behavior in wild calves can not be directly compared with our data on captive animals because a great deal of slow swimming was included in the rest behavior category in the study by Mann and Smuts [29].
The essential point we emphasize here is that the bottlenose dolphin and killer whale mothers and their calves become “obligate swimmers” for the initial postpartum period displaying only one of the cetacean sleep behaviors. Therefore, if EEG defined sleep occurs during the initial period of the calf’s life, it must occur during swimming. Continuous swimming may be required for thermoregulation [12,30] because cetacean neonates have larger surface area to volume ratios than adults and their blubber is not specialized to provide enhanced thermal insulation [31]. Both factors result in greater amounts of heat loss to the environment compared to that in adults. Neonates also appear not to be able to control buoyancy well and are therefore unable to easily float at the surface as adults do [3, 29]. Although we do not know exactly what physiological mechanisms trigger continuous swimming in cetacean mothers and their calves, the ability to remain active and responsive after birth has several advantages for newborn cetaceans. Increased motor activity likely reduces the risk of predation in the wild, and helps the calves to maintain body temperature until their body mass has increased and their insulating blubber has developed. Accordingly, it is reasonable to suggest that large cetaceans do not need to move as much as calves and smaller dolphins do because of enhanced insulation and buoyancy.
The second major finding of the present study is that dolphin mothers and their calves continuously monitored their position relative to each other by sight. This occurred both during active waking and slow resting swimming. Asymmetrical eye closure has been recently observed in slowly swimming bottlenose dolphin neonates and their mothers in a different study [18]. Similar observations were conducted earlier in adult dolphins of two cetacean species – the pacific white-sided dolphin [14] and bottlenose dolphin [8,9,15]. Asymmetrical eye closure was also documented in solitary held adult bottlenose dolphins [12], belugas [11,27] and in a 1-year old gray whale [28] while they were resting at the surface or lying on the bottom of pools. Electrophysiological studies have indicated that the studied beluga and dolphins had unihemispheric EEG slow waves during a significant portion of this time [12,21]. Therefore, maintenance of visual contact with conspecifics or monitoring of the environment is another distinctive feature of cetacean sleep behavior.
Asymmetrical eye state has been shown to be indicative of sleep (unihemispheric or highly asymmetrical slow wave sleep) in adult bottlenose dolphins and belugas with a probability of 80 and 91%, respectively [12]. Electrophysiological studies in the same cetacean species also showed that opening of both eyes is highly indicative of waking (approximately 80% of the time when both eyes were open). Therefore, we can hypothesize that episodes of swimming followed by surfacing with one eye closed and the other eye open, observed in both calves in this study, may have been accompanied by slow waves in EEG during at least some portion of this time. Swimming with asymmetrical eye states in the mothers was rarer than in their calves. These data suggest a high degree of vigilance and a low amount of sleep in two of the studied dolphin mothers during at least the first two months postpartum.
Another feature of continuous swimming in bottlenose dolphin and killer whale neonates is more frequent surfacing for breathing compared to their mothers. Similar findings have been obtained in the wild [29]. Electrophysiological studies have demonstrated that breathing acts in dolphins are fully compatible with uninterrupted unihemispheric EEG sleep [10]. However, the majority of this data were collected in solitary dolphins floating and swimming in shallow pools. The behavior of dolphins under these conditions is stereotypic and monotonous, and the level of their vigilance is likely reduced compared to that in the wild and in females with calves. Our recent recordings indicate that the amplitude of slow wave EEG frequently decreased at the time of respiratory acts in one beluga, which was floating at the surface (unpublished data). Therefore, the likelihood of EEG defined awakening in sleeping cetaceans during surfacing for breathing appears to be related to the nature of the movements they must accomplish in order to surface and the social interactions between dolphins.
It is difficult to estimate total sleep time in freely swimming dolphins, on the basis of behavioral criteria. Asymmetrical eye state in dolphins engaged in resting behavior is the only known behavioral indicator of their sleep. While the opening of one eye and the closure of the opposite eye in dolphins and belugas is highly indicative of sleep, this association is less prominent when the eye state asymmetry is less expressed (one eye open and the other eye in the transitional state) [12]. As we see, dolphin neonate 2 surfaced with both eyes open in 80% of the cases at the postpartum age of 1 week (Fig. 2). It should be emphasized that these data were collected during the nighttime period when captive dolphins are less active compared to the daytime, when they are usually disturbed in captivity. In the majority of the remaining cases, one eye was open in this calf while the opposite eye was in an intermediate position, which is less indicative of sleep in dolphins. In only 5% of the cases one eye was open and the other was closed. Only full closure of one eye was shown to be indicative of sleep in dolphins, with a probability about 80% [12]. It is also quite intriguing that the percentage of surfacing with two eyes open (indicative of waking) was maximal in both calves at the youngest age. We believe that the presence of moderate light illumination at night might have only a minor quantitative effect on the state of eyes during surfacing because the dolphin mothers and calves were adapted to the exposed level of illumination. Moreover, the calves showed behavioral sleep under these conditions based on the presence of asymmetrical eye state during slow swimming next to their mothers.
The data we report here were collected on 3 mother calf-pairs, which is a small sample size. On the other hand, several trends we observed allow us to speculate that the total consolidated sleep time in dolphin neonates may be very small during at least the first 1–2 weeks postpartum. This would be the opposite to the developmental pattern in all previously studied terrestrial mammals, which displayed maximal amounts of rest, sleep and immobility after birth, gradually decreasing with age [1]. Experiments with deprivation of sleep in neonatal terrestrial mammals suggest that sleep serves important functions in developing animals and that a deficit of sleep early in development can produce long lasting behavioral, morphological and biochemical abnormalities [32,33]. Our data indicate that brain development appears to not require consolidated sleep in the cetacean neonate. It is hard to imagine that the calf was continuously asleep when it surfaced to breathe with two eyes open on average every 10–30 sec. Moreover, in cases where calves came to the surface alone breaking synchrony with their mother, they had to accelerate to catch up with their mother and to assume the echelon position. EEG synchrony occurring at this time would likely be brief, breaking sleep into short episodes. No known terrestrial mammal is capable of sleeping and being engaged in visually coordinated locomotion at the same time. Moreover, at least two species of dolphins have been reported to emit sounds while engaged in continuous swimming [18,34]. We neither excluded that EEG slow waves occur in calves and their mother while they were swimming at depth nor suggested this in our earlier publications that calves do not sleep at all [2,3]. However, based on our present knowledge it is very likely that the muscular activation and sensorimotor coordination seen in dolphin calves and their mothers is not possible without extensive activation of the brainstem and forebrain neurons. This is the opposite of what has so far been considered the most essential feature of sleep in terrestrial mammals [35,36]. Therefore, if slow wave EEG activity occurs in the dolphin cortex during the initial postpartum period, the accompanying motor behavior indicates that other brain areas must be active. This pattern is fundamentally different from the extended periods of slow wave sleep observed in terrestrial mammals.
Acknowledgments
The study was supported by DARPA (BAA G412F9034), NIH (NS42947), NSF (0234687) and Utrish Dolphinarium (Moscow, Russia). The authors wish to thank the staff of Utrish Dolphinarium Ltd., Utrish Marine Station (Severtsov Institute of the Russian Academy of Sciences, Novorossiysk, Russia), and Gelendgick Dolphinarium (Gelendgick, Russia) for the help and assistance during the observations. We are also thankful to J. Lapierre for helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zepelin H, Siegel JM, Tobler I. Mammalian sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Saunders; 2005, p. 91–100. [Google Scholar]
- 2.Lyamin OI, Shpak OV, Siegel JM. Ontogenesis of rest behavior in killer whales. Sleep 2003:26:116. [Google Scholar]
- 3.Lyamin OI, Pryaslova J, Lance V, Siegel JM. Animal behaviour: Continuous activity in cetaceans after birth. Nature 2005,435:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick JG. Relationship of sleep, respiration, and anesthesia in the porpoise: a preliminary report. Proc Natl Acad Sci U S A. 1969, 62:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanigan WF. Nocturnal behavior of captive small cetaceans. I. The bottlenose dolphin, Tursiops truncatus. Sleep Research 1974,3:84. [Google Scholar]
- 6.Flanigan WF. More nocturnal observations of captive small cetaceans. I. The killer whale, Orcinus orca. Sleep Research 1975,4:139. [Google Scholar]
- 7.Lyamin OI, Shpak OV, Nazarenko EA. Swimming types and budget of time in bottlenose dolphins in captivity. Biological proceedings of Kharkov State University 1999, 2:103–106 (in Russian). [Google Scholar]
- 8.Gnone G, Benoldi C, Bonsignori B, Fognani P. Observations of rest behaviours in captive bottlenose dolphins (Tursiops truncatus). Aquatic Mammals 2001,27:29–33. [Google Scholar]
- 9.Sekiguchi Y, Kohshima S. Resting behaviors of captive bottlenose dolphins (Tursiops truncatus). Physiol Behav 2003,79:643–653. [DOI] [PubMed] [Google Scholar]
- 10.Mukhametov LM, Supin AY, Polyakova IG. Interhemispheric asymmetry of the electroencephalographic sleep pattern in dolphins. Brain Res 1977,134:581–584. [DOI] [PubMed] [Google Scholar]
- 11.Lyamin OI, Mukhametov LM, Siegel JM, Nazarenko EA, Polyakova IG, Shpak OV. Unihemispheric slow wave sleep and the state of the eyes in a white whale. Behav Brain Res 2000,129:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyamin OI, Mukhametov LM, Siegel LM. Association between EEG asymmetry and eye state in Cetaceans and Pinnipeds. Arch Ital Biol 2004,142:557–568. [PMC free article] [PubMed] [Google Scholar]
- 13.Ridgway SH. Asymmetry and symmetry in brain waves from dolphin left and right hemispheres: some observations after anesthesia, during quiescent hanging behavior, and during visual obstruction. Brain Behav Evol 2002,60:265–274. [DOI] [PubMed] [Google Scholar]
- 14.Goley PD. Behavioral aspects of sleep in pacific white-sided dolphins (Lagenorhynchus obliquidens, Gill 1865). Mar Mamm Sci 1999,15:1054–1064. [Google Scholar]
- 15.Lilly JC. Animals in aquatic environments: adaptations of mammals to the ocean. In: Dill DB, editor. Handbook of Physiology – Environment, Washington, DC: American Physiology Society; 1964, p.741–747. [Google Scholar]
- 16.Mukhametov LM, Lyamin OI. Rest and active states in bottlenose dolphins (Tursiops truncatus). J Sleep Res 1994,3:174. [Google Scholar]
- 17.Gnone G, Moriconi T, Gambini G. Sleep behaviour: activity and sleep in dolphins. Nature 2006,441 (7096):E10–11. [DOI] [PubMed] [Google Scholar]
- 18.Sekiguchi Y, Arai K, Kohshima S. Sleep behaviour: sleep in continuously active dolphins. Nature 2006, 22,441 (7096):E9–10. [DOI] [PubMed] [Google Scholar]
- 19.Reid K, Mann J, Weiner JR, Hecker N Infant development in two aquarium bottlenose dolphins. Zoo Biology 1995, 14: 135–147. [Google Scholar]
- 20.Suchak M, Noonan M. Swimming speed and body position as a function of age on a captive neonatal killer whale (Orcinus orca). Abstract of the 16th Biennial Conference on the Biology of Marine Mammals. 2005:227. [Google Scholar]
- 21.Lyamin OI, Kosenko PO, Lapierre JL, Vyssotski AL, Lipp HP, Mukhametov LM, Siegel JM. Association between behavior and sleep in bottlenose dolphins. Abstract of the 16th Biennial Conference on the Biology of Marine Mammals 2005:174. [Google Scholar]
- 22.Mukhametov LM, Lyamin OI, Shpak OV, Manger P, Siegel JM. Swimming styles and their relationship to rest and activity states in captive Commerson’s dolphins. Abstract of the 14th Biennial Conference on the Biology of Marine Mammals, 2001:152. [Google Scholar]
- 23.Mukhametov LM. Sleep in marine mammals. Exp Brain Res 1984, Suppl.8: 227–327. [Google Scholar]
- 24.Oleksenko AI, Lyamin OI. Rest and activity states in female and baby of harbor porpoise (Phocoena phocoena). J Sleep Res 1996,5:159. [Google Scholar]
- 25.Mukhametov LM. Unihemispheric slow-wave sleep in the Amazonian dolphin, Inia geoffrensis. Neurosci Letters 1987,79:128–132. [DOI] [PubMed] [Google Scholar]
- 26.Perrin FP, Würsig B, Thewissen JGM. Encyclopedia of marine mammals. San Diego: Academic press, 2002. [Google Scholar]
- 27.Lyamin OI, Shpak OV, Nazarenko EA., Mukhametov LM. Muscle jerks during behavioral sleep in a white whale (Delphinapterus leucas L). Physiol Behav 2002,76:265–270. [DOI] [PubMed] [Google Scholar]
- 28.Lyamin OI, Mukhametov LM, Siegel JM, Manger PR, Shpak OV. Resting behavior in a rehabilitating gray whale calf. Aquatic mammals 2001,27:256–266. [Google Scholar]
- 29.Mann J, Smuts B. Behavioral development in wild bottlenose dolphin newborns (Tursiops sp). Behavior 1999, 136:529–566. [Google Scholar]
- 30.Pillay P, Manger PR. Testing thermogenesis as the basis for the evolution of cetacean sleep phenomenology. J Sleep Res 2004,13:353–358. [DOI] [PubMed] [Google Scholar]
- 31.Dunkin RC, McLellan WA, Blum JE, Pabst DA. The ontogenetic changes in the thermal properties of blubber from Atlantic bottlenose dolphin Tursiops truncatus. J Exp Biol. 2005, 208:1469–80. [DOI] [PubMed] [Google Scholar]
- 32.Mirmiran M, Scholtens J, van de Poll NE, Uylings HB, van der Gugten J, Boer GJ. Effects of experimental suppression of active (REM) sleep during early development upon adult brain and behavior in the rat. Brain Res. 1983, 283:277–86. [DOI] [PubMed] [Google Scholar]
- 33.Frank MG, Morrissette R., Heller HC. Effects of sleep deprivation in neonatal rats. Am J Physiol (Regulatory Integrative Comp Physiol) 1998, 275: 148–157. [DOI] [PubMed] [Google Scholar]
- 34.Pillery G, The blind Indus dolphin, Platanista indi. Endeavour 1979,3:48–56. [Google Scholar]
- 35.Siegel JM. Mechanisms of sleep control. Journal of clinical neurophysiology 1990, 7:49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel JM. REM sleep. In: Kryger MK, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine, Fourth Edition. New York: Saunders, 2005, p. 120–135. [Google Scholar]


