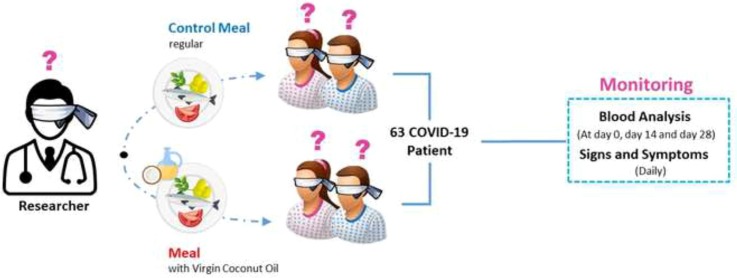
Graphical abstract
Keywords: COVID-19, Virgin coconut oil, C-reactive protein, Lauric acid, Monolaurin
Abstract
Understanding the complex pathogenesis of COVID-19 continues to evolve. With observation and quarantine as the prevailing standard of care, this study evaluated the effects of virgin coconut oil (VCO) in the biochemical markers of suspect and probable cases of COVID-19. A 28-day randomized, double-blind, controlled intervention was conducted among 63 adults in two isolation facilities in Santa Rosa City, Laguna, Philippines. The participants were randomly assigned to receive either a standardized meal (control) or a standardized meal mixed with a predefined dosage of VCO. Changes in clinical markers were measured at three time points (day 0, 14, and 28), with daily monitoring of COVID-19 symptoms. Participants in the intervention group showed a significant decline in the C-reactive protein level, with the mean CRP level normalized to ≤ 5 mg/dL on the 14th day of the intervention. As an adjunct therapy, meals mixed with VCO is effective fostering faster recovery from COVID-19.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory disease that has caused significant morbidity and deaths worldwide. Inflammation is normal in response to injury or pathogenic infection. COVID-19 infection can produce an excessive immune reaction in the host, known as a “cytokine storm” or an overproduction of immune cells and their activating compounds known as cytokines. This surge of activated immune cells causes lung inflammation and fluid build-up that can lead to respiratory distress. The inflammatory chemicals released during COVID-19 infection leads to hyperinflammation forcing the immune cells to destroy healthy cells. Inflammatory response has been associated with the disease severity in COVID-19 (Pamukcu, 2020).
To mitigate the transmission of the SARS Coronavirus2, the causative agent of this disease, current public health strategies towards COVID-19 focus on rapid identification of those who were exposed, followed by eventual isolation, contract tracing, and quarantine (Boulware et al., 2020). With the ongoing drug discovery initiatives, therapeutic options, such as repurposing of existing drugs or combination management regimens consisting of drugs or supplements, and the inclusion of immunomodulatory diet, proper mental support and adherence to standards are being studied and considered to manage and prevent COVID-19 (Lotfi, Hamblin, & Rezaei, 2020).
Considering that inflammation plays a significant role in COVID-19 pathology, it would be important to control hyperinflammation (Zabetakis, Lordan, Norton & Tsoupras, 2020). One intervention pathway is the reduction of viral infection of susceptible cells. Another pathway is the clearance of pro-inflammatory cytokines by anti-inflammatories (Fadai, Sachak-Patwa, Byrne, Maini, Bafadhel & Nicolau, 2021). Virgin coconut oil (VCO) has both antiviral and anti-inflammatory properties.
VCO, an edible oil obtained from the milk of fresh and mature kernel of coconut (Cocos nucifera L.), is largely consumed and used in cooking, bakery, confectionary, and infant foods (Ghani, Channip, Hwa, Ja’afar, Yasin, & Usman, 2018). Recently, VCO emerged as a health supplement owing to its medium-chain fatty acid (MCFA) contents that were found to show potential as anti-obesity treatment and were also shown to heal several minor illnesses such as diarrhoea, skin inflammations, and injuries, among others (Assunção et al., 2009, Nevin and Rajamohan, 2004, Nevin and Rajamohan, 2010). VCO is considered as GRAS (generally recognized as safe) by the US FDA (2020).
There are two types of coconut oil: refined, bleached, and deodorized copra oil (RCO) and virgin coconut oil (VCO). In essence, VCO is produced directly from the fresh endosperm of the coconut while RCO is obtained from copra and requires further processing using chemical treatment and heat. Both RCO and VCO have similar fatty acids profiles. However, VCO retains a higher content of bioactive compounds such as tocopherols, sterols, and polyphenols as refining removes a portion of these compounds (da Silva Lima, & Block, 2019).
Lauric acid (C12) accounts for about 50% of coconut oil by weight (Dayrit, 2015). Lauric acid and its biochemical derivative monolaurin (ML also known as glycerol monolaurate), are naturally released by lipase upon ingestion. The antiviral activity of VCO is attributed to both lauric acid and monolaurin and were found to cause disintegration of the virus envelope (Sands et al., 1979, HIERHOLZER and KABARA, 1982, Thormar et al., 1987), inhibit the late maturation stage in the virus replicative cycle (Bartolotta, García, Candurra, & Damonte, 2001), and prevent the binding of viral proteins to the host cell membrane (Hornung, Amtmann, & Sauer, 1994). Other components of the VCO such as capric acid (C10) and monocaprin also showed promising antiviral properties, particularly against HIV-1 infection (Kristmundsdóttir, Arnadóttir, Bergsson, G.; Thormar, 1999), and other infections caused by respiratory syncytial virus (RSV), human parainfluenza virus type 2 (HPIV2) and influenza-A virus (Hilmarsson, Traustason, Kristmundsdóttir, & Thormar, 2007).
Coconut oil and ML have been shown to have potential anti-HIV properties. In the first clinical trial using coconut oil and monolaurin as monotherapy for HIV, coconut oil (45 mL daily) and monolaurin (95% purity, 7.2–22 g daily) were given to individuals with HIV-AIDS. This study involved 15 HIV patients, aged 22 to 38 years, 5 males and 10 females, for 6 months. There was only one fatality and 11 of the patients showed higher CD4 and CD8 counts after 6 months (Dayrit, 2000). In another study, 40 HIV subjects with CD4 + T lymphocyte counts less than 200 cells/microliter were divided into a virgin coconut oil (VCO) group (45 mL daily) and control group (no VCO). After 6 weeks, the VCO group showed significantly higher average CD4 + T lymphocyte counts versus control (Widhiarta, 2016).
Although C12 accounts for much of the reported antiviral activity of coconut oil, capric acid (C10) and monocaprin have also shown promising activity against viruses, such as HIV-1 (Hornung, Amtmann, & Sauer, 1994). Hilmarsson and co-workers (1999) tested virucidal activities of fatty acids, monoglycerides and fatty alcohols against respiratory syncytial virus (RSV), human parainfluenza virus type 2 (HPIV2) and influenza A virus and reported that the most active compound was monocaprin. C10 accounts for about 7% of coconut oil. Thus, at least two fatty acids in coconut oil (C12 and C10) and their monoglycerides (monolaurin and monocaprin) have antiviral properties.
Moreover, literature studies suggest that medium chain fatty acids (MCFAs) and coconut oil metabolites influence many different aspects of the immune system, starting with their role on the epithelial lining of the intestinal lumen to cytokine secretion to fighting pathogens (Joshi, Kaushik, Gode, & Mhaskar, 2020).
Several published studies have used VCO as food supplement at doses ranging from 30 mL/day to 50 mL/day, for periods from 4 to 6 weeks (Chinwong et al., 2017, Khaw et al., 2018). The outcomes in all studies were favorable and no serious adverse effects were reported.
Although coconut oil and its derivatives have been shown to be safe and effective immunomodulatory agents in both humans and animals, reports on the efficacy of the VCO as used in human trials are few. With earlier reports of its efficacy against various viral infections, this study hypothesized that VCO could potentially be used as an adjunct prophylaxis to prevent the progression of symptoms among suspect or probable cases of COVID-19 in isolation facilities.
2. Materials and methods
The study was conducted in Santa Rosa Community Hospital Isolation Unit (SRCHIU) and Santa Rosa Community Isolation Facility (SRCIF). This was a randomized double-blind controlled intervention trial involving individuals who were considered as suspect and probable cases of COVID-19. The participants in the study were recruited on an enrolment basis taking into consideration the inclusion criteria. Participants were suspect or probable case, aged 20 years and over with no preference for sex, has been admitted ≤ 3 days at the isolation unit/facility, controlled hypertension, and normal or slightly elevated level of liver enzymes where the elevation is due to the administration of antibiotics. The exclusion criteria included: those with history of heart disease, taking statins or any related medications, with hyperlipidaemia, not suffering from COVID-19 signs and symptoms, and pregnant. All qualified cases were asked to provide consent before participating in the study. The study was conducted according to the guidelines of the Declaration of Helsinki, guided by the Council for International Organizations of Medical Sciences Ethical Guidelines for Biomedical Research Involving Human Subjects and the National Guidelines for Biomedical/Behavioural Research and approved by the Institutional Review Board (or Ethics Committee) of the Food and Nutrition Research Institute (protocol code FIERC-2020–009).
The study followed the guidelines of the Department of Health (DOH) in the Philippines for the identification of suspect and probable cases which are defined by Philippine Department of Health Administrative Order 2020–0013 (2020).
The calculated sample size was 56 (28 in each group) computed based on the following formula (Dayrit, 2000):
Where:
n = sample size in each group
µ1 = mean from baseline of CD4+ T lymphocyte = 300
µ2 = mean from endpoint of CD4+ T lymphocyte = 481
σ = standard deviation = 210
Zα/2: 95% confidence interval, alpha 0.05 this is 1.96
Zβ: This depends on power, for 90% this is 1.28
The research team adjusted the sample size to allow for an attrition of 8%. An online randomizer, Research Randomizer (Urbaniak, & Plous, 2013), was used to ascertain the grouping of participants to either Group 1: Intervention (VCO plus meal) or Group 2: Control (meals only).
Demographic information of participants was collected by the attending nurse upon admission in the isolation facility. Weight and height were measured at baseline (day 1), midline (day 14) and endpoint (day 28) using a calibrated Detecto Mechanical Eye-Level Physician Scale with Height Rod (Webb City, Mo. U.S.A.). Occurrence of signs and symptoms (cough, colds, boy aches, headache, loss of taste, fever) was monitored daily. Biochemical markers, in particular lipid profile, fasting blood sugar, liver function test (SGOT/SGPT), white blood cell count, neutrophil count, lymphocyte count, CD4 and C-Reactive Protein (CRP) level were measured at baseline, midline and endpoint.
Registered medical technologists (RMT) in the isolation facilities had extracted blood samples from all the participants via venipuncture vacuum-extraction method. To test the complete blood count (CBC) and platelet of the participants, ethylene diamine tetra acetic acid (EDTA) tube was used in the blood sample collection. For these particular haematological analyses, 2 mL of whole blood was collected. A serum tube with clot activator (gold tube) was used to collect 5 mL of blood to facilitate the analysis of fasting blood glucose (FBG), lipid profile (total cholesterol, HDL, LDL, triglycerides), aspartate aminotransferase (AST/SGOT) and alanine transaminase (ALT/SGPT). Samples for additional analysis such as the measurement of C-reactive protein (CRP) and CD4 count were collected using red top tube (4 mL) and EDTA tube (2 × 2 mL), respectively.
A Sysmex XN1000 (Sysmex Europe GmbH) analyzer was used for CBC analysis. In analysing the CRP level, a Dil-Architect c4000 (Abbott Germany) analyser was used to examine the samples while CD4 count was analysed using Alere Pima (Abbott Germany). Moreover, a Cobas 6000 (Roche, Germany) analyser was used to determine levels of FBG, AST/SGOT and ALT/SGOT, and lipid profile.
A serum CRP threshold of less than 5 mg/L was used to define normal values (WHO, 2020). The normal CD4 count range is between 500 and 1400 cells/microliters (Li, Duffee & Gbadamosi-Akindele, 2020).
The 28-day standardized menu were developed by DOST-FNRI. Meals for patients were prepared in a central contracted catering service away from the isolation facility. Project registered nutritionists – dietitians (RND) oversaw the preparations and coding of the meals. The VCO dosage to be mixed with the meals of the VCO group was based on the actual weight of the patient and the required VCO dosage per day. For day 1 to 3 the added VCO was 0.6 mL per kilogram body weight (kg BW) and was increased to 1.2 mL/kg BW for day 4 to 28. The dose was based on previous studies of HIV patients (Dayrit, 2000). For days 1 to 3, the VCO was only mixed with meals served at breakfast. For days 4 to 28, the calculated VCO was incorporated into meals at breakfast and lunch and dinner or snacks. After each meal, the RND conducted telemonitoring to estimate unconsumed food expressed in tablespoons. It was also during this period that signs and symptoms were monitored and recorded on a daily basis.
The VCO used in the study were strictly analysed by the Laboratory Services Division of the Philippine Coconut Authority (PCA) to ensure product quality and compliance to Philippine National Standards. VCO contained MCFA as the dominant fatty acids. Lauric acid was the most abundant MCFA (47.96%). Myristic acid was the second major fatty acid in VCO sample (20.26%). Palmitic acid (9.08%), caprylic acid (6.99%), capric acid (6.49%), oleic acid (5.18%), stearic acid (3.11%), and linoleic acid (0.81%) and caprioc acid (0.12%) were also identified.
The study endpoint/parameter was assessed at day 1 as baseline, at midline (day 14), and at endpoint (day 28). The absolute changes from baseline in the immune parameters were computed between the three measurement periods, i.e. baseline vs. midline and midline vs. end of intervention. The differences were compared between the two groups unpaired t-test while within groups difference between the periods of measurements were computed. The test of hypotheses used level of significance of 0.05. ANOVA was used to determine significant change between the three periods of measurements both within and between groups. Post hoc analysis was employed using Bonferroni test to determine the significant change in the different clinical biomarkers vs. period of measurements. Descriptive analysis was used to compute the mean and standard error of the anthropometric and biochemical variables. In all analyses, a 2-sided significance level of 5% (p-value < 0.05) was used to determine if the difference between the two treatment groups was statistically significant. The repeated measurements of anthropometric measures and biochemical variables were analysed using one-way repeated measures ANOVA, with Greenhouse-Geisser adjustment to the p-values to account for any violation of the “sphericity” assumption. Further, we performed one-factor ANOVA with Bonferroni to determine significant pairwise difference. Pearson’s chi-square test was used to determine the difference between the two intervention groups for qualitative variables or categorical variables. In the event of small cell frequencies, Fisher’s exact test was utilized. For the qualitative variables, the test of significance will only be exploratory and will not be conclusive. Independent t-test was used to compare the means between VCO and Control groups of anthropometric and biochemical variables. Paired t-test was used to compare two means that are from the same individual or related units. The two means typically represent two different times (e.g., pre-test and post-test with an intervention between the two time points). The purpose of the test is to determine whether there is statistical difference between paired observations.
3. Results
A total of 63 subjects qualified to participate in the study and were randomly allocated into the VCO group (n = 33) and Control group (n = 30). There were six dropouts in the study due to taste intolerance (n = 3) and work-related reasons (n = 3).
The diet of the control and the intervention groups were similar except that VCO provided an additional 34.2–120.48 calories in the intervention group (Table S1 and S2).
The mean ages for the VCO group and Control group were 32.9 and 29.9 years old, respectively. In both groups, most of the participants were single (82.8% in VCO and 78.6% in Control), had college degree (44.8% in VCO and 60.7% in Control) and were working (82.8% in VCO and 78.6% in Control) (Table 1 ).
Table 1.
Demographic characteristics of participants by group.
| Variables |
VCO (n = 29) |
Control (n = 28) |
p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Age, mean ± SE | 32.9 ± 1.7 | 29.9 ± 1.5 | 0.334 |
| Sex | |||
| Male | 15 (53.7) | 12 (42.9) | 0.449 |
| Female | 14 (48.3) | 16 (57.1) | |
| Civil Status | |||
| Single | 24 (82.8) | 22 (78.6) | 0.586 |
| Married | 5 (17.2) | 5 (17.9) | |
| Widowed | 0 | 1 (3.6) | |
| Educational Status | |||
| Elementary Level | 1 (3.5) | 1 (3.6) | 0.672 |
| High School Level | 10 (34.5) | 7 (25.0) | |
| College Level | 13 (44.8) | 17 (60.7) | |
| Vocational Level/Others | 5 (17.2) | 3 (10.7) | |
| Employment Status | |||
| Employed | 24 (82.8) | 22 (78.6) | 0.689 |
| Unemployed | 5 (17.2) | 6 (21.4) |
The mean weight of the participants in the VCO and Control groups at baseline were 64.9 and 66.4 kg, respectively. After the intervention, there was a significant increase of about 0.8 kg in the weight of the participants enrolled in the VCO group. The body mass index (BMI) of the VCO group significantly increased after the intervention but no significant difference between groups was observed (Table 2 ).
Table 2.
Mean anthropometric measurements of participants by group and time period.
|
VCO (n = 29) |
CONTROL (n = 28) |
p-value1 | |
|---|---|---|---|
| mean ± SE | mean ± SE | ||
| Weight, kg | |||
| Baseline | 64.9 ± 2.2 | 66.4 ± 3.4 | 0.706 |
| Midline | 65.4 ± 2.3 | 66.7 ± 3.4 | 0.748 |
| Mean Difference (p-value2) | 0.5 (0.055) | 0.3 (0.148) | 0.541 |
| Baseline | 64.9 ± 2.2 | 66.4 ± 3.4 | 0.706 |
| Endpoint | 65.7 ± 2.3 | 66.6 ± 3.4 | 0.839 |
| Mean Difference (p-value2) | 0.8 (0.045)* | 0.1 (0.601) | 0.164 |
| p-value3 | 0.047* | 0.384 | |
| Height, cm | |||
| Baseline | 160.9 ± 1.5 | 160.4 ± 1.4 | 0.821 |
| Midline | 160.8 ± 1.5 | 160.4 ± 1.4 | 0.858 |
| Mean Difference (p-value2) | 0.1 (0.326) | 0 (1.0) | 0.33 |
| Baseline | 160.9 ± 1.5 | 160.4 ± 1.4 | 0.821 |
| Endpoint | 160.8 ± 1.5 | 160.4 ± 1.4 | 0.846 |
| Mean Difference (p-value2) | 0.1 (0.536) | 0 (1.0) | 0.541 |
| p-value3 | 0.987 | 0.996 | |
| Body Mass Index, kg/m^2 | |||
| Baseline | 25.0 ± 0.7 | 25.9 ± 1.4 | 0.557 |
| Midline | 25.2 ± 0.7 | 26.0 ± 1.4 | 0.605 |
| Mean Difference (p-value2) | 0.2 (0.039)* | 0.1 (0.151) | 0.434 |
| Baseline | 25.0 ± 0.7 | 25.9 ± 1.4 | 0.557 |
| Endpoint | 25.3 ± 0.7 | 25.9 ± 1.4 | 0.685 |
| Mean Difference (p-value2) | 0.3 (0.029)* | 0 (0.573) | 0.141 |
| p-value3 | 0.029* | 0.389 |
Independent t-test,
Paired t-test,
Anova with repeated measure (baseline, midline, and endpoint).
significant at p < 0.05.
The mean Fasting Blood Glucose (FBG) of the Control group was significantly higher than the VCO group at baseline, midline and endpoint. There was no significant difference over time in both groups (Table 3 ).
Table 3.
Mean fasting blood glucose (FBG) of participants by group and time period.
|
VCO (n = 29) |
CONTROL (n = 28) |
p-value1 | |
|---|---|---|---|
| mean ± SE | mean ± SE | ||
| Fasting Blood Glucose, mg/dL | |||
| Baseline | 76.2 ± 2.1 | 83.1 ± 2.4 | 0.037* |
| Midline | 77.3 ± 2.1 | 79.2 ± 2.7 | 0.583 |
| Mean Difference (p-value2) | 1.1 (0.693) | 3.9 (0.133) | 0.191 |
| Baseline | 76.2 ± 2.1 | 83.1 ± 2.4 | 0.037* |
| Endpoint | 74.4 ± 3.0 | 83.2 ± 2.4 | 0.026* |
| Mean Difference (p-value2) | 1.8 (0.595) | 0.1 (0.969) | 0.678 |
| p-value3 | 0.618 | 0.293 |
Independent t-test,
Paired t-test,
Anova with repeated measure (baseline, midline, and endpoint).
significant at p < 0.05.
The mean LDL-cholesterol of the VCO and Control groups at baseline were 96.9 and 108.2 mg/dL, respectively. After the intervention, there was a significant increase in the VCO group. The mean difference of LDL-cholesterol at baseline and endpoint in the VCO group (24.1 mg/dL) was significantly higher than in the Control group (9.8 mg/dL). The HDL-cholesterol in the VCO group also increased significantly after the intervention. The mean difference of HDL-cholesterol in the VCO group was significantly higher compared to the Control group at endpoint. Across all time points, there was no significant difference between the two groups (Table 4 ).
Table 4.
Mean lipid profile of participants by group and time period.
|
VCO (n = 29) |
CONTROL (n = 28) |
p-value1 | |
|---|---|---|---|
| mean ± SE | mean ± SE | ||
| Total Cholesterol, mg/dL | |||
| Baseline | 163.3 ± 6.9 | 184.2 ± 5.3 | 0.021* |
| Midline | 197.6 ± 7.7 | 198.1 ± 7.6 | 0.961 |
| Mean Difference (p-value2) | 34.2 (<0.001)* | 13.9 (0.018)* | 0.012* |
| Baseline | 163.3 ± 6.9 | 184.2 ± 5.3 | 0.021* |
| Endline | 202.7 ± 8.4 | 199.2 ± 7.1bc | 0.7565 |
| Mean Difference (p-value2) | 39.4 (0.001)* | 15.1 (0.011)* | 0.001* |
| p-value3 | <0.001* | 0.009* | |
| LDL- Cholesterol, mg/dL | |||
| Baseline | 96.6 ± 6.1 | 108.2 ± 3.6 | 0.108 |
| Midline | 114.3 ± 6.4 | 113.6 ± 5.5 | 0.9323 |
| Mean Difference (p-value2) | 17.7 (0.001)* | 5.4 (0.288) | 0.071 |
| Baseline | 96.6 ± 6.1 | 108.2 ± 3.6 | 0.108 |
| Endpoint | 120.7 ± 7.2 | 118.0 ± 5.7 | 0.769 |
| Mean Difference (p-value2) | 24.1 (<0.001)* | 9.8 (0.067) | 0.032* |
| p-value3 | <0.001* | 0.137 |
| HDL- Cholesterol, mg/dL | |||
|---|---|---|---|
| Baseline | 42.8 ± 1.8 | 47.1 ± 2.4 | 0.152 |
| Midline | 50.7 ± 2.1 | 47.7 ± 2.6 | 0.369 |
| Mean Difference (p-value2) | 7.9 (0.002)* | 0.6 (0.789) | 0.019* |
| Baseline | 42.8 ± 1.8 | 47.1 ± 2.4 | 0.152 |
| Endpoint | 53.6 ± 2.4 | 50.3 ± 2.7 | 0.365 |
| Mean Difference (p-value2) | 10.8 (<0.001)* | 3.1 (0.076) | 0.006* |
| p-value3 | <0.001* | 0.139 | |
| LDL/HDL ratio | |||
| Baseline | 2.3 ± 0.03 | 2.4 ± 0.7 | 0.561 |
| Midline | 2.3 ± 0.02 | 2.6 ± 0.9 | 0.236 |
| Mean Difference (p-value2) | 0 (0.919) | 0.2 (0.380) | 0.452 |
| Baseline | 2.3 ± 0.03 | 2.4 ± 0.7 | 0.561 |
| Endpoint | 2.3 ± 0.03 | 2.5 ± 0.7 | 0.461 |
| Mean Difference (p-value2) | 0 (0.958) | 0.1 (0.751) | 0.870 |
| p-value3 | 0.998 | 0.867 | |
| Triglycerides, mg/dL | |||
| Baseline | 131.6 ± 9.3 | 131.7 ± 9.0 | 0.996 |
| Midline | 156.4 ± 16.1 | 148.4 ± 13.6 | 0.71 |
| Mean Difference (p-value2) | 24.7 (0.059) | 16.8 (0.128) | 0.632 |
| Baseline | 131.6 ± 9.3 | 131.7 ± 9.0 | 0.996 |
| Endpoint | 142.3 ± 12.5 | 146.5 ± 12.4 | 0.81 |
| Mean Difference (p-value2) | 10.6 (0.431) | 14.8 (0.191) | 0.811 |
| p-value3 | 0.143 | 0.304 |
Independent t-test
Paired t-test.
Anova with repeated measure (baseline, midline, and endpoint).
significant at p < 0.05.
The mean ALT level in the VCO and Control groups were 37.1 and 45.0 L −1 at baseline, respectively. After the intervention, only the VCO group had a significant increase in ALT. The mean difference of ALT in the VCO group (26.4 L −1) was significantly higher than in the Control group (4.5 L −1). More than half of COVID-19 positive patients at baseline had high ALT levels in both the VCO (64.7%) and Control (68.8%) groups until the end of the intervention.
The mean AST level of the Control group (36.0 L −1) was significantly higher than in the VCO group (27.3 L −1) at baseline. A significant increase in the AST level was evident on the intervention group towards the end of the intervention (mean difference 10,1, p < 0.05) (Table 5 ).
Table 5.
Mean ALT and AST levels of participants by group and time period.
|
VCO (n = 29) |
CONTROL (n = 28) |
- | |
|---|---|---|---|
| mean ± SE | mean ± SE | ||
| Alanine Transaminase (ALT), L−1 | |||
| Baseline | 37.1 ± 4.9 | 45.0 ± 5.4 | 0.285 |
| Midline | 71.0 ± 12.4 | 55.2 ± 8.2 | 0.295 |
| Mean Difference (p-value2) | 33.9 (0.001)* | 10.2 (0.161) | 0.049* |
| Baseline | 37.1 ± 4.9 | 45.0 ± 5.4 | 0.285 |
| Endpoint | 63.6 ± 10.2 | 49.5 ± 7.1 | 0.263 |
| Mean Difference (p-value2) | 26.4 (<0.001)* | 4.5 (0.314) | 0.008* |
| p-value3 | <0.001* | 0.268 | |
| Aspartate Aminotransferase (AST), L−1 | |||
| Baseline | 27.3 ± 1.8 | 36.0 ± 2.5 | 0.007* |
| Midline | 36.4 ± 3.6 | 32.7 ± 2.2 | 0.388 |
| Mean Difference (p-value2) | 9.1 (0.004)* | 3.3 (0.175) | 0.001* |
| Baseline | 27.3 ± 1.8 | 36.0 ± 2.5 | 0.007* |
| Endpoint | 37.4 ± 3.3 | 32.7 ± 2.5 | 0.266 |
| Mean Difference (p-value2) | 10.1 (<0.001)* | 3.3 (0.189) | 0.001* |
| p-value3 | 0.001* | 0.242 |
1Independent t-test,
Paired t-test,
Anova with repeated measure (baseline, midline, and endpoint).
significant at p < 0.05.
For the hematological parameters, no significant difference in the white blood cell in-dices was observed (Table 6 ).
Table 6.
Mean levels of the hematology profile of participants by group and time period.
|
VCO (n = 29) |
CONTROL (n = 28) |
p-value1 | |
|---|---|---|---|
| mean ± SE | mean ± SE | ||
| White Blood Cell, uL−1 | |||
| Baseline | 8.1 ± 0.7 | 7.6 ± 0.5 | 0.554 |
| Midline | 7.9 ± 0.3 | 8.1 ± 0.3 | 0.744 |
| Mean Difference (p-value2) | 0.2 (0.734) | 0.5 (0.367) | 0.383 |
| Baseline | 8.1 ± 0.7 | 7.6 ± 0.5 | 0.554 |
| Endpoint | 8.0 ± 0.3 | 8.0 ± 0.3 | 0.95 |
| Mean Difference (p-value2) | 0.1 (0.834) | 0.4 (0.389) | 0.464 |
| p-value3 | 0.839 | 0.485 | |
| Neutrophils, uL−1 | |||
| Baseline | 53.8 ± 2.1 | 55.3 ± 2.1 | 0.606 |
| Midline | 53.3 ± 1.9 | 53.8 ± 1.6 | 0.829 |
| Mean Difference (p-value2) | 0.5 (0.803) | 1.5 (0.464) | 0.725 |
| Baseline | 53.8 ± 2.1 | 55.3 ± 2.1 | 0.606 |
| Endpoint | 51.5 ± 1.7 | 54.9 ± 1.7 | 0.171 |
| Mean Difference (p-value2) | 2.2 (0.328) | 0.4 (0.844) | 0.572 |
| p-value3 | 0.454 | 0.664 | |
| Lymphocytes, uL−1 | |||
| Baseline | 34.2 ± 2.1 | 32.7 ± 1.9 | 0.596 |
| Midline | 34.3 ± 1.9 | 33.3 ± 1.4 | 0.677 |
| Mean Difference (p-value2) | 0.1 (0.962) | 0.6 (0.723) | 0.842 |
| Baseline | 34.2 ± 2.1 | 32.7 ± 1.9 | 0.596 |
| Endpoint | 35.3 ± 1.7 | 32.6 ± 1.4 | 0.225 |
| Mean Difference (p-value2) | 1.1 (0.588) | 1.1 (0.954) | 0.663 |
| p-value3 | 0.724 | 0.841 | |
| Neutrophils/Lymphocytes Ratio | |||
| Baseline | 1.9 ± 0.2 | 2.1 ± 0.3 | 0.577 |
| Midline | 1.8 ± 0.2 | 1.7 ± 0.1 | 0.731 |
| Mean Difference (p-value2) | 0.1 (0.647) | 0.4 (0.203) | 0.383 |
| Baseline | 1.9 ± 0.2 | 2.1 ± 0.3 | 0.577 |
| Endpoint | 1.6 ± 0.1 | 1.8 ± 0.1 | 0.353 |
| Mean Difference (p-value2) | 0.3 (0.212) | 0.3 (0.398) | 0.935 |
| p-value3 | 0.297 | 0.313 |
*significant at p < 0.05.
Independent t-test,
Paired t-test,
Anova with repeated measure (baseline, midline, and endpoint).
A significant decrease in the CRP level was observed in the intervention group (Table 7 ). The mean difference of CRP from baseline and endpoint in the VCO group (4.9 mg/L) was significantly higher as compared to the mean difference of CRP in the Control group (3.2 mg/L). Meanwhile, no significant difference over all time points was observed in the CD4 levels of all the participants.
Table 7.
Mean CRP and CD4 levels of participants by group and time period.
|
VCO (n = 29) |
CONTROL (n = 28) |
p-value1 | |
|---|---|---|---|
| mean ± SE | mean ± SE | ||
| C-reactive protein (CRP), mg/L | |||
| Baseline | 7.4 ± 2.3 | 8.2 ± 2.6 | 0.81 |
| Midline | 3.3 ± 0.7 | 4.9 ± 1.3 | 0.268 |
| Mean Difference (p-value2) | 4.1 (0.067) | 3.3 (0.161) | 0.808 |
| Baseline | 7.4 ± 2.3 | 8.2 ± 2.6 | 0.81 |
| Endpoint | 2.5 ± 0.5 | 5.1 ± 0.9 | 0.017* |
| Mean Difference (p-value2) | 4.9 (0.029)* | 3.2 (0.217) | 0.597 |
| p-value3 | 0.043* | 0.199 | |
| CD4 | |||
| Baseline | 770.8 ± 46.0 | 728.6 ± 51.8 | 0.543 |
| Midline | 833.1 ± 57.6 | 802.4 ± 49.7 | 0.688 |
| Mean Difference (p-value2) | 62.3 (0.081) | 73.8 (0.051) | 0.815 |
| Baseline | 770.8 ± 46.0 | 728.6 ± 51.8 | 0.543 |
| Endpoint | 835.4 ± 55.0 | 801.2 ± 60.1 | 0.675 |
| Mean Difference (p-value2) | 64.6 (0.107) | 72.6 (0.085) | 0.887 |
| p-value3 | 0.127 | 0.074 |
Independent t-test,
Paired t-test,
Anova with repeated measure (baseline, midline, and endpoint).
significant at p < 0.05.
Table 8 depicts the distribution of the C-reactive protein (CRP) level measured on all time points. As suggested by the CRP level, more than half of the participants in both arms of the intervention had no infection or inflammation. Towards the end of the intervention, 82.8% (n = 24) of the participants in the intervention group have normal CRP levels (<5 mg/dL) as compared to the control group, with two more participants having CRP levels beyond the normal cut-off.
Table 8.
Distribution of participants by CRP level and by group and time period.
|
VCO (n = 29) |
Control (n = 28) |
p-value1 | |||
|---|---|---|---|---|---|
|
>5.0 |
<=5.0 |
>5.0 |
<=5.0 |
||
| n (%) | n (%) | n (%) | n (%) | ||
| C-reactive protein, mg/L | |||||
| Baseline | 10 (34.5) | 19 (65.5) | 10 (35.7) | 18 (64.3) | 0.922 |
| Midline | 7 (24.1) | 22 (75.9) | 10 (35.7) | 18 (64.3) | 0.340 |
| Endpoint | 5 (17.2) | 24 (82.8) | 12 (42.9) | 16 (57.1) | 0.035* |
Reference cut offs: Without Infection: </=5.00 mg/dL; With Infection: >5.00 mg/dL.
Pearson Chi-squared test,
Significant at p < 0.05.
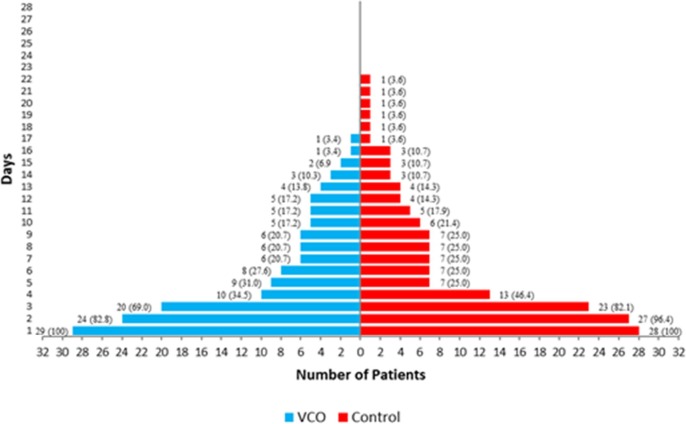
Daily monitoring of signs and symptoms revealed that five out of 29 (17%) patients in the VCO group and one (3.6%) from the Control group manifested improvement in the signs and symptoms as early as the second day of intervention. It should be noted that by day 2, the VCO group was on the low VCO dose. Participants in the VCO group showed no more COVID-19-related symptoms at day 18 while symptoms in the Control group persisted until day 23 (Fig. 1 ).
Fig. 1.
Percentage of participants with diminishing symptoms per group per day.
4. Discussion
This study evaluated the effects of VCO given to suspect and probable cases of COVID-19 in isolation facilities. Overall, the VCO group showed more rapid relief from symptoms of COVID-19 and a significant higher reduction in mean CRP levels compared to the Control group after 28 days. These results are consistent with the anti-viral and anti-inflammatory properties of VCO metabolites as reported in both in vitro and animal studies, bolstering the therapeutic benefit of supplements such as VCO. The results of this study contrast with the findings of a recently published study which found no benefit with high doses of zinc, ascorbic acid, and a combination of these two supplements against COVID-19 compared with usual care (Thomas, Patel, & Bittel, 2021). It is noteworthy to mention that the design of the study is different from our present study. First, their study participants were home-based and were monitored via telephone, email, or virtual communication. Second, interventions were not blinded and there was no control group. Results therefore should be interpreted with caution in the context of overall null findings (Michos, & Cainzos-Achirica, 2021).
The earlier improvement of signs and symptoms in the VCO group (day 18) compared with the Control group (day 23) may be attributed to the anti-inflammatory or immunomodulation property of VCO. It has been observed in numerous in-vitro and in-vivo studies that coconut oil has an immune-nutritive agent (Widhiarta, 2016). It was also inferred that the immunodulatory property of VCO was demonstrated in various in-vitro studies by suppressing inflammatory cytokines, including tumour necrosis factor-α, interferon- γ, interleukin-5, interleukin-6 and interleukin-8 (Varma et al., 2019). VCO metabolites are known to produce highly ordered membranes, which are thought to disrupt pathogens’ cellular function by affecting signal transduction due to blockage of promoters, uncoupling of energy systems, altered respiration state, and altered amino acid uptake. In a comparison among the saturated fatty acids from C10 to C18 against Junin virus (JUNV) infection, C12 showed the strongest effect for altering the cellular distribution of the viral proteins, leading to a blockade in the assembly and/or budding of the viral progeny (Thormar, Isaacs, Brown, Barshatzky, & Pessolano, 1987). Moreover, VCO has been shown to be a potential alternative to antibiotics as a modulator of the cellular immune system (Widianingrum, Noviandi, & Salasia, 2019). Monolaurin has a broad-spectrum activity as an antibacterial, boosts the immune system, and acts as an anti-viral. Its ability to kill various types of viruses, especially enveloped viruses such as influenza viruses and coronavirus 229E, makes monolaurin potentially able to ward off SARS-CoV-2 which is the cause of the COVID-19 (Subroto, & Indiarto, 2020).
Starting from a baseline mean CRP level of 7.4 ± 2.3 in the VCO group and 8.2 ± 2.6 mg/L in the Control group, it can be inferred that the participants were considered to have either an infection or inflammation upon admission. However, the mean CRP levels of the participants in the VCO group have normalized < 5 mg/L as early as day 14 while the Control group remained at the borderline until the end of the study (day 28). Several studies support the role of CRP test as a prognostic indicator of COVID-19. In a study among confirmed COVID-19 cases in early stage, results showed that CRP levels were positively correlated with lung lesions and could reflect disease severity (Wang, 2020). In a retrospective cohort analysis of 268 medical records with at least two CRP values within the first seven days, results showed that CRP levels increased in a linear fashion during the first week of hospitalization and peaked on day 5. Compared to patients who died, those who survived had lower peak CRP levels and earlier declines while CRP levels were significantly higher in patients who died (Sharifpour, Rangaraju, Liu, Alabyad, Nahab, Creel-Bulos, & Jabaley, 2020). The faster normalization of CRP level in the VCO group than in the Control group indicates that VCO is effective in the regulation of inflammatory processes. CRP levels often rises before the manifestation of symptoms such as pain or fever, and eventually drops as the body recovers, making it a useful test for monitoring infections (Sproston and Ashworth, 2018). VCO has anti-inflammatory property which was shown as effective in inhibiting chronic inflammation (Intahpuak, Khonsung, & Panthong, 2010). This study showed a declining trend of CRP in the VCO group which might be attributed to the the phytochemicals present in VCO. Phytochemicals is believed to act as a potential source of antioxidant in scavenging free radicals and toxic intermediates, which is also a factor to its anti-inflammatory property (Chew, 2019). This defence mechanism resulted from the ability of VCO to sustain total glutathione concentration and glutathione during reduced state and hence, decrease inflammation (Nevin, & Rajamohan, 2009).
Further results of this present study show that all the neutrophil count, lymphocyte count, eosinophil count was normal at baseline until endpoint. This is contrary to a previous study on confirmed COVID-19 cases had significantly increased neutrophil count, lymphocyte count, and eosinophil count in the biological analysis (Ahnach, Zbiri, Nejjari, Ousti, & Elkettani, 2020).
CD4 cells are a type of white blood cell, called T-cells that move throughout the body to find and destroy bacteria, viruses, and other invading germ. The CD4 count could increase in response to effective antiretroviral treatment (ART). The VCO as immunomodulator destroys microbial organisms by disturbing their membranes, thus interfering with virus assemble and maturation, and increase macrophage activity which increase the CD4 count (Yuniwarti, Asmara, Artama & Tabbu, 2012). The CD4 count increased gradually from baseline to endpoint in both groups. However, there was a significant increase among COVID-19 positive patients in the VCO group. This means that VCO has a positive effect on CD4 count of positive patients but not to suspected and probable patients.
Intake of meals with VCO seemed to increase the cholesterol level of participants in the VCO group. VCO contains high saturated fatty acids which could significantly increase LDL- and HDL-cholesterol (Cox et al., 1998, Mendis et al., 2001). Both the LDL-cholesterol (96.6–120.7 mg/dL) and HDL-cholesterol (42.8–53.8 mg/dL) significantly increased. However, the significant increase in LDL-cholesterol was still within the normal range (49.03–172.59 mg/dL). Also, there was no significant difference in the LDL/HDL ratio between the two groups across time periods. Moreover, the mean triglycerides were within normal range (<149.57 mg/dL) at baseline until the end of intervention. Furthermore, there was a significant increase in the HDL-cholesterol in the VCO group compared with the Control group. This could be attributed to the high proportion of lauric and myristic acid content of VCO (Chinwong, Chinwong, & Mangklabruks, 2017). Although VCO is high in saturated fat, it is high in MCFA which increases HDL-cholesterol compared to other plant-based oils which have polyunsaturated and monounsaturated fat (Mensink, Zock, Kester & Katan, 2003). This means that the consumption of VCO did not increase the risk of coronary heart disease.
In this study, results show that more than half of COVID-19 positive patients at baseline had high ALT levels in both the VCO (64.7%) and Control (68.8%) groups until the end of the intervention. This might be because of earlier reports explaining the elevated liver enzymes in patients with COVID-19 both with and without chronic liver diseases are common. Existing data suggests that liver injury may be due to drug hepatotoxicity (Cao, Cai, & Chen, 2020), immune dysfunction (Ghoda, & Ghoda, 2020) and immune-mediated inflammation (Zhang, Shi, & Wang, 2020). On the other hand, lymphonemia or immune system overreaction caused by immune dysfunction, accompanied by disease progression, can also independently lead to liver derangement. It has been noted that COVID-19 patients frequently manifest elevated levels of liver enzymes (Quali, Romero-Marrero, & Regueiro, 2020).
The VCO group ingested 34.2–120.48 additional calories from intake of VCO. The increase in weight at endpoint in VCO group may be due to the additional calories and the limited physical activity in the isolation facility. Further, it should be noted that the additional intake of VCO lowered FBG slightly although this was not statistically significant.
5. Conclusions
VCO could be used as an adjunct supplement to probable and suspect cases of COVID-19 due to its anti-viral and immunomodulatory properties. However, more studies are recommended to gather more confirmatory evidence on the benefits of VCO for COVID-19.
Funding
This research was funded by the Department of Science and Technology, Philippine Council for Health Research and Development (DOST PCHRD), the Philippine Coconut Authority (PCA), and DOST CALABARZON.
Ethical statement
The study was conducted according to the guidelines of the Declaration of Helsinki, guided by the Council for International Organizations of Medical Sciences Ethical Guidelines for Biomedical Research Involving Human Subjects and the National Guidelines for Biomedical/Behavioral Research and approved by the Institutional Review Board (or Ethics Committee) of the Food and Nutrition Research Institute (protocol code FIERC-2020-009 approved on 23 April 2020).
8. Author contributions**a
Imelda Angeles-Agdeppa conceptualized, designed, supervised the implementation and acquired funds of the study, interpreted the data, drafted the initial manuscript, and approved the final manuscript as submitted. Jacus S. Nacis contributed in drafting the initial manuscript. Mario V. Capanzana and Fabian M. Dayrit, reviewed and edited the initial manuscript. Keith V. Tanda conducted data analysis. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was in collaboration with the Philippine Coconut Authority (PCA), the Local Government of Sta. Rosa City, DOST CALABARZON, and DOST PCHRD. Authors acknowledge the valuable technical guidance of Dr. Jaime C. Montoya, technical contributions of Carl Vincent D. Cabanilla during the early stage of the study; Edelyn F. Calapano, Carmina Alicia N. Lainez, Nicole Marie A. Lota, Gracielli Anne L. Paunlaqui, and Johnalen Aira S. Soberano for their endearing efforts during the data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jff.2021.104557.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahnach M., Zbiri S., Nejjari S., Ousti F., Elkettani C. C-reactive protein as an early predictor of COVID-19 severity. Journal of Medical Biochemistry. 2020;39(4):500–507. doi: 10.5937/jomb0-27554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção M.L., Ferreira H.S., dos Santos A.F., Cabral C.R., Florêncio T.M.M.T. Effects of dietary coconut oil on the biochemical and anthropometric profiles of women presenting abdominal obesity. Lipids. 2009;44(7):593–601. doi: 10.1007/s11745-009-3306-6. [DOI] [PubMed] [Google Scholar]
- Bartolotta S., Garcí C.C., Candurra N.A., Damonte E.B. Effect of fatty acids on arenavirus replication: Inhibition of virus production by lauric acid. Archives of Virology. 2001;146(4):777–790. doi: 10.1007/s007050170146. [DOI] [PubMed] [Google Scholar]
- Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C.…Hullsiek K.H. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. The New England Journal of Medicine. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Cai X., Chen M. Liver injury in covid-19: Caution and management. Liver Cancer. 2020;9(5):625–626. doi: 10.1159/000508696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew Y.L. The Beneficial Properties of Virgin Coconut Oil in Management of Atopic Dermatitis. Pharmacognosy Reviews. 2019;13:24–27. doi: 10.4103/phrev.phrev_29_18. [DOI] [Google Scholar]
- Chinwong S., Chinwong D., Mangklabruks A. Daily Consumption of Virgin Coconut Oil Increases High-Density Lipoprotein Cholesterol Levels in Healthy Volunteers: A Randomized Crossover Trial. Evidence-based Complementary and Alternative Medicine. 2017;3:1–8. doi: 10.1155/2017/7251562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C., Sutherland W., Mann J., de Jong S., Chisholm A., Skeaff M. Effects of dietary coconut oil, butter and safflower oil on plasma lipids, lipoproteins and lathosterol levels. European Journal of Clinical Nutrition. 1998;52(9):650–654. doi: 10.1038/sj.ejcn.1600621. [DOI] [PubMed] [Google Scholar]
- da Silva Lima R., Block J.M. Coconut Oil and Immunity: What do we really know about it so far? Food Quality and Safety. 2019;3:1–12. doi: 10.1093/fqsafe/fyz004. [DOI] [Google Scholar]
- Dayrit, C. (2000, July). Coconut Oil in Health and Disease: Its and Monolaurin’s Potential as Cure for HIV/AIDS. Paper presented at the 37th Annual Cocotech Meeting, Chennai, India.
- Dayrit F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. Journal of the American Oil Chemists’ Society. 2015;92(1):1–15. doi: 10.1007/s11746-014-2562-7. [DOI] [Google Scholar]
- Department of Health. (2020) Administrative Order 2020-0013. Revised Administrative Order No. 2020-0012 “Guidelines for the Inclusion of the Coronavirus Disease 2019 (COVID-19) in the List of Notifiable Diseases for Mandatory Reporting to the Department of Health” dated March 17, 2020. https://doh.gov.ph/sites/default/files/health-update/ao2020-0013.pdf. Accessed April 9, 2020.
- Fadai N.T., Sachak-Patwa R., Byrne H.M., Maini P.K., Bafadhel M., Nicolau D.V., Jr Infection, inflammation and intervention: Mechanistic modelling of epithelial cells in COVID-19. Journal of the Royal Society Interface. 2021;18:1–8. doi: 10.1098/rsif.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani N.A.A., Channip A.-A., Chok Hwee Hwa P., Ja'afar F., Yasin H.M., Usman A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Science and Nutrition. 2018;6(5):1298–1306. doi: 10.1002/fsn3.2018.6.issue-510.1002/fsn3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoda A., Ghoda M. Liver injury in covid-19 infection: A systematic review. Cureus Journal of Medical Science. 2020;12:1–7. doi: 10.7759/cureus.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIERHOLZER JOHN.C., KABARA JON.J. In-vitro effects of monolaurin compounds on enveloped RNA and DNA viruses. Journal of Food Safety. 1982;4(1):1–12. doi: 10.1111/jfs.1982.4.issue-110.1111/j.1745-4565.1982.tb00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmarsson H., Traustason B.S., Kristmundsdóttir T., Thormar H. Virucidal activities of medium- and long-chain fatty alcohols and lipids against respiratory syncytial virus and parainfluenza virus type 2: Comparison at different pH levels. Archives of Virology. 2007;152(12):2225–2236. doi: 10.1007/s00705-007-1063-5. [DOI] [PubMed] [Google Scholar]
- Hornung B., Amtmann E., Sauer G. Lauric acid inhibits the maturation of vesicular stomatitis virus. Journal of General Virology. 1994;75(2):353–361. doi: 10.1099/0022-1317-75-2-353. [DOI] [PubMed] [Google Scholar]
- Intahpuak S., Khonsung P., Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharmaceutical Biology. 2010;48(2):151–157. doi: 10.3109/13880200903062614. [DOI] [PubMed] [Google Scholar]
- Joshi S., Kaushik V., Gode V., Mhaskar S. Coconut Oil and Immunity: What do we really know about it so far? Journal of Association of Physicians of India. 2020;68:67–72. [PubMed] [Google Scholar]
- Khaw K.-T., Sharp S.J., Finikarides L., Afzal I., Lentjes M., Luben R., Forouhi N.G. Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open. 2018;8(3):e020167. doi: 10.1136/bmjopen-2017-02016710.1136/bmjopen-2017-020167.supp110.1136/bmjopen-2017-020167.supp2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristmundsdóttir T., Árnadóttir S.G., Bergsson G., Thormar H. Development and evaluation of microbicidal hydrogels containing monoglyceride as the active ingredient. Journal of Pharmaceutical Science. 1999;88(10):1011–1015. doi: 10.1021/js9900396. [DOI] [PubMed] [Google Scholar]
- Li, R., Duffee, D., & Gbadamosi-Akindele, M.F. (2020). CD4 Count. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK470231/. Accessed April 9, 2020.
- Lotfi M., Hamblin M.R., Rezaei N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. International Journal of Clinical Chemistry and Diagnostic Laboratory Medicine. 2020;598:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis S., Samarajeewa U., Thattil R.O. Coconut fat and serum lipoproteins: Effects of partial replacement with unsaturated fats. British Journal of Nutrition. 2001;85(5):583–589. doi: 10.1079/BJN2001331. [DOI] [PubMed] [Google Scholar]
- Mensink R.P., Zock P.L., Kester A.D., Katan M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. American Journal of Clinical Nutrition. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Michos E.D., Cainzos-Achirica M. Supplements for the Treatment of Mild COVID-19—Challenging Health Beliefs With Science From A to Z. JAMA Network Open. 2021;4:1–3. doi: 10.1001/jamanetworkopen.2021.0431. [DOI] [PubMed] [Google Scholar]
- Nevin K.G., Rajamohan T. Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clinical Biochemistry. 2004;37(9):830–835. doi: 10.1016/j.clinbiochem.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Nevin K.G., Rajamohan T. Wet And Dry Extraction of Coconut Oil: Impact on Lipid Metabolic and Antioxidant Status in Cholesterol Co-administered Rats. Canadian Journal of Physiology and Pharmacology. 2009;87(8):610–616. doi: 10.1139/Y09-045. [DOI] [PubMed] [Google Scholar]
- Nevin, K.G., & Rajamohan, T. (2010). Effect of topical application of virgin coconut oil on skin components and antioxidant status during dermal would healing in young rats. Skin Pharmacology and Physiology, 23, 290-297. https://doi.org/10.1159.00313516. [DOI] [PubMed]
- Pamukcu B. Inflammation and thrombosis in patients with COVID-19: A prothrombotic and inflammatory disease caused by SARS coronavirus-2. The Anatolian Journal of Cardiology. 2020;24:224–234. doi: 10.14744/AnatolJCardiol.2020.56727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quali S.E., Romero-Marrero C., Regueiro M. Hepatic Manifestations of COVID-19. Cleveland Clinic Journal of Medicine. 2020;1–4 doi: 10.3949/ccjm.87a.ccc061. [DOI] [PubMed] [Google Scholar]
- Sands J.A., Landin P., Auperin D., Reinhardt A. Enveloped Virus Inactivation by Fatty Acid Derivatives. Antimicrobial Agents and Chemotherapy. 1979;15(1):134–136. doi: 10.1128/AAC.15.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifpour, M., Rangaraju, S., Liu, M., Alabyad, D., Nahab, F.B., Creel-Bulos, C.M., & Jabaley, C.S. (2020). C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS One, 15, 1-10. https://doi.org/10.1371/journal.pone.0242400. [DOI] [PMC free article] [PubMed]
- Sproston N.R., Ashworth J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Frontiers in Immunology. 2018;9:1–11. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subroto E., Indiarto R. Bioactive monolaurin as an antimicrobial and its potential to improve the immune system and against COVID-19: A review. Food Research. 2020;4(6):2355–2365. [Google Scholar]
- Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., Il’Giovine Z.J., Mehra R., McWilliams C., Nissen S.E., Desai M.Y. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 InfectionThe COVID A to Z Randomized Clinical Trial. JAMA Network Open. 2021;4(2):e210369. doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormar H., Isaacs C.E., Brown H.R., Barshatzky M.R., Pessolano T. Inactivation of Enveloped Viruses and Killing of Cells by Fatty Acids and Monoglycerides. Antimicrobial Agents and Chemotherapy. 1987;31(1):27–31. doi: 10.1128/AAC.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [computer software] Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0). (2013). http://www.randomizer.org/.
- US FDA. (2020). SCOGS (Select Committee on GRAS Substances). https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=SCOGS&sort=Sortsubstance&order= ASC&startrow=1&type=basic&search=coconut%20oil. Accessed April 9, 2020.
- Varma S.R., Sivaprakasam T.O., Arumugam I., Dilip N., Raghuraman M., Pavan K.B.…Paramesh R. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. Journal of Traditional Complementary Medicine. 2019;9:5–14. doi: 10.1016/j.jtcme.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. C-reactive protein levels in the early stage of COVID-19. Médecine et Maladies Infectieuses. 2020;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widhiarta K.D. Virgin Coconut Oil for HIV - Positive People. Cord. 2016;32:50–57. doi: 10.37833/cord.v32i1.46. [DOI] [Google Scholar]
- Widianingrum, D.C., Noviandi, C.T., & Salasia, S.I.O. (2019). Antibacterial and immunomodulator activities of virgin coconut oil (VCO) against Staphylococcus aureus. Heliyon, 5, 1-5. https://doi.org/10.1016/j.heliyon.2019.e02612. [DOI] [PMC free article] [PubMed]
- World Health Organization (WHO). (2020). C-Reactive Protein Concentrations as a Marker of Inflammation or Infection for Interpreting Biomarkers of Micronutrient Status. WHO Document: Geneva Switzerland.
- Yuniwarti, E.Y.W., Asmara, W., Artama,W.T., & Tabbu, C.R. (2012). The Effect of Virgin Coconut Oil on Lymphocyte and CD4 in Chicken Vaccinated against Avian Influenza Virus. Journal of the Indonesian Tropical Animal Agriculture, 37, 64-69. https://doi.org/ 10.14710/jitaa.37.1.64-69.
- Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: The inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12:2–28. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Chao, Shi Lei, Wang Fu-Sheng. Liver injury in COVID-19: Management and challenges. The Lancet. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.