The novel coronavirus disease 2019 pandemic has disrupted healthcare use, including routine outpatient care. Although many have postulated that major gaps in cancer surveillance are occurring across the United States, few studies have evaluated these changes objectively. This issue is of special concern to patients with cirrhosis who require frequent liver imaging to screen for hepatocellular carcinoma (HCC), a disease with high morbidity, mortality, and cost.1 As health centers begin to reexpand clinical operations, it is critical to understand ongoing trends in imaging resurgence and risk factors for inadequate surveillance to identify high-risk groups for extended lapses in care. We used national Veterans Health Administration (VHA) data to study pandemic-related changes in the volume of HCC surveillance and to identify variables associated with surveillance completion during the period of care resurgence.
Methods
Study Design and Ascertainment of Exposures and Outcomes
We performed a retrospective cohort study using data from the VHA comprising 127 health centers across the United States. We used a longitudinal cohort of patients with cirrhosis identified between 2008 and 2016 using prior methods2, 3, 4 and excluded those with liver transplantation. This study received Institutional Review Board approval from the Corporal Michael J. Crescenz VA Medical Center, Philadelphia.
Completed HCC surveillance studies (ultrasound, contrast-enhanced magnetic resonance imaging, or computed tomography) were ascertained for each patient from September 1, 2018 to September 22, 2020 using previously published methods.5 Patient-level data (demographics, comorbidities, etiology of liver disease, distance to center), center-level data (US region, academic center, rurality), and completed outpatient appointments with primary care providers (PCPs) or gastroenterology/hepatology (GI/Hep) providers in the 6 months before surveillance due date were obtained. Visits were further classified as in-person or telemedicine using designated VHA clinic codes.
The primary outcome was completion of HCC surveillance imaging during the months of the pandemic, defined as March 1, 2020 through September 22, 2020 (date of maximum follow-up). The “due month” for HCC surveillance was determined based on a 6-month interval from the previously completed imaging study (thus requiring imaging data beginning in September 1, 2018), consistent with national guidelines.6 A grace period of 1 month was allowed for classification of completed HCC surveillance.
Statistical Analysis
Weekly volumes of HCC imaging surveillance were plotted for 2019 and 2020; plots stratified by imaging modality and setting (outpatient/inpatient) were also provided in 2020. The proportion of patients completing HCC surveillance for each due month during the pandemic was compared between 2019 and 2020 using bar graphs, and state-level changes in 2020 completion proportions were displayed geographically for the continental United States. Standard descriptive statistics were then reported for patients who di and did not complete HCC surveillance. Linear regression was used to identify shifts in imaging volumes accounting for secular trends, and multivariable logistic regression was used to identify risk factors associated with completion of HCC surveillance. The variables shown in Supplementary Table 1 were considered as potential predictors. Outpatient visits were treated as a multilevel categorical variable (PCP in-person, PCP telemedicine, GI/Hep in-person, GI/Hep telemedicine), where the most recent visit before HCC surveillance due date was considered in models. Further details are provided in the Supplementary Methods. Data management and analyses were performed using Stata 15.1/IC (StataCorp, College Station, TX).
Results
National Changes in HCC Surveillance
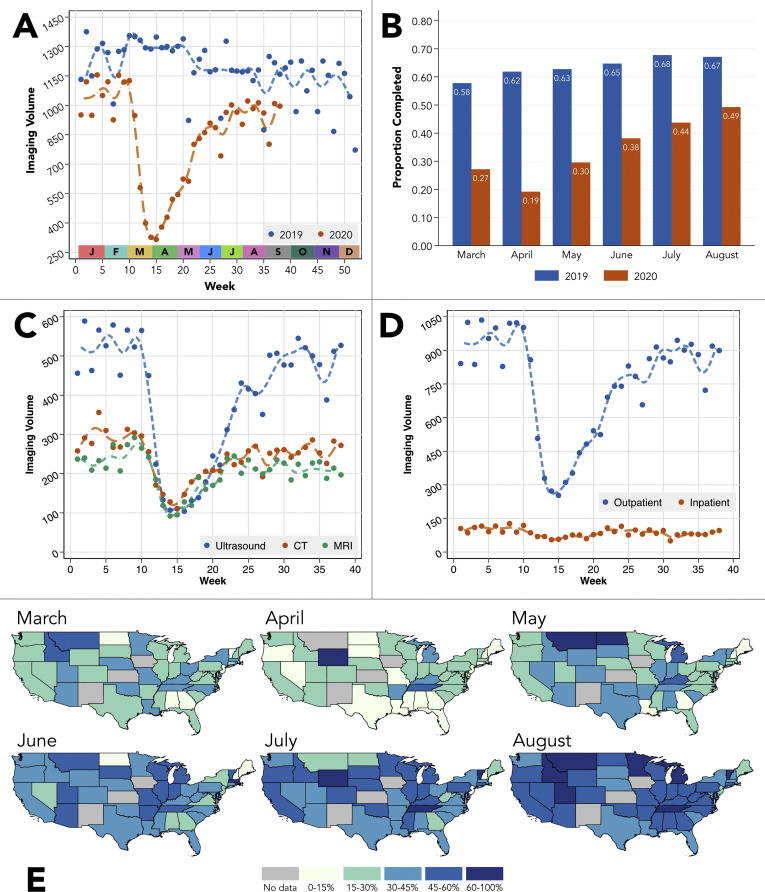
There was a significant decline in weekly HCC surveillance during the early pandemic (–160.6 studies per week, P < .001), with gradual but incomplete return to prepandemic baseline through the date of maximum follow-up (+25.1 studies a week, P < .001, Figure 1 A). When stratified by due month, the proportion of patients completing HCC surveillance remained significantly lower in 2020 vs 2019 for each month (each P < .0001, Figure 1 B). In 2020, declines in each surveillance imaging modality and setting were observed in the early pandemic (each β < 0, P < .001; Figure 1 C and D). Declines and resurgence in surveillance completion were observed in all US regions (Figure 1 E).
Figure 1.
Changes in HCC imaging surveillance studies in the VHA during the coronavirus disease 2019 (COVID-19) pandemic. (A) Changes in weekly volumes of HCC surveillance studies in 2019 and 2020. In 2020, there was a significant decline in surveillance imaging from weeks 10–15 (early COVID-19 period) and a gradual increase in the later COVID-19 period (weeks 15 onward). (B) Proportion of HCC surveillance studies completed by month due in both 2019 and 2020. Calculations incorporate a 1-month grace period for study completion. For example, if HCC surveillance was due in June, it was considered to be completed if performed in July. Each comparison of proportions between 2020 and 2019 was statistically significant at the P < .0001 level. (C) Changes in weekly volume of HCC surveillance studies as stratified by imaging modality in 2020. (D) Changes in weekly volume of HCC surveillance studies as stratified by outpatient vs inpatient imaging location. (E) State-level changes in the proportion of completed HCC surveillance studies during the pandemic months in 2020.
Variables Associated With Surveillance Completion
We identified 15,480 patients due for HCC surveillance during the pandemic (Supplementary Table 1), of whom 5471 (35.3%) completed surveillance on time and 1392 (9.0%) were late (median time from prior imaging, 8.5 months [interquartile range, 7.8–9.7]). In multivariable analysis (Supplementary Table 2), increased odds of surveillance completion were associated with age ≥ 60 years (odds ratio [OR], 1.13 vs <60 years; 95% confidence interval [CI], 1.01–1.27), cirrhosis decompensation (OR, 1.26; 95% CI 1.17–1.36), and later 2020 month (eg, OR for August vs March, 2.85; 95% CI, 2.51–3.23). Patients with a shorter interval from last appointment to due date, in-person visits, and GI/Hep telemedicine visits also had increased odds of surveillance completion, although the most strongly associated was GI/Hep in-person visits (vs none: OR, 2.75; 95% CI, 2.30–3.29).
Discussion
In this nationwide VHA study of patients with cirrhosis, we observed a significant decline in HCC surveillance during the early pandemic. Although the proportion of patients completing surveillance increased each month since April 2020, the rates through August 2020 have remained <50%, far below rates in 2019. Finally, we identified several important risk factors for incomplete surveillance during the pandemic and found that in-person GI/Hep visits were strongly associated with imaging completion.
There are significant clinical implications of our findings. Delays in HCC surveillance are well known to increase the risk of advanced HCC presentations, which may have limited therapeutic options.7 , 8 Thus, it is critical to identify patients for whom targeted outreach efforts may facilitate catch-up surveillance. This may include patients aged < 60 years, those with compensated cirrhosis, care at a nonacademic institution, and those with long intervals from last appointment to surveillance due date. In general, any outpatient contact appeared to improve the odds of surveillance completion, although this was especially true of GI/Hep visits. Although in-person visits were more strongly associated with surveillance completion, it is important to highlight that GI/Hep telemedicine visits conferred a higher odds of completion than in-person PCP visits, underscoring the importance of specialty follow-up where available and the potential role of telemedicine to extend access to care.
This study has limitations, including external validity of findings outside the VHA cohort and possible misclassification of exposures and outcomes (ie, imaging studies performed outside the VHA). Notwithstanding, we observed a significant lapse in HCC surveillance, trends that likely extend to other quality metrics, and have identified patient and practice characteristics that may be used to target surveillance efforts relevant during the pandemic and beyond.
Acknowledgments
CRediT Authorship Contributions
Nadim Mahmud, MD, MS, MPH, MSCE (Conceptualization: Equal; Data curation: Equal; Formal analysis: Lead; Funding acquisition: Lead; Investigation: Equal; Methodology: Equal; Validation: Lead; Visualization: Lead; Writing – original draft: Lead; Writing – review & editing: Equal). David E. Kaplan, MD, MSc (Conceptualization: Supporting; Data curation: Supporting; Investigation: Supporting; Writing – review & editing: Equal). David S. Goldberg, MD, MSCE (Conceptualization: Supporting; Investigation: Supporting; Writing – review & editing: Equal). Tamar H. Taddei, MD (Conceptualization: Supporting; Investigation: Supporting; Writing – review & editing: Equal). Marina Serper, MD, MS (Conceptualization: Equal; Funding acquisition: Equal; Investigation: Equal; Methodology: Equal; Supervision: Lead; Writing – review & editing: Equal).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by resources and facilities available through the Philadelphia Veterans Affairs Healthcare System and the central data repositories maintained by the Veterans Affairs Informatics and Computing Infrastructure and the VA Information Resource Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. Funding/Grant Support: Nadim Mahmud is supported by an American College of Gastroenterology Junior Faculty Development Award (ACG-JR-010-2020). David E. Kaplan has received support from Gilead, Glycotest and Bayer unrelated to the topic of this manuscript. He is also supported by VA Merit Grants (I01-CX-001933, I01-CX-002010). David Goldberg has received support from Gilead, Merck, and AbbVie unrelated to the topic of this manuscript. He is also supported by a National Institutes of Health R01 (DK120561). Marina Serper is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, award #1K23DK115897-03. This project was supported by a pilot grant from the Leonard Davis Institute of Health Economics, University of Pennsylvania, Philadelphia, PA.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org and at https://doi.org/10.1053/j.gastro.2021.01.007.
Supplementary Methods
Additional Cohort Details
The data used in this study were sourced from an established cohort of patients with cirrhosis called the Veterans Outcomes and Costs Associated with Liver Diseases cohort.1 Patients with cirrhosis were originally identified between January 1, 2008 and December 31, 2016 based on a previously validated algorithm.2 Granular, longitudinal data for patients in the Veterans Outcomes and Costs Associated with Liver Diseases cohort are continuously updated and can be queried in near real-time.
Additional Details for Ascertainment of Exposure Data
Decompensated cirrhosis
Decompensated cirrhosis was classified using an algorithm previously validated in the VHA dataset3 and included 1 inpatient or 2 outpatient International Classification of Diseases, 9th or 10th revision codes for hepatic encephalopathy, bleeding esophageal varices, or ascites.
Academic center designation
Indicators in the VHA dataset for affiliation with an academic medical center were used to designate sites as academic vs nonacademic (binary).
Outpatient visits
For each patient we identified completed outpatient visits with PCP or GI/Hep providers in the 6 months before HCC image surveillance due date using primary and secondary stop codes in the VHA outpatient visits table. PCP stop codes included 323 and GI/Hep stop codes included 307 and 337. Telemedicine visits were classified by the presence of 1 of the following additional stop codes: 324 and 338. Regular expression searches for “tel” were also performed to identify additional telemedicine stop codes used in practice, each of which was manually reviewed and verified. These included the following stop codes: 103, 147, 148, 178, 182, 224, 225, 424, 490, 491, 527, 528, 530, 686, and 708.
HCC surveillance imaging studies
To classify imaging studies that would satisfy requirements for HCC surveillance, we followed the American Association for the Study of Liver Diseases guidelines.4 Therefore, we ascertained the following from the VHA dataset for each patient: abdominal ultrasound, right upper quadrant ultrasound, liver ultrasound, contrast-enhanced computed tomography of the abdomen, and contrast-enhanced magnetic resonance imaging of the abdomen. This approach is consistent with previously published work identifying HCC surveillance imaging studies in the VHA dataset.5 For the purpose of descriptive analyses, we categorized imaging modalities as ultrasound, computed tomography, or magnetic resonance imaging. We also classified imaging study location as inpatient or outpatient. Of note, we did not collect data on α-fetoprotein as an indicator of HCC surveillance, because α-fetoprotein is optional per the most recent American Association for the Study of Liver Diseases guidelines4 and there is likely significant practice variability with regard to this biomarker.
Additional Statistical Analysis Details
For analysis of significant shifts in national trends for HCC surveillance imaging volumes in 2020, we used linear regression. Models were adjusted for linear time trends in the pre–coronavirus disease 2019 period (defined as before week 10), early coronavirus disease 2019 period (weeks 10–15), and later coronavirus disease 2019 period (week 15 onward). The β coefficient was presented as changes in volume of imaging studies per week, and an α threshold of 5% was used for statistical significance.
Descriptive statistics are presented as medians and interquartile ranges for continuous variables and as percentages for categorical variables. Exposure variables between patients who completed HCC surveillance and those who did not complete surveillance were compared using Wilcoxon rank-sum or χ2 testing for categorical and continuous variables, respectively, using an α threshold of 5% for statistical significance. Serial 2-sample proportion tests were used to compare differences in HCC surveillance proportions for each pandemic month in 2020 vs equivalent periods in 2019. Because this entailed 6 tests (March through August), we used a Bonferroni-adjusted threshold for statistical significance (α = 0.83%). Finally, to address possible differences in year-over-year surveillance rates because of patient migration to other health systems, loss-to-follow-up, and so on, we repeated the above analysis among only patients who completed visits in the VHA system in both 2019 and 2020. The primary study findings were not substantively changed in this sensitivity analysis (data not shown).
In logistic regression analysis, we began with univariable models testing individual exposures against the outcome of completion of HCC surveillance during the pandemic months. Age was treated as a categorical variable (<60 years vs ≥60 years) based on an apparent difference in outcomes near this threshold on a locally weighted scatterplot smoothing curve. Patient distance to center was computed as a shortest path geographic distance based on zip codes. Variables meeting an α threshold of 10% in univariable analysis were considered as potential predictors in multivariable logistic regression. We used backward stepwise selection using an entry/exit α threshold of 5% to arrive at a candidate final model. We then tested several modified clinician-driven models and selected a final model that minimized the Bayesian information criterion. Of note, the final model also contained a term for month to account for temporal changes in outcomes during the evolution of the pandemic. Odds ratios and 95% confidence intervals were presented for each exposure variable.
Supplementary Table 1.
Patient and Center Characteristics From the Time of Index HCC Surveillance Image
| Factor | HCC Surveillance Not Complete (n = 10,009) | HCC Surveillance Complete (n = 5471) | P |
|---|---|---|---|
| Age, y | 68 (63-71) | 68 (64-71) | .52 |
| Male sex | 9666 (96.6) | 5299 (96.9) | .35 |
| Race | .033 | ||
| White | 5674 (56.7) | 3216 (58.8) | |
| Black | 2591 (25.9) | 1339 (24.5) | |
| Hispanic | 779 (7.8) | 394 (7.2) | |
| Asian | 154 (1.5) | 103 (1.9) | |
| Other | 811 (8.1) | 419 (7.7) | |
| BMI, kg/m2 | 28.5 (25.1-32.6) | 28.6 (25.2-32.6) | .42 |
| Smoking history | .40 | ||
| Never smoker | 2397 (23.9) | 1278 (23.4) | |
| Former smoker | 2489 (24.9) | 1360 (24.9) | |
| Current smoker | 5038 (50.3) | 2773 (50.7) | |
| Unknown | 85 (0.8) | 60 (1.1) | |
| Etiology of liver disease | .77 | ||
| Hepatitis C virus | 2218 (22.2) | 1249 (22.8) | |
| Hepatitis B virus | 333 (3.3) | 193 (3.5) | |
| Alcohol-related liver disease | 2598 (26.0) | 1366 (25.0) | |
| Hepatitis C virus + alcohol-related liver disease | 3146 (31.4) | 1731 (31.6) | |
| Nonalcoholic fatty liver disease | 1268 (12.7) | 690 (12.6) | |
| Other | 446 (4.5) | 242 (4.4) | |
| Hypertension | 8326 (83.2) | 4603 (84.1) | .13 |
| Diabetes mellitus | 4661 (46.6) | 2532 (46.3) | .73 |
| Coronary artery disease | 2192 (21.9) | 1156 (21.1) | .27 |
| Cerebrovascular accident | 1026 (10.3) | 537 (9.8) | .39 |
| Prior decompensation | 3005 (30.0) | 1933 (35.3) | <.001 |
| Patient distance to center, miles | 57.7 (14.8-219.7) | 57.7 (14.7-207.6) | .50 |
| US region | <.001 | ||
| West | 2027 (20.7) | 1167 (21.6) | |
| Midwest | 1746 (17.8) | 1087 (20.2) | |
| Northeast | 1451 (14.8) | 734 (13.6) | |
| South | 4577 (46.7) | 2405 (44.6) | |
| Academic center | 6250 (63.1) | 3757 (69.1) | <.001 |
| Center setting | .47 | ||
| Rural | 358 (3.7) | 186 (3.4) | |
| Urban | 9445 (96.3) | 5242 (96.6) | |
| Outpatient visits 6 months before HCC surveillance due date in pandemic months (3/1/20–9/22/20) | |||
| Most recent visit type | <.001 | ||
| None | 1287 (12.9) | 314 (5.7) | |
| PCP in-person | 3600 (36.0) | 1713 (31.3) | |
| PCP telemedicine | 1475 (14.7) | 727 (13.3) | |
| GI/Hep in-person | 1556 (15.5) | 1293 (23.6) | |
| GI/Hep telemedicine | 2091 (20.9) | 1424 (26.0) | |
| Time from last visit to surveillance due date, days | 87 (30-178) | 68 (24-136) | <.001 |
Values are median (interquartile range) or n (%).
Supplementary Table 2.
Univariable and Multivariable Logistic Regression Models for Completion of HCC Surveillance Due During Pandemic Monthsab
| Univariable Analysis |
Multivariable Analysis |
|||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | P | Odds Ratio | 95% Confidence Interval | P | |
| Age (ref <60 y) | (ref) | (ref) | ||||
| Age ≥ 60 y | 1.10 | (0.99-1.23) | .07 | 1.13 | (1.01-1.27) | .03 |
| Prior decompensation | 1.27 | (1.19-1.37) | <.001 | 1.26 | (1.17-1.36) | <.001 |
| Month due (ref March) | (ref) | (ref) | ||||
| April | 0.64 | (0.55-0.73) | <.001 | 0.64 | (0.56-0.74) | <.001 |
| May | 1.13 | (0.99-1.28) | .07 | 1.16 | (1.01-1.32) | .03 |
| June | 1.65 | (1.46-1.87) | <.001 | 1.77 | (1.56-2.01) | <.001 |
| July | 2.09 | (1.85-2.35) | <.001 | 2.24 | (1.98-2.54) | <.001 |
| August | 2.60 | (2.31-2.94) | <.001 | 2.85 | (2.51-3.23) | <.001 |
| US region (ref West)c | (ref) | (ref) | ||||
| Midwest | 1.08 | (0.97-1.20) | .14 | 1.06 | (0.95-1.19) | .29 |
| Northeast | 0.88 | (0.78-0.99) | .03 | 0.91 | (0.81-1.03) | .13 |
| South | 0.91 | (0.84-1.00) | .04 | 0.92 | (0.84-1.01) | .06 |
| Academic center | 1.31 | (1.22-1.40) | <.001 | 1.29 | (1.20-1.39) | <.001 |
| Time from last appointment to surveillance due date, mo | 0.92 | (0.91-0.93) | <.001 | 0.94 | (0.92-0.96) | <.001 |
| Most recent outpatient visit (ref none) | (ref) | (ref) | ||||
| PCP in-person | 1.95 | (1.70-2.23) | <.001 | 1.61 | (1.35-1.92) | <.001 |
| PCP telemedicine | 2.02 | (1.74-2.35) | .06 | 1.21 | (0.99-1.49) | .06 |
| GI/Hep in-person | 3.41 | (2.95-3.93) | <.001 | 2.75 | (2.30-3.29) | <.001 |
| GI/Hep telemedicine | 2.79 | (2.43-3.21) | <.001 | 1.73 | (1.42-2.09) | <.001 |
| Race/ethnicity (ref white) | (ref) | |||||
| Black | 0.91 | (0.84-0.99) | .02 | ... | ... | ... |
| Hispanic | 0.89 | (0.78-1.01) | .08 | ... | ... | ... |
| Asian | 1.18 | (0.92-1.52) | .20 | ... | ... | ... |
| Other | 0.91 | (0.80 – 1.03) | .15 | ... | ... | ... |
From March 1, 2020 through September 22, 2020. Outpatient visits were categorized as the most recent completed appointment type before HCC surveillance due date.
Variables that did not meet the P < .10 threshold in univariable analysis were sex, smoking history, body mass index, etiology of liver disease, hypertension, diabetes mellitus, coronary artery disease, cerebrovascular accident, patient distance to center, and center setting (rural/urban).
Variable retained on the basis of joint hypothesis test with P < .05.
References
- 1.Tapper E.B., et al. J Hepatol. 2020;73:441–445. doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmud N., et al. Gastroenterology. 2020;159:1134–1136. doi: 10.1053/j.gastro.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan D.E., et al. Development and performance of an algorithm to estimate the Child-Turcotte-Pugh score from a national electronic healthcare database. Clin Gastroenterol Hepatol. 2015;13:2333–2341. doi: 10.1016/j.cgh.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmud N., et al. Hepatology. 2019;69:2150–2163. doi: 10.1002/hep.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg D.S., et al. Hepatology. 2017;65:864–874. doi: 10.1002/hep.28765. [DOI] [PubMed] [Google Scholar]
- 6.Heimbach J.K., et al. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 7.Singal A.G., et al. J Natl Comprehen Cancer Netw. 2014;12:375–382. doi: 10.6004/jnccn.2014.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singal A.G., et al. Am J Gastroenterol. 2013;108:425. doi: 10.1038/ajg.2012.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Kaplan D, et al. Clin Gastroenterol Hepatol. 2015;13:2333–2341. doi: 10.1016/j.cgh.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg D, et al. Pharmacoepidemiol Drug Saf. 2012;21:765–769. doi: 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo Re. V. 3rd., et al. Pharmacoepidemiol Drug Saf. 2011;20:689–699. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heimbach J, et al. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg D, et al. Hepatology. 2017;65:864–874. doi: 10.1002/hep.28765. [DOI] [PubMed] [Google Scholar]