Abstract
Cardiac sarcoidosis is a component of an often multi-organ granulomatous disease of still uncertain cause. It is being recognized with increasing frequency, mainly as the result of heightened awareness and new diagnostic tests, specifically cardiac magnetic resonance imaging and 18F-FDG positron emission tomography scans. The purpose of this case-based review is to highlight the potentially life-saving importance of making the early diagnosis of cardiac sarcoidosis using these new tools, and to provide a framework for the optimal care of patients with this disease. We will review disease mechanisms as currently understood, associated arrhythmias including conduction abnormalities, and atrial and ventricular tachyarrhythmias, guideline-directed diagnostic criteria, screening of patients with extracardiac sarcoidosis, and the use of pacemakers and defibrillators in this setting. Treatment options, including those related to heart failure, and those which may help clarify disease mechanisms are included.
Keywords: Sarcoidosis, Arrhythmias, Implantable Cardioverter Defibrillator
Subject Terms: Arrhythmias, Imaging
Introduction
The diagnosis of cardiac sarcoidosis (CS), part of an often multi-organ granulomatous disease has increased significantly in recent years1, both as a result of increased awareness and improved diagnostic studies, specifically cardiac magnetic resonance imaging (CMR) and positron emission tomography scans (18F-FDG PET)2–5. CS can be seen clinically in isolation as well as in the setting of known involvement of other organs. While it may be associated with severe heart failure, its association with conduction abnormalities and tachyarrhythmias including ventricular arrhythmia (VA), and less often atrial arrhythmia (AA) is its hallmark4. Early recognition of CS, which will alter treatment and may be life-changing or lifesaving is extremely important. This case-based review will highlight clinical presentations, current knowledge and therapies for CS.
Disease Mechanisms
While its cause remains uncertain, sarcoidosis is thought to result from an exaggerated immune response to mostly unidentified environmental antigens in genetically predisposed individuals. Prevalence and incidence vary by age, sex, ethnicity and geographic location. Rates are highest in Northern European and African American populations and lowest in Hispanics and Asians. Cardiac involvement is clinically evident in about 5% of sarcoidosis patients3, but can be occult and may be identified in a much higher percentage of patients based on advanced imaging or autopsy studies5
Although no single occupational or environmental exposure has been identified as causative,6 sarcoidosis has been associated with exposure to insecticides, mold, mildew, birds, radiation and certain bacteria (mycobacteria and proprionibacterium, among others). It has also been linked to certain occupations (automobile manufacturing workers, firefighters and 911 responders). Residual mycobacterial DNA, RNA and non-degradable proteins have been detected in tissues of sarcoidosis patients, who also have increased blood and lung T cell reactivity against these proteins. Proprionibacterium acnes has been detected in Japanese sarcoidosis subjects. However, the presence of viable microbes is unlikely as granulomas are sterile and there is often improvement rather than worsening in response to immunosuppression.
Sarcoidosis, though usually sporadic, is familial in 3.6–9.6% of cases.7 In a twin study, heritability was estimated to be 0.668 and a number of genetic associations have been reported.9 Genes associated with sarcoidosis include class II human leukocyte antigen (HLA) genes (encoding major histocompatibility complex (MHC) molecules that present antigens and are recognized by CD+4 cells) that have connections to immune response or apoptosis pathways.9
The immunopathogenesis of sarcoidosis involves both innate and adaptive immune responses leading to granuloma formation (Figure 1). Antigen presenting cells launch T cell responses, and the balance between pro-inflammatory and suppressive responses may determine whether inflammation persists or resolves. Sarcoidosis is thought to be driven by IFNγ and TNFα secreting T helper (Th)1 cells, influenced by a Th1 promoting milieu rich with cytokines such as interleukin (IL)-12 and IL-18. More recently, a role for Th17 cells has also been implicated.10 Regulatory T cells have been identified in tissues and in the circulation of patients with sarcoidosis. They may help modulate inflammation, although their exact role is still unclear.9
Figure 1:
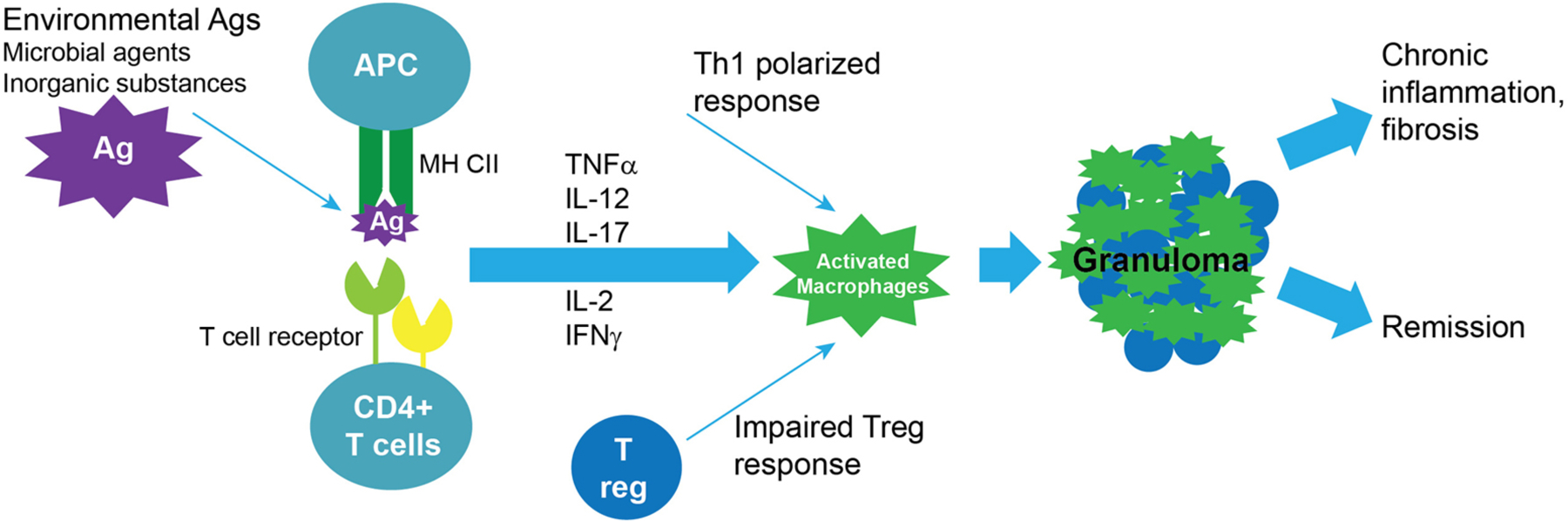
Key events in pulmonary sarcoid pathogenesis. 1) Antigen (Ag) exposure in regional lymph nodes leads to internalization, degradation to peptides and presentation on the surface of antigen presenting cells (APCs), such as dendritic cells in peptide-major histocompatibility complexes (MHC); 2) Recognition by CD4+ T cells that have T cell receptors (TCRs) that recognize the peptide-MHC complex, leads to clonal amplification and activation of these CD4+ T cells and proliferation with Th1 polarization (the detection of clonal amplification of CD4+ T cells support the existence of a pathogenic antigen that is recognized by these cells); 3) Activated circulating CD4+ T cells home via chemokines (e.g. CCL2) to tissue sites with antigen, activating macrophages to organize into granulomas, which consist of multinuclear giant cells and fused highly differentiated macrophages and also include Th1 cells; 4) Resolution of the inflammatory process with remission vs. progression to fibrosis with ongoing chronic inflammation.
Löfgren’s syndrome, a form of sarcoidosis with acute inflammatory onset but a favorable prognosis, is associated with certain HLA genotypes and clonal CD4+ T cells accumulated in the lung suggest local antigen recognition. Relative to other sarcoid presentations, it is characterized by lower Th1 responses, which may promote disease resolution.10 Interestingly, T cell receptor (TCR) sequencing and TCR-MHC modeling have identified a vimentin (cytoskeletal protein)-derived peptide-MHC complex as a proposed autoantigen with the capacity to trigger clonal T cell proliferation.11
Pathogenic mechanisms and identification of causative antigens have been less well studied in CS. Inflammasome activation has been documented in cardiac granulomas in 3 patients.12 Further study is needed to understand key cell response factors and mechanisms of immunopathogenesis in CS which may lead to specific targeted therapies.
Screening and Diagnosis
Case 1
A 38 year-old woman with pulmonary sarcoidosis was referred for CS screening. She noted occasional momentary palpitations but no syncope or heart failure symptoms. An ECG showed a right bundle branch block (RBBB) and normal PR interval, an echocardiogram showed a left ventricular ejection fraction (LVEF) of 48% without regional wall motion abnormalities, Holter monitoring revealed 2% ventricular premature contractions (VPC), but no ventricular tachycardia (VT), a CMR showed no late gadolinium enhancement (LGE) and an 18F-FDG PET showed 18F-FDG uptake in the septum without perfusion abnormalities. Her steroid therapy was intensified, she was started on beta blockers and cardiology follow up was established. Repeat 18F-FDG PET in 3 months showed resolution of the uptake and repeat monitoring showed no progression of her conduction system disease and a decrease in ventricular ectopy. Her steroids were slowly tapered, and repeat surveillance was planned.
Diagnostic Criteria
The most definitive diagnostic criterion for CS remains histological evidence of multinucleated giant cells and noncaseating granulomata on cardiac biopsy. However, endomyocardial biopsy as the sole means for diagnosing CS is problematic because of its risks and low yield due to the heterogeneous involvement of myocardial segments that may not include the right ventricular (RV) septum, the prime biopsy target. Therefore, several diagnostic scoring systems have been developed to help guide clinicians suspecting CS. These include the 2014 Heart Rhythm Society (HRS) Expert Consensus Definition3 (Supplemental Table 1), and the 2019 Japanese Circulation Society (JCS) Criteria5 (Supplemental Table 2), which have significant overlap. Most often the diagnosis of CS is based on the presence of extracardiac histologic findings of sarcoidosis and clinical assessment of cardiac involvement with ECG and multimodality imaging. The HRS Expert Consensus Statement describes two pathways to diagnose CS: a histologic and a clinical pathway. Endomyocardial biopsy showing noncaseating granulomata establishes the histologic diagnosis. The clinical diagnosis of “probable” CS is made by the association of clinical or imaging findings suggestive of cardiac involvement in a patient with histologically proven extracardiac sarcoidosis, provided that alternative causes for the cardiac manifestations are reasonably excluded3.
In the HRS Expert Consensus Document3 histology typical of sarcoidosis-in the heart or elsewhere-is required to establish the diagnosis of CS whereas this is not required by the Joint Committee of the Japan Society of Sarcoidosis & Other Granulomatous Disorders, the Japanese College of Cardiology (2007)2 or the Japanese Society of Nuclear Cardiology (2017)4 criteria. Thus, imaging by CMR and 18F-FDG PET has a key role in establishing the diagnosis of CS in the clinical pathway. There is no pathognomonic CMR finding for CS although LGE in a non-ischemic patchy pattern is typical. Clinically isolated CS is not uncommon13 and indicates that active disease is limited to the heart. In that case the diagnosis can be confirmed by endomyocardial biopsy14, whose sensitivity may be enhanced by advanced imaging15 or electroanatomic mapping16 to identify reachable biopsy targets.
ECG/Monitoring
The ECG and ambulatory monitoring are key tools for the diagnosis and interim reassessment of CS. A probable diagnosis of CS can be made in patients with extra cardiac sarcoidosis based on clinical criteria that include the presence of VA and/or conduction system disease in conjunction with the imaging studies noted above. Conduction abnormalities, especially unexplained heart block in a young patient (≤60 years old), should raise suspicion for CS. As cardiac arrhythmias and conduction abnormalities in patients with CS may include potentially fatal VA and high degree AV block, early detection and treatment during periodic follow up is important3, 17.
Because of its low sensitivity the ECG alone is insufficient as a screening tool for CS in patients with extracardiac sarcoidosis. Mehta and colleagues evaluated a structured screening approach for CS in patients with extracardiac sarcoidosis including obtaining a history of arrhythmia symptoms, ECGs, ambulatory monitoring, and transthoracic echocardiography. Those with symptoms or abnormal test results undergo advanced imaging18. The overall prevalence of CS in the screened group was 39%, with the majority of those with findings on initial screening having abnormalities on advanced imaging18. A similar approach has been suggested in the HRS Consensus document.3
Although it is believed that the atria are affected less often than the ventricles in CS, AA can be seen on monitoring in around 1/3 of patients with CS19 Such arrhythmias may also contribute to the relatively more frequent inappropriate ICD therapies seen in patients with CS20. Other more subtle ECG findings associated with CS include QRS fragmentation, defined as two anatomically contiguous leads demonstrating RSR’ patterns in the absence of bundle branch block, as well as pseudo infarction patterns.21 Supplemental Table 3 lists a number of ECG and ambulatory monitoring abnormalities that suggest CS in patients with extracardiac disease.
Ambulatory monitoring may also be useful in assessing the response to therapy, as arrhythmia burden and conduction disorders can improve with immunosuppressive therapy. As sarcoidosis may be a progressive disease, periodic ECG screening and ambulatory monitoring are important even after a negative initial evaluation, especially in those in whom therapy is deferred or who have active disease elsewhere.22
Imaging
Echocardiography
Echocardiography is an integral component of CS screening and follow up. A main role is to assess patients with extracardiac sarcoidosis for myocardial involvement. CS has a predilection for the basal left ventricle (LV), the basal septum as well as the papillary muscles, the RV free wall, and the pericardium in decreasing frequency23. Regional wall motion abnormalities may mimic the patchy involvement of the disease. Abnormal thinning or thickening in non-coronary distributions especially in the basal septum suggest CS. Areas of thinning are thought to possibly indicate scarring suggesting longer standing disease while areas of thickening may reflect edema and ongoing inflammation as illustrated in Figure 2. LV dilatation, dysfunction, global hypokinesis, aneurysms, diastolic dysfunction, pericardial effusion, and increased echogenicity are other findings that may be indicative of CS. Predominant RV involvement can occur24 and in advanced disease RV dilatation and dysfunction can mimic RV cardiomyopathy25, 26. The echocardiogram is likely to be normal in early disease despite abnormalities in CMR and 18F-FDG PET18. Global longitudinal strain has been described to be impaired in patients with subclinical CS and is correlated with adverse outcomes27. Its reassessment has been suggested as a tool to evaluate response to therapy28; however long-term data are currently lacking.
Figure 2:
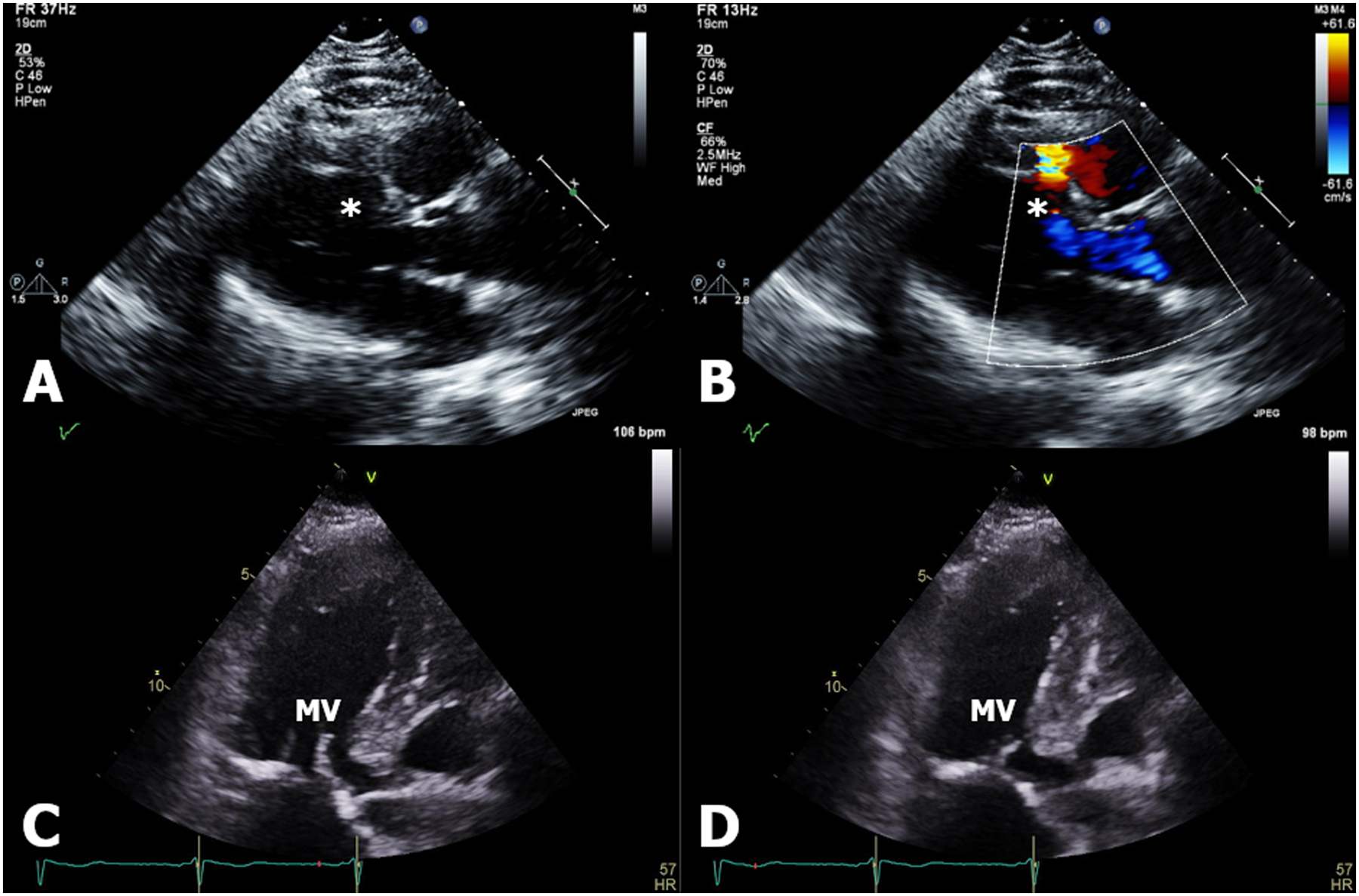
(Panels A-B) Echocardiogram demonstrating a left ventricular basal anteroseptal aneurysm complicated by ventricular septal rupture (denoted by asterisk) with left-to-right shunt seen by color flow. (Panels C-D) Left ventricular basal anteroseptal thickening thought to be due to inflammation, shown in both diastole and systole, that mimics hypertrophic obstructive cardiomyopathy with systolic anterior motion of the mitral valve (MV).
In sum, because of its wide availability, lack of radiation exposure, and low cost, echocardiography is particularly useful as part of an initial or interval testing algorithm to assess for cardiac involvement in patients with extracardiac sarcoidosis but its low sensitivity18 limits its value as a screening tool unless combined with strain analysis or other testing.
Cardiac Magnetic Resonance Imaging (CMR)
As a multi-parametric imaging modality CMR is able to assess both fibrosis and inflammation making it an essential tool for the diagnosis and assessment of treatment response in CS.3, 29, 30
CMR can accurately assess ejection fraction, chamber volume and the structural abnormalities typical of CS30–32 Importantly, LGE allows the detection and quantification of myocardial fibrosis resulting from T-cell mediated necrosis in CS3, 29, 30. While there is no pathognomonic LGE pattern in CS, patchy regions of LGE in a non-vascular distribution frequently involving the mid-myocardium or sub-endocardium of the basal septum or inferolateral LV wall, or occasionally the RV are common.30–32 A perfusion defect (Gradient Echo Perfusion Imaging) and delayed enhancement (Inversion Recovery Imaging) are demonstrated in Figure 3.
Figure 3:
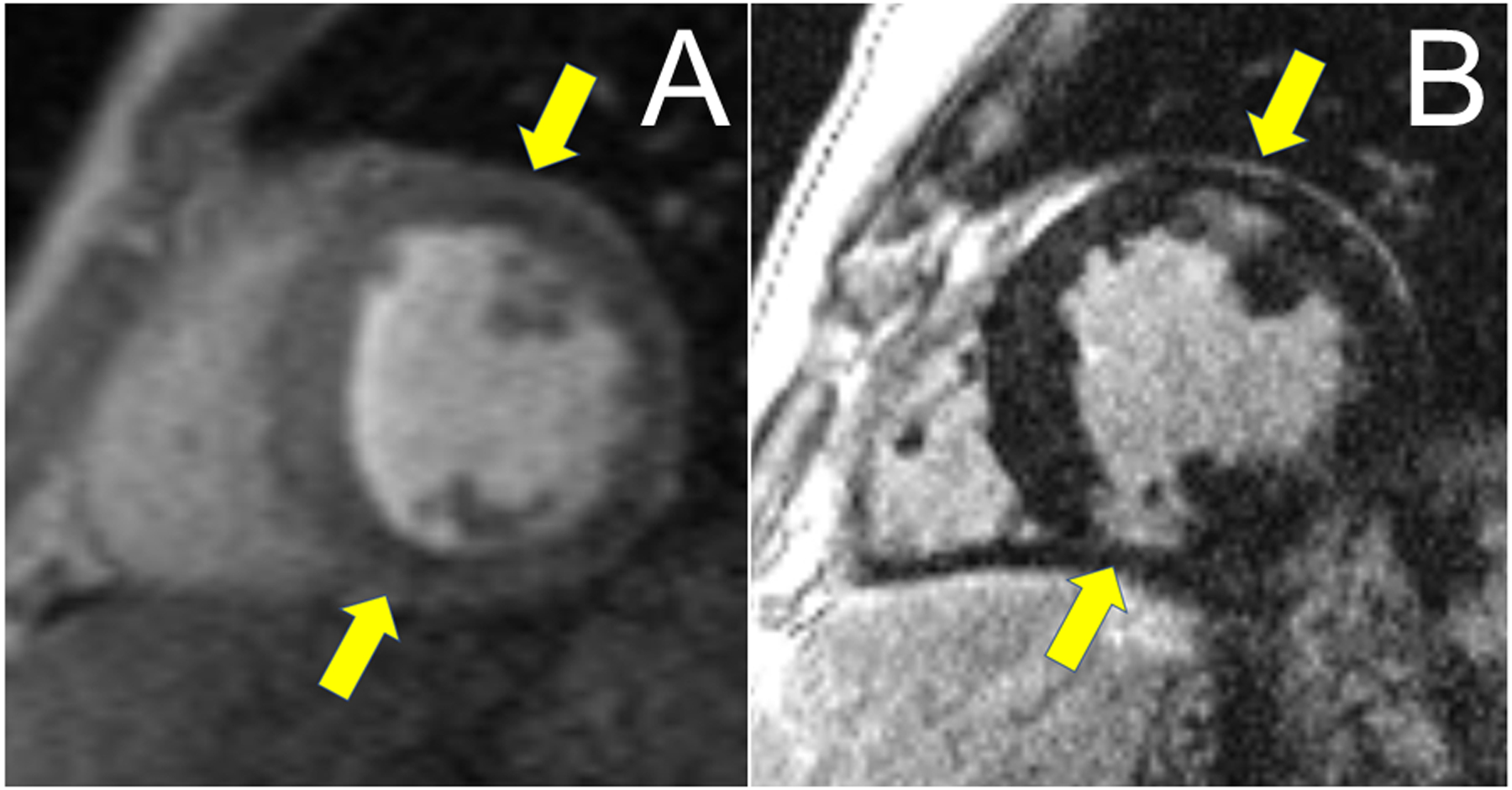
Panel A: CMR with areas of subtle hypoperfusion seen in Gradient Echo Perfusion Imaging (GRE) as areas of decreased signal intensity in endo and mid-myocardium of the LV (yellow arrows). Panel B: Areas of corresponding LGE seen in the anterior (endo/midmyocardial) and inferior (near transmural) wall of the LV (yellow arrows).
As noted, the presence of a typical pattern of LGE for sarcoid in patients with a histological diagnosis of extra-cardiac sarcoid can establish a ‘probable diagnosis’ of CS if other causes have been reasonably excluded.3
CMR has a high sensitivity (75–100%) and specificity (76–78%) for the diagnosis of CS and demonstrates LGE in 19.0–38.7% of patients with extracardiac sarcoidosis without symptoms suggestive of CS.33–36
When screening for CS, CMR can be useful (Class IIa recommendation) in patients with either abnormalities on initial screening using symptoms/ECG/echo or in patients ≤60 years old presenting with unexplained advanced AV block.3
The presence of LGE is considered a high-risk feature for VA37, 38 and even in patients with an LVEF>35% is currently a Class IIa indication for ICD implantation.29
While LGE quantification is still evolving, some studies suggest a cut-off of at least 6% as identifying CS patients with an increased risk of VA/sudden cardiac death (SCD) without other indications for ICD implantation.39, 40
Several studies have also demonstrated the feasibility of detecting acute inflammatory changes (i.e. edema) and/or improvement with immunosuppressive therapy using T1/T2 mapping or T2-weighted CMR. However, clinical applications of these techniques are still evolving (e.g. due to low-signal-to-noise ratio)41–43
18F-Positron Emission Tomography Scans (18F-FDG PET)
To assess cardiac inflammation in CS,30 18F-FDG PET has largely replaced gallium scanning and along with CMR is a preferred method for diagnosis44, 45, prognosis46–48, and evaluating response to therapy49. 18F-FDG PET requires specific patient preparation, such as high-fat/low-carbohydrate diets and/or prolonged fasting, to inhibit normal myocardial glucose uptake and visualize 18F-FDG uptake in inflammatory cells. This preparation is critical, though even under optimal conditions the test-retest accuracy of 18F-FDG PET for CS is not 100%50. In conjunction with 18F-FDG PET, myocardial perfusion imaging with rubidium-82 or N-13 ammonia is commonly performed, which improves characterization of the presence and type of CS involvement51 (Figure 4).
Figure 4:
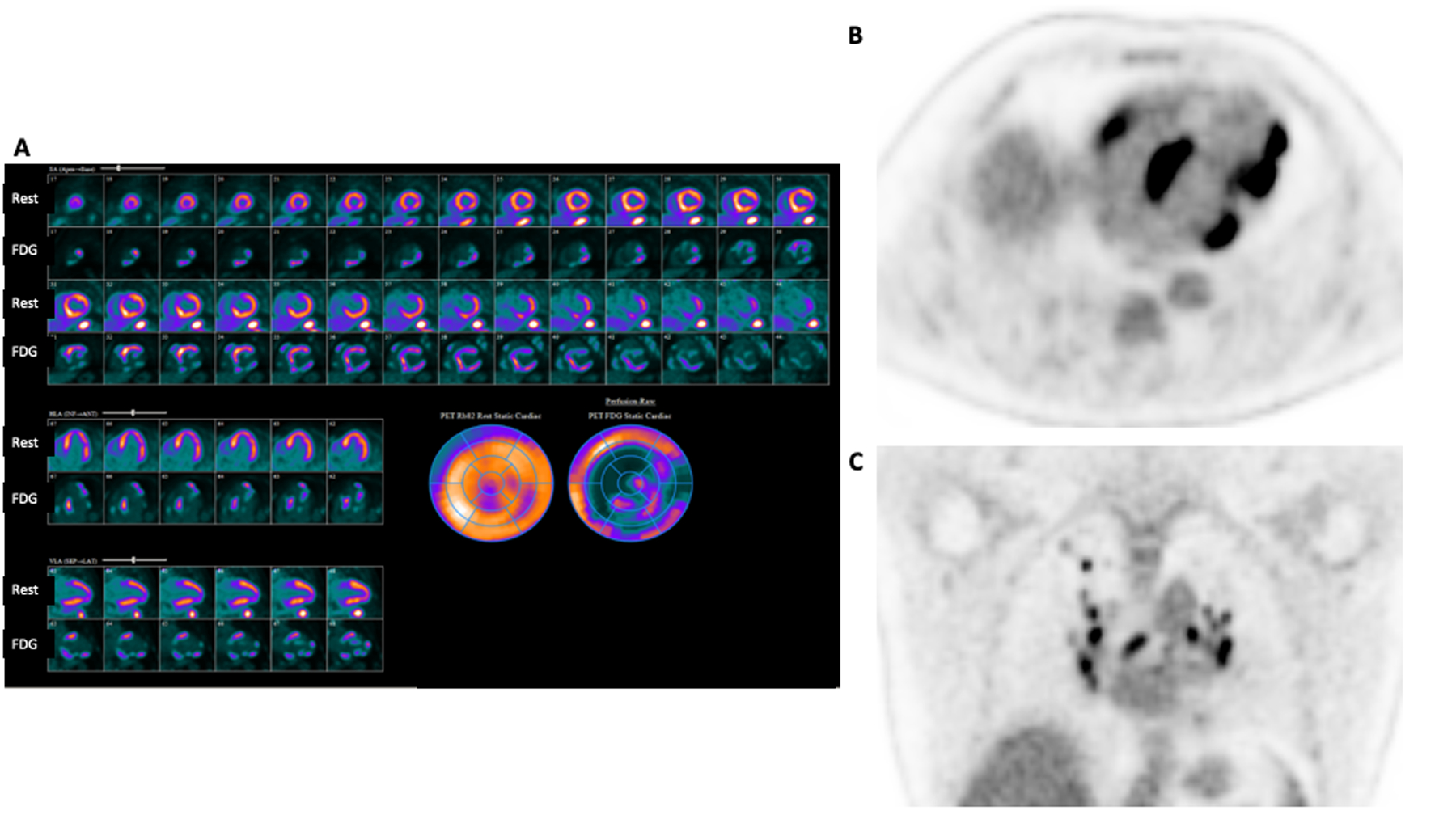
Rest 82-Rubidium perfusion images from Case 2 show perfusion defects in the basal septum, basal anterior and anterolateral segments, as well as apical inferior, apical lateral, and mid inferolateral segments suggesting areas of scar (A). 18F-FDG PET imaging shows a mismatch pattern with increased uptake in these segments, increased uptake in adjacent basal and mid inferior as well as mid anterolateral segments (A), and increased RV uptake (B) consistent with active inflammation in these areas. The combination of perfusion defects (scar) and 18F-FDG uptake (inflammation) allows a more complete understanding of the patient’s burden of disease, prognosis, and pathology. Whole body imaging (C), shows increased 18F-FDG uptake in hilar and mediastinal lymph nodes.
The classic findings of CS on 18F-FDG PET are focal myocardial FDG uptake along with regional perfusion defects in the absence of coronary artery disease45. Specificity is enhanced with hybrid PET/CT imaging that allows quantification of myocardial 18F-FDG uptake52, 53. Review of whole body images can identify extra-cardiac sites of sarcoidosis that are amenable to biopsy, permitting tissue diagnosis54. Even with quantification of 18F-FDG uptake, the interpretation of 18F-FDG PET for CS may be challenging, and the combination of clinical, ECG, CMR and 18F-FDG PET findings in a ‘probabilistic’ framework enhance interpretive certainty55.
In addition, 18F-FDG PET can be used to guide immunosuppressive therapy49, as retrospective studies have shown that a reduction in cardiac 18F-FDG uptake with treatment may be associated with improved LV function56. Moreover, 18F-FDG PET findings have been associated with specific clinical phenotypes. In particular, septal 18F-FDG uptake is associated with CS-mediated acute heart block57–59. As noted, based on these data, the HRS Consensus Statement recommends 18F-FDG PET , used in a complimentary fashion with CMR, in the evaluation of patients with extra-cardiac sarcoidosis and concern for CS, in adults ≤60 years old with newly identified advanced conduction disease and in those with unexplained VA3.
Tissue diagnosis
As noted, the criteria used by the Japanese Ministry of Health and Welfare in 199360 as well as the HRS in 20143 explicitly require histologic evidence of sarcoidosis in the heart or elsewhere before a definitive diagnosis of CS can be made, while the Joint Committee of the Japan Society of Sarcoidosis and Other Granulomatous Disorders and the Japanese College of Cardiology in 20072 and the Japanese Society of Nuclear Cardiology4 have not required this. The need for tissue diagnosis stems from the desire not to overlook other diagnoses and the fact that immunosuppressive treatment of sarcoidosis is not without risk and is often necessary for a prolonged period of time. Available imaging tools should be used to enhance diagnostic yield and identify potential extracardiac sites for biopsy61. The use of bronchioalveolar lavage and ultrasound guided lymph node biopsy during bronchoscopy should also be encouraged.62. However, despite best efforts, biopsies, including those of the heart, may be nondiagnostic or there may be no accessible biopsy targets, and the treating physician will need to make a judgement whether or not sarcoidosis is present in order to initiate or withhold immunosuppressive therapy. In this situation, multidisciplinary institutional boards may be beneficial to discuss management strategies.
Arrhythmias
Conduction Abnormalities
Case 2:
A 35-year-old male with hypertension presented with unheralded syncope. His 12-lead ECG showed sinus tachycardia, high degree heart block, and an escape rhythm with a RBBB, complete and incomplete, and alternating normal and left axis (Figure 5). Transthoracic echocardiogram showed a LVEF of 40%, 18F-FDG PET revealed patchy inflammation in the in basal LV septum, basal anterior and anterolateral segments, as well as apical inferior, apical lateral, and mid inferolateral segments and a whole-body scan demonstrated 18F-FDG uptake in mediastinal and hilar lymph nodes (Figure 4). Transbronchial needle aspiration of hilar lymph nodes revealed noncaseating granulomata. CMR showed mid myocardial hyperintensities in the basal lateral LV and interventricular septum on T2-weighted sequences, without LGE. These findings suggested sarcoidosis with pulmonary involvement and early stage active CS with inflammation and edema, without myocardial fibrosis. A dual chamber biventricular ICD was implanted and immunosuppressive therapy begun with improvement in AV conduction.
Figure 5:
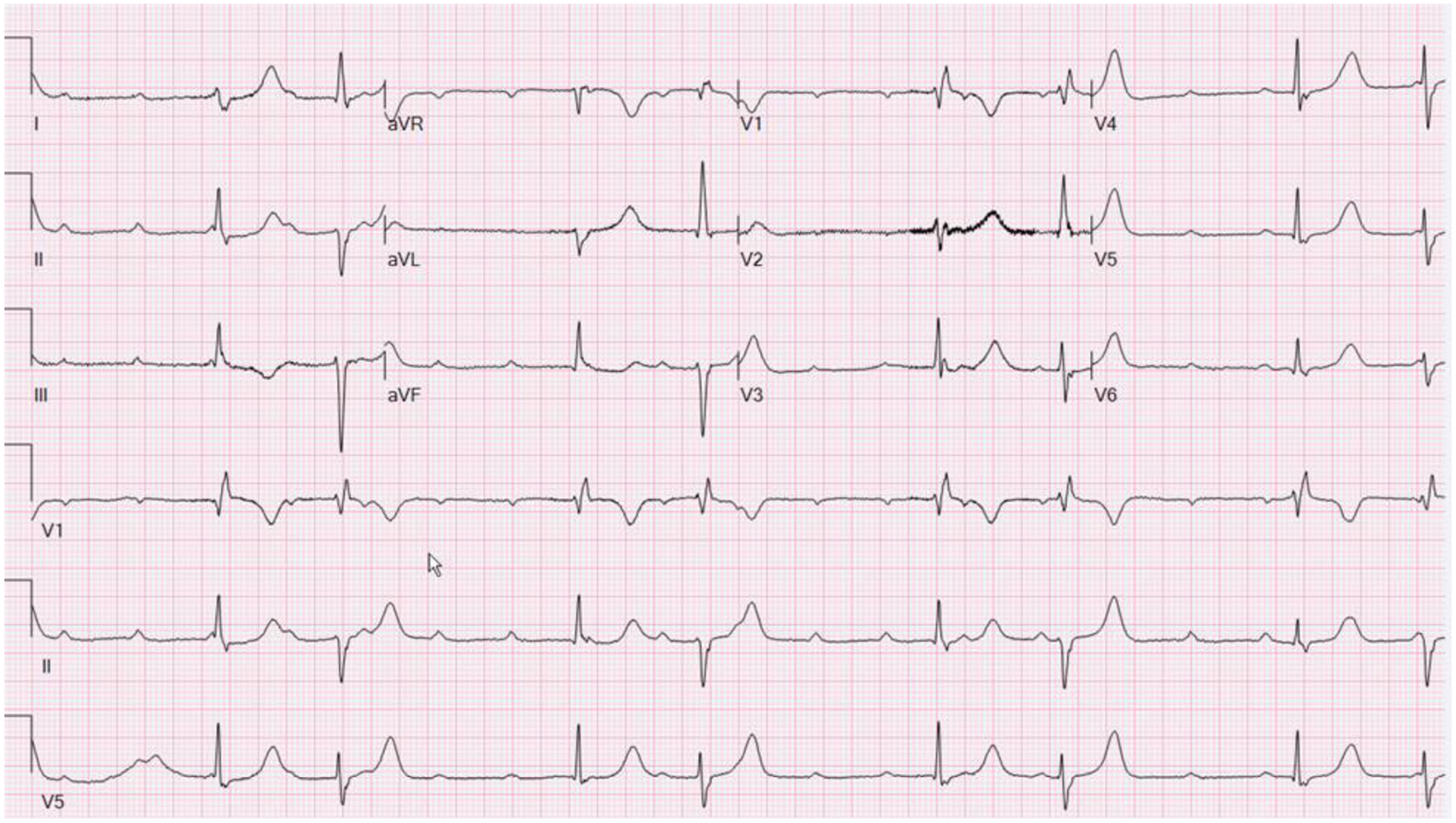
ECG showing sinus tachycardia with high degree heart block and an escape rhythm with a RBBB, complete and incomplete, and alternating normal and left axis.
The presence of conduction abnormalities, but most specifically, first degree AV block, RBBB, and advanced AV block, is one of the diagnostic hallmarks of CS and advanced AV block is one of the criteria for the clinical diagnosis of CS in both the HRS3 and Japanese Guidelines (minor criterion)5. In a study by Nery,et al., CS was identified in 34% of younger patients (<55–60 years old) presenting with heart block without other identified cause63 and should be considered in this situation.
In a study of patients with extracardiac sarcoidosis and no known CS, 25% had ECG abnormalities, and 38% of these had PR prolongation or RBBB64. ECG abnormalities always preceded cardiac symptoms and were associated with an adverse long-term prognosis.
In both Mehta, et al.’s study of patients with extracardiac sarcoidosis18, and Schuller, et al.’s21 series of patients with extra-cardiac sarcoidosis and signs or symptoms suggesting early CS, RBBB was more common in those ultimately found to have CS, than left bundle branch block (LBBB) or other conduction defects (23% vs 8%). Overall, it is estimated that RBBB is seen in 12–66% of patients with CS and complete or advanced AV block in about 25 to 75% of patients presenting with CS5. It is for these reasons, as well as the safety and limited expense associated with routine ECG screening and ambulatory monitoring, that such testing is recommended both at diagnosis and during follow up of patients with extracardiac sarcoidosis3, 5
As advanced AV block in the setting of CS is associated with a high risk for adverse events including VA, an ICD instead of a pacemaker may be considered in this situation even with a normal or near normal LVEF (Class IIa indication).3, 29.
Ventricular Arrhythmias (VA)
Case 3:
A 57 year old male presented with palpitations and near syncope. ECG showed a prolonged PR interval of 370 milliseconds (ms). An echocardiogram demonstrated moderate RV dilation with a LVEF of 45–50% and anteroseptal hypokinesis. Cardiac catheterization showed no obstructive epicardial coronary disease.
An electrophysiology study (EPS) was performed and showed an AH interval of 301 ms. and an HV interval of 69 ms. Monomorphic VT arising from the septum was induced.
A CMR was refused but an 18F-FDG PET was positive only in the heart showing focal 18F-FDG uptake in the septum and diffuse uptake in the RV. An endomyocardial biopsy confirmed CS.
A dual chamber ICD was implanted and a course of steroids was administered after which 18F-FDG PET imaging normalized. Despite this improvement, symptomatic VT of multiple morphologies recurred which was unresponsive to several antiarrhythmic drugs (AAD)s and advancement of immunosuppression. Extensive ablation including the use of bipolar radiofrequency energy (RFA) to target intraseptal VT proved unsuccessful. The patient ultimately succumbed to complications of VT storm while undergoing transplant evaluation.
As noted, VA are commonly seen in CS. In a Finnish study of 110 patients with CS, 31 presented with VT and 5 with ventricular fibrillation (VF) as their first manifestation of CS13. CS and other inflammatory myopathies have been increasingly recognized as the underlying substrate in patients with otherwise unexplained monomorphic VT, multifocal PVCs, or AV block with frequent PVCs.1, 65, 66 The incidence of VA leading to ICD therapies was found to be approximately 15% per year in a single center study of 45 CS patients with ICDs.67
The pathogenesis of VA in CS primarily involves myocardial scarring in the post-inflammatory phase. In the setting of scar the typical arrhythmia is monomorphic VT.68 Because of the frequently extensive and complex substrate, many patients present with several morphologies of VT or VT storm. The incidence of VA can also correlate with disease activity. In areas of active inflammation, arrhythmogenesis can be due to reentry or increased automaticity, manifest as PVCs, PVC-triggered VF, and focal VT. The His-Purkinje system may also participate in ventricular arrhythmogenesis in CS.69
Several studies have identified the presence and extent of LGE by CMR and inflammation by 18F-FDG PET imaging as powerful determinants of VA in CS, but no other established predictors exist. Importantly, LVEF alone is a poor risk marker for VA and patients with a preserved LVEF may still be at risk for life threatening arrhythmias. It remains to be determined whether frequent PVCs can identify a subset of patients at increased risk for sustained VA.
Atrial Arrhythmias (AA)
While the association of CS with VA and SCD is well recognized, AA are often missed3. The exact incidence of AA in CS is unknown, but in a study by Viles-Gonzalez et al., the prevalence of AA in patients with known CS was 32%.19
There are several potential etiologies for AA in CS, including granulomatous involvement of the atria leading to inflammation and scarring. Atrial scar has been shown in patients presenting with reentrant atrial flutter (AFl) and AF using voltage mapping70. This is further supported by atrial LGE positivity on CMR as well as atrial tracer accumulation on 18F-FDG PET71. In addition, elevated atrial pressures in the setting of progressive ventricular dysfunction and/or pulmonary disease or hypertension may contribute.19 Evaluation of CS patients for AA showed that AF was the most common AA (18%), followed by atrial tachycardia (7%), AFl (5%), and AV nodal reentrant tachycardia (2%). The higher incidence of AF is probably related to left ventricular dysfunction and pulmonary disease. Pulmonary hypertension and right atrial dilatation may contribute to typical AFl.72
The relationship of AA to sarcoidosis is primarily based on the identification of AA in patients with CS or non-cardiac systemic sarcoidosis in whom CMR and 18F-FDG PET have been used to specifically look for atrial disease. However, limited data are available about imaging studies in patients with AA without previously identified sarcoidosis.71
The management of CS related AA is essentially the same as in any patient with AA. AADs may be initiated for those with symptomatic AA, however Class I agents should be used with care in the presence of ventricular scar. Catheter ablation (CA) should be considered in symptomatic patients as it has been found to be fairly effective for AA, including AFl and AF in this setting.70 Patients should, in addition, receive guideline directed anticoagulation. The role of steroids specifically and immunosuppression in general has not been systematically evaluated for isolated AA in the setting of sarcoidosis. If immunosuppression is to be used, some studies recommend early initiation of intensive prednisone therapy followed by slow titration to a minimum suppressive dose which is continued indefinitely as there is also concern for VA following cessation of therapy3 although the optimal dose/duration of therapy is not known with certainty.
Cardiac Implantable Electronic Device (CIED) Therapy
CIED therapy has an important role in the management of patients with CS given their risk for VA and heart block. Both the HRS Consensus Statement, (Supplemental Table 4),3 and the 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death, (Supplemental Table 5)29, provide guidance. Both recommend ICDs, with a Class I indication, for secondary prevention as well as in patients with a LVEF ≤35% despite optimal medical therapy.
In CS patients presenting with conduction abnormalities, the decision to proceed with permanent pacing should be guided by standard recommendations. Both documents give a Class IIa recommendation for consideration of an ICD instead of a pacemaker in patients with bradycardia indications for a CIED, even with a LVEF >35%29. This is based on an approximately 24% incidence of significant VA in this group1 and emphasizes the importance of having a high suspicion for CS in younger patients (age ≤ 60) with advanced AV block.
The two guidelines couch their emphasis on EPS as a risk discriminator in the population with confirmed or probable CS but without bradycardia indications for a CIED and a normal or near normal ejection fraction somewhat differently3, 29. Risk assessment in this group remains especially challenging and a number of additional modalities have been proposed to refine the discrimination of the current guidelines including, in addition to EPS, voltage mapping, 18F-FDG PET response to therapy, amount of LGE on CMR40 and levels of a variety of biomarkers. Further prospective studies are warranted to identify the ideal risk assessment strategy in this group.
When Devices are not Enough
Case 4:
A 56-year-old man with pulmonary sarcoidosis CMR showing LGE, an LVEF of 30%, an initial 18F-FDG PET without evidence of inflammation, and a primary prevention ICD presented with ICD shocks several months after device implantation. His medications included carvedilol, spironolactone, furosemide, lisinopril and low dose prednisone. Device interrogation at that time showed 3 appropriate shocks for monomorphic VT as well as successful antitachycardia pacing (Figure 6). Repeat 18F-FDG PET showed new focal myocardial 18F-FDG uptake. High dose prednisone, methotrexate and amiodarone were initiated. The patient had no further arrhythmias or ICD shocks. A repeat 18F-FDG PET 6 months later showed no 18F-FDG uptake. Prednisone was stopped, methotrexate was continued and amiodarone was gradually tapered and stopped.
Figure 6:
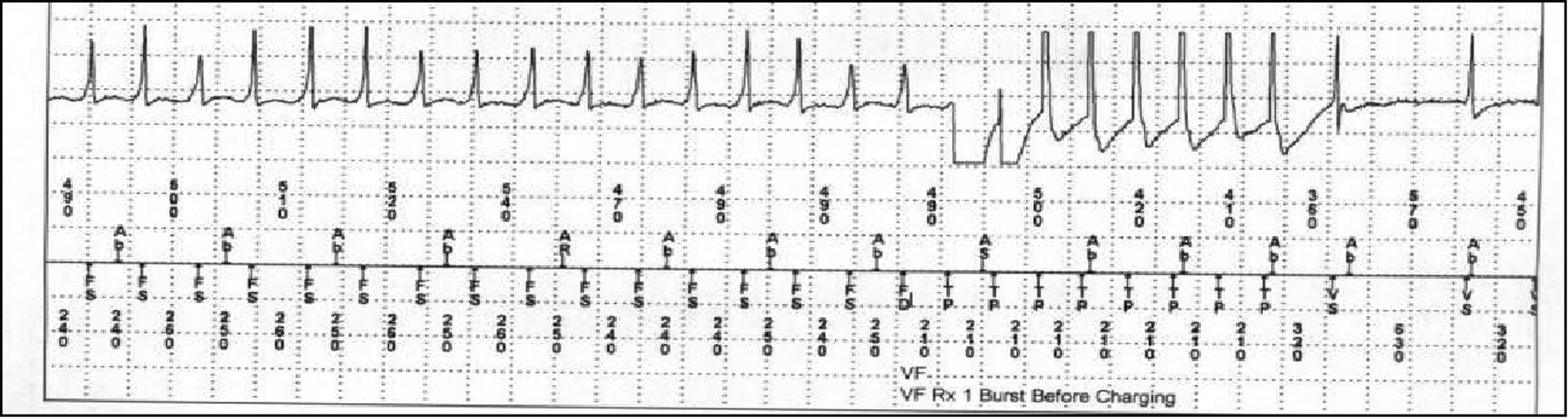
ICD interrogation showing rapid ventricular tachycardia (FS), meeting criteria for the “ventricular fibrillation” (VF) zone, terminated by burst pacing (TP) prior to device charging. AR/Ab=markers of atrial activity, VS=Ventricular sensed event.
Antiarrhythmic Drugs (AAD)s
Few observational and no randomized data exist regarding the effectiveness of AADs in patients with CS. Many of the available case series are small. A study of 7 CS patients presenting with VT reported recurrent VT in 4 patients and sudden death in 2 despite EPS-guided use of AADs.73 Among the 4 patients receiving both an ICD and AADs, all had appropriate shocks. In a series of 8 CS patients presenting with VT, 6 received a variety of AADs in addition to immunosuppression.74 Among these, 4 underwent ICD placement, all of whom had ICD therapies.
A multicenter report described a stepwise approach for managing VT in CS, beginning with ICD implantation, followed by immunosuppression, a single AAD, a second AAD, and finally CA.75 Of 42 patients included, all were treated with immunosuppression, 21 required AADs, and 9 of those required CA. VT control was achieved with medication in 76%. Specific AADs used included amiodarone, sotalol, mexiletine, quinidine, dofetilide, or a combination of AADs. In a series of 37 CS patients presenting with VT and followed for 39 months,69 23 patients (62%) were rendered free of VT with medical therapy—either AADs (1 patient) or AADs and immunosuppression (22). Patients were treated with amiodarone, sotalol, or procainamide. In another study of 30 CS patients with ICDs, 20 received AADs: class III agents in 17 and class I agents in 576. Appropriate ICD therapies occurred in 9/21 (42%) patients treated with AADs, compared with 2/9 (22%) not treated with AADs. Finally, a study of 18 CS patients presenting with VT included 4 without active inflammation who were treated with AADs (amiodarone or sotalol) without concomitant immunosuppression. All had recurrent VT and ultimately required CA.77 The same study described successful adjunctive use of intravenous amiodarone and lidocaine for the management of unstable VA. Thus, current limited data suggest that a patient specific, multimodality approach is necessary.
Catheter Ablation (CA)
As with other types of myocardial scarring, VT in CS is most often caused by reentry68 and the principles of ablation (guided by substrate-, activation-, and entrainment-mapping) are similar.75 However, the nature and distribution of the CS substrate poses distinct challenges.
As illustrated by the accompanying case, ablation is not recommended as first line therapy if 18F-FDG PET indicates the presence of active inflammation as the arrhythmia substrate is changeable and mechanisms may differ. Edema may also limit the penetration of thermal energy, and recurrence is higher in this setting.3, 78 However, ablation has a role when immunosuppression and AADs fail, and for recurrent VA in the absence of active disease.69, 77
Multiple inducible VTs are usual (a median of 3 morphologies reported in two studies), with 59% having a LBBB pattern (suggesting RV or septal origin), 32% RBBB (suggesting LV free wall), and 9% indeterminate.68, 75 Approximately 20% of patients require epicardial ablation.75, 79 In the RV, areas of scar can be widespread, confluent, and transmural; most can be reached with a combined endo/epicardial approach. Conversely, scar in the LV is usually patchy and can affect any part of the ventricle (most commonly the septum and anterior wall).68 A particular challenge is the presence of mid-mural scar, especially in the septum, which is difficult to identify and reach using conventional endo/epicardial mapping techniques. Scar imaging with CMR is invaluable in procedure planning.3, 78
The efficacy of ablation in CS is modest when defined as complete freedom from VA. Reported outcomes in part reflect the extent of disease, with better results in patients with preserved LV function.75, 80 In a meta-analysis, recurrence occurred in 54% of patients; 31% underwent a second procedure, and 5% a third.79 However, taking a wider view of success, ablation significantly reduces VA and resultant ICD therapies in 88% of patients and is a successful treatment for VT storm in 78%.68, 79 Major procedural complications occur in around 5% of this sick group of patients.79
Steroids and Beyond
Corticosteroids are the drugs of choice for treating most forms of sarcoidosis. However, the effectiveness of corticosteroids in suppressing VA in CS is not clear cut and in a meta-analysis, the data quality was too limited to draw conclusions.81 Corticosteroids may be particularly beneficial in early disease, especially early after presentation with heart block22, but may paradoxically worsen VA and may be associated with aneurysm formation in a minority of patients.3
Most experts initiate prednisone therapy at a dose of 30–60 mg a day for several months68, 77, 80. In the acute setting, methylprednisolone 10–15mg/kg per day for 3 days may be considered80. Gradual weaning of steroids followed by repeat 18F-FDG PET imaging to establish treatment response is recommended82.
Corticosteroid-sparing agents, most commonly methotrexate, are often used with or without corticosteroids to reduce the risk of corticosteroid-related side effects. In one study of 36 CS patients, the clinical relapse rate was 46% in the corticosteroid group versus 17% in the corticosteroid with immunosuppressive (azathioprine, methotrexate, or cyclophosphamide) group (p=0.048).83 A small retrospective analysis showed that corticosteroid-sparing regimens containing methotrexate with or without adalimumab are effective maintenance therapy after initial response, but patients must be monitored closely for relapse both on and off immunosuppression.84 Infliximab has been used for CS despite concerns about cardiotoxicity and worsening of heart failure that have made its role less certain. A recent study evaluated the efficacy of infliximab in 36 patients with refractory CS who had failed therapy with corticosteroids and corticosteroid-sparing agents.85 Two thirds improved, with half the responders showing a >50% reduction in arrhythmia on ICD or other monitoring. Six patients (17%) had an adverse event, and 3 patients stopped infliximab for a prolonged period. Other biologic agents are also being evaluated. The currently-enrolling CHASM-CS RCT (NCT03593759) is the first randomized controlled treatment study for CS and will compare standard dose prednisone versus low dose prednisone with a taper over 3 months plus methotrexate.86 While the primary endpoint will be summed perfusion rest score on 18F-FDG PET, secondary endpoints will include arrhythmia burden, mortality, and quality of life.
The Need to Treat the Whole Patient: Heart Failure Management
Case 5:
A 51-year-old man presented with worsening appetite and shortness of breath and was diagnosed with gastrointestinal and pulmonary sarcoidosis prompting initiation of corticosteroid therapy. Initial and periodic screening for CS was negative. He initially did well, but later presented with bradycardia and was found to have a new cardiomyopathy with an LVEF of 20% and 18F-FDG uptake concerning for active CS. He received a biventricular ICD, his steroids were adjusted, and he remained stable for several years on medical therapy. He then received multiple shocks for VT/VF. Episodes of VT storm with associated multisystem organ failure prompted peripheral veno-arterial extracorporeal membrane oxygenation. He was urgently evaluated for dual heart/kidney transplantation, underwent heart transplantation followed by kidney transplantation and was ultimately discharged home. Cardiac tissue obtained at transplant showed noncaseating granulomata consistent with CS (Figure 7).
Figure 7:
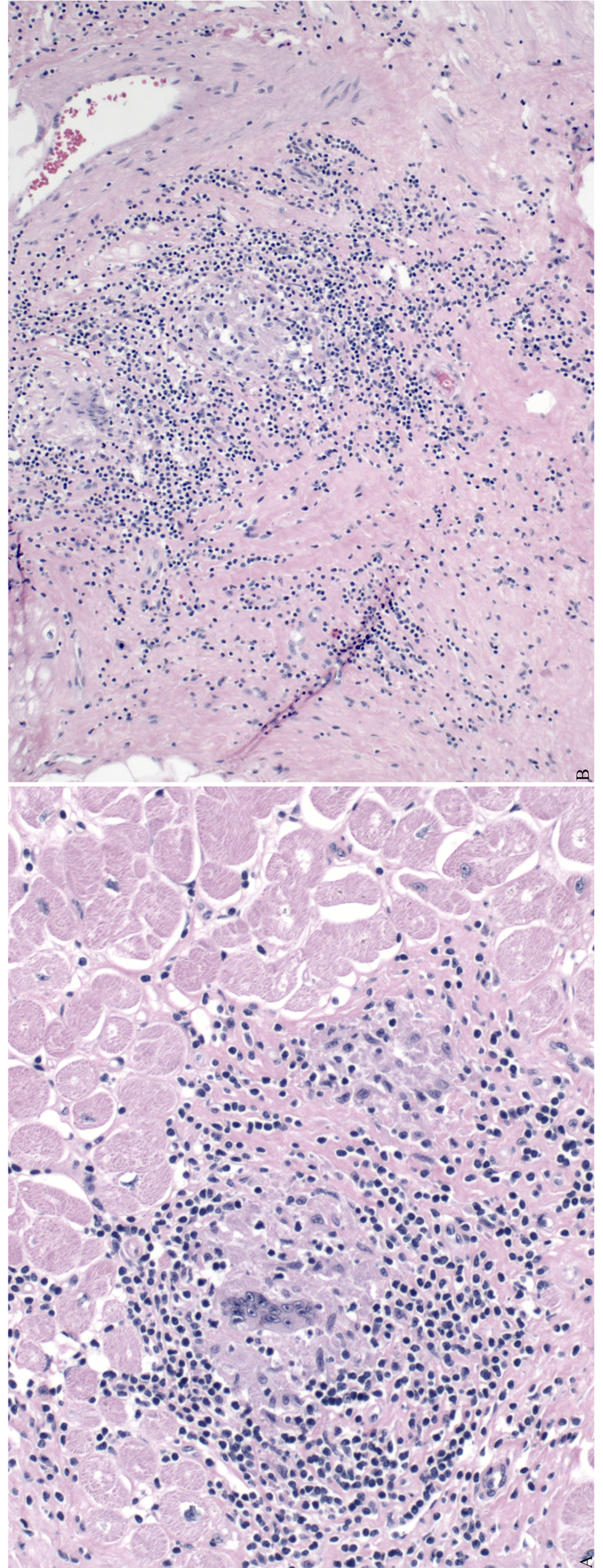
Panels A and B: Pathology of explanted heart of a patient with cardiac sarcoidosis. Giant cells and focal granulomata are seen.
Medical Therapy of Heart Failure
CS can present as either a restrictive or dilated cardiomyopathy. The prognosis is significantly worse in those presenting with heart failure13. Restrictive cardiomyopathy with inflammation is treated with immunosuppression and diuretics to manage volume status and improve symptoms. Medical management of patients presenting with dilated cardiomyopathy includes appropriate neurohormonal blockade along with immunosuppression87. While dedicated trials addressing the efficacy of neurohormonal blockade in patients with CS are lacking, the overwhelming evidence that it improves survival88, reduces hospitalizations and improves LVEF in patients with heart failure and reduced LVEF has been extrapolated to this setting89, 90.
Advanced Therapy
Most CS patients do not progress to advanced heart failure. For those with refractory disease, advanced heart failure therapies (AHFT), specifically heart transplantation or mechanical circulatory support may be considered. Three principal questions guide consideration of AHFT:
Is the patient sick enough to benefit?
What is the burden of co-morbid disease? With sarcoidosis, what is the extent of extracardiac involvement?
What are the anticipated long-term outcomes?
The International Society of Heart and Lung Transplantation Guidelines do not make recommendations specific to CS, but do discuss restrictive cardiomyopathies. Patients with refractory heart failure should be considered for AHFT if their extracardiac involvement is not significant91. Other indications for AHFT include severe LV and RV failure and intractable VT.
The number of CS patients undergoing left ventricular assist device (LVAD) placement and heart transplantation has increased over the last decade92. Potential issues with LVADs include: (1) Immunosuppression predisposing to infection, (2) Small LV size leading to pump dysfunction, and (3) The risk of RV failure. Data on LVAD use specific to CS are limited. An INTERMACS analysis of patients with restrictive cardiomyopathy included 17 CS patients. The overall restrictive cardiomyopathy cohort had survival similar to non-restrictive patients, although LV end diastolic dimension < 5.0 cm resulted in increased mortality93.
Considerations for heart transplantation also include the risk for CS recurrence in the allograft. In most instances, immunosuppression after heart transplantation prevents progression of systemic disease, although patients with advanced disease in other organs may not be heart transplant candidates. Case reports of recurrence in the allograft exist, but this is uncommon. To minimize risk of recurrence, post-heart transplantation patients with a history of CS are continued on chronic corticosteroids. Survival after heart transplantation in selected CS patients is similar to other cohorts92.
It is important to document restrictive hemodynamics as this finding, if present, qualifies CS patients listed for heart transplantation as Status 4 which may increase their priority for this lifesaving operation. Problematically, a single center study showed 18% discordance between clinical and pathologic diagnosis overall in patients listed for heart transplantation, with CS being the most frequently misclassified entity94. This, again, supports the importance of pursuing the pathologic diagnosis of CS, as it may be critical to avoid inadvertent underutilization of treatment options and, if necessary, underprioritization for transplantation.
Conclusions
While the exact cause of CS remains undefined, this review describes the great strides that have been made in recent years in its recognition, treatment and prognosis. Armed with heightened awareness, and knowledge of current care guidelines, it is hoped that more patients will receive appropriate treatment and have improved outcomes, and that ongoing research will ultimately lead to a better understanding of disease mechanisms.
Supplementary Material
Disclosures:
Dr Chung has received grants from the NIH and AHA. Dr Grunewald has received grants from the Karolinska Instituet. Dr Miller has received grants, “other” and done consulting for Bracco, Eidos, Ainylan, Pfizer Inc., and has received “other” and done consulting for General Electric. Dr Kron has been supported by the Virginia Commonwealth University Wright Center for Clinical and Translational Research. Dr Singh has done consulting and received personal fees from Abbott, Boston Scientific, Biotronik, EBR, Medtronic, Microport, Cardiologs, Impulse Dynamics, Toray and OrchestraBioMed. Dr Ellenbogen has received grants from Medtronic and Boston Scientific and has received personal fees from Medtronic, Boston Scientific, Abbott, and Biotronik. Dr Callahan has received personal fees and served as a consultant for Biotronik. Dr Blankstein has received royalties from Up To Date. Dr Estep has received personal fees and served as a medical advisor for Abbott. He has also received personal fees and served as a consultant for Getinge. The other authors have no relevant conflicts to disclose.
Non-standard Abbreviations and Acronyms
- AA
Atrial arrhythmia
- AAD
Antiarrhythmic drug
- AF
Atrial fibrillation
- AFl
Atrial flutter
- AHFT
Advanced heart failure therapies
- CA
Catheter ablation
- CIED
Cardiac implantable electronic device
- CMR
Cardiac magnetic resonance imaging
- CS
Cardiac sarcoidosis
- EPS
Electrophysiology Study
- 18F-FDG PET
Fluorodeoxyglucose F-18 positron emission tomography scan
- HLA
Human leukocyte antigen
- HRS
Heart Rhythm Society
- ICD
Implantable cardioverter defibrillator
- IL
Interleukin
- JCS
Japanese Circulation Society
- LBBB
Left bundle branch block
- LGE
Late gadolinium enhancement
- LV
Left ventricle
- LVAD
Left ventricular assist device
- LVEF
Left ventricular ejection fraction
- MHC
Major histocompatibility complex
- RBBB
Right bundle branch block
- RFA
Radiofrequency ablation
- RV
Right ventricle
- SCD
Sudden cardiac death
- TCR
T cell receptor
- Th
T helper
- VA
Ventricular arrhythmia
- VF
Ventricular fibrillation
- VPC
Ventricular premature contraction
- VT
Ventricular tachycardia
References:
- 1.Nordenswan HK, Lehtonen J, Ekstrom K, Kandolin R, Simonen P, Mayranpaa M, Vihinen T, Miettinen H, Kaikkonen K, Haataja P, et al. Outcome of Cardiac Sarcoidosis Presenting With High-Grade Atrioventricular Block. Circ Arrhythm Electrophysiol. 2018;11:e006145. [DOI] [PubMed] [Google Scholar]
- 2.Diagnostic standard and guidelines for sarcoidosis. Japanese Journal of Sarcoidosis Granulomatous Disorders. 2007;27:89–102. [Google Scholar]
- 3.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Nielsen JC, et al. HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated With Cardiac Sarcoidosis. Heart Rhythm. 2014;11:1304–1323. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinaga K, Miyagawa M, Kiso K, Ishida Y. Japanese Guidelines for Cardiac Sarcoidosis. Annals of Nuclear Cardiology. 2017;3:121–124. [Google Scholar]
- 5.Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, Inomata T, Ishibashi-Ueda H, Eishi Y, Kitakaze M, et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis- Digest Version. Circ J. 2019;83:2329–2388. [DOI] [PubMed] [Google Scholar]
- 6.Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, Terrin ML, Weinberger SE, Moller DR, McLennan G, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324–30. [DOI] [PubMed] [Google Scholar]
- 7.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–67. [DOI] [PubMed] [Google Scholar]
- 8.Sverrild A, Backer V, Kyvik KO, Kaprio J, Milman N, Svendsen CB, Thomsen SF. Heredity in sarcoidosis: a registry-based twin study. Thorax. 2008;63:894–6. [DOI] [PubMed] [Google Scholar]
- 9.Moller DR, Rybicki BA, Hamzeh NY, Montgomery CG, Chen ES, Drake W, Fontenot AP. Genetic, Immunologic, and Environmental Basis of Sarcoidosis. Ann Am Thorac Soc. 2017;14:S429–s436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miedema JR, Kaiser Y, Broos CE, Wijsenbeek MS, Grunewald J, Kool M. Th17-lineage cells in pulmonary sarcoidosis and Lofgren’s syndrome: Friend or foe? J Autoimmun. 2018;87:82–96. [DOI] [PubMed] [Google Scholar]
- 11.Grunewald J, Kaiser Y, Ostadkarampour M, Rivera NV, Vezzi F, Lotstedt B, Olsen RA, Sylwan L, Lundin S, Kaller M, et al. T-cell receptor-HLA-DRB1 associations suggest specific antigens in pulmonary sarcoidosis. Eur Respir J. 2016;47:898–909. [DOI] [PubMed] [Google Scholar]
- 12.Kron J, Mauro AG, Bonaventura A, Toldo S, Salloum FN, Ellenbogen KA, Abbate A. Inflammasome Formation in Granulomas in Cardiac Sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12:e007582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–32. [DOI] [PubMed] [Google Scholar]
- 14.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–31. [DOI] [PubMed] [Google Scholar]
- 15.Kandolin R, Lehtonen J, Graner M, Schildt J, Salmenkivi K, Kivisto SM, Kupari M. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270:461–8. [DOI] [PubMed] [Google Scholar]
- 16.Nery PB, Keren A, Healey J, Leug E, Beanlands RS, Birnie DH. Isolated cardiac sarcoidosis: establishing the diagnosis with electroanatomic mapping-guided endomyocardial biopsy. Can J Cardiol. 2013;29:1015.e1–3. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Kanda T, Kubota S, Imai S, Murata K. Holter monitoring as a noninvasive indicator of cardiac involvement in sarcoidosis. Chest. 1994;106:1021–4. [DOI] [PubMed] [Google Scholar]
- 18.Mehta D, Lubitz SA, Frankel Z, Wisnivesky JP, Einstein AJ, Goldman M, Machac J, Teirstein A. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426–1435. [DOI] [PubMed] [Google Scholar]
- 19.Viles-Gonzalez JF, Pastori L, Fischer A, Wisnivesky JP, Goldman MG, Mehta D. Supraventricular arrhythmias in patients with cardiac sarcoidosis prevalence, predictors, and clinical implications. Chest. 2013;143:1085–1090. [DOI] [PubMed] [Google Scholar]
- 20.Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, Rosenfeld L, Mitiku TY, Cooper JM, Mehta D, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace. 2013;15:347–54. [DOI] [PubMed] [Google Scholar]
- 21.Schuller JL, Olson MD, Zipse MM, Schneider PM, Aleong RG, Wienberger HD, Varosy PD, Sauer WH. Electrocardiographic characteristics in patients with pulmonary sarcoidosis indicating cardiac involvement. J Cardiovasc Electrophysiol. 2011;22:1243–8. [DOI] [PubMed] [Google Scholar]
- 22.Padala SK, Peaslee S, Sidhu MS, Steckman DA, Judson MA. Impact of early initiation of corticosteroid therapy on cardiac function and rhythm in patients with cardiac sarcoidosis. International journal of cardiology. 2017;227:565–570. [DOI] [PubMed] [Google Scholar]
- 23.Roberts WC, McAllister HA Jr., Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). The American journal of medicine. 1977;63:86–108. [DOI] [PubMed] [Google Scholar]
- 24.Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol. 2009;104:571–7. [DOI] [PubMed] [Google Scholar]
- 25.Vasaiwala SC, Finn C, Delpriore J, Leya F, Gagermeier J, Akar JG, Santucci P, Dajani K, Bova D, Picken MM, et al. Prospective study of cardiac sarcoid mimicking arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2009;20:473–6. [DOI] [PubMed] [Google Scholar]
- 26.Patel MB, Mor-Avi V, Murtagh G, Bonham CA, Laffin LJ, Hogarth DK, Medvedofsky D, Lang RM, Patel AR. Right Heart Involvement in Patients with Sarcoidosis. Echocardiography. 2016;33:734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joyce E, Ninaber MK, Katsanos S, Debonnaire P, Kamperidis V, Bax JJ, Taube C, Delgado V, Ajmone Marsan N. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. European journal of heart failure. 2015;17:51–62. [DOI] [PubMed] [Google Scholar]
- 28.Lo A, Foder K, Martin P, Younger JF. Response to steroid therapy in cardiac sarcoidosis: insights from myocardial strain. European heart journal cardiovascular Imaging. 2012;13:E3. [DOI] [PubMed] [Google Scholar]
- 29.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 30.Slart R, Glaudemans A, Lancellotti P, Hyafil F, Blankstein R, Schwartz RG, Jaber WA, Russell R, Gimelli A, Rouzet F, et al. A joint procedural position statement on imaging in cardiac sarcoidosis: from the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J Nucl Cardiol. 2018;25:298–319. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez R, Trivieri M, Fayad ZA, Ahmadi A, Narula J, Argulian E. Advanced Imaging in Cardiac Sarcoidosis. J Nucl Med. 2019;60:892–898. [DOI] [PubMed] [Google Scholar]
- 32.Yatsynovich Y, Valencia D, Petrov M, Linares JD, Rahman MM, Dittoe N. Updates on the Role of Imaging in Cardiac Sarcoidosis. Current treatment options in cardiovascular medicine. 2018;20:74. [DOI] [PubMed] [Google Scholar]
- 33.Greulich S, Deluigi CC, Gloekler S, Wahl A, Zurn C, Kramer U, Nothnagel D, Bultel H, Schumm J, Grun S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovascular imaging. 2013;6:501–11. [DOI] [PubMed] [Google Scholar]
- 34.Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, Miura M, Sakaue S, Tamaki N, Nishimura M. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–41. [DOI] [PubMed] [Google Scholar]
- 35.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45:1683–90. [DOI] [PubMed] [Google Scholar]
- 37.Coleman GC, Shaw PW, Balfour PC Jr., Gonzalez JA, Kramer CM, Patel AR, Salerno M. Prognostic Value of Myocardial Scarring on CMR in Patients With Cardiac Sarcoidosis. JACC Cardiovascular imaging. 2017;10:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hulten E, Agarwal V, Cahill M, Cole G, Vita T, Parrish S, Bittencourt MS, Murthy VL, Kwong R, Di Carli MF, et al. Presence of Late Gadolinium Enhancement by Cardiac Magnetic Resonance Among Patients With Suspected Cardiac Sarcoidosis Is Associated With Adverse Cardiovascular Prognosis: A Systematic Review and Meta-Analysis. Circ Cardiovasc Imaging. 2016;9:e005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, Gu X, Schuller J, Kron J, Nour KA, Cheng A, et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2014;7:1109–15. [DOI] [PubMed] [Google Scholar]
- 40.Kazmirczak F, Chen KA, Adabag S, von Wald L, Roukoz H, Benditt DG, Okasha O, Farzaneh-Far A, Markowitz J, Nijjar PS, et al. Assessment of the 2017 AHA/ACC/HRS Guideline Recommendations for Implantable Cardioverter-Defibrillator Implantation in Cardiac Sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12:e007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amano Y, Tachi M, Tani H, Mizuno K, Kobayashi Y, Kumita S. T2-weighted cardiac magnetic resonance imaging of edema in myocardial diseases. ScientificWorldJournal. 2012;2012:194069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med. 2014;189:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puntmann VO, Isted A, Hinojar R, Foote L, Carr-White G, Nagel E. T1 and T2 Mapping in Recognition of Early Cardiac Involvement in Systemic Sarcoidosis. Radiology. 2017;285:63–72. [DOI] [PubMed] [Google Scholar]
- 44.Takeda N, Yokoyama I, Hiroi Y, Sakata M, Harada T, Nakamura F, Murakawa Y, Nagai R. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation. 2002;105:1144–5. [DOI] [PubMed] [Google Scholar]
- 45.Yamagishi H, Shirai N, Takagi M, Yoshiyama M, Akioka K, Takeuchi K, Yoshikawa J. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44:1030–6. [PubMed] [Google Scholar]
- 46.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muser D, Santangeli P, Castro SA, Liang JJ, Enriquez A, Werner TJ, Nucifora G, Magnani S, Hayashi T, Zado ES, et al. Prognostic role of serial quantitative evaluation of (18)F-fluorodeoxyglucose uptake by PET/CT in patients with cardiac sarcoidosis presenting with ventricular tachycardia. Eur J Nucl Med Mol Imaging. 2018;45:1394–1404. [DOI] [PubMed] [Google Scholar]
- 48.Sperry BW, Tamarappoo BK, Oldan JD, Javed O, Culver DA, Brunken R, Cerqueira MD, Hachamovitch R. Prognostic Impact of Extent, Severity, and Heterogeneity of Abnormalities on (18)F-FDG PET Scans for Suspected Cardiac Sarcoidosis. JACC Cardiovascular imaging. 2018;11:336–345. [DOI] [PubMed] [Google Scholar]
- 49.Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL, Birnie DH, Chen ES, Cooper LT, Tung RH, et al. Joint SNMMI-ASNC Expert Consensus Document on the Role of (18)F-FDG PET/CT in Cardiac Sarcoid Detection and Therapy Monitoring. J Nucl Med. 2017;58:1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvi RM, Young BD, Shahab Z, Pan H, Winkler J, Herzog E, Miller EJ. Repeatability and Optimization of FDG Positron Emission Tomography for Evaluation of Cardiac Sarcoidosis. JACC Cardiovascular imaging. 2019;12:1284–1287. [DOI] [PubMed] [Google Scholar]
- 51.Berman JS, Govender P, Ruberg FL, Mazzini M, Miller EJ. Scadding revisited: a proposed staging system for cardiac sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:2–5. [PubMed] [Google Scholar]
- 52.Ahmadian A, Brogan A, Berman J, Sverdlov AL, Mercier G, Mazzini M, Govender P, Ruberg FL, Miller EJ. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2014;21:925–39. [DOI] [PubMed] [Google Scholar]
- 53.Varghese M, Smiley D, Bellumkonda L, Rosenfeld LE, Zaret B, Miller EJ. Quantitative Interpretation of FDG PET for cardiac sarcoidosis reclassifies visually interpreted exams and potentially impacts downstream interventions. Sarcoidosis Vasculitis and Diffuse Lung Disease. 2018;35:342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simonen P, Lehtonen J, Kandolin R, Schildt J, Marjasuo S, Miettinen H, Airaksinen J, Vihinen T, Tuohinen S, Haataja P, et al. F-18-fluorodeoxyglucose positron emission tomography-guided sampling of mediastinal lymph nodes in the diagnosis of cardiac sarcoidosis. Am J Cardiol. 2015;116:1581–5. [DOI] [PubMed] [Google Scholar]
- 55.Vita T, Okada DR, Veillet-Chowdhury M, Bravo PE, Mullins E, Hulten E, Agrawal M, Madan R, Taqueti VR, Steigner M, et al. Complementary Value of Cardiac Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Assessment of Cardiac Sarcoidosis. Circ Cardiovasc Imaging. 2018;11:e007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, Hainer J, Murthy VL, Skali H, Dorbala S, et al. Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2014;21:166–74. [DOI] [PubMed] [Google Scholar]
- 57.Ohira H, Birnie DH, Pena E, Bernick J, Mc Ardle B, Leung E, Wells GA, Yoshinaga K, Tsujino I, Sato T, et al. Comparison of (18)F-fluorodeoxyglucose positron emission tomography (FDG PET) and cardiac magnetic resonance (CMR) in corticosteroid-naive patients with conduction system disease due to cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2016;43:259–269. [DOI] [PubMed] [Google Scholar]
- 58.Orii M, Hirata K, Tanimoto T, Ota S, Shiono Y, Yamano T, Matsuo Y, Ino Y, Yamaguchi T, Kubo T, et al. Comparison of cardiac MRI and 18F-FDG positron emission tomography manifestations and regional response to corticosteroid therapy in newly diagnosed cardiac sarcoidosis with complet heart block. Heart Rhythm. 2015;12:2477–85. [DOI] [PubMed] [Google Scholar]
- 59.Mc Ardle BA, Birnie DH, Klein R, de Kemp RA, Leung E, Renaud J, DaSilva J, Wells GA, Beanlands RS, Nery PB. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by (1)(8)F- fluorodoexyglucose positron emission tomography? Circ Cardiovasc Imaging. 2013;6:617–26. [DOI] [PubMed] [Google Scholar]
- 60.Hiraga H, Yuwai K, Hiroe M. Guidelines for diagnosis of Cardiac Sarcoidosis: Study report on Diffuse Pulmonary Diseases. Japanese Ministry of Health and Welfare. 1993:23–24. [Google Scholar]
- 61.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65. [DOI] [PubMed] [Google Scholar]
- 62.von Bartheld MB, Dekkers OM, Szlubowski A, Eberhardt R, Herth FJ, in ‘t Veen JC, de Jong YP, van der Heijden EH, Tournoy KG, Claussen M, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. Jama. 2013;309:2457–64. [DOI] [PubMed] [Google Scholar]
- 63.Nery PB, Beanlands RS, Nair GM, Green M, Yang J, McArdle BA, Davis D, Ohira H, Gollob MH, Leung E, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25:875–881. [DOI] [PubMed] [Google Scholar]
- 64.Nagao S, Watanabe H, Sobue Y, Kodama M, Tanaka J, Tanabe N, Suzuki E, Narita I, Watanabe E, Aizawa Y et al. Electrocardiographic abnormalities and risk of developing cardiac events in extracardiac sarcoidosis. International journal of cardiology. 2015;189:1–5. [DOI] [PubMed] [Google Scholar]
- 65.Nery PB, Mc Ardle BA, Redpath CJ, Leung E, Lemery R, Dekemp R, Yang J, Keren A, Beanlands RS, Birnie DH. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2014;37:364–74. [DOI] [PubMed] [Google Scholar]
- 66.Tung R, Bauer B, Schelbert H, Lynch JP 3rd, Auerbach M, Gupta P, Schiepers C, Chan S, Ferris J, Barrio M, et al. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: The potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015;12:2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Betensky BP, Tschabrunn CM, Zado ES, Goldberg LR, Marchlinski FE, Garcia FC, Cooper JM. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm. 2012;9:884–91. [DOI] [PubMed] [Google Scholar]
- 68.Kumar S, Barbhaiya C, Nagashima K, Choi EK, Epstein LM, John RM, Maytin M, Albert CM, Miller AL, Koplan BA, et al. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol. 2015;8:87–93. [DOI] [PubMed] [Google Scholar]
- 69.Naruse Y, Sekiguchi Y, Nogami A, Okada H, Yamauchi Y, Machino T, Kuroki K, Ito Y, Yamasaki H, Igarashi M, et al. Systematic treatment approach to ventricular tachycardia in cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2014;7:407–13. [DOI] [PubMed] [Google Scholar]
- 70.Willner JM, Viles-Gonzalez JF, Coffey JO, Morgenthau AS, Mehta D. Catheter ablation of atrial arrhythmias in cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2014;25:958–963. [DOI] [PubMed] [Google Scholar]
- 71.Terasaki F, Fujita S, Miyamura M, Kuwabara H, Hirose Y, Torii I, Nakamura T, Hoshiga M. Atrial Arrhythmias and Atrial Involvement in Cardiac Sarcoidosis. Int Heart J. 2019;60:788–795. [DOI] [PubMed] [Google Scholar]
- 72.Mehta D, Willner JM, Akhrass PR. Atrial Fibrillation in Cardiac Sarcoidosis. J Atr Fibrillation. 2015;8:1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winters SL, Cohen M, Greenberg S, Stein B, Curwin J, Pe E, Gomes JA. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol. 1991;18:937–43. [DOI] [PubMed] [Google Scholar]
- 74.Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol. 2004;27:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jefic D, Joel B, Good E, Morady F, Rosman H, Knight B, Bogun F. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009;6:189–95. [DOI] [PubMed] [Google Scholar]
- 76.Mohsen A, Jimenez A, Hood RE, Dickfeld T, Saliaris A, Shorofsky S, Saba MM. Cardiac sarcoidosis: electrophysiological outcomes on long-term follow-up and the role of the implantable cardioverter-defibrillator. J Cardiovasc Electrophysiol. 2014;25:171–6. [DOI] [PubMed] [Google Scholar]
- 77.Yalagudri S, Zin Thu N, Devidutta S, Saggu D, Thachil A, Chennapragada S, Narasimhan C. Tailored approach for management of ventricular tachycardia in cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2017;28:893–902. [DOI] [PubMed] [Google Scholar]
- 78.Muser D, Santangeli P, Liang JJ, Castro SA, Magnani S, Hayashi T, Garcia FC, Frankel DS, Dixit S, Zado ES, et al. Characterization of the Electroanatomic Substrate in Cardiac Sarcoidosis: Correlation With Imaging Findings of Scar and Inflammation. JACC Clin Electrophysiol. 2018;4:291–303. [DOI] [PubMed] [Google Scholar]
- 79.Papageorgiou N, Providencia R, Bronis K, Dechering DG, Srinivasan N, Eckardt L, Lambiase PD. Catheter ablation for ventricular tachycardia in patients with cardiac sarcoidosis: a systematic review. Europace. 2018;20:682–691. [DOI] [PubMed] [Google Scholar]
- 80.Koplan BA, Soejima K, Baughman K, Epstein LM, Stevenson WG. Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006;3:924–9. [DOI] [PubMed] [Google Scholar]
- 81.Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29:1034–41. [DOI] [PubMed] [Google Scholar]
- 82.Tan JL, Fong HK, Birati EY, Han Y. Cardiac Sarcoidosis. Am J Cardiol. 2019;123:513–522. [DOI] [PubMed] [Google Scholar]
- 83.Ballul T, Borie R, Crestani B, Daugas E, Descamps V, Dieude P, Dossier A, Extramiana F, van Gysel D, Papo T, et al. Treatment of cardiac sarcoidosis: A comparative study of steroids and steroids plus immunosuppressive drugs. International journal of cardiology. 2019;276:208–211. [DOI] [PubMed] [Google Scholar]
- 84.Rosenthal DG, Parwani P, Murray TO, Petek BJ, Benn BS, De Marco T, Gerstenfeld EP, Janmohamed M, Klein L, Lee BK, et al. Long-Term Corticosteroid-Sparing Immunosuppression for Cardiac Sarcoidosis. J Am Heart Assoc. 2019;8:e010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harper LJ, McCarthy M, Ribeiro Neto ML, Hachamovitch R, Pearson K, Bonanno B, Shaia J, Brunken R, Joyce E, Culver DA. Infliximab for Refractory Cardiac Sarcoidosis. Am J Cardiol. 2019;124:1630–1635. [DOI] [PubMed] [Google Scholar]
- 86.Birnie D, Beanlands R, Nery P, Aaron S, Culver D, DeKemp R, Gula L, Ha A, Healey H, Inoue Y, et al. Cardiac Sarcoidosis Multi-Center Randomized Controlled Trial (CHASM CS-RCT). Am Heart J. 2019, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 88.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. Am Heart J. 2011;161:1024–30 e3. [DOI] [PubMed] [Google Scholar]
- 89.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 90.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 91.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016;35:1–23. [DOI] [PubMed] [Google Scholar]
- 92.Crawford TC, Okada DR, Magruder JT, Fraser C, Patel N, Houston BA, Whitman GJ, Mandal K, Zehr KJ, Higgins RS, et al. A Contemporary Analysis of Heart Transplantation and Bridge-to-Transplant Mechanical Circulatory Support Outcomes in Cardiac Sarcoidosis. J Card Fail. 2018;24:384–391. [DOI] [PubMed] [Google Scholar]
- 93.Patel SR, Saeed O, Naftel D, Myers S, Kirklin J, Jorde UP, Goldstein DJ. Outcomes of Restrictive and Hypertrophic Cardiomyopathies After LVAD: An INTERMACS Analysis. J Card Fail. 2017;23:859–867. [DOI] [PubMed] [Google Scholar]
- 94.Raeisi-Giglou P, Rodriguez ER, Blackstone EH, Tan CD, Hsich EM. Verification of Heart Disease: Implications for a New Heart Transplantation Allocation System. JACC Heart Fail. 2017;5:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


