 Ischemic stroke is the second most common cause of death worldwide and cerebral ischemia/reperfusion (I/R) injury also leads to serious tissue damage.
Ischemic stroke is the second most common cause of death worldwide and cerebral ischemia/reperfusion (I/R) injury also leads to serious tissue damage.
Abstract
Ischemic stroke is the second most common cause of death worldwide and cerebral ischemia/reperfusion (I/R) injury also leads to serious tissue damage. Astilbin, a natural bioactive flavonoid compound, has been reported to have protective effects on neurological diseases. This study aims to investigate the effects of astilbin on cerebral I/R injury and determine the mechanisms involved. The results demonstrated that, in cerebral I/R rats, astilbin could attenuate I/R injury in the hippocampal region, decreasing the activity of lactate dehydrogenase (LDH) and malondialdehyde (MDA) in the rat brain. Astilbin also inhibited the I/R-induced upregulation of pro-inflammatory mediators (TNFα, IL-1β, IL-6). Similarly, in hypoxia/reperfusion (H/R) treated human neuroblastoma cells, astilbin could increase the cell viability of SH-SY5Y, decrease the activity of LDH and MDA, and inhibit the H/R-induced upregulation of pro-inflammatory mediators. For the mechanism study, western blot results indicated that astilbin could inhibit the expression of Toll-like receptor 4 (TLR4), myeloid differential protein 88 (MYD88) and phosphorylated NF-κB p65 in H/R treated SH-SY5Y cells. The research indicated that astilbin ameliorated cerebral I/R injury partly via the TLR4/MyD88/NF-κB pathway. Astilbin may have potential therapeutic effects on cerebral ischemia.
Introduction
Cerebral ischemia, the second cause of death worldwide, is characterized by neuronal dysfunction and nerve cell damage and is usually caused by a sudden obstruction of arterial blood flow to the brain.1,2 Previous studies revealed that about 10 risk factors contribute to the chance of having a stroke, including high blood pressure, dyslipidemias, alcohol and tobacco, obesity, diabetes mellitus, etc.3 Although thrombolysis therapy for the restoration of blood flow is effective for brain injury, blood reperfusion in an ischemic brain leads to further serious damage, namely ischemia/reperfusion (I/R) injury.4The pathogenesis of I/R injury is complex, including inflammation, apoptosis, energy autophagy, calcium-overload, oxidative stress, etc.5,6 Recently, more and more pieces of evidence have shown that the I/R induced inflammatory cascade plays a role in the further damage in cerebral I/R injury.7 Researchers found that in a middle cerebral artery occlusion (MCAO) model, the nuclear factor κB (NF-κB) signaling pathway was activated in brain tissue, and inhibiting the excess inflammatory responses could attenuate I/R injury.8 Furthermore, a previous study confirmed that in a MCAO rat model, the TLR4/MyD88/NF-κB signaling pathway was activated, and inhibition of TLR4/MyD88/NF-kB-induced inflammation could attenuate ischemic stroke-induced inflammation and cerebral I/R injury.9
Astilbin, a major bioactive flavonoid compound isolated from the Smilax china L., Engelhardtia chrysolepis, Hypericum perforatum and other plants, has been reported to exert many biological activities including anti-inflammation, anti-oxidation, anti-fibrosis, renal protection etc. In potassium oxonate treated mice, astilbin attenuated kidney pathological changes through inhibiting oxidative stress and inflammation.10 In an transgenic mouse model of Alzheimer's Disease, astilbin could enhance cognitive ability partly by the CREB/BDNF signalling pathway and further decrease the accumulation of Ab.11 In bleomycin-treated mice and mouse alveolar epithelial cells, astilbin could ameliorate pulmonary fibrosis by the Hedgehog signaling pathway.12 Many studies have shown that astilbin could alleviate tissue injury through anti-inflammatory or immuno-modulatory effects under various pathological conditions. In experimental autoimmune myasthenia gravis (MG), astilbin attenuated the clinical symptom by inhibiting Th17 cytokines and up-regulating T regulatory cells.13 In in vitro experiments, astilbin inhibited high glucose-induced inflammation of glomerular mesangial cells through the TLR4/MyD88/NF-κB pathway.14 However, there are few reports on the effects of astilbin on cerebral ischemia, and the mechanism is still unclear. This study investigates the effect of astilbin on cerebral I/R injury and the related mechanisms. The results may provide experimental support of the pharmacological effects of astilbin and provide a potential therapeutic drug for cerebral ischemia.
Materials and methods
Cerebral ischemia/reperfusion (I/R) injury model and drug administration
Twenty adult male rats (Sprague – Dawley, 220 to 240 g), purchased from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China), were divided into 4 groups (sham, sham + astilbin, I/R and I/R + astilbin) (5 rats in each group). Rats were anesthetized with chloral hydrate. The left common carotid artery and external and internal carotid artery were separated out after the skin incision. A 3-0 nylon suture was inserted from the right common carotid artery to the internal carotid artery. The occlusion was maintained for 2 hours. Then the suture was removed and reperfusion was continued for 24 hours. Sham rats also underwent the same procedure, but the middle cerebral artery was not blocked. Body temperatures were maintained at about 37 °C. Rats were subjected to occlusion for 2 h, and 24 h reperfusion. Local cerebral blood flow was monitored by using a laser Doppler cerebral flow meter (moorVMS-LDF, Delaware, DE, USA). The local blood flow of the brain was reduced by 80%, and 70% after reperfusion, indicating that the model was successful.
Astilbin (CAS#29838-67-3, no. 111798), purchased from the National Institutes of Food and Drug Control (Beijing, China), was dissolved in DMSO at a concentration of 20 mg mL–1 and stored at –80 °C. The astilbin treatment group was pretreated with 50 mg kg–1 for 2 h before surgery. Neurologic deficit and infarct volume were analyzed after 24 h of reperfusion. All the animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals15 and approved by the animal ethics committee of Changchun University of Traditional Chinese Medicine (Approval no. 20190117).
Cells and the in vitro cerebral ischemia/reperfusion model
The human neuroblastoma cell line (SH-SY5Y), was purchased from American Type Culture Collection (CRL-2266, ATCC, Rockville, MD, USA), and cultured in DMEM/F12 medium supplemented with 15% fetal bovine serum at 37 °C with 5% CO2. The cells were adhered and cultured for 24 h prior to treatment. To establish the I/R model in vitro, SH-SY5Y cells were cultured in an anoxic environment in a hypoxia chamber for 4 h, and then under normal conditions (H/R group). The stock solution of astilbin was diluted with complete culture medium to a final concentration of 20 μg mL–1. Then, cells were cultured with astilbin added medium for 24 hours (H/R + astilbin group). The cell viability and MDA and LDH activity were analyzed.
Measurement of neurological score
Neurological scores were evaluated by a researcher blinded to the treatment according to a previously published method.16 Scores were as follows: grade 0, no neurological deficits; grade 1, failure to lift forepaw fully; grade 2, circling continuously to the left side; grade 3, falling to the injured side; grade 4, failure to walk spontaneously and/or depressed level of consciousness.
Determination of infarction volume
The brain infarction volume was measured by TTC staining as previously reported.17 Briefly, the animals were sacrificed after neurological deficit evaluation and the brains were removed. Brains were frozen at –20 °C for 24 h and then sliced into coronal slices about 2 mm thick. Then, brain slices were stained with 1% TTC at 37 °C for 30 min and fixed in paraformaldehyde. The stained brain slice was imaged by using a digital camera. The infarct volume was calculated as a percentage of the contralateral hemisphere with a correction for edema.
Measurement of the activity of MDA and LDH
The brain tissue was dissociated and stored at –80 °C for further analysis. The activity of MDA and LDH was detected by using commercial biochemistry assay kits according to the instructions (Nianjing Jiancheng Bioengineering Institute, Nanjing, China).
Nissl staining
Nissl staining was used to detect the nerve injury in the hippocampus and to analyze the density of neurons. Brain tissues were removed quickly and fixed in formalin (0.4%), sectioned into slices and stained with tolridine blue. The staining images were acquired by using a light microscope. In the hippocampal CA1 region, neurons with round and palely stained nuclei were identified as surviving cells.
Western blot
The brains cortices were lysed in RIPA buffer. After being centrifuged (12 000g, 10 min), the supernatant was collected. The protein samples were separated and electrotransferred to PVDF membranes. After blocking, the membranes were incubated with primary antibodies (purchased from Cell Signaling Technology, at a dilution of 1 : 1000) at 4 °C overnight, and then, incubated with HRP-conjugated secondary antibody. Protein bands were detected by using an enhanced chemiluminescence system and the intensity was normalized to GADPH.
Quantitative real-time PCR
The total RNA was extracted by using Trizol reagent (Invitrogen Life Technologies) according to the manufacturer's protocol. The reverse transcription of mRNA was performed using the GoScript™ Reverse Transcription System (Promega Corporation) following the manufacturer's instructions. Quantitative RT-PCR was performed by real-time PCR with SYBR PCR reagents and a Bio-Rad system. The mRNA levels were normalized to GADPH and the relative expression was analyzed by the 2–ΔΔCT method. The primers used in the RT-PCR were as previously reported.18
Measurements of TNFα, IL-1β and IL-6 in the hemisphere
Inflammatory mediators (TNFα, IL-1β and IL-6) in brain homogenate supernatants were determined by ELISA following the manufacturer's protocol. The production of the inflammatory mediator was quantified with a microplate reader.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) and analyzed by GraphPad software. Data were analyzed by one-way ANOVA followed by the Student–Newman–Keuls test and p < 0.05 was considered statistically significant.
Results
Astilbin attenuates cerebral I/R injury in vivo
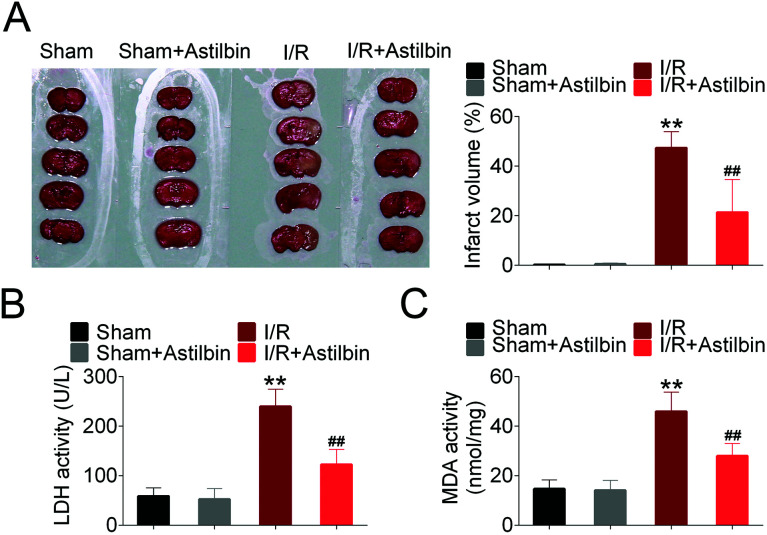
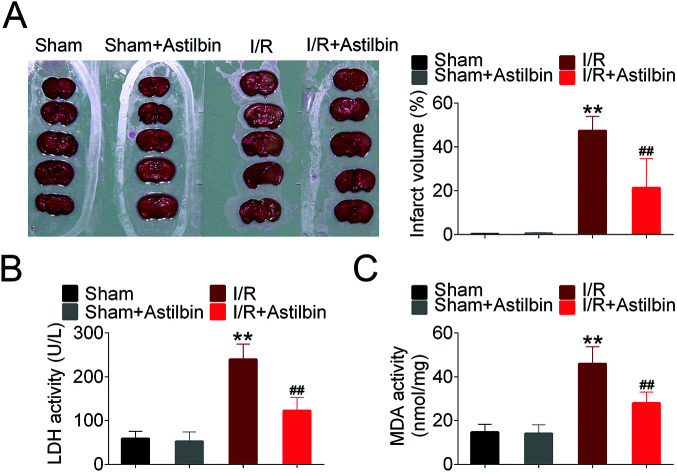
The brain infarction volume was measured by TTC staining and the results are shown in Fig. 1A. The infarction volume of sham and astilbin (sham + astilbin) group had no significant difference. While after 24 h of reperfusion, a significant infarction appeared in the I/R group (I/R vs. sham, p < 0.01). However, the infarct volume after astilbin treatment was significantly reduced compared with the I/R group (I/R + astilbin vs. I/R, p < 0.01). Furthermore, the activity of LDH and MDA was also measured to evaluate I/R injury (Fig. 1B and C). The activity of LDH and MDA significantly increased in I/R rats compared with the sham group (I/R vs. sham, p < 0.01) and was markedly decreased by astilbin treatment (I/R + astilbin vs. I/R, p < 0.01).
Fig. 1. Astilbin ameliorates cerebral I/R injury. (A) Photographs of TTC staining (top panel) and the statistical column. (B) The activity of LDH in brain tissues. (C) The activity of MDA in brain tissues. **p < 0.01 vs. sham, ##p < 0.01 vs. I/R group.
Astilbin attenuates I/R induced neuropathological changes in rats
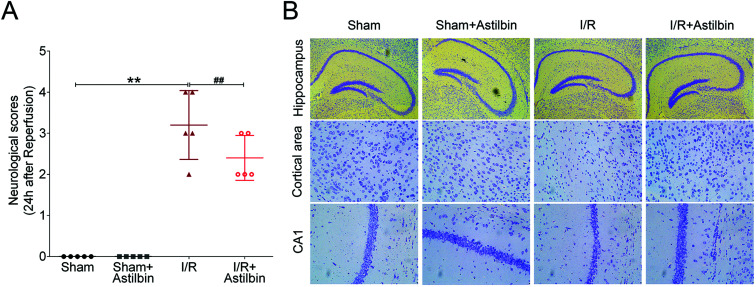
Neuropathological alterations in the brain were evaluated by the neurological score and Nissl staining. I/R increased the neurological scores compared with the sham group (I/R vs. sham, p < 0.01), while astilbin when pretreated could decreased the scores (I/R + astilbin vs. I/R, p < 0.01). Consistently, I/R remarkably induced the decrease of neuron density, which was inhibited by astilbin after reperfusion (Fig. 2B). Taken together, these results indicated that astilbin could attenuate I/R induced neuropathological changes and neuronal loss in rats.
Fig. 2. Astilbin attenuates I/R induced neuropathological changes in rats. (A) The neurological score was measured at 48 h after reperfusion. (B) The density of neurons measured by Nissl staining. **p < 0.01 vs. sham, ##p < 0.01 vs. I/R group.
Astilbin inhibits I/R induced inflammatory mediator expression in the hemisphere
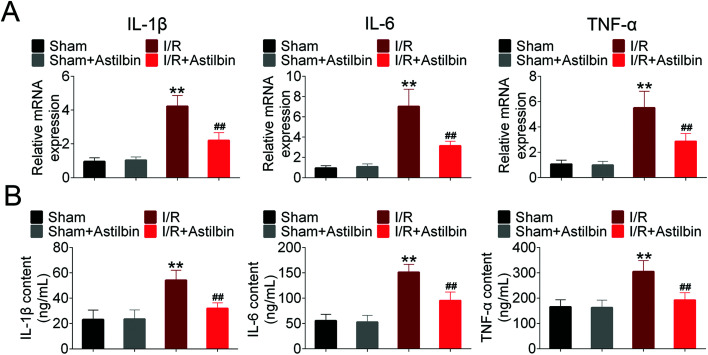
To determine the inflammatory responses in I/R induced injury and the effects of astilbin, the mRNA and protein levels of TNFα, IL-1β, IL-6 were measured by RT-PCR and ELISA. As shown in Fig. 3A, I/R increased the mRNA expression of TNFα, IL-1β, and IL-6 (I/R vs. sham, p < 0.01), which was inhibited by astilbin treatment (I/R + astilbin vs. I/R, p < 0.01). Similarly, the protein levels in the hemisphere were increased about 2 times (I/R vs. sham, p < 0.01), and the enhancements were inhibited by astilbin (I/R + astilbin vs. I/R, p < 0.01) (Fig. 3B). To sum up, the results indicated that astilbin could alleviate I/R-induced injury by decreasing inflammatory cytokines.
Fig. 3. Astilbin inhibits I/R induced inflammatory mediator expression in the hemisphere. (A) The relative mRNA expression of TNFα, IL-1β and IL-6 analyzed by RT-PCR. (B) The level of TNFα, IL-1β and IL-6 analyzed by ELISA. **p < 0.01 vs. sham, ##p < 0.01 vs. I/R group.
Astilbin ameliorates hypoxia/reperfusion (H/R) induced injury of SH-SY5Y cells
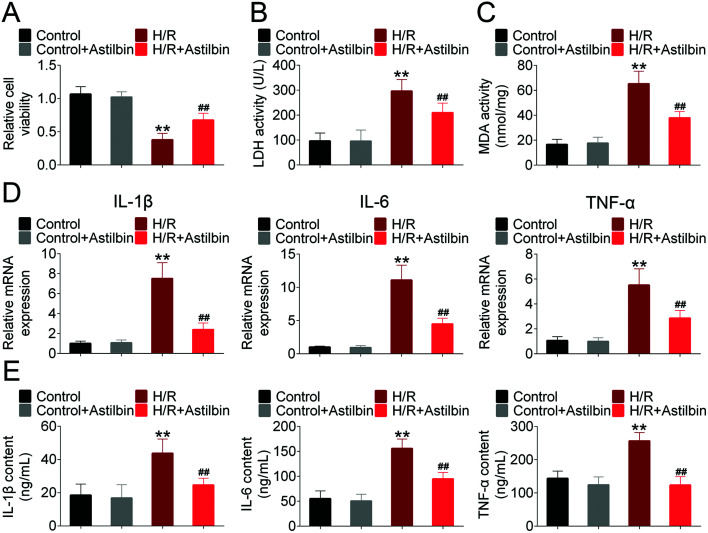
The in vitro model was established by hypoxia and reperfusion of SH-SY5Y cells. As shown in Fig. 4A, the cell viability of SH-SY5Y was decreased in the H/R group (H/R vs. control, p < 0.01), and astilbin treatment reversed the H/R induced injury of SH-SY5Y cells (I/R + astilbin vs. I/R, p < 0.01). The activity of LDH and MDA is shown in Fig. 4B and C. H/R induced the activity of LDH and MDA (H/R vs. control, p < 0.01), while the increases were inhibited by astilbin (I/R + astilbin vs. I/R, p < 0.01). To determine the inflammatory responses in the H/R induced injury of SH-SY5Y cells, the mRNA and protein levels of TNFα, IL-1β, and IL-6 were measured. As shown in Fig. 4D, H/R significantly increased the gene expression of TNFα, IL-1β, and IL-6 (H/R vs. control, p < 0.01), which was inhibited by astilbin treatment (I/R + astilbin vs. I/R, p < 0.01). Similarly, the protein levels in the culture medium of H/R treated cells were increased about 2–3 times (H/R vs. control, p < 0.01), and the increases were inhibited by astilbin treatment (I/R + astilbin vs. I/R, p < 0.01) (Fig. 4E). To sum up, the results indicated that astilbin may alleviate H/R-induced injury in vitro by inhibiting cytokines.
Fig. 4. Astilbin ameliorates hypoxia/reperfusion (H/R) induced injury of SH-SY5Y cells. (A) The relative cell viability measured by the CCK-8 method. (B) The activity of LDH in SH-SY5Y cells. (C) The activity of MDA in SH-SY5Y cells. (D) The relative mRNA expression of TNFα, IL-1β and IL-6 analyzed by RT-PCR. (E) The level of TNFα, IL-1β and IL-6 analyzed by ELISA. **p < 0.01 vs. sham, ##p < 0.01 vs. I/R group.
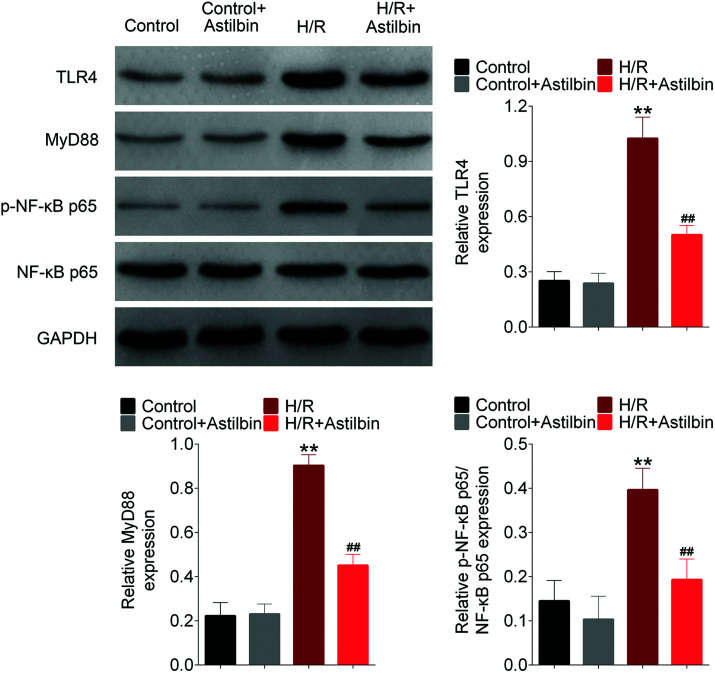
Astilbin inhibits the activation of the TLR4/MyD88/NF-κB pathway induced by H/R in vitro
To investigate the mechanism of astilbin in attenuating H/R injury, NF-κB pathway activation was analyzed by western blot analysis. The levels of TLR4, MyD88 and p-NF-κB-p65 were increased markedly in the H/R group (H/R vs. control, p < 0.01), and inhibited by astilbin (H/R vs. control, p < 0.01), which indicated that astilbin could inhibit the activation of the NF-κB pathway induced by H/R in SH-SY5Y cells (Fig. 5).
Fig. 5. Astilbin inhibits the activation of the TLR4/MyD88/NF-κB pathway of SH-SY5Y cells (analyzed by western blot). **p < 0.01 vs. sham, ##p < 0.01 vs. I/R group.
Discussion
Cerebral I/R is a major cause of disability and death and commonly results in irreversible brain injury, which originates from a sudden blocking of cerebral blood and is characterized by neuronal dysfunction.19 Excess inflammatory responses have been reported to aggravate cerebral I/R injury.20 Researchers found that I/R triggered neuro-inflammation, which induced the production of reactive oxygen species and inflammatory mediators, and further led to blood–brain barrier disruption and neuronal death.21 The P2X7 receptor (P2X7R), predominantly expressed in microglia in the central nervous system (CNS), was reported to participate in cerebral I/R injury, and the inhibition of P2X7Rs attenuates global cerebral I/R injury by inhibiting inflammatory response.22The present study is in accordance with the previous research, showing that I/R injury induced the over-expression of pro-inflammatory mediators and the activation of NF-κB signaling in vivo and in vitro. Thus, anti-inflammation therapy may be an important method for the treatment of cerebral I/R injury. Some active compounds isolated from herbal medicines exert anti-inflammatory effects and recent research indicated that these compounds could attenuate I/R injury via regulating inflammatory responses. For example, Lifa Huang et al. found that curcumin, a polyphenolic compound extracted from Curcuma longa, exerts neuro-protective effects by inhibiting autophagy and inflammatory mediator production via the PI3K/Akt/mTOR and TLR4/p38/MAPK pathways.23
Astilbin, a natural bioactive flavonoid compound, has been reported to exert many biological activities including anti-inflammation, anti-oxidation, etc.10,13 Recently, astilbin has been reported to have certain effects on neurological diseases in vivo. For example, Dongmei Wang indicated that astilbin can alleviate cognitive dysfunction (lessening learning and memory deficits) in transgenic mice with Alzheimer's disease.24 Qiong-Qiong et al. found the antidepressant-like effects of astilbin on the mice chronic unpredictable mild stress (CUMS) model of depression. Astilbin could increase the concentration of 5-HT and dopamine in CUMS mice through the extracellular signal-regulated kinase (ERK) 1/2 – AKT signaling pathway.25 However, there are few studies on the effects of astilbin on cerebral I/R injury and its mechanism is still unclear. Hence, this research investigated the effects of astilbin on cerebral I/R injury in vivo and in vitro. The present results indicated that astilbin could attenuate cerebral I/R injury in the rat hippocampal region and in human neuroblastoma cells, and inhibit the production of pro-inflammatory cytokines. Furthermore, the mechanism of astilbin in attenuating cerebral I/R injury was investigated.
NF-κB is a key transcription factor in inflammatory response, and plays an important role in the neuro-inflammation in I/R injury. The research found that in H/R injured cells, NF-κB was up-regulated, as well as the NF-κB pathway related proteins. TLR4, a transmembrane receptor that mainly distributes on microglial cells and astrocytes in the central nervous system, could activate the NF-κB pathway to enhance the expression of cytokines.26 Here, astilbin could inhibit the expression of TLR4 and MyD88 and the phosphorylation of NF-κB-p65, which indicated that the underlying mechanism for the protective effects of astilbin on cerebral I/R injury, was partly, through regulating the TLR4/MyD88/NF-κB pathway. The results were partly in accordance with the previous reports, revealing that astilbin protects diabetic rat heart against I/R injury by inhibiting the NF-κB signaling pathway.27
To sum up, astilbin attenuates cerebral I/R injury partly through inhibiting the activation of the TLR4/MyD88/NF-κB pathway, and more detailed mechanisms need further experiments. The research indicated that astilbin has potential therapeutic effects for cerebral ischemia.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author contributions
JL and ZWG conceived and designed the experiments, YL and YW analyzed and interpreted the results of the experiments, and MZ performed the experiments.
Ethics approval and consent to participate
The animal use protocol listed has been reviewed and approved by the Animal Ethical and Welfare Committee. Approval No. 20190117.
Informed consent
Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
Conflicts of interest
There are no conflicts of interest to declare.
References
- Katan M., Luft A. Semin. Neurol. 2018;38:208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- He L., He R., Liang R., Li Y., Li X., Li C., Zhang S. J. Integr. Neurosci. 2018;17:277–292. doi: 10.31083/JIN-170047. [DOI] [PubMed] [Google Scholar]
- Fodor K., Tit D. M., Pasca B., Bustea C., Uivarosan D., Endres L., Iovan C., Abdel-Daim M. M., Bungau S. Oxid. Med. Cell. Longevity. 2018;2018:10. doi: 10.1155/2018/4147320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abas F., Alkan T., Goren B., Taskapilioglu O., Sarandol E., Tolunay S. Turk. Neurosurg. 2010;20:1–8. [PubMed] [Google Scholar]
- Pan J., Konstas A. A., Bateman B., Ortolano G. A., Pilespellman J. Neuroradiology. 2007;49:93–102. doi: 10.1007/s00234-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M. S., Al Mamun A., Asaduzzaman M., Hosn F., Abu Sufian M., Takeda S., Herrera-Calderon O., Abdel-Daim M. M., Uddin G. M. S., Noor M. A. A., Begum M. M., Kabir M. T., Zaman S., Sarwar M. S., Rahman M. M., Rafe M. R., Hossain M. F., Hossain M. S., Ashraful Iqbal M., Sujan M. A. R. Ann. Neurosci. 2018;25:25–37. doi: 10.1159/000481812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha P., Tocharus J., Janyou A., Jittiwat J., Changtam C., Suksamrarn A., Tocharus C. PLos One. 2017;12:e0189211. doi: 10.1371/journal.pone.0189211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huiqing L., Xinbing W., Lingjun K., Xiaoqian L., Li C., Shi Y., Xiumei Z., Lin C. Int. J. Biol. Sci. 2015;11:525–535. [Google Scholar]
- Zhu S., Tang S., Su F. Mol. Med. Rep. 2017;17:660–666. doi: 10.3892/mmr.2017.7900. [DOI] [PubMed] [Google Scholar]
- Wang M., Zhao J., Zhang N., Chen J. Biomed. Pharmacother. 2016;83:975–988. doi: 10.1016/j.biopha.2016.07.025. [DOI] [PubMed] [Google Scholar]
- Wang D., Li S., Chen J., Liu L., Zhu X. Cell. Mol. Neurobiol. 2016;37:1–12. doi: 10.1007/s10571-016-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liu H., Song C., Zhang J., Wang Y., Lv C., Song X. Pulm. Pharmacol. Ther. 2018;50:19–27. doi: 10.1016/j.pupt.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Meng Q. F., Zhang Z., Wang Y. J., Chen W., Li F. F., Yue L. T., Zhang C. J., Li H., Zhang M., Wang C. C. J. Neuroimmunol. 2016;298:138–145. doi: 10.1016/j.jneuroim.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Chen F., Zhu X., Sun Z., Ma Y. Front. Pharmacol. 2018;18:1187. doi: 10.3389/fphar.2018.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council N., Guide for the Care and Use of Laboratory Animals, J Publication No. 85-23, 8th edn, 2010, vol. 327, pp. 963–965. [Google Scholar]
- Longa E. Z., Weinstein P. R., Carlson S., Cummins R. Stroke. 1989;20:84. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- He Q., Li Z., Wang Y., Hou Y., Li L., Zhao J. Int. Immunopharmacol. 2017;50:208–215. doi: 10.1016/j.intimp.2017.06.029. [DOI] [PubMed] [Google Scholar]
- Warisa A., Rachana S. Molecules. 2015;20:17288–17308. [Google Scholar]
- Kurokawa Y., Tranmer B. I. Neurosurgery. 1995;37:750–757. doi: 10.1227/00006123-199510000-00020. [DOI] [PubMed] [Google Scholar]
- Ahmad M., Graham S. H. Transl. Stroke Res. 2010;1:74–84. doi: 10.1007/s12975-010-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G., Kleinschnitz C., Nieswandt B. Ann. N. Y. Acad. Sci. 2010;1207:149–154. doi: 10.1111/j.1749-6632.2010.05730.x. [DOI] [PubMed] [Google Scholar]
- Chu K. J. Neuroinflammation. 2012;9:69. doi: 10.1186/1742-2094-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Chen C., Zhang X., Li X., Chen Z., Yang C., Liang X., Zhu G., Xu Z. J. Mol. Neurosci. MN. 2017;64:129–139. doi: 10.1007/s12031-017-1006-x. [DOI] [PubMed] [Google Scholar]
- Wang D., Li S., Chen J., Liu L., Zhu X. Cell. Mol. Neurobiol. 2016;37:1–12. doi: 10.1007/s10571-016-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiong-Qiong L., Wen-Jie W., Xiao-Liang G., Rui-Li L., Yu-Ping Y., Du-Shuang Z., Ji-Xia Z., Ju-Yuan L. Biol. Pharm. Bull. 2014;37:987–995. [Google Scholar]
- Zhang B., Choi J. J., Eum S. Y., Daunert S., Toborek M. J. P. O. PLoS One. 2013;8:e63159. doi: 10.1371/journal.pone.0063159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H., Kang Z. C., Han F., Jiang W. L. Food Chem. Toxicol. 2014;63:104–110. doi: 10.1016/j.fct.2013.10.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.