Figure 2.
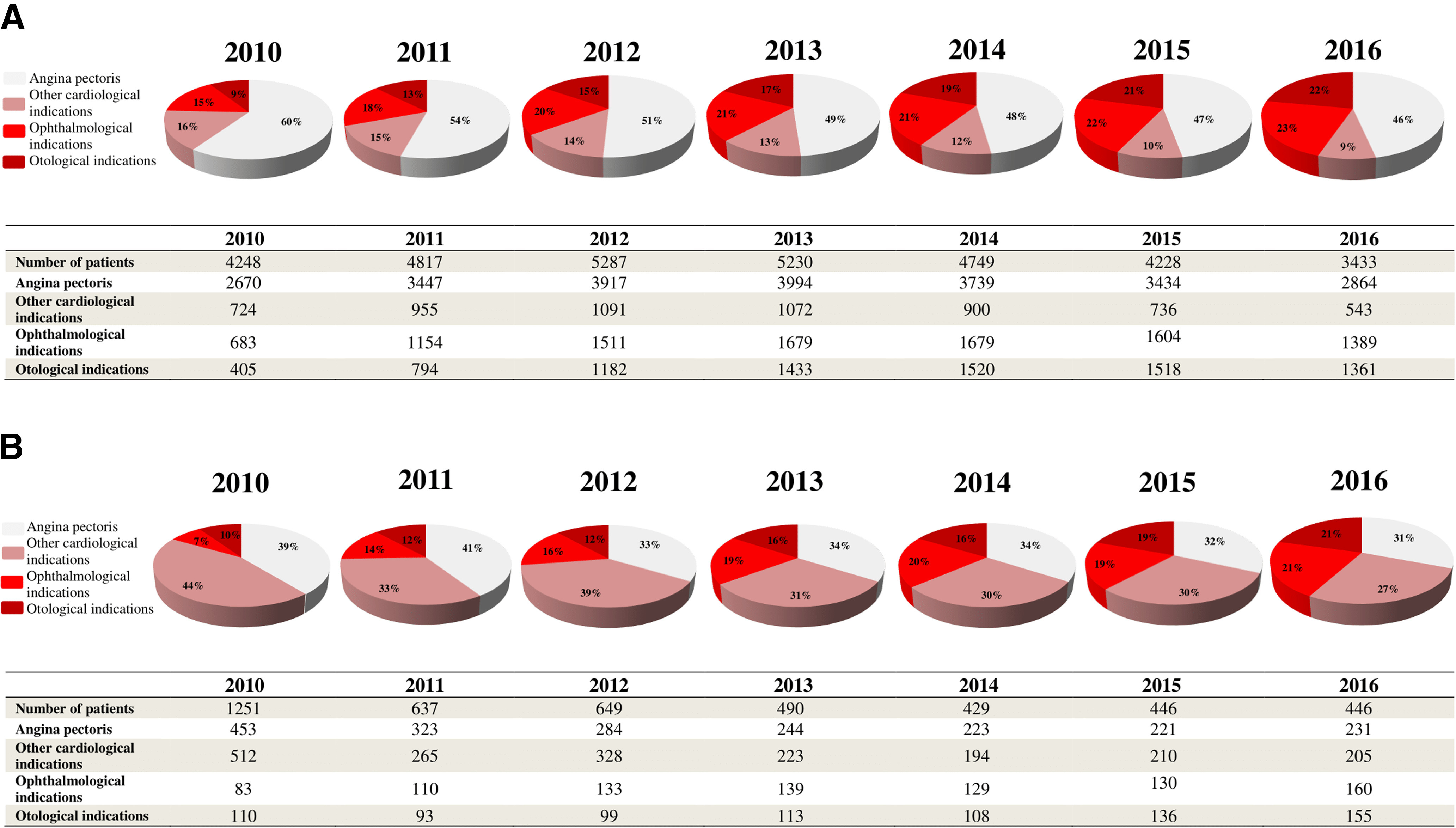
Possible indications for ongoing TMZ treatment (A) and new initiations on the drug (B) in PD from 2010 to 2016. The following categorizations were used: (1) antianginal indication (ICD-10-CM I20, on-label prescriptions); (2) other cardiological indications (ICD-10-CM I00-I99 with the exemption of ICD-10-CM I20, possibly off-label prescriptions after the EMA warning); (3) ophthalmological indications (ICD-10-CM H30-H36, definitely off-label prescriptions after the EMA warning); and (4) otological indications (ICD-10-CM H80-H83, definitely off-label indications after the EMA warning). Other non-investigated disorders might have also served as the basis of TMZ use or initiation. One patient might have had more than one diagnosis. ICD-10-CM, International Classification of WHO Diseases, 10th Revision, Clinical Modification.
