Abstract
Background:
Cancer immunotherapy is associated with several immune-related adverse events, but the relationship between immunotherapy and venous thromboembolism has not been thoroughly studied.
Methods:
We conducted a retrospective cohort study of 1,686 patients who received immunotherapy for a variety of malignancies to determine the incidence of venous thromboembolism and the impact of venous thromboembolism on survival. To examine the potential role of inflammation in venous thromboembolism, we also profiled immune cells and plasma cytokines in blood samples obtained prior to initiation of immunotherapy in a sub-cohort of patients treated on clinical trials who subsequently did (N = 15), or did not (N = 10) develop venous thromboembolism.
Findings:
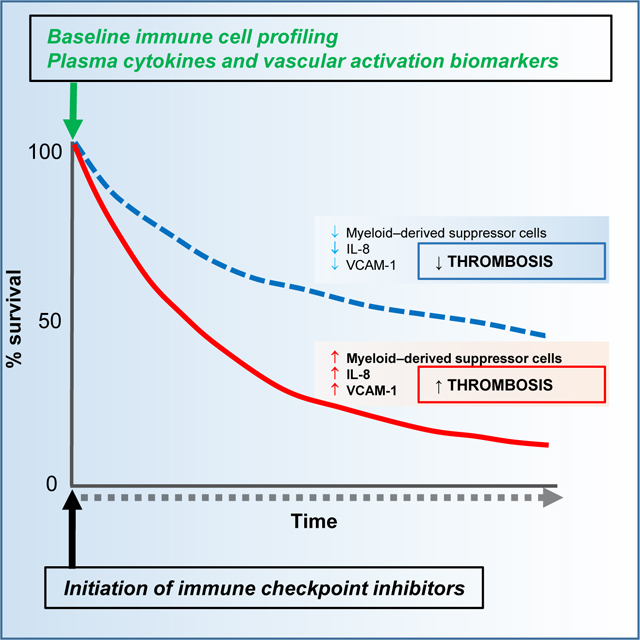
Venous thromboembolism occurred while on immunotherapy in 404/1686 patients (24%) and was associated with decreased overall survival [HR=1.22 (95% CI 1.06–1.41), p<0.008]. Patients that developed venous thromboembolism had significantly higher pretreatment levels of myeloid-derived suppressor cells (5.382 ± 0.873 vs. 3.341 ± 0.3402, mean ± SEM; p=0.0045), interleukin 8 (221.2 ± 37.53 vs. 111.6 ± 25.36, mean ± SEM; p=0.016), and soluble vascular cell adhesion protein 1 (1210 ± 120.6 vs. 895.5 ± 53.34, mean ± SEM; p=0.0385).
Conclusions:
These findings demonstrate that venous thromboembolism is an underappreciated and important immune-related adverse event associated with cancer immunotherapy, and may implicate an interleukin 8 and myeloid-derived suppressor cell-driven pathway in pathogenesis.
eTOC blurb
Venous thrombosis is an underappreciated adverse event associated with cancer immunotherapy. Roopkumar et al describe the incidence of venous thrombosis in cancer patients receiving immunotherapy and suggest that elevated levels of myeloid-derived suppressor cells, interleukin 8 and soluble vascular cell adhesion molecule 1 before immunotherapy correlates with development of venous thrombosis.
Graphical Abstract
Introduction
Cancer immunotherapy disables pathways that regulate the immune response, offering a novel approach to the treatment of malignancy 1, 2. The immune response is normally regulated by binding of CD80/CD86 and programmed death-ligand 1 (PD-L1) to their cognate receptors, cytotoxic T-lymphocyte–associated-4 (CTLA-4) and programmed cell death protein 1 (PD-1) expressed on the surface of cytotoxic T cells 3, 4. Immune checkpoint inhibitors (ICIs) block these ligand-receptor interactions, releasing an immune response toward the tumor 2. There are currently six ICIs approved for treatment of advanced malignancies: ipilimumab, which targets CTLA-4, nivolumab and pembrolizumab, directed against PD-1, and atezolizumab, avelumab and durvalumab, which bind PD-L1. While these agents have demonstrated substantial efficacy in some cases, ICIs are associated with a variety of immune-related adverse effects (irAEs) that differ from those associated with traditional cytotoxic chemotherapeutic agents 5, 6. These irAEs may involve the skin, gastrointestinal tract, lungs, endocrine, musculoskeletal, renal, nervous, hematologic, cardiovascular and ocular systems. Hematologic irAEs include neutropenia 7, immune thrombocytopenia 8, acquired hemophilia 9, autoimmune hemolytic anemia 10, 11 and aplastic anemia 12. While the mechanisms underlying these toxicities are not well understood, evidence implicates a role for cellular immune responses, inflammatory cytokines and complement-mediated inflammation 5, 13, 14. Interestingly, some studies suggest that occurrence of irAEs may be associated with improved cancer prognosis 15–17.
Thromboembolism, including venous and arterial thromboembolism (VTE and ATE, respectively) is a leading cause of death in cancer patients 18. Patients with cancer that develop a VTE such as deep-vein thrombosis (DVT), pulmonary embolism (PE) or visceral vein thrombosis (VVT) may experience decreased survival 19, 20. The incidence of VTE is increased in patients with certain types of malignancies 21, as well as in those treated with specific cytotoxic chemotherapies and targeted agents 22–25. However, despite the expanding use of ICIs, there is little information available concerning their association with thrombosis 26, 27. Moreover, whether the occurrence of VTE in patients receiving ICIs is associated with longer or shorter survival is unknown. We hypothesized that the pro-inflammatory effects of immunotherapy might initiate a thromboinflammatory response that augments the already-elevated risk of VTE in malignancy 22, 28, and therefore conducted a retrospective cohort study to determine the incidence of VTE in cancer patients receiving immunotherapy. We also assessed the association of VTE with overall survival, and to obtain insight into potential mechanisms, probed for biomarkers predictive of VTE.
Results
Patient characteristics
The study population comprised 1,686 patients with a mean age of 64.5 years (range, 18–93 years; Table 1). Most patients were male (n = 1014, 60.1%), white (n =1464, 86.8%), non-Hispanic (n = 1610, 96.4%), and had metastatic disease (n = 1523, 90.3%). The most common types of primary cancers were lung (49.6%) and melanoma (13.2%) (Table 1 and Table S1). Nivolumab was the most commonly used immunotherapy, followed by pembrolizumab, atezolizumab, ipilimumab, avelumab and durvalumab. The most common combination regimen was nivolumab and ipilimumab. The median patient follow-up was 438 days from the initiation of immunotherapy (range: 7–1,971 days). Data concerning concurrent chemotherapy was not collected, but it is unlikely that many patients were receiving chemotherapy given the types of malignancies included in this cohort and the fact that chemotherapy is not often used for these types of tumors per current guidelines. We attempted to define a concurrent control group with similar cancers not receiving immunotherapy, however this was not possible given that immunotherapy represents the current standard of care.
Table 1.
Patient characteristics by VTE status
| Characteristic | VTE (N = 404) | No VTE (N = 1282) | Total (N =1686) | P value | |
|---|---|---|---|---|---|
| Age | 0.02* | ||||
| Median | 63.5 | 65 | 64.5 | ||
| Range | 23 – 90 | 18 – 93 | 18 – 93 | ||
| Sex – no. (%) | 0.054 | ||||
| Male | 226 (55.9) | 788 (61.5) | 1014 (60.1) | ||
| Female | 178 (44.1) | 494 (38.5) | 672 (39.9) | ||
| Race – no. (%) | 0.1 | ||||
| Asian | 3 (0.7) | 5 (0.4) | 8 (0.5) | ||
| African American | 46 (11.4) | 108 (8.4) | 154 (9.1) | ||
| Caucasian | 341 (84.5) | 1123 (87.6) | 1464 (86.9) | ||
| Multiracial / Other | 5 (1.2) | 21 (1.7) | 26 (1.5) | ||
| Unknown | 9 (2.2) | 25 (1.9) | 34 (2.0) | ||
| Stage – no. (%) | 0.03 | ||||
| Stage I - III | 28 (6.9) | 135 (10.5) | 163 (9.6) | ||
| Stage IV | 376 (93.1) | 1147 (89.5) | 1523 (90.4) | ||
| Number of IO drug – no. (%) | >0.99 | ||||
| >1 | 8 (24.2) | 25 (75.8) | 33 (2.0) | ||
| 1 | 396 (24.0) | 1257 (76.0) | 1653 (98.0) | ||
| Type of Immunotherapy – no. (%) | 0.58 | ||||
| Ipilimumab | 8 (2.0) | 40 (3.1) | 48 (2.8) | ||
| Nivolumab | 214 (53.0) | 655 (51.1) | 869 (51.5) | ||
| Pembrolizumab | 106 (26.2) | 350 (27.3) | 456 (27.1) | ||
| Atezolizumab | 40 (9.9) | 135 (10.6) | 175 (10.4) | ||
| Durvalumab | 5 (1.2) | 14 (1.1) | 19 (1.1) | ||
| Avelumab | 8 (2.0) | 12 (0.9) | 20 (1.2) | ||
| Nivolumab + ipilimumab | 23 (5.7) | 76 (5.9) | 99 (5.9) | ||
| Current status – no. (%) | |||||
| Alive | 153 (37.9) | 627 (48.9) | 780 (43.36) | ||
| Dead | 251 (62.1) | 655 (51.1) | 906 (53.74) |
p-value by Wilcoxon rank sum test
VTE events
VTE occurred in 404 of 1,686 patients (24%). Of patients with VTE, 42.6% developed DVT (n=172), 33.2% PE (n=134), 15.3% both DVT and PE (n=63) and 5.4% VVT (n=24) (Table 2). However, the incidence of concurrent PE and DVT may have been higher, since patients with clinically-diagnosed PE did not routinely undergo lower extremity ultrasound. There were no significant differences observed between the rates of VTE in patients treated with different immunotherapies (Table 1). Likewise, the incidence of VTE in patients receiving single versus combination immunotherapy did not differ.
Table 2.
Types of venous thromboembolic events
| Site(s) of VTE | N (%) |
|---|---|
| Deep-vein thrombosis | 172 (42.6) |
| Pulmonary embolism | 137 (33.2) |
| Visceral vein thrombosis | 24 (5.9) |
| Deep vein thrombosis with pulmonary embolism | 63 (15.6) |
| Deep vein thrombosis with visceral vein thrombosis | 2 (0.5) |
| Pulmonary embolism with visceral vein thrombosis | 3 (0.7) |
| Deep vein thrombosis with pulmonary embolism and visceral vein thrombosis | 6 (1.5) |
The six-month and one-year cumulative VTE incidence rates for all immunotherapies were 7.13% and 10.86%, respectively (time to event analysis is shown in Table S2). We did not discriminate between symptomatic versus asymptomatic events, since many patients with incidental and apparently asymptomatic thrombi might have experienced symptoms that would be apparent only upon careful questioning, but cannot be captured in a retrospective study.
Clinical predictors of VTE
In univariate analysis, age and the presence of advanced tumor stage and/or metastatic disease was significantly associated with VTE (p <0.05 for each), while gender, race, ethnicity, number of immunotherapy treatments and type of immunotherapy were not. In multivariate analysis, only younger age at diagnosis [odds ratio (OR) per one year increase in age = 0.99 (95% CI, 0.980–0.998), p=0.015] and metastatic disease [OR 1.71 (95% CI 1.12—2.61), p=0.013] were significantly associated with VTE. Given the high incidence of irAEs and the retrospective nature of our study, we were unable to assess the relationship between thrombosis and other irAEs.
Relationship between VTE and overall survival
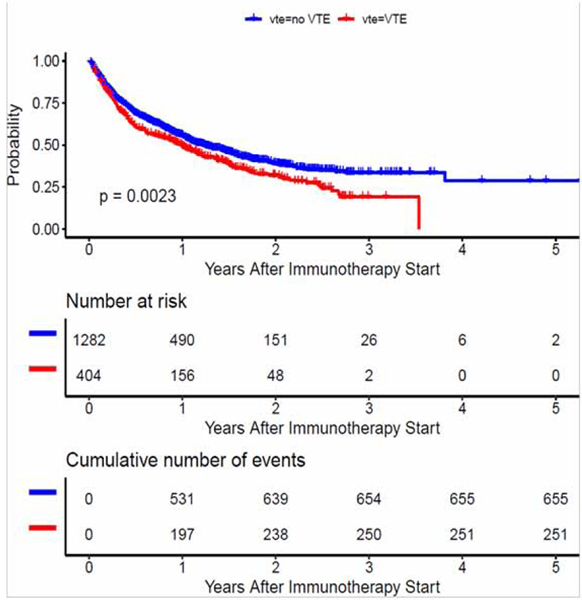
Of the 1,686 study patients, the median overall survival was 416 days (95% CI, 380–471 days), and 906 patients died by the end of the first year of follow-up. The median survival in patients with VTE was significantly decreased: 365 days (95% CI 288, 438 days) compared to 453 days (95% CI 398, 529 days) for patients without VTE (p= 0.002) by log rank test (Figure 1). Decreased survival in patients with VTE was also observed when the two most common tumors in the study, lung cancer and melanoma, were examined individually (Figure S1).
Figure 1. Overall survival of patients on immunotherapy by occurrence of VTE.
Kaplan-Meier curve for landmark analysis (overall survival) for patients on immunotherapy by occurrence of venous thromboembolism demonstrating reduced survival in those with VTE.
In a multivariate Cox proportional hazard analysis for overall survival, occurrence of VTE remained significantly associated with decreased survival [HR = 1.22 (95% CI 1.06–1.41), p < 0.008] (Table 3). Other factors significantly associated with decreased survival included stage IV or metastatic disease [HR = 6.16 (95% CI 1.54–24.71) p < 0.001], older age at diagnosis [HR = 1.01 (95% CI 1.00–1.01) p < 0.006] and single agent immunotherapy [HR = 1.37 (95% CI 1.01–1.85), p = 0.04].
Table 3.
Multivariate Cox model analysis for overall survival
| Clinical characteristic | Comparison | Hazard Ratio | 95% LCL | 95% UCL | P value |
|---|---|---|---|---|---|
| VTE | VTE vs. No VTE | 1.22 | 1.06 | 1.41 | 0.008 |
| Stage | Stage II vs. I | 4.89 | 1.06 | 22.65 | 0.01 |
| Stage III vs. I | 1.58 | 0.37 | 6.72 | 0.49 | |
| Stage IV vs. I | 6.16 | 1.54 | 24.71 | < 0.0001 | |
| Use of combination immunotherapy | No vs. Yes | 1.37 | 1.01 | 1.85 | 0.04 |
| Age at diagnosis | 1 year increase | 1.01 | 1.00 | 1.01 | 0.006 |
Biomarkers Predictive of VTE
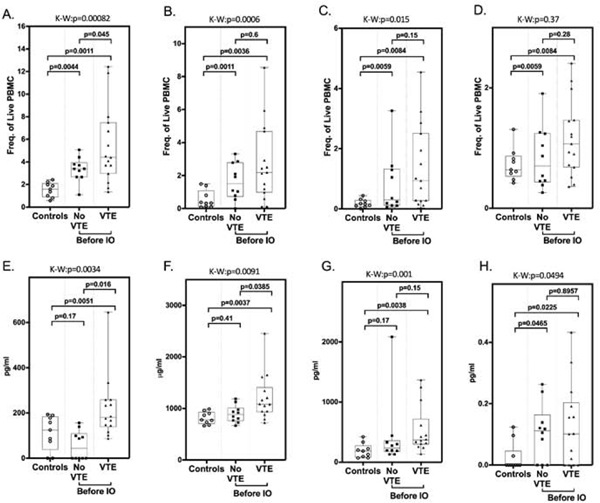
Given the association of inflammation with ICI-associated irAEs as well as thrombotic disease, we hypothesized that thrombosis in immunotherapy-treated patients may have an inflammatory etiology. Therefore, we analyzed the phenotype of circulating peripheral blood mononuclear cells (PBMC) in blood samples obtained prospectively from a cohort of 25 patients (15 of whom subsequently developed VTE and 10 who did not, matched for age, sex, site and stage of cancer) at the time of entry into immunotherapy clinical trials. Of these individuals, 7 had bladder carcinoma, 6 had renal cell carcinoma, 8 had melanoma, and 4 had lung cancer (Table 4). In terms of PBMC, the only phenotypic difference observed between these cell populations was a significant elevation in numbers of total MDSCs (% frequency of live PBMC) in patients who subsequently developed VTE compared to those who did not (mean ± SEM: 5.382 ± 0.873 vs. 3.341 ± 0.3402, p = 0.0045) (Figure 2A). There were also higher levels of the polymorphonuclear (mean ± SEM: 2.720 ± 0.6119 vs. 1.641 ± 0.3520, p = 0.6), monocytic (mean ± SEM: 1.363 ± 0.3561 vs. 0.8114 ± 0.3184, p = 0.15) and early (mean ± SEM: 1.163 ± 0.1624 vs 0.8406 ± 0.1647, p = 0.28) MDSC subtypes in patients who developed VTE (Figure 2B–D), though these differences were not statistically significant.
Table 4:
Characteristics of patients in the subcohort used to define VTE biomarkers
| Characteristic | VTE (N = 15) | No VTE (N = 10) | Total (N =25) |
|---|---|---|---|
| Age | |||
| Median | 69 | 63 | 66 |
| Range | 42–84 | 51–85 | 42–85 |
| Sex – no. (%) | |||
| Male | 10 | 7 | 17 |
| Female | 5 | 3 | 8 |
| Type of Immunotherapy – no. (%) | |||
| Nivolumab | 3 (20) PE | 0(0) | |
| Pembrolizumab | 6 (40) 2 DVT, 4 PE | 2 (20) | 7 |
| Atezolizumab | 2 (13.3) DVT | 2 (20) | 5 |
| Durvalumab | 1 (6.6) DVT | 5(50) | 6 |
| Nivolumab + ipilimumab | 3 (20) DVT | 1 (10) | 4 |
| Current status – no. (%) | |||
| Alive | 3 | 9 | 12 |
| Dead | 12 | 1 | 13 |
Figure 2. Elevation of total MDSC and soluble biomarkers correlate with VTE after immunotherapy.
MDSC within peripheral blood mononuclear cell fractions from healthy, age-matched donors (controls), and cancer patients before initiation of immunotherapy were quantified by flow cytometry analysis. (A) Total MDSC (CD33+/HLADR−), (B) polymorphonuclear MDSC (CD14−/CD15+), (C) monocytic MDSC (CD14+CD15−), (D) early stage MDSC (CD14−CD15−). Plasma concentrations of immune/inflammatory biomarkers from healthy, age-matched donors “controls” and cancer patients before initiation of immunotherapy. (E) IL-8 (pg/ml), (F) sVCAM-1 (μg/ml), (G) IL-1ra (pg/ml), (H) GM-CSF (pg/ml). Box-and whisker plots show median and 25th and 75th percentiles, minimum and maximum. Kruskal-Wallis (K-W) rank-sum test was used to categorize statistical differences seen between the three groups (VTE, no VTE, controls) with results shown above each graph. Pair-wise two-group analysis was performed using the Wilcoxon rank-sum test. p values are indicated. These studies demonstrate a significantly increased rate of thrombosis in patients with elevated levels of total MDSC, IL-8, sVCAM-1, and GM-CSF compared to normal controls and patients with no VTE.
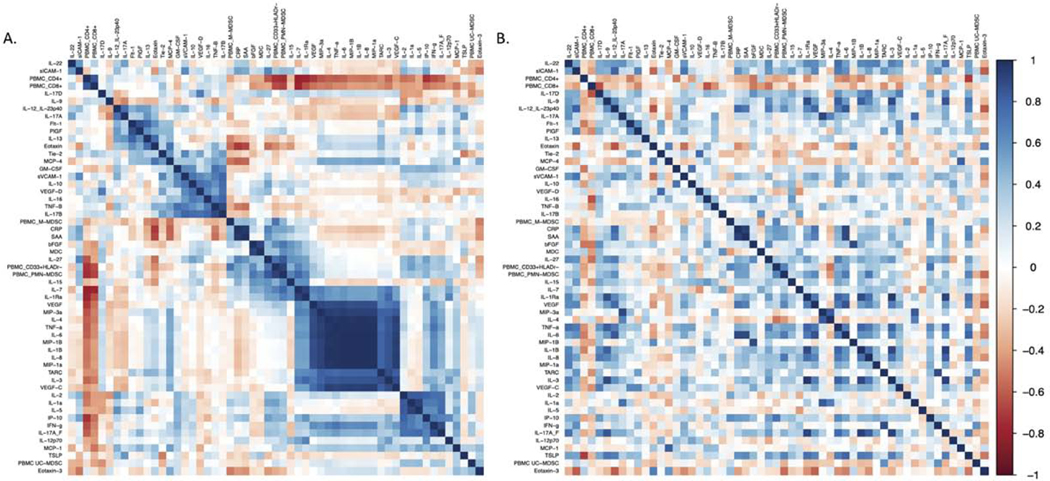
We also assessed levels of 51 plasma cytokines and chemokines associated with inflammation and/or vascular damage in the same pretreatment samples (Table S3). Significantly elevated levels of interleukin 8 (IL-8) (p = 0.016), and soluble vascular cell adhesion molecule 1 (sVCAM-1) (p = 0.038) were present in patients who developed VTE (Figure 2 E, F). Other biomarkers including granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-1 receptor antagonist (Figure 2G, H) (Table S3) were also elevated in VTE patients. When we examined correlations between levels of different inflammatory cytokines using correlation matrices, a large cluster of correlated cytokines that included IL-8, TNFα, IL-6, IL-1β and several others was detected in the group of patients that developed VTE, but not in those who did not (Figure 3). This cytokine cluster also correlated positively with PMN-MDSCs and negatively with CD4+ and CD8+ T cells, although given the small sample size across several tumor types these correlations were not statistically significant.
Figure 3: Correlation matrix of MDSCs and soluble plasma analytes associated with VTE in immunotherapy-treated patients with (A) and without (B) VTE.
Pairwise Pearson’s correlations with hierarchical clustering among the absolute plasma analytes and MDSC populations (n=55 parameters in total). Key to the right of the correlation matrix represents the correlation coefficient [ρ= −1 negative correlation (red) to ρ=1 positive correlation (blue)]. The prominent blue square in the right lower quadrant of the matrix in the VTE patients (A) indicates that elevated levels of IL-8 correlate with elevated levels of several other inflammatory cytokines including IL-1β, IL-6 and TNFα. This clustering is not observed in patients without VTE.
We also used non-metric multi-dimensional analysis was used to visualize the distributions of the 55 biomarker assays (plasma analytes and MDSC populations) across the three sample populations. Using the PERMANOVA approach, significant differences in the multivariate distributions across the three populations were found (p=0.001, R2=0.32) (Figure S2).
Discussion
The association of VTE with standard cancer therapies is well-established 21, 24, 29, 30. However, in this retrospective cohort study, we report that VTE also developed in nearly one-fourth of all cancer patients treated with immunotherapy. These VTE were clinically important, particularly since nearly one-half were due to pulmonary emboli. Moreover, VTE was associated with decreased overall survival. Hence, although immune checkpoint inhibitors have emerged as powerful agents in the treatment of cancer 2, 31–33, VTE should be added to the expanding list of immune-related adverse events associated with these therapies 5, 13.
These findings are novel since there is little information in the literature concerning the association of cancer immunotherapy with VTE. Kunimasa et al first described a single case of VTE in a patient treated with pembrolizumab 27. Ando expanded on this report, describing VTE and arterial thromboembolism in 8.2% of a retrospective cohort of 122 patients treated with pembrolizumab or nivolumab 26. Gutierrez-Sainz et al described VTE in 16 of a cohort of 229 patients with primarily lung cancers and melanoma over a median follow-up period of 9.8 months 34, and Moik et al. described a cumulative incidence of VTE and ATE in 12.9 and 1.8% of a cohort of 662 patients with a median follow up of 8.5 months 35. In our substantially-larger analysis, we have observed a similar incidence of VTE when comparable follow-up periods are examined, although our studies demonstrate that the risk of VTE extends beyond the initial 6–12 months of therapy.
Our study is the first to define potential biomarkers of thrombotic risk in ICI-treated patients. These immune biomarkers differ from those that have been identified in thrombosis associated with standard cancer therapies, such as tissue factor-bearing microparticles 36, 37, D-dimer and P-selectin 38–40; suggesting a novel mechanism of thrombosis in ICI-treated patients. However, it remains possible that some of the same biomarkers identified as risk factors for cancer thrombosis in patients treated with standard cancer therapies may also contribute to thrombosis in immunotherapy-treated patients, a hypothesis that we did not address in this study. Likewise, the biomarkers and mechanisms identified here have yet to be thoroughly tested in patients treated with standard chemotherapy approaches.
The six-month and one-year cumulative incidence of VTE in our population is similar to that observed in cancer patients at high risk for VTE, such as those with pancreas cancer 41, 42. This is remarkable given the fact that the primary sites of cancer in most patients in this study are associated with a low or intermediate VTE risk 22, suggesting that the high incidence of VTE was indeed immunotherapy-related.
The biomarkers observed in this study are potentially informative concerning the pathogenesis of VTE in immunotherapy-treated patients. MDSCs are associated with tumor progression, metastasis, and reduced survival 43–46 and are key targets of immunotherapy 47, 48, but have not been linked to cancer thrombosis. However, an association of MDSCs with activation of coagulant pathways is suggested by the observation that these cells may impair anti-tumor immunity through modulation of procoagulant activity 49. Moreover, MDSC exposed to metastatic tumors in vitro secrete proteins that promote platelet aggregation 50.
Another intriguing mechanism that our studies suggest may contribute to immunotherapy-associated thrombosis involves the IL-8/CXCR1/2 axis, an important inflammatory pathway. IL-8 released by tumor and other cells in the tumor microenvironment is strongly linked to accumulation of MDSCs within tumors 51, and elevated plasma levels of IL-8 have been associated with resistance to atezolizumab 52 as well as nivolumab and ipilimumab 53 in large retrospective analyses. Moreover, IL-8, through activation of CXCR1/2, induces release of neutrophil extracellular traps (NETs) by tumor-infiltrating MDSCs, and these NETs may shield tumor cells from the cytotoxic effects of CD8+ T cells and NK lymphocytes 52, 53. However, NETs also play a critical role in thromboinflammatory disorders through activation of the complement, contact activation 54, 55, and other systems, and have been implicated in the prothrombotic mechanisms underlying disorders such as heparin-induced thrombocytopenia 56, antiphospholipid syndrome 57, and SARS-COV2 infection 58, among others. We hypothesize that like these non-malignant disorders, immunotherapy-induced thrombosis is largely an immune-mediated thromboinflammatory disorder 28, and that NETs may play a central role.
In addition to NETs, our data suggests that other inflammatory pathways may contribute to immunotherapy-associated thrombosis; indeed, a correlation matrix (Figure 4) taken together with the NMDA analysis (Figure 5) suggests that in immunotherapy patients who develop thrombosis, elevated levels of IL-8 are associated with elevations of a number of other inflammatory cytokines and identify a patient subgroup with increased thrombotic risk. These cytokines may act directly on endothelial and other cells to induce proinflammatory and procoagulant responses 59, 60. Indeed the elevated levels of sVCAM-1 (Figure 2 and Table S3) observed in immunotherapy-treated patients who develop thrombosis support the argument that systemic endothelial activation may be present in these individuals 61–63. Moreover, IL-6, IL-8 and IL-1β increase the viscoelasticity of blood, and the combination of these three cytokines induce platelet activation and spreading 64.
These results have significant implications for patient care, since recent studies demonstrate that VTE in cancer patients can be prevented by prophylactic anticoagulation 65, 66. Confirmation of our findings might suggest that thromboprophylaxis should be considered for patients receiving immunotherapy. However, the safety of prophylactic anticoagulation in immunotherapy-treated patients must be established before such treatment can be routinely recommended.
Limitations of study
Our study has several limitations, including the fact that our data is derived from a single-health system-derived retrospective cohort. The retrospective nature of the study precluded us from thoroughly assessing other comorbidities that may have contributed to thrombotic risk in individual patients. However, the large sample size and detailed patient follow-up enhance the fidelity of our observations. We also did not include arterial events in our analysis, though this may have led us to underestimate the overall thrombotic rate. Due to the evolution of cancer therapy, we were not able to identify a contemporary control cohort treated with non-immunotherapy approaches. However, studies performed prior to the immunotherapy era suggest that the odds ratio for thrombosis in patients with melanoma was 0.78 (95% CI 0.29–2.09), for GU cancer 2.72 (1.80–4.12) and for lung cancer 3.87 (2.73–5.49) 67; though it is difficult to translate these odds ratios into incidence rates, these historical results suggest that the thrombosis incidence in our immunotherapy patients is substantially increased compared to patients with similar cancers not treated with immunotherapy. Finally, the predictive biomarkers reported here must be considered exploratory given the relatively small sample size, may be potentially prone to artifacts related to false discovery rates with multiple testing, and require confirmation in a prospective study.
STAR ★ METHODS
RESOURCE AVAILABILITY
Materials Availability
The study did not generate any new materials or reagents
Data and Code Availability
Data involved in this research is available from the corresponding authors upon request. The request should be in the form of a proposal with aims, a statistical approach and other information that will provide assurance of data confidentiality. Patient data will be shared after review and approval of the request by the Cleveland Clinic Institutional Review Board. No data that includes patient names, birthdates, or other potentially identifying information can be shared.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Study Design and participants
We performed a retrospective single-institution cohort study of cancer patients treated with immunotherapy at the Taussig Cancer Institute, Cleveland Clinic, between July, 2015 and December, 2017. Inclusion criteria included 1) a biopsy proven diagnosis of malignancy, and 2) active treatment with an FDA-approved immunotherapy agent. Patients were identified through a pharmacy database and clinical data were collected directly from the electronic medical record by manual review and stored in a secure web-based server. We included all adult patients who received immunotherapy for their cancer diagnosis and were followed at Cleveland Clinic.
We identified and confirmed all VTE events (including DVT, PE and VVT) through physician notes in the electronic medical record, and confirmed that all VTE events were documented by imaging studies. Since this was a retrospective study in which all included patients received their care through the Cleveland Clinic health system, no patients were lost to follow-up. The study protocol was approved by the Cleveland Clinic Institutional Review Board.
METHOD DETAILS
Biomarker Analysis
For biomarker analysis, we identified a sub-cohort of patients that had been treated on clinical trials of cancer immunotherapies for whom pretreatment analysis of peripheral blood leukocyte populations, including myeloid-derived suppressor cells (MDSC), had been performed (n=25). Pre-treatment plasma from these patients was also available for cytokine analysis. Of these individuals, 7 had bladder carcinoma, 6 had renal cell carcinoma, 8 had melanoma, and 4 had lung cancer. Fifteen of these patients developed VTE during administration of immunotherapy (cases) and ten did not. The third quartile cutoff for time on immunotherapy was 26.75 months for the no VTE group and 10 months for patients with VTE (p = 0.945). Nine healthy individuals without cancer were included as controls.
Phenotyping of MDSCs was performed on fresh peripheral blood samples immediately after phlebotomy. Briefly, peripheral blood mononuclear cells (PBMC) were purified from whole blood using Ficoll density centrifugation (10 minutes, 1200 x g), then stained using a panel of nine fluorochrome-conjugated monoclonal antibodies that included anti-CD14-APC H7, anti-CD15-PE CF594, anti-CD33-PE, and anti-HLA-DR-APC (Supplementary Table 4). Stained cells were analyzed using an LSR Fortessa flow cytometer (BD Biosciences) with compensation for spectral overlap and auto-fluorescence determined using single-stained and unstained PBMC. The compensation matrix was calculated using FACSDiva software (BD Biosciences), with manual fine-tuning to align the median fluorescence intensities of negative and positive populations. Analysis of cell populations was performed using FlowJo v.10.4.1 (FlowJo LLC, Ashland, OR), with PBMC gating strategies as previously described 68. Total MDSC were defined as CD33+HLADR−. MDSC subsets were defined as: monocyte (M-MDSC) = CD14+CD15−, polymorphonuclear (PMN-MDSC) = CD14−CD15+, and early-stage (e-MDSC) = CD14−CD15− 44.
Analysis of soluble plasma biomarkers was performed using clinical-grade electrochemiluminescence assays (V-PLEX Human Biomarkers). Briefly, pre-coated electrochemiluminescence assay plates were incubated with a blocking solution. Plates were then washed, and incubated with diluted plasma samples and/or calibrators. Bound analytes were detected using sulfo-TAG detection antibodies and plates were analyzed on a SECTOR S 120 plate reader (MSD) 69. Data was analyzed using MSD Discovery Workbench Version 4.0.
Quantification and statistical analysis
Statistical analysis including overall survival (OS) was performed using the Kaplan-Meier method, and its association with VTE following immunotherapy was estimated using Cox proportional hazard regression adjusted for age at diagnosis and presence/absence of metastases. We compared the incidence of VTE following immunotherapy between single and combination therapy cohorts using the Fisher’s exact test.
A multivariate logistic regression model was constructed to identify clinical variables associated with VTE. The cumulative incidence rate of VTE was estimated using the method of Fine and Gray 70. Survival and logistic regression analyses were performed using SAS Studio version 3.7 (SAS Institute, Cary, NC) and R version 3.6.3 (R Foundation, Vienna, Austria). All tests were two-sided and P-values of 0.05 or less were considered statistically significant.
Correlative analysis was performed using GraphPad Prism. A non-parametric factorial Kruskal-Wallis (KW) rank-sum test was used to categorize statistical differences between three groups (i.e. VTE, no VTE and control). Pair-wise, two group analysis was performed using the Wilcoxon rank-sum test 71. Kruskal-Wallis and Wilcoxon rank-sum tests were adjusted by the Benjamini and Hochberg method 72. V-PLEX assay data was normalized using the varstab method implemented in R. The Bray-Curtis method was used to calculate dissimilarities between samples 73.
Non-metric multidimensional scaling (NMDS) plots were generated in the ggplot2 package using Bray-Curtis dissimilarity matrices 74. PERMANOVA with the Bray-Curtis dissimilarities was used to compare the multivariate assay distributions across the 3 sample groups (Vegan R package). We also generated correlation plots using R version 3.6.3 (https://cran.case.edu/bin/macosx/R-3.6.3.nn.pkg). The R package corrplot was used to generate each correlation matrix. Hierarchical clustering75 was determined using hclust (http://search.r-project.org/R/library/stats/html/hclust.html).
Supplementary Material
Key Resources Table
| Resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| anti-CD14-APC H7 (MΦP9) | BD Biosciences (Franklin Lakes, NJ) | Catalog #560180 |
| anti-CD15-PEC F594 (W6D3) | BD Biosciences (Franklin Lakes, NJ) | Catalog #562372 |
| anti-HLA-DR-APC (G46-6) | BD Biosciences (Franklin Lakes, NJ) | Catalog #559866 |
| anti CD33-PE (WM53) | BD Biosciences (Franklin Lakes, NJ) | Catalog #555450 |
| anti-CD4-PE (RPA-T4) | BD Biosciences (Franklin Lakes, NJ) | Catalog #555347 |
| anti-CD8-APC (RPA-T8) | BD Biosciences (Franklin Lakes, NJ) | Catalog #555369 |
| anti-PD1-FITC (MIH4) | eBioscience (San Diego, CA) | Catalog #11-9969-42 |
| anti-PDL1-PerCPeF710 (MIH1) | eBioscience (San Diego, CA) | Catalog #46-5983-42 |
| anti-human VISTA/B7-H5/PD-1H AF 488 | R&D Systems (Minneapolis, MN) | Catalog #FAB71261RG-100UG |
| Assay kits | ||
| Human biomarker 46-PLEX assay (V-PLEX) | MesoScale Discovery | K15088D |
| V-PLEX vascular injury panel 2 (V-PLEX) | MesoScale Discovery | K15198D |
| Software | ||
| FACS-Diva | Becton-Dickinson | https://www.bdbiosciences.com/en-us/instruments/research-instruments/research-software/flow-cytometry-acquisition/facsdiva-software |
| Flow-Jo | Becton Dickinson | https://www.flowjo.com/ |
| R 3.6.3 (including corrplot and hclust)) | R Foundation for statistical computing | https://www.r-project.org/ |
| Graph Pad Prism | GraphPad.com | https://www.graphpad.com/scientific-software/prism/ |
Highlights.
Cancer immunotherapy blocks physiologic immune regulatory pathways
Immune regulatory blockade is associated with immune-related adverse events
Venous thrombosis is an underappreciated immune-related adverse event
Novel biomarkers may predict thrombosis in patients receiving immunotherapy
Context and Significance.
Immunotherapy is a promising new treatment approach for cancer. Researchers from the Cleveland
Clinic demonstrate that venous thrombosis (the formation of blood clots in veins) occurs in up to 25% of patients receiving this treatment, and thus must be considered to be a common immune-related adverse event in immunotherapy-treated cancer patients. The authors also identified biomarkers that may recognize patients receiving immunotherapy who are at highest risk of thrombosis. These findings suggest that venous thrombosis is an underappreciated toxicity of cancer immunotherapy, and may guide future studies that focus on the validation of the biomarkers demonstrated here, as well as the role of prophylactic anticoagulation to prevent thrombosis in high-risk cancer patients considered for immunotherapy.
Acknowledgements:
This work was supported by U01 HL143402 to KRM and AAK, and a Cleveland Clinic VeloSano award.
Funding: NIH U01 HL143402
Declaration of Interest Statement:
JR, LG, BT, SS, JR, WW, NS, MDR, PR, PGP, SK and PF have no competing interests. KRM served on a data safety monitoring board on a study sponsored by Halozyme and received fees from Paraxel for this service. AK reports personal fees and non-financial support from Janssen, personal fees and non-financial support from Bayer, personal fees and non-financial support from Sanofi, personal fees from Parexel, personal fees and non-financial support from Halozyme, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Seattle Genetics, personal fees from Pharmacyclics, personal fees from Pharmacyte, personal fees and non-financial support from AngioDynamics, personal fees from Leo Pharma, personal fees from TriSalus, personal fees from Medscape, grants from Merck, grants from Array, grants from Bristol Myers Squibb, and grants from Leap, outside the submitted work. BH has research funds from Amgen, is a consultant to SimulStat, and a scientific advisor to Presagia.
Footnotes
Inclusion and Diversity Statement:
We worked to ensure gender balance in the recruitment of human subjects. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. The author list of this paper includes contributors from the location where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma P and Allison JP (2015). Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y (2015). Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest 125, 3335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchbinder EI and Desai A (2016). CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 39, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukari A, Nagasaka M, Al-Hadidi A and Lum LG (2016). Cancer Immunol Immunotherapy. Anticancer Res 36, 5593–5606. [DOI] [PubMed] [Google Scholar]
- 5.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A (2016). Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54, 139–148. [DOI] [PubMed] [Google Scholar]
- 6.Postow MA, Sidlow R and Hellmann MD (2018). Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 378, 158–168. [DOI] [PubMed] [Google Scholar]
- 7.Petrelli F, Ardito R, Borgonovo K, Lonati V, Cabiddu M, Ghilardi M and Barni S (2018). Haematological toxicities with immunotherapy in patients with cancer: a systematic review and meta-analysis. Eur J Cancer 103, 7–16. [DOI] [PubMed] [Google Scholar]
- 8.Nie M, Liu Y, Li XX, Min YN, Yang DD, Li Q, Feng Q, Hou Y, Li GS, Sun JZ, Hou M and Shi Y (2019). PD-1/PD-L Pathway Potentially Involved in ITP Immunopathogenesis. Thromb Haemost 119,758–765. [DOI] [PubMed] [Google Scholar]
- 9.Gokozan HN, Friedman JD, Schmaier AH, Downes KA, Farah LA and Reeves HM (2019). Acquired Hemophilia A After Nivolumab Therapy in a Patient With Metastatic Squamous Cell Carcinoma of the Lung Successfully Managed With Rituximab. Clin Lung Cancer 20, e560–e563. [DOI] [PubMed] [Google Scholar]
- 10.Leaf RK, Ferreri C, Rangachari D, Mier J, Witteles W, Ansstas G, Anagnostou T, Zubiri L, Piotrowska Z, Oo TH (2019). Clinical and laboratory features of autoimmune hemolytic anemia associated with immune checkpoint inhibitors. Am J Hematol 94, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanios GE, Doley PB and Munker R (2019). Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol 102, 157–162. [DOI] [PubMed] [Google Scholar]
- 12.Comito RR, Badu LA and Forcello N (2019). Nivolumab-induced aplastic anemia: A case report and literature review. Journal Oncol Pharm Pract 25, 221–225. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese LH, Calabrese C and Cappelli LC (2018). Rheumatic immune-related adverse events from cancer immunotherapy. Nature Rev Rheum 14, 569–579. [DOI] [PubMed] [Google Scholar]
- 14.Weinmann SC and Pisetsky DS (2019). Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford, England) 58, vii59–vii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das S and Johnson DB (2019). Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 7, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M and Nakagawa K (2018). Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol 4, 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, Arranz R, Lorenzo A, Gullón P, Donnay O (2019). Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer 109, 21–27. [DOI] [PubMed] [Google Scholar]
- 18.Khorana AA, Francis CW, Culakova E, Kuderer NM and Lyman GH (2007). Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy 5, 632–634. [DOI] [PubMed] [Google Scholar]
- 19.Khorana AA, Carrier M, Garcia DA and Lee AY (2016). Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis 41, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khorana AA, Dalal MR, Lin J and Connolly GC (2013). Health care costs associated with venous thromboembolism in selected high-risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clinicoecon Outcomes Res 5, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khorana AA, Kuderer NM, Culakova E, Lyman GH and Francis CW (2008). Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111, 4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim AS, Khorana AA and McCrae KR (2020). Mechanisms and biomarkers of cancer-associated thrombosis: Cancer thrombosis mechanisms and biomarkers. Trans Res 225, 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campia U (2020). Vascular effects of cancer treatments. Vasc Med 25, 226–234. [DOI] [PubMed] [Google Scholar]
- 24.Gervaso L, Montero AJ, Jia X and Khorana AA (2020). Venous thromboembolism in breast cancer patients receiving cyclin-dependent kinase inhibitors. J Thromb Haemost 18, 162–168. [DOI] [PubMed] [Google Scholar]
- 25.Hisada Y, Geddings JE, Ay C and Mackman N (2015). Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost 13, 1372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ando Y, Hayashi T, Sugimoto R, Nishibe S, Ito K, Kawada K, Ikeda Y, Yamada S and Imaizumi K (2020). Risk factors for cancer-associated thrombosis in patients undergoing treatment with immune checkpoint inhibitors. Invest New Drugs 38, 1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunimasa K, Nishino K, Kimura M, Inoue T, Tamiya M, Kumagai T and Imamura F (2018). Pembrolizumab-induced acute thrombosis: A case report. Medicine (Baltimore) 97, e10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson SP, Darbousset R and Schoenwaelder SM (2019). Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 133, 906–918. [DOI] [PubMed] [Google Scholar]
- 29.Connolly GC and Khorana AA (2009). Risk stratification for cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 22, 35–47. [DOI] [PubMed] [Google Scholar]
- 30.Khorana AA and Connolly GC (2009). Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol, 27:4839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribas A (2015). Releasing the Brakes on Cancer Immunotherapy. N Engl J Med 373, 1490–2. [DOI] [PubMed] [Google Scholar]
- 32.Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, Chiarion Sileni V, Schachter J, Garbe C, Bondarenko I (2019). Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med 381, 626–636. [DOI] [PubMed] [Google Scholar]
- 33.Ribas A and Wolchok JD (2018). Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez-Sainz L, Martinez-Marin V, Viñal D, Martinez-Perez D, Pedregosa J, Garcia-Cuesta JA, Villamayor J, Zamora P, Pinto A, Redondo A (2020). Incidence of venous thromboembolic events in cancer patients receiving immunotherapy: a single-institution experience. Clin Transl Oncol Nov 24 (online ahead of print). [DOI] [PubMed] [Google Scholar]
- 35.Moik F, Chan WE, Wiedemann S, Hoeller C, Tuchmann F, Aretin MB, Fuereder T, Zöchbauer-Müller S, Preusser M, Pabinger I et al. (2020). Incidence, risk factors and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood Oct 16 (online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharthuar A, Khorana AA, Hutson A, Wang JG, Key NS, Mackman N and Iyer RV (2013). Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res 132, 180–184. [DOI] [PubMed] [Google Scholar]
- 37.van Es N, Hisada Y, Di Nisio M, Cesarman G, Kleinjan A, Mahé I, Otten HM, Kamphuisen PW, Berckmans RJ, Büller HR, Mackman N and Nieuwland R (2018). Extracellular vesicles exposing tissue factor for the prediction of venous thromboembolism in patients with cancer: A prospective cohort study. Thromb Res 166, 54–59. [DOI] [PubMed] [Google Scholar]
- 38.Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, Quehenberger P, Wagner O, Zielinski C and Pabinger I (2009). D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 27, 4124–9. [DOI] [PubMed] [Google Scholar]
- 39.Grilz E, Marosi C, Königsbrügge O, Riedl J, Posch F, Lamm W, Lang IM, Pabinger I and Ay C (2019). Association of complete blood count parameters, d-dimer, and soluble P-selectin with risk of arterial thromboembolism in patients with cancer. J Thromb Haemost. 17, 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pabinger I, Thaler J and Ay C (2013). Biomarkers for prediction of venous thromboembolism in cancer. Blood 122, 2011–2018. [DOI] [PubMed] [Google Scholar]
- 41.Campello E, Ilich A, Simioni P and Key NS (2019). The relationship between pancreatic cancer and hypercoagulability: a comprehensive review on epidemiological and biological issues. Br J Cancer 121, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsen AC, Brøndum Frøkjaer J, Wishwanath Iyer V, Vincents Fisker R, Sall M, Yilmaz MK, Kuno Møller B, Kristensen SR and Thorlacius-Ussing O (2015). Venous thrombosis in pancreaticobiliary tract cancer: outcome and prognostic factors. J Thromb Haemost 13, 555–62. [DOI] [PubMed] [Google Scholar]
- 43.Tobin RP, Jordan KR, Robinson WA, Davis D, Borges VF, Gonzalez R, Lewis KD and McCarter MD (2018). Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipilimumab. Int Immunopharmacol 63, 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munn DH and Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Current Opin Immunol 39, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruffell B and Coussens LM (2015). Macrophages and therapeutic resistance in cancer. Cancer Cell 27, 462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrilovich DI (2017). Myeloid-Derived Suppressor Cells. Cancer Immunol Res 5, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy CB, Pavelko KD, Li Y, O’Brien D, Wang C (2020). Targeting tumor-associated macrophages and granulocytic-myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest 130, 5380–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Wei G, Cheng WA, Dong Z, Sun H, Lee VY, Cha SC, Smith DL, Kwak LW and Qin H (2018). Targeting myeloid-derived suppressor cells for cancer immunotherapy. Cancer Immunol immunother 67, 1181–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graf C, Wilgenbus P, Pagel S, Pott J, Marini F, Reyda S, Kitano M, Macher-Göppinger S, Weiler H and Ruf W (2019). Myeloid cell-synthesized coagulation factor X dampens antitumor immunity. Sci Immunol 4 (39), eaaw8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boutté AM, McDonald WH, Shyr Y, Yang L and Lin PC (2011). Characterization of the MDSC proteome associated with metastatic murine mammary tumors using label-free mass spectrometry and shotgun proteomics. PloS One 6, e22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alfaro C, Teijeira A, Oñate C, Pérez G, Sanmamed MF, Andueza MP, Alignani D, Labiano S, Azpilikueta A, Rodriguez-Paulete A (2016). Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin Cancer Res 22, 3924–36. [DOI] [PubMed] [Google Scholar]
- 52.Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C, Rishipathak D, Williams P, Kadel EE 3rd, Koeppen H, Chen YJ, Modrusan Z (2020). High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med 26, 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, Walsh AM, Baxi V, Pandya D, Baradet T (2020). Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med 26, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ekdahl KN, Teramura Y, Hamad OA, Asif S, Duehrkop C, Fromell K, Gustafson E, Hong J, Kozarcanin H, Magnusson PU, Huber-Lang M, Garred P and Nilsson B (2016). Dangerous liaisons: complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol Rev 274, 245–269. [DOI] [PubMed] [Google Scholar]
- 55.Foley JH and Conway EM (2016). Cross Talk Pathways Between Coagulation and Inflammation. Circ Res 118, 1392–408. [DOI] [PubMed] [Google Scholar]
- 56.Gollomp K, Kim M, Johnston I, Hayes V, Welsh J, Arepally GM, Kahn M, Lambert MP, Cuker A, Cines DB et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight 3, e99445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Núñez-Álvarez C, Hernández-Ramírez D, Bockenstedt PL, Liaw PC, Cabral AR and Knight JS (2015). Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheum 67, 2990–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, Cody MJ et al. (2020). Neutrophil Extracellular Traps (NETs) Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood 136, 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sturtzel C (2017). Endothelial Cells. Adv Exp Med Biol 1003, 71–91. [DOI] [PubMed] [Google Scholar]
- 60.Boulanger CM (2016). Endothelium. Arterioscler Thromb Vasc Biol 36, e26–31. [DOI] [PubMed] [Google Scholar]
- 61.Silvestro A, Brevetti G, Schiano V, Scopacasa F and Chiariello M (2005). Adhesion molecules and cardiovascular risk in peripheral arterial disease. Soluble vascular cell adhesion molecule-1 improves risk stratification. Thromb Haemost 93, 559–63. [DOI] [PubMed] [Google Scholar]
- 62.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM and Stehouwer CD (2000). Increased levels of soluble vascular cell adhesion molecule 1 are associated with risk of cardiovascular mortality in type 2 diabetes: the Hoorn study. Diabetes 49, 485–91. [DOI] [PubMed] [Google Scholar]
- 63.Edlinger C, Lichtenauer M, Wernly B, Pistulli R, Paar V, Prodinger C, Krizanic F, Thieme M, Kammler J, Jung C (2019). Disease-specific characteristics of vascular cell adhesion molecule-1 levels in patients with peripheral artery disease. Heart Vessels 34, 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bester J and Pretorius E (2016). Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep 6, 32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, Streiff MB, Garcia DA, Liebman HA, Belani CP (2019) et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med 380, 720–728. [DOI] [PubMed] [Google Scholar]
- 66.Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, Kuruvilla P, Hill D, Spadafora S, Marquis K et al. (2019). Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med 380, 711–719. [DOI] [PubMed] [Google Scholar]
- 67.Paneesha S, McManus A, Arya R, Scriven N, Farren T, Nokes T, Bacon S, Nieland A, Cooper D, Smith H et al. (2010). Frequency, demographics and risk (according to tumour type or site) of cancer-associated thrombosis among patients seen at outpatient DVT clinics. Thromb Haemost 103, 338–43. [DOI] [PubMed] [Google Scholar]
- 68.Tzeng A, Diaz-Montero CM, Rayman PA, Kim JS, Pavicic PG Jr., Finke JH, Barata PC, Lamenza M, Devonshire S, Schach K et al. (2018). Immunological Correlates of Response to Immune Checkpoint Inhibitors in Metastatic Urothelial Carcinoma. Targeted Oncology 13, 599–609. [DOI] [PubMed] [Google Scholar]
- 69.Brown D, Zingone A, Yu Y, Zhu B, Candia J, Cao L and Ryan BM (2019). Relationship between Circulating Inflammation Proteins and Lung Cancer Diagnosis in the National Lung Screening Trial. Cancer Epidemiol, Biomarkers Prev 28, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fine JP and Gray RJ (1999). A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 94, 496–509. [Google Scholar]
- 71.Haynes W. Wilcoxon Rank Sum Test (2013). In: Dubitzky W, Wolkenhauer O, Cho K-H and Yokota H, eds. Encyclopedia of Systems Biology New York, NY: Springer New York, 2354–2355. [Google Scholar]
- 72.Ghosh D (2012). Incorporating the empirical null hypothesis into the Benjamini-Hochberg procedure, in Statistical Applications in Genetics and Molecular Biology, DeGruyter vol 11, 1–21. [DOI] [PubMed] [Google Scholar]
- 73.Bray JRC (1957). An ordination of the upland forest communities of Southern Wisconsin. Ecol Monographies 27, 325–349. [Google Scholar]
- 74.Wickham H (2016). ggplot2-Elegant graphics for data analysis, Springer, 11–31. [Google Scholar]
- 75.Murtagh F, Legendre P (2014). Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J Classification 31, 274–295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data involved in this research is available from the corresponding authors upon request. The request should be in the form of a proposal with aims, a statistical approach and other information that will provide assurance of data confidentiality. Patient data will be shared after review and approval of the request by the Cleveland Clinic Institutional Review Board. No data that includes patient names, birthdates, or other potentially identifying information can be shared.