Abstract
Prolonged immobilization from a critical illness can result in significant muscle atrophy. Whole-body vibration (WBV) could potentially attenuate the issue of muscle atrophy; however, there exists no device that could potentially provide WBV in supine position that is suitable for critically ill patients. Hence, the purpose of this study was to develop a new wearable suit, called therapeutic vibration device (TVD), that can provide WBV in supine position and test its effects on physiologic markers of physical activity including muscle activation, oxygen consumption (VO2), and regional hemoglobin oxygen saturation (rSO2). The prototype TVD delivered multi-frequency WBV axially to 19 healthy participants in supine position for 10 minutes simultaneously at 25 Hz/4.2 grms on the feet and 15 Hz/0.7 grms on the shoulders. Muscle activation was recorded by electromyography (EMG), VO2 was measured by indirect calorimetry and rSO2 was recorded by near-infrared spectroscopy. Recordings were collected from each participant from multiple body locations, on three separate days, at baseline and during the intervention. Acceleration was also recorded to gain insight into transmissibility and coherence. Repeated-measures ANOVA using Bonferroni correction revealed that the muscle activity significantly increased by 4% - 62% (p < 0.05), VO2 improved by 22.3% (p < 0.05) and rSO2 increased by 1.4% - 4.5% (p < 0.05) compared to baseline. WBV provided by the TVD is capable of producing physiologic responses consistent with mild physical activity. Such effects could potentially be valuable as an adjunct to physical therapy for early mobilization to prevent atrophy occurring from prolonged immobilization.
Keywords: Early mobilization, exoskeleton, muscle atrophy, post intensive care syndrome, rehabilitation, tonic vibration reflex
I. INTRODUCTION
During critical illness, patients who are immobilized or incapacitated for prolonged periods develop severe complications including neuromuscular weakness, contractures, and muscle atrophy [1]-[3]. Complete immobilization leads to complications such as loss of muscle strength by 10%-15% each week [1], [3], approximately 40% strength decline within a month [1], [3], and up to a 27% reduction in maximal O2 uptake [1]. Despite the reduction in mortality rates [4]-[6], ICU-acquired weakness, functional impairment, and cognitive dysfunction continue to increase [3]-[6]. These sequelae are aspects of the recently defined post-intensive care syndrome (PICS) [4] and may contribute to increased duration of mechanical ventilation, increased length of hospital stay, poor quality of life among survivors, and an increased rate of readmissions [1], [4], [5]. To address PICS, an institutional culture of early mobility and activity is advocated [2], [4], [6] in addition to proactively educating patients and their families [4]-[6]. Early mobilization in the form of walking and cycle ergometry has been demonstrated to significantly reduce polyneuropathy and atrophy associated with PICS, which in turn has demonstrated a reduction in the time patients spend on mechanical ventilation and the overall length of hospital stay [2], [3]. However, these approaches to early mobilization are labor-intensive and cannot be introduced in heavily sedated or unconscious patients.
Whole-body vibration (WBV) may facilitate the rehabilitation process by enhancing muscle activity [7]-[19], increasing metabolic expenditure [20]-[32], improving blood flow and tissue oxygenation [22], [32]-[40], and improving bone mineral density and content [41]-[45]. An increase in muscle activity has been attributed to the tonic vibration reflex [13], [20], [46] or a similar characteristic activity [8], [13], [47]. Evidence suggests that vibrations may produce adequate muscle contraction via spinal reflex loops [13], [15] that may be sufficient to reduce or prevent muscle weakness caused by prolonged immobilization [15], [16]. Thus, WBV may serve as an effective treatment in rehabilitating immobilized patients. Commercially available vibration platforms and pads apply vibrations for alert subjects in a standing or seated position. However, for supine and immobilized patients, these devices have no or very limited body preloading capability and cannot target the upper-extremities. Moreover, the vibration transmissibility to remote parts of the body is attenuated due to the adopted posture and the vibration delivery mechanism. This, in turn, may reduce the effects of vibration on the ability to engage a larger percentage of muscle groups. The use of these existing devices in a clinical environment for immobilized patients poses a significant challenge.
To address these issues, we created a prototype therapeutic vibration device capable of delivering WBV at multiple frequencies simultaneously at distal ends of the body, i.e., feet and shoulders, under axial loading. We also tested the effects of therapeutic vibration device on physiologic markers such as muscle activity, regional hemoglobin oxygen saturation, and whole-body metabolism including oxygen consumption as indicators of physical activity. We hypothesized that low frequency WBV applied axially to large muscle groups in the supine position will increase muscle activation, regional tissue oxygenation, and oxygen consumption.
II. MATERIALS AND METHODS
A. PARTICIPANTS
The study was conducted at the University of Michigan, Ann Arbor by the Michigan Center for Integrative Research in Critical Care with approval from the Institutional Review Board of the University of Michigan Medical School (IRBMED). The study was also registered as a clinical trial (clinicaltrials.gov, identifier: NCT03479008). All participants provided written informed consent. The exclusion criteria were: known pregnancy, acute spinal cord injury, acute vertebral body fracture or injury, acute stroke or intracerebral hemorrhage, hemodynamic instability or other events/conditions (e.g., deep vein thrombosis, neurological disorders, and other heart conditions) believed by the care team to warrant exclusion. Out of the 22 participants who were enrolled in the study, 3 participants withdrew due to nausea and discomfort after attending the first session. Data from these participants were excluded from the analysis. 19 medically healthy participants, [9 women and 10 men, age, 51.7 ± 19.5 yr; height, 172.9 ± 8 cm; mass, 76.2 ± 13.9 kg; body mass index (BMI), 25.4 ± 3.6 kg/m2 (mean ± SD)] successfully completed the study. An a priori power analysis using G*Power 3.1 with an effect size specification as in SPSS indicated that this sample size yielded a power (β) > 80% to detect statistical significance at α = 0.05. We used repeated measures (within factors) statistical test for the power analysis with an effect size f(U) = 0.734 and nonsphericity correction ε = 1. The effect size f(U) was computed directly in G*Power 3.1 using previously reported effect size (partial η2 = 0.35) for changes in oxygen consumption due to WBV in active young adults [28].
B. THERAPEUTIC VIBRATION DEVICE
The device contains four commercially available inertial actuators (ButtKicker Concert and ButtKicker mini Concert, Guitammer Company, USA) housed in custom-designed enclosures. These are assembled with their respective body interfaces, i.e. ankle braces and shoulder cups. A shoulder cup with actuator assembly was mounted on each shoulder. Similarly, an ankle brace with actuator assembly was strapped onto each foot. The device was preloaded using a non-elastic, non-rigid strap restraint mechanism. The inertial actuators were capable of generating vibrations at frequencies between 10 and 100 Hz (Fig. 1). We specifically chose the shoulder and feet combination as it is less obtrusive to bedridden patients. This is also the best configuration for longitudinal transmission of WBV, which has been shown to induce many positive physiologic effects. Actuators at the back, waist, or hips could induce shear forces/stresses that could potentially be detrimental to the patient (e.g., skin breakdown) and hence, those sites were not chosen.
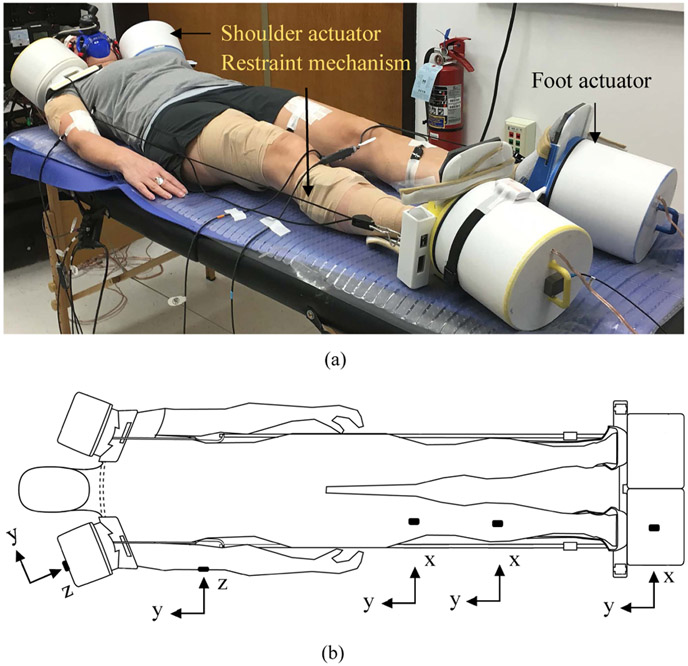
FIGURE 1.
(a) A picture showing the therapeutic vibration device delivering whole-body vibration using inertial actuators to a participant in a supine position. The actuators are preloaded using a restraint mechanism while the participant remains passive. (b) Triaxial accelerometer mounting and coordinate axes orientation with respect to the direction of vibration – i.e., in y-axis on feet and z-axis on shoulders, respectively. They are mounted on foot shaker, tibialis anterior, rectus femoris, brachialis and shoulder shaker.
C. PROTOCOL
The schematic of the experimental protocol is provided in Fig. 2. Three sessions were conducted with each participant on three separate days. During the first session, participants were familiarized with the protocol and the device and were trained to carry out maximal voluntary isometric contractions (MVIC) of several muscle groups, in addition to delivering WBV. Participants performed two sets of MVIC tests for 5 seconds per muscle while verbal encouragement was given. Between each protocol activity, a break interval of at least 3 minutes was provided. Participants then laid passively in a relaxed supine position, with their hands on either side of the trunk, without any voluntary contraction for the duration of vibration for 10 minutes (Fig. 1). Based on our pilot experiments, the vibration frequencies and acceleration amplitudes of 25 Hz/4.2 grms on feet actuators and 15 Hz/0.7 grms on shoulder actuators were chosen as vibration stimuli. This is because we found that multi-frequency excitation at the distal end of the body stimulated upper extremity muscles to a greater extent than single frequency WBV. There was no slip between the device and body at the interfaces on feet and shoulders. Each of the interfaces had high-density minicell closed cell polyethylene foam lining for better grip and to avoid slip. To reduce the friction between the bed and the participants’ posterior surface, a commercially available smooth plastic sheet was used. LabVIEW (National Instruments, version 2017) was used to simultaneously program and generate frequencies of 25 and 15 Hz. The acceleration amplitudes were controlled from the actuator amplifiers.
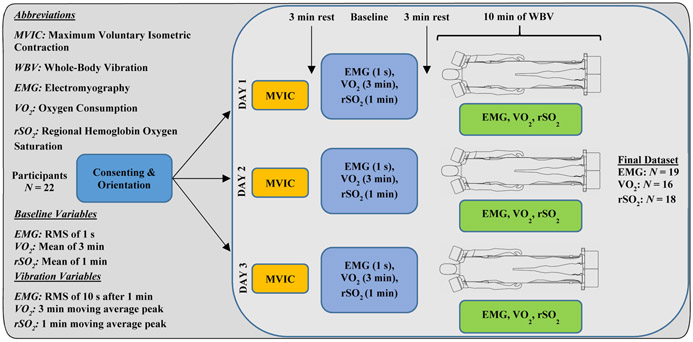
FIGURE 2.
A schematic of the study design.
D. ACCELERATION
Shimmer3 wireless inertial measurement units (Shimmer, Ireland), each containing a triaxial wide range accelerometer (±16 g), were used to measure accelerations on the actuators and tissue. They were mounted using self-adhesive elastic bandages on the foot actuator, shoulder actuator, tibialis anterior (TA), rectus femoris (RF) and brachialis (BC) muscles. Each sensor has a sampling frequency of 512 Hz, weighs 23.6 grams, 14-bit resolution, and a root mean square noise of 0.6 mg at ±2 g. The data were detrended and then low pass filtered using a 6th order Butterworth filter with a cut-off frequency of 35 Hz. Intra-subject mean root mean square values for 4-second segments for each individual were calculated, and then the inter-subject mean RMS was computed. Using the cross-spectral density method, the transmissibility H(f) was defined as [48]
where Gio(f) is the cross-spectral density between the acceleration on the actuators (input) and the tissue acceleration (output), Gii(f) is the power spectral density of the actuator acceleration (input). The transmissibility was interpreted assuming linear elastic behavior of the tissue so that the amplitude of the input did not have to be intentionally varied. The coherence was also calculated to examine the relationship between the output and input signals. The signal coherence was defined as [48]
where Goo(f) is the power spectral density of the tissue acceleration. The spectral densities were calculated using the mean acceleration values with Welch’s power spectral density estimate at a resolution of 0.25 Hz (Fig. 3).
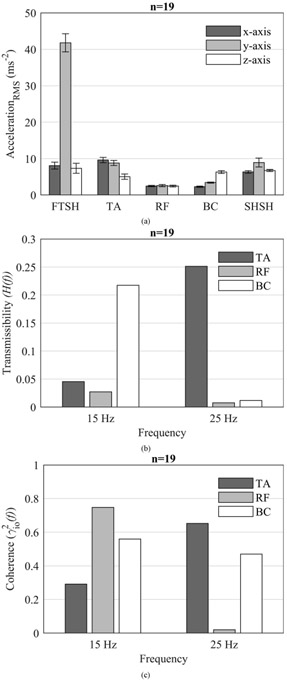
FIGURE 3.
(a) Mean ± SEM of the low pass filtered acceleration on foot shaker (FTSH), tibialis anterior (TA), rectus femoris (RF), brachialis (BC) and shoulder shaker (SHSH). (b) Transmissibility, and (c) Coherence computed using the cross spectral density method from the mean acceleration of all participants in the direction of vibration (refer Fig. 1(b) for the coordinate axes of the accelerometers) at simultaneous excitation frequencies of 15 Hz on shoulders and 25 Hz on feet.
E. MUSCLE ACTIVATION
A BTS FREEEMG 1000 (BTS S.p.A., Italy) system containing wireless EMG probes (13 grams, CMRR >110db at 50-60 Hz, input impedance 100 MΩ) was used to measure muscle activity from eight anatomical sites on the body. The probes were directly attached to bipolar Ag/AgCl pre-gelled disposable snap electrodes (Covidien Kendall disposable surface electrodes H124SG 30 mm ×24 mm, Covidien, USA) and affixed using self-adhesive elastic bandage. The anatomical sites were prepared using an abrasive skin gel (Nuprep, Weaver and Company, USA). The probes were positioned on the muscle bellies of the soleus (SO), tibialis anterior (TA), gastrocnemius lateralis (GL), vastus medialis (VM), vastus lateralis (VL), rectus femoris (RF), semitendinosus (ST) and deltoideus medius (DM). The electrodes were aligned with the muscle axis and placed on the muscle with an inter electrode distance of 20 mm in accordance with the SENIAM (surface electromyography for the non-invasive assessment of muscles) recommendations [49]. Baseline EMG data were recorded prior to commencement of vibration, and a 1 second segment was extracted for post processing. For computing muscle activation during vibration, a 10 second EMG segment was extracted after 1 minute of start of the vibration. The extracted signals were filtered using an 8th order Butterworth digital band-pass filter from 10-400 Hz followed by zero-phase digital filtering to preserve the waveform. A linear interpolation method [50] which filters out only the artifact spikes from the signal in the Welch’s power spectral density was implemented [7], [14], [50]. The spikes corresponding to excitation frequencies of 15 Hz, 25 Hz, 60 Hz (line frequency) and their harmonics up to 400 Hz were filtered out. A uniform spike width of ± 2 Hz around 15 Hz, 25 Hz, 60 Hz and their harmonics were replaced by interpolated straight line. Similar filtering procedures were carried out for EMG signals recorded during MVIC tests and baseline recording. The root-mean square values of the EMG signals of vibration and MVIC were directly calculated from the Welch’s power spectral density. Normalization to MVIC was carried out using the formula, (Vibration EMGRMS)/(MVIC EMGRMS) × 100. Bias was calculated using the formula (Filtered EMGRMS at baseline)/(Unfiltered EMGRMS at baseline), and the bias-corrected EMG during vibration was computed using (Vibration EMGRMS /Bias) [50].
F. INDIRECT CALORIMETRY
A COSMED K5 wearable metabolic system (COSMED srl, Italy) was used to measure oxygen uptake (VO2), carbon dioxide production (VCO2), energy expenditure (EE), minute ventilation (VE) and tidal volume (VT). Testing was conducted in a mixing chamber mode which uses the measuring principle of proportional sampling and collection of exhaled gas via a miniaturized mixing chamber. Based on the manufacturer’s guidelines, the calibration of the device was carried out at recommended intervals. The turbine flowmeter was calibrated before each test using the manufacturer’s supplied 3-liter calibration syringe. The zero of the CO2 analyzer and detection of environmental air composition was performed using the manufacturer’s supplied scrubber. The oxygen sensors were calibrated using the manufacturer’s supplied calibration gas cylinder with recommended concentration of 16% oxygen, 5% carbon dioxide, and balance nitrogen. Data were sampled at 0.1 Hz (i.e., every 10 seconds) and updated using a 30 second moving average. The calorimetry data were recorded in supine position both at baseline and during vibration. For the baseline data, a mean of 3 minutes (18 data points) of the segment preceding vibration was computed. For the data collected during vibration, a moving average peak analysis for every 3 minutes for 10 minutes of VO2, VCO2, EE, VE, and VT data was carried out. The maximum value of the moving average was selected as the mean value of VO2. This methodology of segment extraction precluded the possibility of picking up short transient changes in metabolic data and helped in ensuring selection of steady set of values of metabolic variables which estimated the true response of the participant.
G. REGIONAL HEMOGLOBIN OXYGEN SATURATION
A Nonin SenSmart Model X-100 Universal Oximetry System (Nonin Medical, Inc., USA) was used to measure rSO2 using near-infrared spectroscopy. Oxygenation sensors were placed on three anatomical sites i.e., the gastrocnemius lateralis (GL), rectus femoris (RF) and biceps brachii (BB). The rSO2 data were recorded at a sampling frequency of 0.25 Hz (i.e., once every 4 seconds). The data were filtered by the manufacturer’s signal processing algorithms to reduce interference from external sources and motion artifacts. The mean value of rSO2 for 1 minute (15 data points) preceding vibration was computed as the baseline value. For the data collected during vibration, a moving average peak analysis for every 1 minute for 10 minutes of rSO2 data was carried out. The maximum value of the moving average was selected as the mean value of vibration. The moving average peak analysis was independently conducted for all three measurements from GL, RF and BB.
H. DATA PROCESSING AND STATISTICS
Data processing of EMG, rSO2 and metabolic data were performed using MATLAB version 9.4 R2019a (MathWorks, MA, USA). All values are expressed as mean ± SEM (standard error of the mean) unless otherwise stated. Statistical analyses were performed using IBM SPSS Statistics version 25 (SPSS Inc., Chicago, IL). A one-way repeated measures analysis of variance (ANOVA) with condition (baseline and vibration) as a repeated measure was carried out to test the effect of WBV on muscle activation, metabolic parameters, and rSO2%. Statistical significance level was set at p < 0.05 for all tests.
III. RESULTS
A. MUSCLE ACTIVATION
There was a significant effect of condition on muscle activation [F (8, 11) = 17.17, p < 0.0001, Wilk’s Λ = 0.074, partial η2 = 0.926]. Post hoc analysis with Bonferroni correction indicated that the mean activation was significantly higher during WBV for the SO (p = 0.011), TA (p = 0.012), GL (p = 0.003), VM (p < 0.001), VL (p < 0.0001), DM (p = 0.006), but not for RF (p = 0.59) and ST (p = 0.86) (Fig. 4).
FIGURE 4.
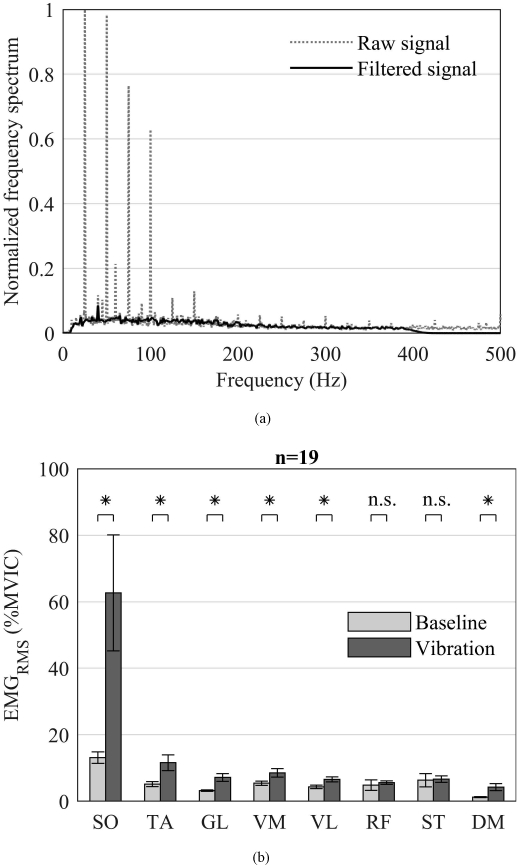
(a) A sample EMG frequency spectrum of the soleus (SO) muscle from a single participant in a single session at multi-frequency synchronous excitation of 15 Hz on shoulders and 25 Hz on feet. The gray dotted line depicts the raw EMG signal and the black solid line depicts the band-pass (8th order Butterworth filter 10-400 Hz), linear interpolated filtered EMG signal. The motion artifacts (sharp spikes) at excitation frequencies and corresponding harmonics up to 400 Hz have been removed using the linear interpolation method. (b) Mean ± SEM of the filtered EMG RMS at baseline and during vibration normalized to the maximum voluntary isometric contraction (MVIC) at excitation frequencies of 15 Hz and 25 Hz. EMG measured from the muscle bellies of soleus (SO), tibialis anterior (TA), gastrocnemius lateralis (GL), vastus medialis (VM), vastus lateralis (VL), rectus femoris (RF), semitendinosus (ST) and deltoideus medius (DM). Asterisks indicate statistical significance at p < 0.05 and n.s. indicates not significant.
B. INDIRECT CALORIMETRY
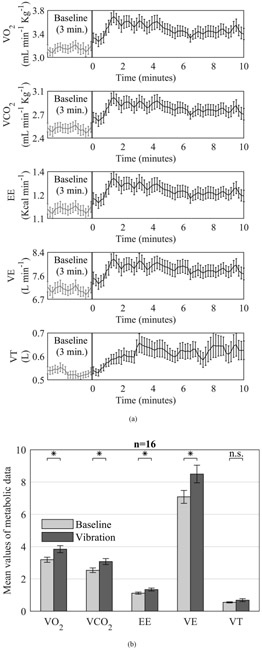
There was a significant effect of condition on metabolic data [F (5, 11) = 7.42, p = 0.003, Wilk’s Λ = 0.229, partial η2 = 0.771]. Post hoc analysis with Bonferroni correction indicated significant increases in VO2, VCO2, EE, VE (p < 0.0001) but not for VT (p = 0.094) during vibration compared with baseline period (Fig. 5).
FIGURE 5.
(a) Mean ± SEM of each metabolic data point in time series at baseline and during vibration. (b) Mean ± SEM of oxygen uptake (VO2), carbon dioxide production (VCO2), energy expenditure (EE), minute ventilation (VE), and tidal volume (VT) recorded at rest and during vibration. Asterisks indicate statistical significance at p < 0.05 and n.s. indicates not significant.
C. REGIONAL HEMOGLOBIN OXYGEN SATURATION
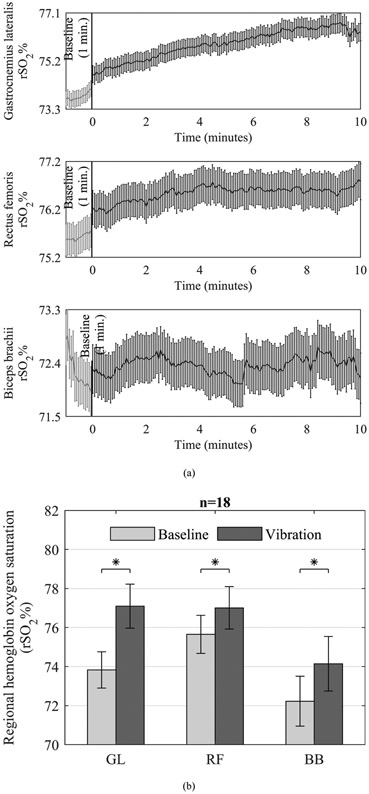
There was a significant effect of condition on rSO2 data [F (3, 15) = 20.93, p < 0.0001, Wilk’s Λ = 0.193, partial η2 = 0.807]. Post hoc analysis with Bonferroni correction indicated that the mean rSO2 of GL (p < 0.0001), RF (p < 0.0001) and BB (p < 0.001) were significantly higher during vibration compared with baseline period (Fig. 6).
FIGURE 6.
(a) Mean ± SEM of each regional hemoglobin oxygen saturation (rSO2%) data point in time series at baseline and during vibration. (b) Comparison of rSO2% (mean ± SEM) at rest and during vibration. Asterisks indicate statistical significance at p < 0.05.
IV. DISCUSSION
The purpose of this study was to test the effects of simultaneous multi-frequency WBV delivered axially using a therapeutic vibration device on several important physiological markers of physical activity in healthy participants as a pilot study in prelude to a future study on critically ill immobilized subjects. Results indicate that WBV using the therapeutic vibration device induced modest but significant increases in muscle activation, regional muscle oxygenation, and metabolic parameters. These increases were similar to those observed during mild physical activity, suggesting that WBV might serve as a suitable approach in assisting to combat muscle weakness and associated dysfunctions of PICS due to prolonged immobilization.
A. MUSCLE ACTIVATION DURING WBV
Several studies have evaluated the effects of WBV on muscle activation and have shown that WBV increases EMG activation of both the upper- and lower-extremity muscles [7]-[19]. However, such WBV protocols are often carried out in standing position or with added loads through active contraction of the individual’s muscles, which may not be applicable to critically ill patients who are often sedated and unconscious. Moreover, WBV while standing or exercising is often accompanied by posture dependent voluntary contractions, which may confound the results by masking the actual muscle activity induced by the vibration. To our knowledge, this is the first study to characterize the effects of WBV on muscle activation using a novel therapeutic vibration device that was specifically designed for the treatment of immobilized critically ill patients in the intensive care unit. We found that WBV using the therapeutic vibration device significantly increased the muscle activation of upper and several lower-extremity muscles. We note that the observed increase in muscle activation was much larger in the distal leg muscles than in the proximal muscles, which is a finding that is consistent with previous studies [7], [12]. A key reason for this observation is that the acceleration amplitude on body segments was substantially dampened as the distance from the source of vibration increased (Fig. 3). Further, it is possible that the preloading of the muscles using the restraint mechanism may not have been adequate for the proximal muscles.
It is important to note that the EMG values were expressed as a percentage of MVIC values. Therefore, a formal statistical comparison between the EMG values recorded during MVIC and during baseline or vibration was not possible. While the increase in EMG due to WBV did not reach MVIC values (as shown in Fig. 4), it is unrealistic to expect such a drastic increase in EMG activation via WBV without any added exercise. We did not incorporate any additional exercise and sought to study the isolated effects of WBV because patients who are in a critical condition are often sedated and paralyzed, and hence, will not have the capability to perform any volitional exercise. Moreover, they will not respond to other promising interventions such as neuromuscular electrical stimulation. Thus, WBV is the only alternative for providing mechanical stimulus to these patients, and this study provides the initial foundation for future studies that are planned on critically-ill patients. Further, a small change in muscle activation in multiple muscles could be strongly beneficial to critically ill patients, albeit, this remains to be tested. Thus, future research should focus on optimizing the vibration frequency and loading of the muscles to maximize the clinical potential of the therapeutic vibration device.
B. INDIRECT CALORIMETRY DURING WBV
WBV, when combined with exercise, is known to augment VO2 and energy expenditure [28]-[31]. However, the ability of WBV to improve metabolic consumption while lying relaxed has remained somewhat unclear. Here, we show for the first time that meaningful improvements in metabolic consumption (22.3%) can be induced by WBV in recumbent position using the therapeutic vibration device. Previous studies have suggested that an increase in metabolic rate during WBV is due to increased muscle activation [20]-[22], [32] and tissue oxygenation [22], [32]. Additionally, the application of external loads during WBV has been shown to enhance metabolic rate [21], [25]-[27]. The results of the current study using preloading are in general, consistent with these previous reports demonstrating an increase in VO2, VCO2, energy expenditure, and minute ventilation, which can be interpreted as a response to increased musculature metabolic demand. Apart from the increase in metabolic demand due to WBV, changes in skin/body temperature could also explain the observed increase in VO2 [51]. Unfortunately, we did not evaluate changes in skin/body temperature due to vibration in this study. While there is no reason to believe that skin/body temperature could have changed due to extraneous factors, as the room temperature was maintained constant and the subjects were resting in recumbent position throughout the experiment, WBV in itself could likely have elevated skin/body temperature [47], [52], [53]. Future research should explore the associations and interactions between muscle activation, skin/body temperature, and VO2 to determine the causal relationship between these variables and to establish the mechanistic underpinnings of increased VO2 due to WBV.
C. REGIONAL HEMOGLOBIN OXYGEN SATURATION DURING WBV
Several studies have investigated the acute effects of vibration on peripheral blood flow, venous return, muscle oxygenation, and total hemoglobin and have reported conflicting or contrasting conclusions [22], [32]-[40], [54], [55]. Our results indicate that WBV increases regional hemoglobin oxygen saturation of the upper- and lower-extremity muscles, which is consistent with several previous studies [22], [33]-[35], [38], [40]. While the exact mechanism for the increased oxygen saturation is not clear, it has been previously hypothesized that vibration applied to the bottom of the foot creates a “skeletal muscle pumping” action resulting in displacement of blood, peripheral lymphatic transport, and an increased arteriovenous pressure gradient, which consequently elevates regional oxygen saturation in the tissues [22], [33], [34], [36]-[38]. Additionally, muscle activation due to tonic vibration reflex may trigger metabolic demand and vasodilation, thus leading to enhancement in blood oxygenation and perfusion [32], [36]. Hence, we believe that both mechanisms (i.e., skeletal muscle pumping and increased muscle activation) could have contributed to the observed increase in regional oxygen saturation.
D. STUDY LIMITATIONS
There are some potential limitations to this study. This study did not examine the isolated effects of shoulder (15 Hz) and foot (25 Hz) vibration because our primary question was related to whole body vibration. Moreover, our pilot testing indicated that some of the measured parameters did not return to baseline even after 10 minutes of completion of the vibration, which would necessitate a large amount of washout period. However, because of our experimental study design, it is not clear if the effects of shoulder and foot vibration differ from each other and if the effect of the 15 Hz is masked, amplified, or negated by the 25 Hz frequency. Further research is needed to study the isolated effects of shoulder and foot vibration on various physiological parameters as this will help in optimizing vibration dosage for upper and lower extremity muscles. Another limitation of this study is that it may be difficult to fully extrapolate the results of the present study to patients at risk for PICS from prolonged immobilization. The participants in this study were medically healthy and led relatively active lifestyles. It is not clear if critically ill and injured patients who are immobilized would realize physiologic responses similar to or greater than those of this healthy cohort. Further, it is not known from this study whether the degree of muscle activation changes observed with WBV would be sufficient to act as a significant mitigator of atrophy. We also do not know what effect it may have at the center of the body, as we did not measure any parameters (e.g., EMG or rSO2%) at this region. As can be seen in Fig. 3, the acceleration amplitude substantially dampens in proximal muscles, which is due to the nonlinear behavior of the human body [7], [12] and inadequate preloading of the trunk/center of the body muscles. Based on this observation, we speculate that the transmission to the center of the body is relatively low compared to the extremities, which could result in low muscle activation and other physiologic responses. Future studies should evaluate the effects of WBV at the trunk region as it could have meaningful therapeutic implications for lung health in critically ill patients (e.g., sputum clearance). Additionally, measurement of myoglobin would have provided insight into the muscle metabolism due to muscle activation. Unfortunately, this study did not measure myoglobin due to the Nonin SenSmart X-100 NIRS device limitations. However, in our future studies we will measure myoglobin to gain insight into muscle metabolism due to therapeutic vibration device. Finally, this study did not address dosing in terms of frequency or duration of vibration on various physiologic parameters. Thus, future studies are needed to address the issue of dosage and also to examine both acute and long-term effects of WBV using the therapeutic vibration device in critically ill and injured patients to determine its potential to counteract PICS due to prolonged immobilization.
V. CONCLUSION
This study tested the effects of simultaneous multi-frequency axial WBV using inertial actuators on physiological parameters in healthy individuals. The results of the study indicate that WBV induces significant changes in muscle activation, metabolic parameters of oxygen consumption, and tissue oxygenation that are consistent with mild physical activity. Additional studies on individuals who are immobilized due to critical illness are needed to understand if WBV using the therapeutic vibration device may act as a sufficient countermeasure to prevent PICS associated atrophy and its complications.
TABLE 1.
Abbreviation list.
| Abbreviation | Description |
|---|---|
| WBV | Whole body vibration |
| TVD | Therapeutic vibration device |
| ICU | Intensive care unit |
| PICS | Post-intensive care syndrome |
| MVIC | Maximal voluntary isometric contraction |
| EMG | Electromyography |
| rSO2% | Regional hemoglobin oxygen saturation |
| SO | Soleus |
| TA | Tibialis anterior |
| GL | Gastrocnemius lateralis |
| VM | Vastus medialis |
| VL | Vastus lateralis |
| DM | Deltoideus medius |
| RF | Rectus femoris |
| ST | Semitendinosus |
| BB | Biceps brachii |
| BC | Brachialis |
| VO2 | Oxygen uptake |
| VCO2 | Carbon dioxide production |
| EE | Energy expenditure |
| VE | Minute ventilation |
| VT | Tidal volume |
| FTSH | Foot shaker |
| SHSH | Shoulder shaker |
| SENIAM | Surface electromyography for the non-invasive assessment of muscles |
| EMGRMS% | RMS of EMG values expressed as a percentage of MVIC |
| SEM | Standard error of the mean |
ACKNOWLEDGMENT
The authors would like to thank all the participants for volunteering in this study. They would also like to thank A. J. Pennington, Clinical Project Manager for helping us in recruiting and consenting participants.
This work was supported in part by the Michigan Center for Integrative Research in Critical Care, in part by the University of Michigan Coulter Translational Research Partnership Program, in part by the University of Michigan’s MCubed program, and in part by the National Institutes of Health (Grant #R21 HD092614).
Biography

HIMANSHU SAXENA received the B.Tech. degree in mechanical engineering from Jawaharlal Nehru Technological University, Hyderabad, India, in 2005, and the M.S. degree in mechanical engineering from the University of Michigan, Ann Arbor, USA, in 2016.
He is currently an Engineer in Research with the Department of Mechanical Engineering, University of Michigan. His research interests include the research and development of vibration device technology for patient rehabilitation and investigating the influence of neck muscles on head acceleration during sport-associated impact events in high school athletes.

KEVIN R. WARD received the degree in medicine from Tulane University, New Orleans, LA, in 1989. He then completed a residency in emergency medicine at the University of Pittsburgh followed by a Resuscitation Research fellowship at Ohio State University, Columbus, OH.
Prior to joining the University of Michigan, in 2012, he was a Professor and an Associate Chair of emergency medicine with Virginia Common-wealth University (VCU) were he also founded and directed the VCU Reanimation Engineering Science Center (VCURES). He is currently a Professor of emergency medicine and biomedical engineering with the University of Michigan, where he serves as the Executive Director of the Michigan Center for Integrative Research in Critical Care. His research interests are in developing platform technologies that improve the care of critically ill and injured patients. In collaboration with the U.S. Army and its Joint Special Operations Training Medical Center, he developed and medically directed special training programs, which have been responsible for providing clinical training to over 1000 Special Operation Combat Medics. For this work, he was awarded a Certificate for Patriotic Civilian Service by the Department of the Army and the Joint Special Operations Training Center. Serving on numerous editorial and review boards in the field of resuscitation, emergency and critical care medicine, he also serves on the executive committee for the Trauma Hemostasis and Oxygenation Research (THOR).

CHANDRAMOULI KRISHNAN received the bachelor’s degree in physiotherapy from the Tamil Nadu Dr. M.G.R. Medical University, in 1998, and the master’s degree in physical therapy and the Ph.D. degree in physical therapy and rehabilitation science from the University of Iowa, in 2006 and 2009, respectively. He then pursued a postdoctoral fellowship for three years at the Rehabilitation Institute of Chicago focusing on rehabilitation robotics and noninvasive brain stimulation.
Since 2012, he has been Directing the Neuromuscular and Rehabilitation Robotics Laboratory (NeuRRo Lab), University of Michigan. He is currently an Associate Professor with the Department of Physical Medicine and Rehabilitation, Biomedical Engineering, Robotics Institute, and School of Kinesiology at the University of Michigan. His research examines the alterations in neuromuscular function, plasticity, and movement control after an injury to the neuro-musculoskeletal system and connects how these insights can be applied to rehabilitation using robotics, motor learning, and exercise interventions. He performs both basic mechanistic/device development studies and interventional studies (i.e., clinical trials) to improve motor outcomes and function. Dr. Krishnan’s NeuRRo lab has developed several novel low-cost devices and software programs for the assessment and treatment of upper- and lower-extremity neuromuscular impairments. His research program is funded through intramural and extramural grants, including support from the National Institutes of Health and National Science Foundation. He has over 20 years of clinical and research experience in physical therapy and rehabilitation sciences, has published more than 50 articles in peer-reviewed journals, and has presented at over 100 national and international meetings. He is an Associate Editor of the IEEE Transactions on Neural Systems and Rehabilitation Engineering. He is also an Associate Editor of the Restorative Neurology and Neuroscience and a Section Editor of Measurement in Physical Education and Exercise Science. He is an ad hoc reviewer for more than 40 international journals spanning across the field of engineering, biomechanics, and rehabilitation, and a regular reviewer of the National Institutes of Health and various international grant panels.

BOGDAN I. EPUREANU received the Ph.D. degree from Duke University.
He is currently an Arthur F. Thurnau Professor with the College of Engineering, University of Michigan, and the Director of the Automotive Research Center (ARC). During his tenure at the University of Michigan, since 2002, he also served as Associate Chair of the Division of Integrative Systems and Design and was a founding Program Director of the Systems Engineering and Design Master Program. He focuses on understanding dynamic phenomena in teaming of autonomous vehicles, enhanced aircraft safety and performance, early detection of neurodegenerative diseases, forecasting tipping points in disease epidemics and ecology. He has published over 350 articles in journals, conferences, and books. His research interests include nonlinear dynamics of complex systems, particularly those with behaviors leading to catastrophes. He is a Fellow of the ASME and Associate Fellow of AIAA, an Associate Editor of ASME Journal of Computational and Nonlinear Dynamics.
Footnotes
The associate editor coordinating the review of this manuscript and approving it for publication was Jenny Mahoney.
DISCLOSURES
Kevin R. Ward, Bogdan I. Epureanu and Himanshu Saxena have a patent pending for the therapeutic vibration device through the University of Michigan. The funding sources had no roles in the study design, collection, analysis or interpretations of the data or in the publication process. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- [1].Dittmer DK and Teasell R, “Complications of immobilization and bed rest. Part 1: Musculoskeletal and cardiovascular complications,” Can Fam Physician, vol. 39, pp. 1428–1432 and 1435–1437, 1993. [PMC free article] [PubMed] [Google Scholar]
- [2].Hunter A, Johnson L, and Coustasse A, “Reduction of intensive care unit length of stay: The case of early mobilization,” Health Care Manager, vol. 33, no. 2, pp. 128–135, 2014. [DOI] [PubMed] [Google Scholar]
- [3].Garzon-Serrano J, Ryan C, Waak K, Hirschberg R, Tully S, Bittner EA, Chipman DW, Schmidt U, Kasotakis G, Benjamin J, Zafonte R, and Eikermann M, “Early mobilization in critically ill patients: Patients’ mobilization level depends on health care provider’s profession,” PM&R, vol. 3, no. 4, pp. 307–313, April. 2011. [DOI] [PubMed] [Google Scholar]
- [4].Elliott D, Davidson JE, Harvey MA, Bemis-Dougherty A, Hopkins RO, Iwashyna TJ, Wagner J, Weinert C, Wunsch H, Bienvenu OJ, and Black G, “Exploring the scope of post–intensive care syndrome therapy and care: Engagement of non–critical care providers and survivors in a second stakeholders meeting,” Crit. Care Med, vol. 42, no. 12, pp. 2518–2526, 2014. [DOI] [PubMed] [Google Scholar]
- [5].Davidson JE, Harvey MA, Bemis-Dougherty A, Smith JM, and Hopkins RO, “Implementation of the pain, agitation, and delirium clinical practice guidelines and promoting patient mobility to prevent post-intensive care syndrome,” Crit. Care Med, vol. 41, no. 9, pp. S136–S145, September. 2013. [DOI] [PubMed] [Google Scholar]
- [6].Needham DM, Feldman DR, and Kho ME, “The functional costs of ICU survivorship: Collaborating to improve post-ICU disability,” Amer. J. Respiratory Crit. Care Med, vol. 183, no. 8, pp. 962–964, 2011. [DOI] [PubMed] [Google Scholar]
- [7].Abercromby AFJ, Amonette WE, Layne CS, Mcfarlin BK, Hinman MR, and Paloski WH, “Variation in neuromuscular responses during acute whole-body vibration exercise,” Med. Sci. Sports Exerc, vol. 39, no. 9, pp. 1642–1650, September. 2007. [DOI] [PubMed] [Google Scholar]
- [8].Rittweger J, Mutschelknauss M, and Felsenberg D, “Acute changes in neuromuscular excitability after exhaustive whole body vibration exercise as compared to exhaustion by squatting exercise,” Clin. Physiol. Funct. Imag, vol. 23, no. 2, pp. 81–86, March. 2003. [DOI] [PubMed] [Google Scholar]
- [9].Marín PJ and Hazell TJ, “Effects of whole-body vibration with an unstable surface on muscle activation,” J. Musculoskelet Neuronal Interact, vol. 14, no. 2, pp. 213–219, 2014. [PubMed] [Google Scholar]
- [10].Morel DS, Marín PJ, Moreira-Marconi E, Dionello CF, and Bernardo-Filho M, “Can whole-body vibration exercises in different positions change muscular activity of upper limbs? A randomized trial,” Dose-Response, vol. 16, no. 4, October. 2018, Art. no. 155932581880436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oliveira MP, Menzel H-J-K, Cochrane DJ, Drummond MDM, Demicheli C, Lage G, and Couto BP, “Individual responses to different vibration frequencies identified by electromyography and dynamometry in different types of vibration application,” J. Strength Conditioning Res, March. 2019, doi: 10.1519/JSC.0000000000002985. [DOI] [PubMed] [Google Scholar]
- [12].Pollock RD, Woledge RC, Mills KR, Martin FC, and Newham DJ, “Muscle activity and acceleration during whole body vibration: Effect of frequency and amplitude,” Clin. Biomech, vol. 25, no. 8, pp. 840–846, October. 2010. [DOI] [PubMed] [Google Scholar]
- [13].Pollock RD, Woledge RC, Martin FC, and Newham DJ, “Effects of whole body vibration on motor unit recruitment and threshold,” J. Appl. Physiol, vol. 112, no. 3, pp. 388–395, February. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mischi M and Cardinale M, “The effects of a 28-Hz vibration on arm muscle activity during isometric exercise,” Med. Sci. Sports Exerc, vol. 41, no. 3, pp. 645–652, 2009. [DOI] [PubMed] [Google Scholar]
- [15].Miokovic T, Armbrecht G, Gast U, Rawer R, Roth HJ, Runge M, Felsenberg D, and Belavý DL, “Muscle atrophy, pain, and damage in bed rest reduced by resistive (vibration) exercise,” Med. Sci. Sports Exerc, vol. 46, no. 8, pp. 1506–1516, August. 2014. [DOI] [PubMed] [Google Scholar]
- [16].Delecluse C, Roelants M, and Verschueren S, “Strength increase after whole-body vibration compared with resistance training,” Med. Sci. Sports Exerc, vol. 35, no. 6, pp. 1033–1041, June. 2003. [DOI] [PubMed] [Google Scholar]
- [17].Hazell TJ, Jakobi JM, and Kenno KA, “The effects of whole-body vibration on upper-and lower-body EMG during static and dynamic contractions,” Appl. Physiol., Nutrition, Metabolism, vol. 32, no. 6, pp. 1156–1163, December. 2007. [DOI] [PubMed] [Google Scholar]
- [18].Boeselt T, Nell C, Kehr K, Holland A, Dresel M, Greulich T, Tackenberg B, Kenn K, Boeder J, Klapdor B, Kirschbaum A, Vogelmeier C, Alter P, and Koczulla R, “Whole-body vibration therapy in intensive care patients: A feasibility and safety study,” J. Rehabil. Med, vol. 48, no. 3, pp. 316–321, 2016. [DOI] [PubMed] [Google Scholar]
- [19].Marín PJ, García-Gutiérrez MT, Da Silva-Grigoletto ME, and Hazell TJ, “The addition of synchronous whole-body vibration to battling rope exercise increases skeletal muscle activity,” J. Musculoskelet Neuronal Interact, vol. 15, no. 3, p. 240, 2015. [PMC free article] [PubMed] [Google Scholar]
- [20].Rittweger J, Beller G, and Felsenberg D, “Acute physiological effects of exhaustive whole-body vibration exercise in man,” Clin. Physiol, vol. 20, no. 2, pp. 134–142, March. 2000. [DOI] [PubMed] [Google Scholar]
- [21].Rittweger J, Schiessl H, and Felsenberg D, “Oxygen uptake during whole-body vibration exercise: Comparison with squatting as a slow voluntary movement,” Eur. J. Appl. Physiol, vol. 86, no. 2, pp. 169–173, December. 2001. [DOI] [PubMed] [Google Scholar]
- [22].Rittweger J, Moss AD, Colier W, Stewart C, and Degens H, “Muscle tissue oxygenation and VEGF in VO2-matched vibration and squatting exercise,” Clin. Physiol. Funct. Imag, vol. 30, no. 4, pp. 269–278, May 2010. [DOI] [PubMed] [Google Scholar]
- [23].Sá-Caputo D, Paineiras-Domingos LL, Francisca-Santos A, dos Anjos EM, Reis AS, Neves MFT, Oigman W, Oliveira R, Brandão A, Machado CB, Chiementin X, Taiar R, Sartório A, and Bernardo-Filho M, “Whole-body vibration improves the functional parameters of individuals with metabolic syndrome: An exploratory study,” BMC Endocrine Disorders, vol. 19, no. 1, p. 6, December. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sousa-Gonçalves CR, Renata C, Tringali G, Tamini S, De Micheli R, Soranna D, Taiar R, Sá-Caputo D, Moreira-Marconi E, Paineiras-Domingos L, Bernardo-Filho M, and Sartorio A, “Acute effects of whole-body vibration alone or in combination with maximal voluntary contractions on cardiorespiratory, musculoskeletal, and neuromotor fitness in obese male adolescents,” Dose-Response, vol. 17, no. 4, pp. 1–7, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rittweger J, Ehrig J, Just K, Mutschelknauss M, Kirsch KA, and Felsenberg D, “Oxygen uptake in whole-body vibration exercise: Influence of vibration frequency, amplitude, and external load,” Int. J. Sports Med, vol. 23, no. 6, pp. 428–432, August. 2002. [DOI] [PubMed] [Google Scholar]
- [26].Friesenbichler B, Nigg BM, and Dunn JF, “Local metabolic rate during whole body vibration,” J. Appl. Physiol, vol. 114, no. 10, pp. 1421–1425, May 2013. [DOI] [PubMed] [Google Scholar]
- [27].Wollersheim T, Haas K, Wolf S, Mai K, Spies C, Steinhagen-Thiessen E, Wernecke K-D, Spranger J, and Weber-Carstens S, “Whole-body vibration to prevent intensive care unit-acquired weakness: Safety, feasibility, and metabolic response,” Crit. Care, vol. 21, no. 1, p. 9, December. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Milanese C, Cavedon V, Sandri M, Tam E, Piscitelli F, Boschi F, and Zancanaro C, “Metabolic effect of bodyweight whole-body vibration in a 20-min exercise session: A crossover study using verified vibration stimulus,” PLoS ONE, vol. 13, no. 1, January. 2018, Art. no. e0192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kang J, Porfido T, Ismaili C, Selamie S, Kuper J, Bush JA, Ratamess NA, and Faigenbaum AD, “Metabolic responses to whole- body vibration: Effect of frequency and amplitude,” Eur. J. Appl. Physiol, vol. 116, no. 9, pp. 1829–1839, September. 2016. [DOI] [PubMed] [Google Scholar]
- [30].Gojanovic B, Feihl F, Gremion G, and Waeber B, “Physiological response to whole-body vibration in athletes and sedentary subjects,” Physiol. Res, vol. 63, no. 6, pp. 92–779, 2014. [DOI] [PubMed] [Google Scholar]
- [31].Hazell TJ and Lemon PWR, “Synchronous whole-body vibration increases VO2 during and following acute exercise,” Eur. J. Appl. Physiol, vol. 112, no. 2, pp. 413–420, February. 2012. [DOI] [PubMed] [Google Scholar]
- [32].Yarar-Fisher C, Pascoe DD, Gladden LB, Quindry JC, Hudson J, and Sefton JE, “Acute physiological effects of whole body vibration (WBV) on central hemodynamics, muscle oxygenation and oxygen consumption in individuals with chronic spinal cord injury,” Disabil. Rehabil, vol. 36, no. 2, pp. 136–145, 2014. [DOI] [PubMed] [Google Scholar]
- [33].Zange J, Molitor S, Illbruck A, Müller K, Schönau E, Kohl-Bareis M, and Rittweger J, “In the unloaded lower leg, vibration extrudes venous blood out of the calf muscles probably by direct acceleration and without arterial vasodilation,” Eur. J. Appl. Physiol, vol. 114, no. 5, pp. 1005–1012, May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Çakar HI, Doğan S, Kara S, Rittweger J, Rawer R, and Zange J, “Vibration-related extrusion of capillary blood from the calf musculature depends upon directions of vibration of the leg and of the gravity vector,” Eur. J. Appl. Physiol, vol. 117, no. 6, pp. 1107–1117, June. 2017. [DOI] [PubMed] [Google Scholar]
- [35].Calvisi V, Angelozzi M, Franco A, Mottola L, Crisostomi S, Corsica C, Ferrari M, and Quaresima V, “Influence of whole-body vibration static exercise on quadriceps oxygenation,” in Oxygen Transport to Tissue XXVII (Advances in Experimental Medicine and Biology), vol. 578. Boston, MA, USA: Springer, 2006, pp. 137–141. [DOI] [PubMed] [Google Scholar]
- [36].Lythgo N, Eser P, de Groot P, and Galea M, “Whole-body vibration dosage alters leg blood flow,” Clin. Physiol. Funct. Imag, vol. 29, no. 1, pp. 53–59, January. 2009. [DOI] [PubMed] [Google Scholar]
- [37].Stewart JM, Karman C, Montgomery LD, and McLeod KJ, “Plantar vibration improves leg fluid flow in perimenopausal women,” Amer. J. Physiol.-Regulatory, Integrative Comparative Physiol, vol. 288, no. 3, pp. R623–R629, March. 2005. [DOI] [PubMed] [Google Scholar]
- [38].Coza A, Nigg BM, and Dunn JF, “Effects of vibrations on gastrocnemius medialis tissue oxygenation,” Med. Sci. Sports Exerc, vol. 43, no. 3, pp. 509–515, March. 2011. [DOI] [PubMed] [Google Scholar]
- [39].Games KE and Sefton JM, “Whole-body vibration influences lower extremity circulatory and neurological function,” Scandin. J. Med. Sci. Sports, vol. 23, no. 4, pp. 516–523, August. 2013. [DOI] [PubMed] [Google Scholar]
- [40].Herrero AJ, Menendez H, Gil L, Martin J, Martin T, Garcia-Lopez D, Gil-Agudo A, and Marin PJ, “Effects of whole-body vibration on blood flow and neuromuscular activity in spinal cord injury,” Spinal Cord, vol. 49, no. 4, pp. 554–559, 2011. [DOI] [PubMed] [Google Scholar]
- [41].Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, and Judex S, “Low-level mechanical vibrations can influ- ence bone resorption and bone formation in the growing skeleton,” Bone, vol. 39, no. 5, pp. 1059–1066, November. 2006. [DOI] [PubMed] [Google Scholar]
- [42].Rittweger J, Beller G, Armbrecht G, Mulder E, Buehring B, Gast U, Dimeo F, Schubert H, de Haan A, Stegeman DF, Schiessl H, and Felsenberg D, “Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise,” Bone, vol. 46, no. 1, pp. 137–147, January. 2010. [DOI] [PubMed] [Google Scholar]
- [43].Armbrecht G, Belavý DL, Gast U, Bongrazio M, Touby F, Beller G, Roth HJ, Perschel FH, Rittweger J, and Felsenberg D, “Resistive vibration exercise attenuates bone and muscle atrophy in 56 days of bed rest: Biochemical markers of bone metabolism,” Osteoporosis Int., vol. 21, no. 4, pp. 597–607, April. 2010. [DOI] [PubMed] [Google Scholar]
- [44].Lai C-L, Tseng S-Y, Chen C-N, Liao W-C, Wang C-H, Lee M-C, and Hsu P-S, “Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: A randomized controlled trial,” Clin. Intervent. Aging, vol. 8, pp. 1603–1609, December. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Von Stengel S, Kemmler W, Bebenek M, Engelke K, and Kalender WA, “Effects of whole-body vibration training on different devices on bone mineral density,” Med. Sci. Sports Exerc, vol. 43, no. 6, pp. 1071–1079, June. 2011. [DOI] [PubMed] [Google Scholar]
- [46].Zaidell LN, Mileva KN, Sumners DP, and Bowtell JL, “Experimental evidence of the tonic vibration reflex during whole-body vibration of the loaded and unloaded leg,” PLoS ONE, vol. 8, no. 12, December. 2013, Art. no. e85247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cochrane DJ, Stannard SR, Firth EC, and Rittweger J, “Acute whole-body vibration elicits post-activation potentiation,” Eur. J. Appl. Physiol, vol. 108, no. 2, pp. 311–319, January. 2010. [DOI] [PubMed] [Google Scholar]
- [48].Mansfield NJ and Griffin MJ, “Non-linearities in apparent mass and transmissibility during exposure to whole-body vertical vibration,” J. Biomech, vol. 33, no. 8, pp. 933–941, August. 2000. [DOI] [PubMed] [Google Scholar]
- [49].Hermens HJ, Freriks B, Disselhorst-Klug C, and Rau G, “Development of recommendations for SEMG sensors and sensor placement procedures,” J. Electromyogr. Kinesiol, vol. 10, no. 5, pp. 361–374, October. 2000. [DOI] [PubMed] [Google Scholar]
- [50].Lienhard K, Cabasson A, Meste O, and Colson SS, “Comparison of sEMG processing methods during whole-body vibration exercise,” J. Electromyogr. Kinesiol, vol. 25, no. 6, pp. 833–840, December. 2015. [DOI] [PubMed] [Google Scholar]
- [51].Podstawski R, Borysławski K, Laukkanen JA, Clark CCT, and Choszcz D, “The effect of prolonged thermal stress on the physiological parameters of young, sedentary men and the correlations with somatic features and body composition parameters,” HOMO, vol. 70, no. 2, pp. 119–128, October. 2019. [DOI] [PubMed] [Google Scholar]
- [52].Cochrane DJ, Stannard SR, Firth EC, and Rittweger J, “Comparing muscle temperature during static and dynamic squatting with and with- out whole-body vibration,” Clin. Physiol. Funct. Imag, vol. 30, no. 4, pp. 223–229, May 2010. [DOI] [PubMed] [Google Scholar]
- [53].Cochrane DJ, Stannard SR, Sargeant AJ, and Rittweger J, “The rate of muscle temperature increase during acute whole-body vibration exercise,” Eur. J. Appl. Physiol, vol. 103, no. 4, pp. 441–448, July. 2008. [DOI] [PubMed] [Google Scholar]
- [54].Cardinale M, Ferrari M, and Quaresima V, “Gastrocnemius medialis and vastus lateralis oxygenation during whole-body vibration exercise,” Med. Sci. Sports Exerc, vol. 39, no. 4, pp. 694–700, April. 2007. [DOI] [PubMed] [Google Scholar]
- [55].Yamada E, Kusaka T, Miyamoto K, Tanaka S, Morita S, Tanaka S, Tsuji S, Mori S, Norimatsu H, and Itoh S, “Vastus lateralis oxygenation and blood volume measured by near-infrared spectroscopy during whole body vibration,” Clin. Physiol. Funct. Imag, vol. 25, no. 4, pp. 203–208, July. 2005. [DOI] [PubMed] [Google Scholar]