ABSTRACT
To protect against water loss, land plants have developed the cuticle; however, the cuticle strongly restricts CO2 uptake for photosynthesis. Controlling this trade-off relationship is an important strategy for plant survival, but the extent to which the changes in cuticle affects this relationship is not clear. To evaluate this, we measured CO2 assimilation rate and transpiration rate together in the Arabidopsis thaliana mutant excessive transpiration1 (extra1), which exhibited marked evaporative water loss due to an increased cuticle permeability caused by a new allele of ACETYL-COA CARBOXYLASE 1 (ACC1). Under high humidity (85%) conditions, the extra1 mutant exhibited higher CO2 assimilation rate in exchange for decreasing water use efficiency by one-third compared to the slow anion channel-associated 1 (slac1) mutant, whose stomata are continuously open. Our results indicate that the increased cuticle permeability in extra1 affects transpiration rate more than CO2 assimilation rate, but the effect on CO2 assimilation rate is larger than the effect of open stomata in slac1, suggesting that the cuticle permeability is an important parameter for the trade-off relationship between drought tolerance and CO2 uptake in land plants.
KEYWORDS: carbon dioxide, cuticle, stomata, Arabidopsis, water use efficiency
Land plants absorb carbon dioxide (CO2) for photosynthesis from the atmosphere; however, CO2 uptake from the air poses a risk of evaporative water loss to plants. Excessive water loss is fatal to plants, and therefore, plants cover their aerial epidermis with a thick waxy layer, called cuticle, and access atmospheric CO2 mostly through stomatal pores, which make up only about 2% per leaf area.1 The cuticle limits transpiration through plant surfaces other than through the stomatal pores to less than 10% of the total,2 in exchange for strongly restricted CO2 uptake efficiency. The trade-off between drought tolerance and CO2 uptake efficiency by developing cuticles is an essential strategy for plant survival.
Cuticle limits gas exchange but does not completely shut it off. A previous study reported that the cuticle increasingly affects gas exchange as stomata close.3 However, although there are many reports on the extent of the relationship between cuticle characteristics and water loss, much less attention has been paid to the relationship between the cuticle and CO2 uptake efficiency, despite its substantial role in photosynthesis. One reason is that CO2 uptake seems to be more strictly restricted than emission of water vapor in gas exchange through cuticle, due to the differences in molecular size and diffusion paths between CO2 and H2O. Studies on cuticle conductances in grape (Vitis vinifera) and sunflower (Helianthus annuus)3,4 reported that the cuticle and epidermis transport 20–40 times more water than CO2; the ratio is vastly different from 1.6, the ratio of the stomatal conductances for H2O and CO2. However, our recent study revealed that increased cuticle permeability strongly enhances CO2 uptake efficiency under non-drought stress conditions.5 The Arabidopsis thaliana excessive transpiration1 (extra1) mutant, exhibiting remarkable transpirational water loss due to an increased cuticle permeability (Figure 1(a, b)) caused by a new allele of ACETYL-COA CARBOXYLASE 1 (ACC1), demonstrated a higher CO2 uptake efficiency (e.g., CO2 assimilation rate) than did the wild-type (WT) plant and the stomatal mutant slow anion channel-associated 1 (slac1),6,7 whose stomata are continuously open. However, the study does not address the precise relationship between CO2 uptake and water loss in extra1, hence, the present study investigated this relationship between CO2 uptake and water loss through the permeable cuticle in extra1.
Figure 1.
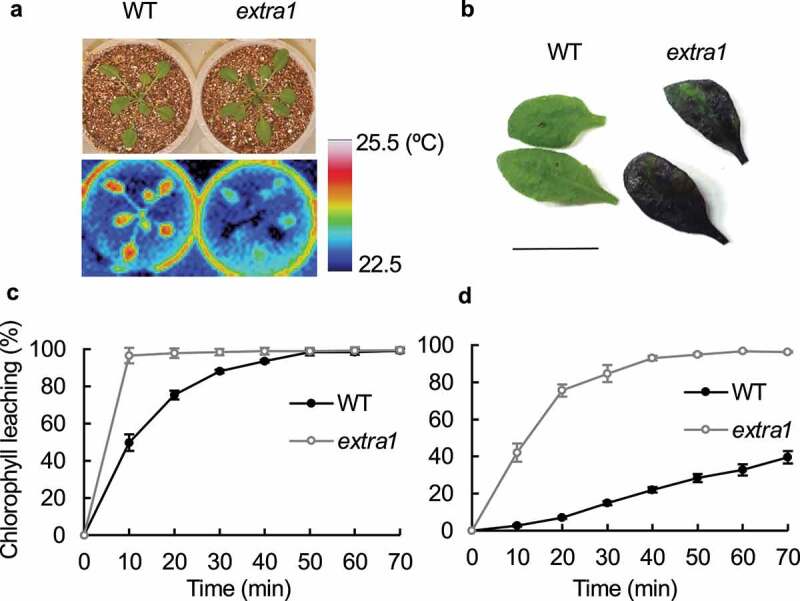
The cuticle mutant extra1 has an extremely permeable cuticle. (a) Thermal imaging of wild type (WT) of Col-0 and extra1. Compared to WT, extra1 exhibited significantly low leaf temperature, indicating excessive transpiration. (b) Toluidine blue staining. The WT plant with normal cuticle was not stained by immersing in 0.1% (w/v) toluidine blue for 5 minutes, whereas extra1 was well stained. Scale bar = 1 cm. (c, d) Chlorophyll leaching analysis. Plants were grown in petri dishes at saturated water vapor conditions for 3 weeks (c) or on pot in a growth chamber at 60% relative humidity for a week after this 3-week growth (d). Chlorophyll efflux at each time point was expressed as a percentage of total chlorophyll extracted after 80 minutes (c) or 30 hours (d) in 80% (v/v) ethanol. Data presented are means ± SE (n = 4)
First, the cuticle permeability in extra1 was quantified by chlorophyll leaching (Figure 1(c, d)). When plants were grown at saturated water vapor conditions, the chlorophyll in the WT leaves almost completely leaked 50 minutes after immersion in 80% (v/v) ethanol, whereas that in extra1, it leaked within 10 minutes (Figure 1c). In short, the extraction rate of chlorophyll in extra1 was over 5 times higher than that of WT. This difference in chlorophyll leaching rate is larger when plants were exposed at a little drier condition (60% relative humidity, RH) that induces cuticular modifications (Figure 1d). We then investigated the extent to which this permeable cuticle of extra1 affects the relationship between CO2 assimilation rate and transpiration rate (Figure 2). Under 85% RH, which is not saturated but a high water vapor condition, the extra1 mutant exhibited 1.47 times higher CO2 assimilation rate and 2.95 times higher transpiration rate than WT. The water use efficiency (WUE), calculated by the CO2 assimilation rate and transpiration rate, was 52.6% in extra1 compared to that of WT. On the other hand, the slac1-2 mutant exhibited 1.23 times higher CO2 assimilation rate and 1.59 times higher transpiration rate, and 80.6% WUE compared to that of WT. In other words, the permeable cuticle in extra1 decreases WUE by one-third but increases CO2 assimilation rate by 1.2 times, compared to the continuously open stomata in slac1-2. We conclude that while the effect of changes in cuticle permeability on CO2 assimilation rate is smaller than on transpiration rate as previous study reported,3,4 the effect on CO2 assimilation rate is too large to ignore. There is a concern that the transpiration rates are low at 85% RH condition; however, the ratio of transpiration rate among extra1, slac1-2, and WT appears not to be markedly different from the ratio of transpiration rate measured in our previous study (at 40% RH condition).5 We also have to consider the difference in plant species having various cuticle characteristics8-11 and the effects of drought stress caused by a permeable cuticle on CO2 assimilation.12–15 However, our results suggest that not only stomatal characteristics but also cuticle permeability is an important parameter for the trade-off strategy between drought tolerance and CO2 uptake in land plants. Generally, increasing WUE improves drought tolerance. However, the previous report of low WUE and high drought tolerance of shrubs suggests that maintaining high WUE under competitive water-limited conditions may not be advantageous.16 Moreover, there is a negative correlation between WUE and photosynthetic nitrogen use efficiency.16–18 Further studies exploring the effects of changing cuticle permeability under varying environmental conditions could allow us to gain better insights into this relationship and could possibly facilitate extending such insights to regular agricultural production.
Figure 2.

The extra1 mutant exhibited higher CO2 assimilation rate in exchange for decreasing water use efficiency (WUE) by about half compared to wild-type (WT). The CO2 assimilation rate (a), transpiration rate (b), and WUE (c) in WT, slac1-2, extra1 are shown. Plants were grown under the same conditions in Fig. 1d. The CO2 concentration (400 μL L−1), light intensity (30 μmol m−2 s−1), and humidity condition (85% relative humidity) were kept constant throughout the measurements. Data presented are means ± SE (n ≥ 16). Asterisks indicate statistical significance by Dunnett’s test (*P < 0.05)
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana wild-type accessions Columbia (Col-0) and the slac1-2 mutant were obtained from our laboratory stock. The extra1 mutant was isolated in our recent study.5 The plants of extra1 and slac1-2 were derived from the Col-0 ecotype. The Arabidopsis plants were sowed and grown on Murashige and Skoog (MS) medium as described previously.5 Plants were grown in a growth chamber at 22°C, 60% RH, and at continuous light condition with a light intensity of 30 μmol m−2 s−1. The seedlings at 13 d after sowing (DAS) were transplanted into a new MS medium plate, and the plants at 21–22 DAS were used for the chlorophyll leaching analysis (Figure 1c). For other measurements, the 3-week-old seedlings were transplanted into pots filled with a mixture of vermiculite and perlite supplemented with mineral nutrients. Plants at 24 DAS were used for the thermal imaging (Figure 1a) and toluidine blue staining (Figure 1b), and 4-week-old plants were used for the analyses of chlorophyll leaching (Figure 1d) and the rates of CO2 assimilation and transpiration (Figure 2).
Thermal imaging
The analysis was performed as previously described.5 Plants were incubated under the conditions of constant white light of 100 μmol m−2 s−1 at 22°C, 60% RH, and 400 μL L−1 [CO2] for 2 hours and the thermal image was captured.
Toluidine blue staining
Toluidine blue staining was performed exactly as previously described.5 After 5 minutes of staining by 0.1% (w/v) toluidine blue, mature rosette leaves were rinsed with water and then photographed on a filter paper.
Measurement of chlorophyll leaching
Chlorophyll leaching assays were performed as described in Lolle et al. (1997) and Lü et al. (2011).19,20 Approximately 100 mg of leaves per assay was weighed and immersed in tubes containing 30 ml of 80% ethanol at room temperature. Tubes were incubated in the dark with gentle agitation, 200 μL aliquots were taken at the indicated times and the absorbance was measured at 664 and 647 nm by using UV-2400PC (Shimadzu). The concentrations of total chlorophyll per fresh weight of leaf tissue were calculated using the following formula:19 total micromoles chlorophyll = 7.93 (A664) + 19.53 (A647). Chlorophyll efflux at each time point was expressed as a percentage of total chlorophyll extracted after 80 minutes (Figure 1c) or 30 hours (Figure 1d).
Measurements of CO2 assimilation rate and transpiration rate
The CO2 assimilation rate and transpiration rate were measured simultaneously using a portable gas-exchange fluorescence system (GFS-3000; Heinz Walz). One mature leaf was used per measurement. To avoid drought and light stress, the measurements were performed under a high humidity condition (85% RH) and a weak light intensity (30 μmol m−2 s−1). The cuvette temperature (22°C), flow rate (750 μmol s−1), and CO2 concentration (400 μL L−1) were kept constant throughout the gas-exchange experiments. The values measured by GFS-3000 was per unit of leaf area.
Funding Statement
This work was supported by JSPS KAKENHI, Japan (grant nos. JP20K15820 to K.M., JP18K06293 to J.N., and JP20H03279 to K.I.) and by JST CREST, Japan (grant no. JPMJCR15O5 to K.I.), and by Kyushu University Qdai-jump Research Program, Japan (grant no. 02306 to A.M.).
Author contributions
K.M. and K.I. designed the studies; K.M. performed most of the experiments with A.M. and J.N.; K.M. wrote the article with contributions from all the authors.
References
- 1.Willmer C, Fricker M.. Stomata. 2nd. Berlin (Germany): Springer Science & Business Media; 1996. [Google Scholar]
- 2.Mohr H, Schopfer P.. Physiology of xylem transport. In: Mohr H, Schopfer P, editors. Plant physiology. Berlin (Heidelberg, Germany): Springer; 1995. p. 1–4. [Google Scholar]
- 3.Boyer JS. Turgor and the transport of CO2 and water across the cuticle (epidermis) of leaves. J Exp Bot. 2015;66(9):2625–2633. doi: 10.1093/jxb/erv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer JS, Chin Wong S, Farquhar GD. CO2 and water vapor exchange across leaf cuticle (Epidermis) at various water potentials. Plant Physiol. 1997;114:185–191. doi: 10.1104/pp.114.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monda K, Mabuchi A, Takahashi S, Negi J, Tohmori R, Terashima I, Yamori W, Iba K. Increased cuticle permeability caused by a New Allele of ACETYL COA CARBOXYLASE1 enhances CO 2 Uptake. Plant Physiol. 2020;184(4):1917–1926. doi: 10.1104/pp.20.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- 7.Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bengtson C, Larsson S, Liljenberg C. Effects of water stress on cuticular transpiration rate and amount and composition of epicuticular wax in seedlings of six oat varieties. Physiol Plant. 1978;44:319–324. doi: 10.1111/j.1399-3054.1978.tb01630.x. [DOI] [Google Scholar]
- 9.O’Toole JC, Cruz RT, Seiber JN, Wax E. Cuticular resistance in rice. Physiol Plant. 1979;47:239–244. doi: 10.1111/j.1399-3054.1979.tb06520.x. [DOI] [Google Scholar]
- 10.Kerstiens G. Cuticular water permeability and its physiological significance. J Exp Bot. 1996;47:1813–1832. doi: 10.1093/jxb/47.12.1813. [DOI] [Google Scholar]
- 11.Lee SB, Suh MC. Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Rep. 2015;34:557–572. doi: 10.1007/s00299-015-1772-2. [DOI] [PubMed] [Google Scholar]
- 12.Lawlor DW, Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell Environ. 2002;25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- 13.Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashraf M, Harris PJC. Photosynthesis under stressful environments: an overview. Photosynthetica. 2013;51:163–190. doi: 10.1007/s11099-013-0021-6. [DOI] [Google Scholar]
- 16.DeLucia EJ, Schlesinger WH. Resource-use efficiency and drought tolerance in adjacent great basin and sierran plants. Ecology. 1991;72:51–58. doi: 10.2307/1938901. [DOI] [Google Scholar]
- 17.Field C, Merino J, Mooney HA. Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia. 1983;60(3):384–389. doi: 10.1007/BF00376856. [DOI] [PubMed] [Google Scholar]
- 18.Limousin JM, Yepez EA, Mcdowell NG, Pockman WT, Tjoelker M. Convergence in resource use efficiency across trees with differing hydraulic strategies in response to ecosystem precipitation manipulation. Funct Ecol. 2015;29:1125–1136. doi: 10.1111/1365-2435.12426. [DOI] [Google Scholar]
- 19.Lolle SJ, Berlyn GP, Engstrom EM, Krolikowski KA, Reiter W-D, Pruitt RE. Developmental regulation of cell interactions in the Arabidopsis fiddlehead-1mutant: a role for the epidermal cell wall and cuticle. Dev Biol. 1997;189(2):311–321. doi: 10.1006/dbio.1997.8671. [DOI] [PubMed] [Google Scholar]
- 20.Lü S, Zhao H, Parsons EP, Xu C, Kosma DK, Xu X, Chao D, Lohrey G, Bangarusamy DK, Wang G, et al. The glossyhead1 Allele of ACC1 reveals a principal role for multidomain acetyl-coenzyme A carboxylase in the biosynthesis of cuticular waxes by arabidopsis. Plant Physiol. 2011;157:1079–1092. doi: 10.1104/pp.111.185132. [DOI] [PMC free article] [PubMed] [Google Scholar]


