Abstract
Background
Andexanet alfa (andexanet) and prothrombin complex concentrate (PCC) are both reversal agents for major bleeding in patients using factor Xa inhibitors (FXaIs). Our aim was to evaluate the current evidence for the effectiveness and safety of andexanet and PCC in a systematic review and meta‐analysis.
Objectives
Primary objective was hemostatic effectiveness. Secondary objectives were thromboembolic event rate and mortality.
Methods
A systematic review was performed in PubMed and Embase. Studies describing the effectiveness and/or safety of PCC or andexanet in patients with major bleeding using FXaIs were included. Meta‐analysis was performed using a random‐effects model.
Results
Seventeen PCC studies, 3 andexanet studies, and 1 study describing PCC and andexanet were included, comprising 1428 PCC‐treated and 396 andexanet‐treated patients. None of the included studies had control groups, hampering a pooled meta‐analysis to compare the two reversal agents. Separate analyses for andexanet and PCC were performed. In subgroup analysis, the pooled proportion of patients with effective hemostasis in studies that used Annexa‐4 criteria demonstrated a hemostatic effectiveness of 0.85 (95% confidence interval [CI], 0.80‐0.90) in PCC and 0.82 (95% CI, 0.78‐0.87) in andexanet studies. The pooled proportion of patients with thromboembolic events was 0.03 (95% CI, 0.02‐0.04) in PCC and 0.11 (95% CI, 0.04‐0.18) in andexanet studies.
Conclusion
Based on the available evidence with low certainty from observational studies, PCC and andexanet demonstrated a similar, effective hemostasis in the treatment of major bleeding in patients using FXaIs. Compared to PCC, the thromboembolic event rate appeared higher in andexanet‐treated patients.
Essentials.
Andexanet alfa and prothrombin complex concentrate (PCC) are both used for major bleeding.
We evaluated the current evidence for the effectiveness and safety of andexanet alfa and PCC.
No direct comparisons of both reversal agents were available.
Both reversal agents demonstrated good hemostatic effectiveness.
1. INTRODUCTION
Since the introduction of factor Xa inhibitors (FXaIs; rivaroxaban in 2008, apixaban in 2011, and edoxaban in 2015), there has been an urgent need for a reversal agent with proven effectiveness and safety. Although not authorized for this indication, extensive experience has been gained with prothrombin complex concentrate (PCC), both in real life and in clinical trials. Therefore, current guidelines recommend PCC as a therapeutic option in the reversal of FXaI‐related major bleeding, based on expert opinion.1, 2, 3, 4, 5
PCC is known to provide a rapid and complete reversal of vitamin K antagonist (VKA)‐induced coagulopathy and is recommended in current guidelines for VKA‐associated major bleeding.1, 5, 6, 7, 8 Its safety has been studied extensively. A meta‐analysis reported a thromboembolic event rate of 1.9% in patients with VKA coagulopathy treated with PCC. 9 In 2019, a systematic review and meta‐analysis was published concerning the effectiveness and safety of PCC in treating FXaI‐related major bleeding. 10 The pooled proportion of patients with hemostatic effectiveness using the ISTH criteria was 0.69 (95% CI, 0.61‐0.76), based on two studies involving 150 patients.10, 11 The pooled proportion of patients with hemostatic effectiveness determined by non‐ISTH criteria was 0.77 (95% CI, 0.63‐0.92), based on eight studies involving 190 patients. 10
Recently, andexanet alfa (andexanet) was registered by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the indication “neutralization of the anticoagulant activity of apixaban and rivaroxaban in life‐threatening or uncontrolled bleeding.” This was done by an accelerated procedure based on the registration study because no alternatives for this life‐threatening indication were registered.12, 13 The registration study of andexanet demonstrated a hemostatic effectiveness in 208 of 254 (82%) patients.12, 13 However, andexanet is not widely available due to its high costs, which form an important obstacle to adding it to the hospital formulary.
Since the registration of andexanet, numerous observational PCC and andexanet studies were published that have further explored their effectiveness and/or safety. Up to now, no meta‐analyses are available on the effectiveness and safety of andexanet and PCC for the stated indication. Therefore, the aim of this meta‐analysis was to evaluate the current evidence of the effectiveness and safety of andexanet and PCC in the treatment of FXaI‐related major bleeding.
2. METHODS
2.1. Search strategy
The databases of PubMed and Embase were systematically searched, with articles included up to June 26, 2020. The search strategy can be found in Appendix S1. Inclusion criteria were cohort studies and randomized controlled trials examining the effectiveness and/or safety of andexanet or PCC.
2.2. Study selection
All studies examining hemostatic effectiveness, thromboembolic events, and/or mortality of andexanet or PCC for the indication major bleeding in patients treated with oral FXaIs were included. As randomized controlled trials on this indication are challenging and therefore could be scarce or absent, observational studies could also be included. Studies with a sample size ≤10 patients were excluded, as well as studies from which outcome measures could not be extracted for the subgroup of patients with major bleeding (in studies that also included perioperative use of PCC/andexanet). Two authors (TJ and KS) independently reviewed the title, abstract, and full text of the articles. Disputes were resolved by joint review and consensus. If consensus was not reached, a third author (NK) made the decision whether to include articles.
2.3. Data extraction
Data of the final set of articles were extracted by TJ and independently checked by KS. Extracted data of interest were study characteristics such as number of patients, study design, inclusion and exclusion criteria, follow‐up, and outcomes of interest. For the definition of major bleeding, it was verified whether standardized criteria were used.14, 15
2.4. Risk of bias and study quality assessment
Two authors (TJ and KS) assessed the quality of the observational studies according to two methods. The methodological index for nonrandomized studies (MINORS) method was developed and validated to assess the methodological quality of nonrandomized studies on a 16‐point scale. 16
The risk of bias assessment tool for nonrandomized studies (RoBANS) method was developed and validated to determine the bias risk for nonrandomized studies. 17 According to the RoBANS method, the bias risk was scored in six different domains: patient selection, risk of confounding, intervention measurement, blinding of assessors, incomplete outcome data, and selective outcome reporting. For each domain, the risk of bias was scored as low, medium, or high. The overall bias risk of each study was classified by showing the mean score as the final score. If the mean score was between two classifications, both classifications were mentioned. Important criteria for the quality assessment were, among others, the inclusion of consecutive patients, usage of standardized criteria to define a major bleeding, prospective collection of data, unbiased evaluation of end points, usage of ISTH criteria to define hemostatic effectiveness, an appropriate follow‐up period of ≥14 days, and a small loss to follow‐up of ≤5%.
2.5. Outcome measures
The primary outcome was hemostatic effectiveness as assessed in the included studies. The safety parameters thromboembolic events and mortality were reviewed as secondary outcomes. Thromboembolic events included deep vein thrombosis, pulmonary embolism, myocardial infarction, and/or stroke. Mortality was defined as death from any cause.
2.6. Meta‐analysis
Because no direct comparative studies were found, the safety and effectiveness of andexanet and PCC could not be compared directly by meta‐analysis. Therefore, single‐arm event rates on hemostatic effectiveness, thromboembolic events and mortality were pooled, using the DerSimonian‐Laird random effects model.
Crude pooled proportions with 95% confidence intervals (CIs) of patients with the outcomes were calculated. Forest plots were used to display the results graphically. Subgroup analysis was performed on the used criteria for hemostatic effectiveness: ISTH, Annexa‐4, and other nonstandardized criteria. Heterogeneity among studies was assessed with the I2 statistic. Interpretation of I2 of 30% to 60% may represent moderate heterogeneity, and substantial heterogeneity was defined as I2 > 60%. 18 All analyses were performed with Jamovi (version 1.2, Sydney, Australia).
The Preferred Reporting Items for Systematic Reviews and Meta‐Analysis guideline was followed. The review protocol of this meta‐analysis was not registered prospectively in an international database.
3. RESULTS
3.1. Study selection
Using the search criteria, a total of 551 articles were retrieved. After screening, 21 studies were included in the systematic review. The study selection is provided in Figure 1.
FIGURE 1.
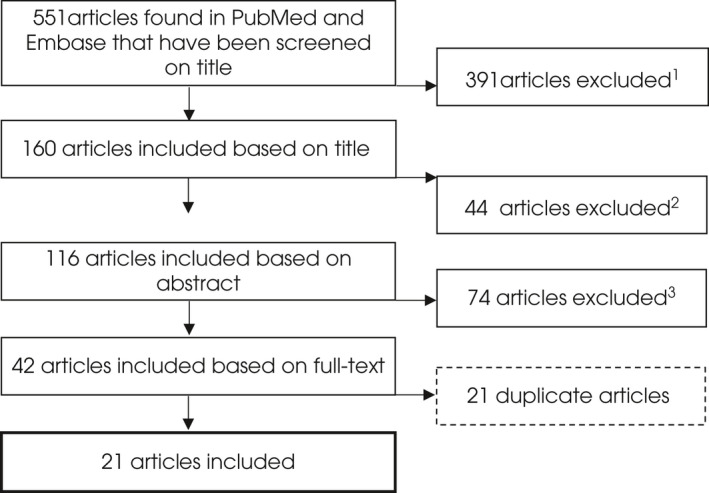
Study selection. 1 Reason for exclusion: updates/reviews: 132, editorial/expert opinion: 50, ex vivo/in vitro study: 44, perioperative usage: 35, no major bleeding in FXa‐I users: 30, no PCC or and exanet used: 25, study in healthy volunteers: 19, case reports: 18, guideline: 18, study in animals: 12, cost‐efficacy analysis: 4, erratum: 2, survey: 1, preliminary results: 1. 2 Reason for exclusion: review: 26, no major bleeding in FXa‐I users: 7, ex vivo/in vitro study: 3, healthy volunteers: 2, no relevant outcome measures: 2, preliminary results: 1, no PCC or andexanet used: 1, ≤10 patients: 1, survey: 1. 3 Reason for exclusion: Outcome measures could not be extracted for the study population ‘major bleeding while using FXa‐I, treated with andexanet or PCC’: 27, review: 17, editorial/expert opinion: 11, case reports: 6, ≤10 patients: 5, no major bleeding in FXa‐I users: 5, perioperative usage: 2, in vitro study: 1
3.2. Study characteristics
Seventeen PCC studies, 3 andexanet studies and 1 study describing PCC and andexanet both were included, comprising 1428 PCC‐treated patients and 396 andexanet‐treated patients. No direct comparative studies were found, and none of the studies had control groups. Only 4 PCC studies and 1 andexanet study had a prospective design. Of the PCC studies, 7 had a follow‐up until discharge, and 10 had a follow‐up of ≥30 days. All the andexanet studies had a 30‐day follow‐up. One article (Barra et al 35 ) studied both PCC and andexanet in an observational, retrospective study design with a follow‐up of 30 days. Study details are included in Table 1.
TABLE 1.
Baseline characteristics of the studies included
| Ref. | Treatment | Dose | Study design | No. of FXaI patients | Type of bleeding, % | Type of FxaI, % | Age, y, mean (SD) or median [IQR] | Additional inclusion and exclusion criteria | Definition MBE | Follow‐up |
|---|---|---|---|---|---|---|---|---|---|---|
| Grandhi 19 | PCC | 25‐50 U/kg | Retrospective, observational, single‐center |
18 |
100.0: ICH |
88.9: rivaroxaban 11.1: apixaban |
79.7 (10.9) |
Inclusion: only patients with an ICH Exclusion: use of plasma |
ISTH | Until discharge |
| Majeed 20 | PCC | 25 U/kg |
Prospective, observational, multicenter |
84 |
70.2: ICH 15.5: GIH 14.3: other |
53.6: rivaroxaban 46.4: apixaban |
75.0 [70.0–83.0] | Exclusion: use of other hemostatics (plasma, platelets, aPCC, or factor VIIa), ACS or CVA in the past 30 d, Hb decline without focus, and a do not resuscitate policy | ISTH | 30 d |
| Gerner 21 | PCC | 25‐50 U/kg | Retrospective, observational, multicenter | 94 | 100.0: ICH |
86.2: rivaroxaban 13.8: apixaban |
77.5 (7.6) |
Inclusion: only patients with an ICH Exclusion: ICH related to trauma, tumor, arteriovenous malformation, aneurysmal subarachnoid hemorrhage, acute thrombolysis, or other coagulopathies |
ISTH | 3 months |
| Schulman 22 | PCC |
±25 U/kg (2000 U fixed dose) |
Prospective, observational, multicenter |
66 |
55.0: ICH 24.0: GIH 21.0: other |
56.1: rivaroxaban 43.9: apixaban |
76.9 (10.4) | Exclusion: use of other hemostatics (plasma, platelets, aPCC or factor VIIa), ACS or CVA in past 30 d, Hb decrease without focus and a do not resuscitate policy | ISTH | 30 d |
| Harrison 23 | PCC | 25‐50 U/kg | Retrospective, observational, multicenter | 14 | 100.0: ICH | NR | 74 (8) |
Inclusion: only patients with an ICH Exclusion: usage of fresh frozen plasma |
ISTH | Unknown |
| Testa 24 | PCC | NR | Prospective, observational, multicenter |
27 |
45.3: ICH 35.9: GIH 18.8: other |
74.1: rivaroxaban 25.9: apixaban |
79 [74–85] | NA | ISTH |
Until discharge and after six months |
| Allison 25 | PCC | 35 U/kg | Retrospective, observational, single‐center | 33 |
90.9 ICH 6.1: GIH 3.0 other |
81.8: rivaroxaban 18.2: apixaban |
73 (14.8) | Exclusion: pregnant and incarcerated patients | A life‐threatening or potentially life‐threatening bleed requiring emergency surgery or invasive procedure, acute bleeding associated with a fall in Hb ≥2 g/dL within 48 h, and bleeding requiring blood product transfusion | Until discharge |
| Sheikh‐Taha (PCC) 26 | PCC | 50 U/kg | Retrospective, observational, single‐center |
29 |
72.4: ICH 13.8: GIH 13.8: other |
55.2: rivaroxaban 44.8: apixaban |
73.8 (12.0) | NA | ISTH | Until discharge |
| Arachchillage 27 | PCC | 25 U/kg | Retrospective, observational, single‐center |
80 |
57.5: ICH 30.0: GIH 12.5: other |
50.0: rivaroxaban 50.0: apixaban |
76.3 (7.9) | NA | MBE was related to receiving PCC; per protocol, only patients with a MBE (defined by ISTH) were eligible for PCC | 30 d |
| Stevens 28 | Andexanet | PP | Retrospective, observational, single‐center |
13 |
46.2: ICH 53.8: other |
30.8: rivaroxaban 69.2: apixaban |
69 (10) | NA | A potentially life‐threatening bleeding with signs of hemodynamic instability, bleeding with Hb drop ≥2 g/dl (or Hb ≤8 g/dl if no baseline), or bleeding in a critical area or organ (retroperitoneal, intra‐articular, pericardial, epidural, intracranial, or intramuscular with compartment syndrome) |
30 d |
| Dybdahl 29 | PCC | 50 U/kg | Retrospective, observational, multicenter |
35 |
100.0: ICH |
51.4: rivaroxaban 48.6: apixaban |
78.9 (8.9) | Inclusion: only patients with an ICH | ISTH | Until discharge |
| Connolly 12 | Andexanet | PP, which changed during the study period | Prospective, observational, multicenter |
352 for safety analysis 254 for effectiveness analysis |
Safety analysis: 64.5: ICH 25.6: GIH 9.9: other Efficacy analysis: 67.3: ICH 24.4: GIH 8.3: other |
Safety analysis: 36.4: rivaroxaban 55.1: apixaban 2.8: edoxaban Efficacy analysis: 39.4: rivaroxaban 52.8: apixaban 1.6: edoxaban |
Safety analysis: 77.4 (10.8) Efficacy analysis: 77.1 (11.1) |
Exclusion: ICH patients with a GCS score ≤7 or a hematoma volume of ≥60 mL. Patients with scheduled surgery within 12 h of andexanet, life expectancy <1 mo, thrombotic event in the past 14 d. Patients who have received vitamin K antagonists, dabigatran, PCC, factor VIIa, blood or plasma Additional exclusion criteria for the efficacy analysis: patients with an anti–factor Xa activity <75 ng/mL or patients without a confirmed MBE |
A potentially life‐threatening bleeding with signs of hemodynamic instability, bleeding with Hb drop ≥2 g/dl (or Hb ≤8 g/dl if no baseline), or bleeding in a critical area or organ (retroperitoneal, intra‐articular, pericardial, epidural, intracranial, or intramuscular with compartment syndrome) | 30 d |
| Smith 30 | PCC | 25 – 50 U/kg | Retrospective, observational, single‐center |
31 |
58.1: ICH 3.2: GIH 38.6: other |
45.2: rivaroxaban 54.8: apixaban |
74 [69–84] | Exclusion: ICH and GCS score ≤7 or ACS or CVA in the past 30 d | Bleed in a critical location, a life‐threatening bleed that requires surgery or an invasive procedure, or a bleed that requires a blood transfusion | Until discharge |
| Sheikh‐Taha (aPCC) 31 | PCC | 25 −50 U/kg | Retrospective, observational, single‐center |
35 |
51.4: ICH 28.6: GIH 20.0: other |
42.9: rivaroxaban 57.1: apixaban |
75.9 (14) | NA | ISTH | Until discharge |
| Brown 32 | Andexanet | PP | Retrospective, observational, multicenter |
13 for safety analysis∆ 11 for effectiveness analysis |
100.0: ICH |
Safety analysis: 23.1: rivaroxaban 76.9: apixaban |
Safety analysis: 75.2 (13.5) |
NA | ISTH | 30 d |
| Reynolds 33 | PCC | 25 – 50 U/kg | Retrospective, observational, single‐center |
31 for safety analysis 28 for effectiveness analysis |
100.0: ICH |
Safety‐analysis: 54.8: rivaroxaban 45.2: apixaban |
Safety analysis: 77 [68–84] |
NA | ISTH | 7 d |
| Panos 34 | PCC | 25 – 50 U/kg | Retrospective, observational, multicenter |
663 for safety analysis 433 for effectiveness analysis |
100.0: ICH |
Safety analysis: 44.8: rivaroxaban 55.2: apixaban Efficacy analysis: 46.0: rivaroxaban 54.0: apixaban |
Safety analysis: >65: 14.6% 65–75: 26.4% >75: 59.0% Efficacy analysis: >65: 12.5% 65–75: 24.5% >75: 63.0% |
Inclusion: only patients with an ICH Exclusion: pregnant or lactating patients, withdrawal of care within 24 h of admission. Exclusion from effectiveness analysis when a follow‐up image was not present within the first 24 h of PCC administration Additional exclusion criteria for the efficacy analysis: patients without a follow‐up image of the brain within 24 h of PCC administration |
ISTH |
Mortality: until discharge TE: 30 d or until discharge |
| Barra 35 | PCC | 25 – 50 U/kg | Retrospective, observational, two‐arm, single‐center |
11 PCC |
100.0: ICH |
72.7: rivaroxaban 27.3: apixaban |
71.0 [68.7–73.2] | Inclusion: only patients with an ICH | ISTH |
Mortality: until discharge TE: 30 d or until discharge |
| Andexanet | PP | 18 andexanet |
16.7: rivaroxaban 83.3: apixaban |
83.4 [70.3–88.8] | ||||||
| Bavalia 36 | PCC | 50 U/kg | Prospective, observational, multicenter | 51 |
59.2: ICH 36.8: GIH 13.2: other |
71.0: rivaroxaban 21.1: apixaban 7.9: edoxaban |
75 (11) | NA | ISTH | 30 d |
| Korobey 37 | PCC | 50 U/kg | Retrospective, observational, single‐center | 59 |
100.0: ICH |
32.2: rivaroxaban 67.8: apixaban |
78.5 (10.9) |
Inclusion: only patients with an ICH Exclusion: neuro intervention prior to repeat imaging, died or had care withdrawn prior to repeat imaging |
ISTH |
Mortality: until discharge TE: 30 d or until discharge |
| Castillo 38 | PCC | 25 – 50 U/kg | Retrospective, observational, multicenter | 67 |
100.0: ICH |
56.7: rivaroxaban 43.3: apixaban |
77.6 (13) |
Inclusion: only patients with an ICH Exclusion: (acute on) chronic ICH, neurosurgical intervention between baseline and 12‐h follow‐up scan, or an ICH volume ≥60 mL |
ISTH | Until discharge |
ISTH, MBE in nonsurgical patients is defined as having a symptomatic presentation and: fatal bleeding; and/or bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular, pericardial, or intramuscular with compartment syndrome; and/or bleeding giving a hemoglobin drop of 2 g/dL (1.24 mmol/L) or more, or resulting in transfusion of two of more units of whole blood or red cells. 14 ∆ Only patients with ICH are shown; 3 surgical patients and 9 patients of which the severity of the bleed is not described are not shown.
Abbreviations: ACS, acute coronary syndrome; aPCC, activated prothrombin complex concentrate; CVA, cerebrovascular accident; GCS, Glascow Coma Scale; GIH, gastrointestinal hemorrhage; Hb, hemoglobin; ICH, intracranial hemorrhage; ISTH, International Society on Thrombosis and Haemostasis, IQR, interquartile range; MBE, major bleeding event (uncontrolled or life‐threatening bleeding); NA, not applicable; NR, not reported; PCC, prothrombin complex concentrate; PP, per protocol (low‐dose 400 mg in 30 min, followed by 480 mg in 2 h, high‐dose 800 mg in 30 min, followed by 960 mg in 2 h); SD, standard deviation; TE, thromboembolic event.
Nine PCC studies, one andexanet study, and the study with both reversal agents included only patients with intracerebral hemorrhage (ICH). The vast majority of the included studies had a mean or median age ≥75 years. Fifteen PCC studies and two andexanet studies used standardized criteria of the ISTH to define a major bleeding. No other standardized definitions for a major bleeding were used. The registration study of andexanet (Annexa‐4), which comprises 352 patients, did not use a standardized definition for a major bleeding.
For hemostatic effectiveness, the standardized ISTH criteria were adopted in five PCC studies (Table 3). In Annexa‐4, self‐defined criteria to assess hemostatic effectiveness were used. After its publication, three PCC and two other andexanet studies used the Annexa‐4 criteria for assessment of hemostatic effectiveness.
TABLE 3.
Outcome measures of the included studies
| Ref. | Hemostatic effectiveness, n (%) | Criteria for hemostatic effectiveness | Mortality, n (%) | Cause of death and time of onset | Thromboembolic events, n (%) | Definition thromboembolic event and time of onset |
|---|---|---|---|---|---|---|
| PCC studies | ||||||
| Grandhi 19 | 11 (61.1) | No bleeding progression on CT scan | 6 (33.3) | 4 MBE, 2 pneumonia | 1 (5.6) | 1 VTE within 24 h |
| Majeed 20 | 58 (69.1) | ISTH | 27 (32.1) | 18 MBE, 7 multiorgan failure, 1 cardiac arrest, 1 PE. 56% 0–7 d, 41% 7–30 d, 3% >30 d | 3 (3.6) | 2 iCVAs after 5 and 10 d; 1 PE after 15 d |
| Gerner 21 | 61 (64.9) | No hemorrhagic expansion | NR | NR | NR | NR |
| Schulman 22 |
56 (85.0) 45 (68.0) |
Sarode a ISTH |
9 (13.6) | 8 MBE, 1 stab wound. Within 30 d | 4 (6.1) | 3 iCVAs, 1 DVT, on days 1, 2, 9, 12 |
| Harrison 23 | 13 (92.9) | No hemorrhagic expansion | 2 (14.3) | NR | 0 (0.0) | NA |
| Testa 24 | NR | NR | 4 (14.8) d | 100% within 3 d | NR | NR |
| Allison 25 | 26 (83.8) | No bleeding progression on CT scan | 5 (15.2) | NR | 0 (0.0) | NA |
| Sheikh‐Taha (PCC) 26 | 21 (72.4) | ISTH | 6 (20.7) | 6 MBE. 5 within 6 d, 1 on day 14 | 1 (3.4) | 1 iCVA on day 6 |
| Arachchillage 27 | 59 (73.4) | No recurrent bleeding after 48 h and a patient surviving the MBE | 26 (33.0) | NR | 3 (3.8) | 3 iCVAs within 30 d |
| Dybdahl 29 | NA | NA | 8 (22.9) | NR | 1 (2.9) | 1 VTE |
| Smith 30 | 25 (80.6) | Sarode a | 5 (16.1) | 5 MBE | 0 (0.0) | NA |
| Sheikh‐Taha (aPCC) 31 | 24 (68.6) | ISTH | 7 (20.0) | 6 MBE, 1 septic shock, within 9 d | 3 (8.6) | 2 DVTs on days 2 and 4, 1 MI on day 2 |
| Reynolds 33 | 19 (67.9) | Decreased/stable hemorrhage on CT for patients with ICH and ≤20% decrease in Hb 12 h after PCC and no additional blood or factor products within 24 h after PCC for patients without ICH | 5 (16.1) | NR | 4 (12.9) | 3 iCVA, 1 DVT within 7 d |
| Panos 34 | 354 (81.8) | Annexa‐4 b | 126 (19.0) | Unknown cause. 48% 0‐5 d, 39% 6‐14 d, 10% 15‐30 d | 25 (3.8) | 15 DVT, 1 PE, 8 iCVAs, 2 MIs; 50% 0‐5 d, 35% 6‐14 d, and 15% 15‐30 d |
| Barra 35 | 6 (60.0) | <35% increase in hematoma volume, SAH thickness, or SDH thickness at 24 h | 7 (63.6) | NR | 0 (0.0) | NA |
| Bavalia 36 | 35 (69.6) | ISTH | 9 (17.6) | NR | 1 (2.0) | 1 PE after 10 d |
| Korobey 37 | 52 (88.1) | Annexa‐4 b | 6 (10.2) | NR | 7 (11.9) | 4 DVTs, 1 PE, 2 iCVAs |
| Castillo 38 | 59 (88.1) | Annexa‐4 b | 5 (7.5) | NR | 0 (0.0) | NA |
| Andexanet studies | ||||||
| Stevens 28 | 10 (77.0) | Annexa‐4 b | 2 (15.4) | 2 MBEs within 3 d | 4 (30.8) | 1 MI, 1 iCVA, 1 DVT, 1 PE, within 30 d |
| Connolly 12 | 208 (81.9) | Annexa‐4 b | 49 (13.9) | 35 CV cause, 12 non‐CV cause, 2 unknown cause. | 40 (11.4) e | 7 MIs, 15 iCVAs, 13 DVTs, 5 PEs; 32% 0‐5 d, 32% 6‐14 d, 35% 15‐30 d |
| Brown 32 | 10 (90.9) c | Annexa‐4 b | 4 (30.8) f | 50% 0‐7 d, 50% 7‐30 d |
0 (0.0) f |
NA |
| Barra 35 | 16 (88.9) | <35% increase in hematoma volume, SAH thickness, or SDH thickness at 24 h | 4 (22.2) | NR | 3 (16.7) | 3 DVTs after 1, 6 and 14 d |
Abbreviations: CT, computed tomography; CV, cardiovascular; DVT, deep vein thrombosis; Hb, hemoglobin; Ht, hematocrit; iCVA, ischemic cerebrovascular accident; MI, myocardial infarction; NA, not applicable; NR, not reported; PCC, prothrombin complex concentrate; PE, pulmonary embolism; VTE, venous thromboembolism.
The criteria by Sarode et al were compiled in 2013 with US Food and Drug Administration approval, as there was no standardized method to assess hemostatic effectiveness. 37 Its definition is included in Appendix S2
The criteria for effective hemostasis used in Annexa‐4 are adjusted Sarode criteria and included in Appendix S2. 38
Patients who died were excluded from the effectiveness analysis.
Mortality rate was the same at discharge and at 6 months.
Forty thromboembolic events occurred in 34 patients.
Only patients with ICH are shown. Three surgical patients and nine patients for which the severity of the bleed is not described are not shown.
3.3. Study quality
All included studies lacked a control group and therefore had a high risk of confounding and selection bias. The majority of studies had a retrospective design; only four PCC studies and one andexanet study had a prospective patient enrollment. Study quality of observational studies was assessed according to the MINORS and RoBANS methods and are shown in Table 2. Detailed information about the assessment of each included study is included in Appendix S4.
TABLE 2.
The quality of the included studies according to the MINORS and RoBANS methods
| Reference | MINORS | RoBANS | ||||||
|---|---|---|---|---|---|---|---|---|
| MINORS score |
Selection |
Confounding |
Intervention |
Blinding assessor |
Incomplete outcome data |
Selective reporting |
Risk of bias (mean RoBANS score) |
|
| Prothrombin complex concentrate studies | ||||||||
| Grandhi 19 | 10/16 | Medium | Medium | Low | Medium | Medium | Low | Medium |
| Majeed 20 | 13/16 | Medium | Medium | Low | Low | Low | Low | Low |
| Gerner 21 | 12/16 | Medium | Medium | Medium | Low | Low | High | Medium |
| Schulman 22 | 13/16 | Medium | Low | Low | Low | Low | Low | Low |
| Harrison 23 | 6/16 | Medium | High | Low | High | High | High | High |
| Testa 24 | 12/16 | Low | Medium | High | Low | Low | High | Medium |
| Allison 25 | 7/16 | Medium | Medium | Low | High | Medium | High | Medium |
| Sheikh‐Taha (PCC) 26 | 7/16 | Medium | Medium | Medium | Medium | Medium | Low | Medium |
| Arachchillage 27 | 10/16 | High | Medium | Low | High | Low | Low | Medium |
| Dybdahl 29 | 11/16 | Medium | Low | Medium | Medium | Low | Low | Low‐Medium |
| Smith 30 | 11/16 | Medium | High | Low | High | Medium | Low | Medium |
| Sheikh‐Taha (aPCC) 31 | 8/16 | Medium | Medium | Medium | Medium | Medium | Low | Medium |
| Reynolds 33 | 9/16 | Medium | Low | Low | High | High | High | High |
| Panos 34 | 11/16 | Medium | Low | Low | Medium | High | High | Medium |
| Bavalia 36 | 15/16 | Low | Low | Low | Low | Medium | High | Low |
| Korobey 37 | 12/16 | Medium | Low | Low | Medium | Medium | High | Medium |
| Castillo 38 | 8/16 | Medium | Low | Low | Medium | High | High | Medium |
| Andexanet Studies | ||||||||
| Stevens 28 | 11/16 | Medium | Medium | Low | Medium | Low | Low | Low‐Medium |
| Connolly 12 | 9/16 | High | High | Medium | High | High | Medium | High |
| Brown 32 | 8/16 | High | High | Low | High | Medium | High | High |
| PCC and andexanet studies | ||||||||
| Barra 35 | 10/16 | High | High | Low | Medium | Medium | Low | Medium |
Of the PCC studies, eight were of moderate quality with a low to medium bias risk, and six were of low quality with a medium to medium‐high bias risk. The PCC studies by Majeed et al,20 Schulman et al,22 and Bavalia et al36 were high‐quality studies with a low risk of bias.
Of the andexanet studies, none were graded as high quality. The andexanet studies by Stevens et al28 and Barra et al35 were graded of moderate quality with a low‐medium and medium bias risk, respectively. The studies by Connolly et al12 and Brown et al32 were graded of low quality with a high bias risk. This was mainly due to strict selection criteria, not using an intention‐to‐treat analysis, and not using standardized criteria for defining major bleeding and assessing hemostatic effectiveness.
3.4. Outcome measures
The study results based on hemostatic effectiveness, thromboembolic events, and mortality are shown in Table 3. Forest plots of the pooled outcome proportions are provided in Figure 2. Forest plots of the subgroup analysis for hemostatic effectiveness are provided in Appendix S3.
FIGURE 2.
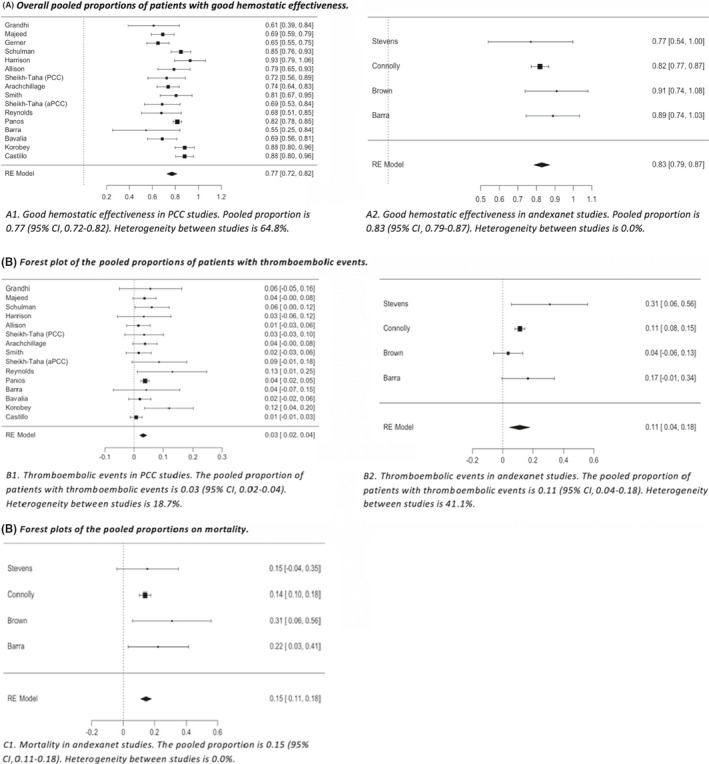
Forest plots of the pooled outcome proportions. aPCC, activated prothrombin complex concentrate; PCC, prothrombin complex concentrate
3.4.1. Prothrombin complex concentrate studies
Two hundred thirty‐three of 1428 PCC‐treated patients from safety analyses were excluded from hemostatic effectiveness analyses. In total, hemostatic effectiveness was assessed in 1195 PCC‐treated patients. The hemostatic effectiveness ranged from 60.0% to 92.9%. The pooled proportion of patients with good hemostatic effectiveness was 0.77 (95% CI, 0.72‐0.82; I2=64.8%), demonstrating substantial heterogeneity hampering pooled analysis.
Subgroup analysis for hemostatic effectiveness based on the assessment criteria used demonstrated a pooled proportion of 0.69 (95% CI, 0.64‐0.75; I2 = 0.0%) in the three high‐quality PCC studies that assessed hemostatic effectiveness by ISTH criteria, 0.85 (95% CI, 0.80‐0.90; I2 = 41.5%) in the three PCC studies that assessed hemostatic effectiveness by Annexa‐4 criteria and 0.75 (95% CI, 0.68‐0.83; I2 = 60.8%) in the nine PCC studies that used self‐defined criteria for hemostatic effectiveness. There was no heterogeneity between the PCC studies that used ISTH criteria for the definition of hemostatic effectiveness. Moderate heterogeneity was demonstrated between the PCC studies that used Annexa‐4 criteria and a substantial heterogeneity between studies that used self‐defined criteria.
Safety outcome measures were analyzed in 1428 PCC‐treated patients. Incidence of thromboembolic events ranged from 0.0% to 12.9%, demonstrating a pooled proportion of 0.03 (95% CI, 0.02‐0.04; I2 = 18.7%). The mortality rate ranged from 13.6% to 63.6%. The pooled analysis on mortality demonstrated substantial heterogeneity and was therefore not further analyzed.
3.4.2. Andexanet studies
One hundred of 396 andexanet‐treated patients from safety analyses were excluded from hemostatic effectiveness analyses. In total, hemostatic effectiveness was assessed in 296 andexanet‐treated patients. The hemostatic effectiveness ranged from 77.0% to 90.9%. The pooled proportion of patients with good hemostatic effectiveness was 0.83 (95% CI, 0.79‐0.87; I2 = 0.0%), demonstrating no heterogeneity. Subgroup analysis in the andexanet studies that used Annexa‐4 criteria demonstrated a hemostatic effectiveness of 0.82 (95% CI, 0.78‐0.87; I2 = 0.0%). No heterogeneity was observed in the andexanet studies that used Annexa‐4 criteria.
Thromboembolic events and mortality were assessed in 396 andexanet‐treated patients. Incidence of thromboembolic events ranged from 0.0% to 30.8% in andexanet studies, demonstrating a pooled proportion of 0.11 (95% CI, 0.04‐0.17; I2 = 35.7%). The mortality rate ranged from 13.9% to 24.0%, demonstrating a pooled proportion of 0.15 (95% CI, 0.11‐0.18; I2 = 0.0%).
4. DISCUSSION
In this meta‐analysis, similar hemostatic effectiveness for PCC and andexanet was observed with pooled proportions of 0.77 and 0.83 in PCC and andexanet studies, respectively. This was also demonstrated in subgroup analysis in studies that used Annexa‐4 criteria for hemostatic effectiveness; a similar hemostatic effectiveness of 0.85 in PCC studies and 0.82 in andexanet studies was demonstrated. The pooled proportion of patients with thromboembolic events was 0.03 (95% CI, 0.02‐0.04) in PCC studies and 0.11 (95% CI, 0.04‐0.18) in andexanet studies. The mortality rate in PCC studies ranged from 13.6% to 63.6%. The mortality pooled proportion in andexanet studies was 0.15 (95% CI, 0.11‐0.18).
All of the included studies lacked a control group, making it difficult to isolate the effect of the reversal agents. A meta‐analysis of the registry studies of the direct oral anticoagulants described a case‐fatality rate of major bleeding of 7.57% (95% CI, 6.53‐8.68). 39 This may be underestimated, because patients with comorbidities and concomitant use of antiplatelet agents were excluded in the registration studies. In a prospective study of 732 patients with major bleeding treated with direct oral anticoagulants, the case‐fatality rate was 14%. 40 However, this study does not purely reflect the case‐fatality rate without a reversal agent, because 38.4% were treated with PCC. 40 Nevertheless, the case‐fatality rate of 14% was similar to the mortality rates of PCC and andexanet in this meta‐analysis. Therefore, it remains uncertain whether PCC and andexanet are of added value in addition to supportive care.
While no difference in hemostatic effectiveness was observed, the incidence of thromboembolic events was higher in the andexanet studies than PCC studies. The pooled proportion of thromboembolic events in PCC‐treated patients in this study are consistent with previous studies. Dentali et al 9 described a thromboembolic event rate of 1.9% in 631 patients who were treated with PCC in VKA‐related major bleeding. Piran et al 10 described a 3.0% thromboembolic event rate in 216 patients who were treated with PCC in FXaI‐related major bleeding. Connolly et al 12 stated that the majority of thromboembolic events in Annexa‐4 occurred in patients in whom resumption of oral anticoagulation was delayed or in patients who did not restart anticoagulation. However, 32% of the thromboembolic events occurred 0 to 5 days after andexanet administration, and 23.5% occurred after restarting anticoagulation. Furthermore, some hypothesize that andexanet may block tissue factor pathway inhibitor, an endogenous inhibitor of factor Xa, which may lead to thrombosis. 41 A difference in mortality may result in a different ascertained thromboembolic event rate; the thromboembolic event rate is lower in studies with higher mortality, probably due to the competing risk. We conclude that, due to the lack of direct comparative studies, a heterogeneous patient inclusion, and different follow‐up periods in included studies, no strong statements can be made about a difference in thromboembolic events between PCC and andexanet treatment.
Our review demonstrates the methodological limitations of the available evidence for effectiveness and safety of PCC and andexanet in FXaI‐related major bleeding. All studies were observational without a comparator group, leading to a high risk of selection bias. Furthermore, the majority of the included studies had retrospective data collection, leading to an even higher risk of bias. Only four PCC studies20, 22, 24, 36 minimized the risk of bias through prospective, consecutive patient enrollment, while the only andexanet study with prospective patient enrollment 12 likely suffered a high risk of bias due to strict inclusion criteria, excluding patients with more critical bleeding (eg, patients with a planned intervention within 12 hours after andexanet or an ICH with score <7 on the Glasgow Coma Scale or a hematoma volume >60 mL). Regarding these methodological challenges, we applied two scoring systems to obtain the most objective assessment of the study quality and risk of bias. However, due to the few andexanet studies included, there were insufficient data for a subgroup analysis on moderate/high‐quality studies. Eliminating low‐quality studies with a high risk of bias would eliminate the studies of Connolly et al12, 32 and Brown et al,12, 32 eliminating the vast majority of andexanet‐treated patients.12, 32
We applied several measures to enhance interpretation of the data on hemostatic effectiveness of PCC or andexanet in management of anticoagulation‐related major bleeding, such as uniformity in study design characteristics (ISTH criteria for major bleeding) and standardized definitions for hemostatic effectiveness.11, 14, 15 Despite this, heterogeneity among studies was large, hampering pooled analyses.
In the Annexa‐4 study, self‐defined criteria to assess hemostatic effectiveness were used (included in Appendix S2). The main difference with the ISTH major bleeding criteria is that the assessment time was changed to 12 hours instead of 24 hours after infusion. In addition, no restrictions were made on the administration of hemostatic agents, coagulation factors, or blood products. Moreover, pericardial and intraspinal hemorrhages have their own criteria for effective hemostasis in the Annexa‐4 scoring system. These changes may lead to a more favorable outcome of hemostatic effectiveness using the Annexa‐4 criteria in comparison to the ISTH criteria.
Annexa‐4 is the only study that included anti–factor Xa <75 ng/mL as an exclusion criterion for assessing hemostatic effectiveness. However, FXaIs have short half‐lives and may no longer be present in patients with major bleeding, obviating the need for treatment with a reversal agent. Because of this bias, most studies may have overrated the therapy as being effective since anti–factor Xa levels are not usually measured before treatment.
In conclusion, the available evidence makes it challenging to choose a preferred FXaI reversal agent. All studies lacked control groups, and the majority were retrospectively conducted with strict selection criteria, without use of standardized definitions for major bleeding, and without standardized scoring methods to define hemostatic effectiveness. Randomized controlled trials with less stringent inclusion criteria and usage of standardized scoring methods are therefore urgently needed. For andexanet, a conditional marketing authorization was given by the EMA and FDA with the condition that a direct comparative study would be conducted to assess the effectiveness and safety of andexanet versus “usual care.” This study, ANNEXA‐I, is currently recruiting patients with ICH and is expected to be completed in 2023. 42
5. CONCLUSION
Our systematic review and meta‐analysis is the first that we are aware of to evaluate the current evidence for the effectiveness and safety of andexanet and PCC in patients with major bleeding. Separately performed analyses for both reversal agents demonstrated them to be similar in hemostatic effectiveness, while the thromboembolic event rate appeared higher in andexanet. None of the included studies had control groups, hampering a pooled meta‐analysis to compare the two reversal agents. There is an urgent need for a randomized clinical trial comparing both reversal agents.
RELATIONSHIP DISCLOSURE
TJ and KS declare no conflicts of interest. NK has no conflicts of interest for this manuscript. In the past, NK led the ISTH Scientific and Standardization Committee working group, which compiled the ISTH definition for hemostatic effectiveness. MVH has no conflicts of interest for this manuscript. Outside the work, he received grants from ZONMW, Bayer Health Care, Pfizer‐BMS, Boehringer‐Ingelheim, and Leo Pharma. KM received research grants from Pfizer, Bayer, and Sanquin; lecturing fees from Bayer, Sanquin, Boehringer‐Ingelheim, BMS, and Aspen; and consulting fees from Uniqure. All fees were paid to the institute. No other potential conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
TJ contributed to study design, study selection, collection of data, statistical analyses, and writing the initial draft of the manuscript; KS contributed to study design, study selection, and collection of data and provided vital reviews of the manuscript; MVH and KM contributed to study design, interpreted the data, and provided vital reviews of the manuscript. NK contributed to study design and writing the initial draft of the manuscript, interpreted the data, and provided vital reviews of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank B.S. Bhoelan for her support with the meta‐analysis.
Handling Editor: Dr Mary Cushman.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
REFERENCES
- 1. Christensen H, Cordonnier C, Korv J et al. European stroke organisation guideline on reversal of oral anticoagulants in acute intracerebral haemorrhage. Eur Stroke J. 2019;4:294‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Makris M, Van Veen JJ, Tait CR, Mumford AD, Laffan M. British Committee for Standards in Haematology. Guideline on the management of bleeding in patients on antithrombotic agents. Br J Haematol. 2013;160:35‐46. [DOI] [PubMed] [Google Scholar]
- 3. Steffel J, Verhamme P, Potpara TS et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330‐1393. [DOI] [PubMed] [Google Scholar]
- 4. Tomaselli GF, Mahaffey KW, Cuker A et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:3042‐3067. [DOI] [PubMed] [Google Scholar]
- 5. Witt DM, Nieuwlaat R, Clark NP et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2:3257‐3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holbrook A, Schulman S, Witt DM et al. Evidence‐based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e152S‐e184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keeling D, Baglin T, Tait C et al. Guidelines on oral anticoagulation with warfarin ‐ fourth edition. Br J Haematol. 2011;154(3):311‐324. [DOI] [PubMed] [Google Scholar]
- 8. Khorsand N, Kooistra HA, van Hest RM, Veeger NJ, Meijer K. A systematic review of prothrombin complex concentrate dosing strategies to reverse vitamin K antagonist therapy. Thromb Res. 2015;135:9‐19. [DOI] [PubMed] [Google Scholar]
- 9. Dentali F, Marchesi C, Giorgi Pierfranceschi M et al. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. A meta‐analysis. Thromb Haemost. 2011;106:429‐438. [DOI] [PubMed] [Google Scholar]
- 10. Piran S, Khatib R, Schulman S et al. Management of direct factor Xa inhibitor‐related major bleeding with prothrombin complex concentrate: a meta‐analysis. Blood Adv. 2019;3:158‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khorsand N, Majeed A, Sarode R et al. Assessment of effectiveness of major bleeding management: proposed definitions for effective hemostasis: communication from the SSC of the ISTH. J Thromb Haemost. 2016;14:211‐214. [DOI] [PubMed] [Google Scholar]
- 12. Connolly SJ, Crowther M, Eikelboom JW et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380:1326‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. U.S. Food & Drug Administration . Accelerated approval. FDA.gov. https://www.fda.gov/media/113285/download Published May 3, 2018. Accessed July 1, 2020.
- 14. Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692‐694. [DOI] [PubMed] [Google Scholar]
- 15. Palareti G, Leali N, Coccheri S et al. Bleeding complications of oral anticoagulant treatment: an inception‐cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348:423‐428. [DOI] [PubMed] [Google Scholar]
- 16. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712‐716. [DOI] [PubMed] [Google Scholar]
- 17. Kim SY, Park JE, Lee YJ et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408‐414. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions [Internet] Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Chapter 9.5.2, Identifying and measuring heterogeneity. Cited December 1, 2020. Available from: www.handbook.cochrane.org
- 19. Grandhi R, Newman WC, Zhang X et al. Administration of 4‐Factor prothrombin complex concentrate as an antidote for intracranial bleeding in patients taking direct factor Xa inhibitors. World Neurosurg. 2015;84:1956‐1961. [DOI] [PubMed] [Google Scholar]
- 20. Majeed A, Agren A, Holmstrom M et al. Management of rivaroxaban‐ or apixaban‐associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130:1706‐1712. [DOI] [PubMed] [Google Scholar]
- 21. Gerner ST, Kuramatsu JB, Sembill JA et al. Association of prothrombin complex concentrate administration and hematoma enlargement in non‐vitamin K antagonist oral anticoagulant‐related intracerebral hemorrhage. Ann Neurol. 2018;83:186‐196. [DOI] [PubMed] [Google Scholar]
- 22. Schulman S, Gross PL, Ritchie B et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb. Haemost. 2018;118:842‐851. [DOI] [PubMed] [Google Scholar]
- 23. Harrison SK, Garrett JS, Kohman KN, Kline JA. Comparison of outcomes in patients with intracranial hemorrhage on factor Xa inhibitors versus vitamin K antagonists treated with 4‐factor prothrombin complex concentrate. Proc (Bayl Univ Med Cent). 2018;31:153‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Testa S, Ageno W, Antonucci E et al. Management of major bleeding and outcomes in patients treated with direct oral anticoagulants: results from the START‐Event registry. Intern Emerg Med. 2018;13:1051‐1058. [DOI] [PubMed] [Google Scholar]
- 25. Allison TA, Lin PJ, Gass JA et al. Evaluation of the use of low‐dose 4‐factor prothrombin complex concentrate in the reversal of direct oral anticoagulants in bleeding patients. J Intensive Care Med. 2020;35(9):903‐908. [DOI] [PubMed] [Google Scholar]
- 26. Sheikh‐Taha M. Treatment of apixaban‐ and rivaroxaban‐associated major bleeding using 4‐factor prothrombin complex concentrate. Intern Emerg Med. 2019;14:265‐269. [DOI] [PubMed] [Google Scholar]
- 27. Arachchillage DRJ, Alavian S, Griffin J et al. Effectiveness and safety of prothrombin complex concentrate in patients treated with rivaroxaban or apixaban compared to warfarin presenting with major bleeding. Br J Haematol. 2019;184:808‐816. [DOI] [PubMed] [Google Scholar]
- 28. Stevens VM, Trujillo T, Mueller SW et al. Coagulation factor Xa (recombinant), inactivated‐Zhzo (andexanet alfa) hemostatic outcomes and thrombotic event incidence at an academic medical center. Clin Appl Thromb Hemost. 2019;25:1076029619896619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dybdahl D, Walliser G, Chance Spalding M, Pershing M, Kincaid M. Four‐factor prothrombin complex concentrate for the reversal of factor Xa inhibitors for traumatic intracranial hemorrhage. Am J Emerg Med. 2019;37:1907‐1911. [DOI] [PubMed] [Google Scholar]
- 30. Smith MN, Deloney L, Carter C, Weant KA, Eriksson EA. Safety, effectiveness, and cost of four‐factor prothrombin complex concentrate (4F‐PCC) in patients with factor Xa inhibitor‐related bleeding: a retrospective study. J Thromb Thrombolysis. 2019;48:250‐255. [DOI] [PubMed] [Google Scholar]
- 31. Sheikh‐Taha M, Crawley RM. Reversal of apixaban and rivaroxaban using activated prothrombin complex concentrates in patients with major bleeding. Am J Cardiovasc Drugs. 2020;20:295‐299. [DOI] [PubMed] [Google Scholar]
- 32. Brown CS, Scott RA, Sridharan M, Rabinstein AA. Real‐world utilization of andexanet alfa. Am J Emerg Med. 2020;38:810‐814. [DOI] [PubMed] [Google Scholar]
- 33. Reynolds TR, Gilbert BW, Hall KM. Utilization of 4‐factor prothrombin complex concentrate for reversal of oral factor Xa inhibitor‐associated acute major bleeding. J Pharm Pract. 2020:897190020907012. [DOI] [PubMed] [Google Scholar]
- 34. Panos NG, Cook AM, John S, Jones GM. Neurocritical Care Society Pharmacy Study Group. Factor Xa inhibitor‐related intracranial hemorrhage: results from a multicenter, observational cohort receiving prothrombin complex concentrates. Circulation. 2020;141:1681‐1689. [DOI] [PubMed] [Google Scholar]
- 35. Barra ME, Das AS, Hayes BD et al. Evaluation of andexanet alfa and four‐factor prothrombin complex concentrate (4F‐PCC) for reversal of rivaroxaban‐ and apixaban‐associated intracranial hemorrhages. J Thromb Haemost. 2020;18:1637‐1647. [DOI] [PubMed] [Google Scholar]
- 36. Bavalia R, Abdoellakhan R, Brinkman HJM et al. Emergencies on direct oral anticoagulants: Management, outcomes, and laboratory effects of prothrombin complex concentrate. Res Pract Thromb Haemost. 2020;4:569‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Korobey MJ, Sadaka F, Javed M, Moynihan M, Alsaei A. Efficacy of 4‐factor prothrombin complex concentrates in factor Xa inhibitor‐associated intracranial bleeding. Neurocrit Care. 2021;34(1):112–120. [DOI] [PubMed] [Google Scholar]
- 38. Castillo R, Chan A, Atallah S et al. Treatment of adults with intracranial hemorrhage on apixaban or rivaroxaban with prothrombin complex concentrate products. J Thromb Thrombolysis. 2021;51(1):151‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chai‐Adisaksopha C, Hillis C, Isayama T, Lim W, Iorio A, Crowther M. Mortality outcomes in patients receiving direct oral anticoagulants: a systematic review and meta‐analysis of randomized controlled trials. J Thromb Haemost. 2015;13:2012‐2020. [DOI] [PubMed] [Google Scholar]
- 40. Albaladejo P, Samama CM, Sié P et al. Management of severe bleeding in patients treated with direct oral anticoagulants: an observational registry analysis. Anesthesiology. 2017;127:111‐120. [DOI] [PubMed] [Google Scholar]
- 41. Levy JH, Ageno W, Chan NC et al. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:623‐627. [DOI] [PubMed] [Google Scholar]
- 42. U.S. National Library of Medicine . Trial of Andexanet in ICH Patients Receiving an Oral FXa Inhibitor. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03661528. Published September 7, 2018. Updated [July 28, 2020. Accessed August 1, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


