Abstract
Many studies indicate that diets including carotenoid-rich foods have positive effects on human health. Some of these compounds are precursors of the essential nutrient vitamin A. The present work is aimed at implementing a database of carotenoid contents of foods available in the European market. Factors affecting carotenoid content were also discussed. Analytical data available in peer-reviewed scientific literature from 1990 to 2018 and obtained by HPLC/UHPLC were considered. The database includes foods classified according to the FoodEx2 system and will benefit compilers, nutritionists and other professionals in areas related to food and human health. The results show the importance of food characterization to ensure its intercomparability, as large variations in carotenoid levels are observed between species and among varieties/cultivars/landraces. This highlights the significance of integrating nutritional criteria into agricultural choices and of promoting biodiversity. The uncertainty quantification associated with the measurements of the carotenoid content was very rarely evaluated in the literature consulted. According to the EuroFIR data quality evaluation system for food composition tables, the total data quality index mean was 24 in 35, reflecting efforts by researchers in the analytical methods, and less resources in the sampling plan documentation.
Keywords: agro-food, agronomy, food composition, fruits and vegetables
1. Introduction
The main dietary sources of carotenoids for humans are in general fruits and vegetables, although they are also present in other plant products (herbs, legumes, cereals or even oils), algae, animal foods, additives or supplements [1].
There are many studies indicating that carotenoid-rich diets can have a positive role in human health and contribute to reduce the risk of diseases associated with aging, such as certain cancers, cardiovascular disease, bone, skin or eye disorders and may be beneficial for mental and metabolic health, during pregnancy and early life. In addition to their role as colorants and bioactive compounds with possible positive effects on human health, some carotenoids also have a well-defined role as provitamin A nutrient, being key in combating vitamin A deficiency, especially in areas with low consumption of animal foods [2,3].
However, neither the European Food Safety Authority (EFSA) [4] nor the U.S. Food and Nutrition Board [5] have established official recommended daily intakes/nutritional reference values for the main dietary carotenoids. Indeed, there is still insufficient categorical evidence of the amounts needed to help promote health, even for a widely studied non-provitamin A carotenoid such as lutein, which has been positively associated to eye health and other benefits [6,7].
According to the European labeling requirement [8], the daily reference intake for vitamin A is 800 µg, with 15% (120 µg) being considered significant to the label’s nutritional table. The equivalence between carotenoids provitamin A and retinol is an issue on which a scientific consensus has not yet been established. According to the Food and Nutrition Board of the Institute of Medicine, in USA, for provitamin A carotenoids from food, 6 µg of β-carotene or 12 µg of α-carotene or 12 µg of β-cryptoxanthin are equivalent to 1 µg of retinol. In terms of activity, twice those amounts are needed to obtain that of 1 µg of retinol [9,10]. The first equivalence is named retinol equivalent (RE) and the second retinol activity equivalent (RAE). The Food and Agriculture Organization of the United Nations/International Network of Food Data Systems (FAO/INFOODS), adopted these equivalences with the note that they may be specific for each country [11]. The equivalence factors are estimated with large intervals of confidence due to factors that lead to high standard deviations, including large interindividual response, differences in food matrix and processing or analytical errors [12].
Apart from retinoids, carotenoids can also be enzymatically cleaved by humans into apocarotenoids and other derivatives, which are eliciting increasing interest, as they may be involved in biological actions. Given their versatility, carotenoids are importance continues growing in the era of sustainable healthy diets and for the development of innovative products such as novel foods or nutricosmetics, among others [2,3,13].
The promotion of currently underutilized local foods is increasingly a priority to improve environmental sustainability and reducing in particular, the carbon footprint as local foods have been often replaced by processed unhealthy foods due to its convenience. Frequently, mainly in low-income countries, the latter foods are produced far from the place of consumption having negative impacts in health and the environment. According to FAO, in 2019, more than 6000 plant species have been cultivated for food. Of these less than 200 make major contributions to food production globally, regionally or nationally and only nine species account for 66% of total crop production [14]. In this context, food composition databases are important tools for the adequate choice of foods and to promote biodiversity by highlighting neglected yet nutritionally rich species that lost importance with growing industrialization.
Food composition data are used in different fields of knowledge, mainly in the areas of agro-food, nutrition and health [15]. They are often the basis of studies that link the ingestion of carotenoids with health outcomes, so the quality of the data is of particular importance. The uncertainty of these data influences that of studies made from those and their conclusions drastically. This is not positive not only in scientific terms but also in order to support the implementation of public health policies.
The determination of the different components of foodstuffs is particularly time consuming and expensive due to their diversity and the different analytical methods involved. Carotenoids are particularly difficult to analyze accurately due to their lipophilicity, instability, structural similarity or scarcity of certified reference materials, which poses problems of accuracy and comparability of results. On the other hand, there is a clear dispersion of food carotenoids composition data. In general, food composition databases only include β-carotene, the carotenoid with the highest theoretical vitamin A activity and probably the most ubiquitous in foods [1,2].
In general, variations in the profile of carotenoids within a species are not observed with the naked eye unless there are color mutants. However, great variations are perceived in each carotenoid content and in the total carotenoid content in the different varieties of a species. This variability requires high resources in the design and implementation of sampling plans and highly specialized analytical and human resources for obtaining high quality data.
Several original data quality assessment systems were developed by several entities, such as the United States Department of Agriculture (USDA), the Agence Française de Sécurité des Produits Alimentaires (AFSSA), the Bioactive Substances in Food Information Systems (BASIS), the Centro per lo Studio e la Prevenzione Oncologica (CSPO) and the German Food Code and Nutrient Data Base (BLS). More recently the European Food Information Resource (EuroFIR) developed a system based on those in order to harmonize food composition data evaluation, by compilers [16]. Although its importance and usefulness are indisputable it is still not widely. Regarding carotenoids the first works on data quality assessment dates back to 1993, although only the analytical steps were considered [17,18].
This work is aimed at developing a comprehensive database of analytical data on carotenoid contents in plant foods produced/marketed in Europe reported in peer reviewed primary scientific sources. The quality of the data was assessed following current EuroFIR AISBL (International Non-Profit Organization) recommendations for compilers [16]. On the other hand, factors influencing carotenoid levels in foods are discussed.
2. Material and Methods
2.1. Data Collection
The identification of the papers for data collection was done through the b-on (Online Knowledge Library), a search engine that pre index metadata in a single central index. It contains over 16,750 scientific international publications from 16 publishers, through subscriptions negotiated on a national (Portugal) basis with these publishers.
The following keywords were searched in titles: carotenoid OR carotene OR xanthophyll OR lutein OR zeaxanthin OR cryptoxanthin OR lycopene OR phytoene OR phytofluene. Thereafter filters related to European countries were applied: Albania OR Armenia OR Austria OR Azerbaijan OR Belarus OR Belgium OR Bosnia OR Bulgaria OR Croatia OR Cyprus OR Czech Republic OR Denmark OR Estonia OR Europe* OR Finland OR France OR Georgia OR Germany OR Greece OR Hungary OR Iceland OR Ireland OR Italy OR Kazakhstan OR Kosovo OR Latvia OR Liechtenstein OR Lithuania OR Luxembourg OR Macedonia OR Malta OR Moldova OR Monaco OR Montenegro OR Netherlands OR Norway OR Poland OR Portugal OR Romania OR Russia OR Serbia OR Slovakia OR Slovenia OR Spain OR Sweden OR Switzerland OR Turkey OR Ukraine OR United Kingdom OR UK were searched in all fields. From this search, using filters, papers containing Food OR Vegetable OR Fruit were selected. Papers about biological fluids and food of animal origin were excluded manually.
The research was conducted in papers published in the period 1990–2018 and only original research full-text papers were scrutinized. Only analytical data obtained by HPLC or UHPLC systems equipped with at least spectrophotometric detector were considered. The use or not of the saponification step during extraction method was registered since this step affects the analytical measurement results [1]. Only food items intended for human consumption produced and/or marketed in Europe were considered. Food supplements were not in the scope of this collection.
Each paper was analyzed by researchers working in the area of carotenoids, in particular with knowledge of the analytical methods. All carotenoids reported in each paper were collected and the water content, when available. References to carotenoids content expressed as RE, RAE and % of the total, presented only in the graphic form and indicating only the minimum and maximum were discarded. The different units referred in the papers were converted to µg/100 g. The tables were organized to document the food, common name, scientific name including species and varieties/cultivars/landraces/accessions (whenever known). Other descriptors, including countries of origin and purchase, general food processing method, color and part analyzed, were also registered when available.
All foods items were classified according to the FoodEx2 hierarchical system developed by the European Food Safety Authority (EFSA), at least at level 1 (twenty food groups). When the number of foods at a given level was high, lower levels were used in order to distinguish them. That is, being a hierarchical system, when the group level comprised a large amount of foods (for instance, in the case of fruits and vegetables), the main sources of carotenoids, levels 3 and 4 were reached in order to obtain a greater distinction between the foods of these groups. Facet descriptors were used to provide additional information for processed foods.
2.2. Data Quality Assessment
The quality of data published was evaluated using the methodology developed in the scope of EuroFIR. This includes the evaluation of the measurement and the sampling methods [16]. Briefly, this method evaluates seven categories: food description, component identification, sampling plan, number of analytical samples, sample handling, sample analysis and analytical quality control. Quality was scored in each one by answering a total of 28 predefined questions (criteria). For each category several criteria were defined and for each one the evaluator should answer “Yes”, “No” or “Not Applicable” (when the criterion is not relevant for the nutrient in appreciation). These answers were translated quantitatively, accordingly the following answers points scheme: “Yes”, 5 points; “No” and “Not Applicable”, 0 points. The total points in each category were divided by the total number of “Yes” and “No”; by definition when one category gets only “No” or “Not Applicable” answers, the score should be 1. Through this evaluation, each category received a score between 1 (low quality) and 5 (high quality). At the end, the scores of each category were added and the overall quality index ranged between 7 (low quality) and 35 (high quality). The evaluation was done for all papers collected and, for each category and overall quality index, means were calculated.
3. Results and Discussion
3.1. Data Collection
The search tools of b-on was not selective enough and a general review of the abstracts resulting from the search, which returned initially >1000 results, was necessary. After this review, 373 references were collected and from these 25 were rejected, the main cause being the determination of carotenoids by spectrophotometric method instead of HPLC/UHPLC. The time frame was from 1990 to 2018, with emphasis on the most recent years. The year with the most papers was 2016. The references were from Archives of Biochemistry and Biophysics, Crop Science, European Food Research and Technology, Food and Nutrition Research, Food Chemistry, Food Research International, Food Science and Technology International, International Journal of Food Science and Technology, International Journal of Food Sciences and Nutrition, Journal of Agricultural and Food Chemistry, Journal of Chromatography A, Journal of Food Composition and Analysis, Journal of Food Science, Journal of Food, Agriculture and Environment, Journal of Separation Science, Journal of the Science of Food and Agriculture, LWT-Food Science and Technology, Nutrición Hospitalaria, Plant Foods for Human Nutrition, Planta, Postharvest Biology and Technology, Archivos Latinoamericanos de Nutrición, Food and Nutrition Bulletin, Revista do Instituto Adolfo Lutz and Scientia Horticulturae. Although b-on has a high journal coverage, digital object identifier (DOI) was not available in some of the oldest references, forcing a new internal referencing of these papers in order to create a unique identification.
To facilitate comparisons, all carotenoid content units were converted to µg/100 g, preferably in fresh weights, unless water content was not given, and in this case, the dry basis was flagged. The vast majority of papers did not report water content despite this parameter is key in order to be able to relate compositions in food composition databases.
The information about the use or not of saponification during the analytical process was collected as this reaction can lead to important losses of carotenoids or even the formation of artifacts, decreasing the accuracy of the analysis. To monitor this process, an internal standard is often added by some researchers, for example β-apo-8′-carotenal or echinenone. Despite being a lengthy step, saponification is often essential to hydrolyze the carotenoid esters, to eliminate lipids and eliminate chlorophylls [1,2]. However, only 10% of the papers refer the inclusion/not inclusion of this step.
In general, the part of the food analyzed was mentioned in the papers. The descriptor “edible part” was used very often. However, in foods that may generate ambiguities, a more detailed description will be an asset. This is the case, for example, of apple, pear, tomato and apricot, in which the peel may or may not be considered an edible part, influencing the carotenoid content.
All food items were codified by FoodEx2, using the exposure hierarchy, including facets for processed food. This classification system, initially developed for exposure evaluation to contaminants, is currently increasingly used in the area of food composition and mandatory in projects supported by EFSA. It is less complex in relation to other systems (e.g., LanguaL) and it is used by EFSA to facilitate the compatibility/comparability among different domains of food databases and from different countries at the EU level.
Supplementary Tables S1–S10 [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180], corresponding to 10 level 1 FoodEX2 groups, Coffee, cocoa, tea and infusions; Seasoning, sauces and condiments; Composite dishes; Fruit and vegetable juices and nectars; Animal and vegetable fats and oils and primary derivatives thereof; Fruit and fruit products; Legumes, nuts, oilseeds and spices; Starchy roots or tubers and products thereof, sugar plants; Vegetables and vegetable products; Grains and grain-based products present the carotenoid content of food items. Sub tables by FoodEX2 subgroups (levels 2–4) and the respective codes, including facets (represented by #) were also used to obtain more discrimination and facilitate consultation.
The lack of quantitative data for a carotenoid in the tables meant in many cases that the carotenoid was not reported in the paper and not that the food did not contain that carotenoid. This has been common for the colorless carotenoids phytoene and phytofluene, which have been very often ignored [2].
Supplementary Tables S1–S10 contain 3507 values of carotenoid content, with 29.4% referring to β-carotene, 18.8% to lutein, 10.3% to β-cryptoxanthin, 8.8% to zeaxanthin, 7.0% to α-carotene and 3.9% to lycopene.
Excluding foods analyzed in their dry form, and according to this collection, the major sources of the carotenoids above were, in µg/100 g fresh weight, in descending order (extracts of Tables S1–S10 for some of these foods area presented on Appendix A):
-
–
Carrot, spinach, goosefoot, peppers (red) and sheep sorrel for β-carotene.
-
–
Spinach, goosefoot, sheep sorrel, Indian spinach and amaranth for lutein.
-
–
Peppers (red), apricot, sarsaparilla, tamarillo and mandarin for β-cryptoxanthin.
-
–
Peppers (red), goosefoot, duckweed, goji berries and maize for zeaxanthin.
-
–
Carrot, pumpkin, carrot greens, cowpea and Ceylon spinach for α-carotene.
-
–
Tomato, rosehip, ketchup, watermelon and tomato sauce for lycopene.
3.2. Data Quality Index
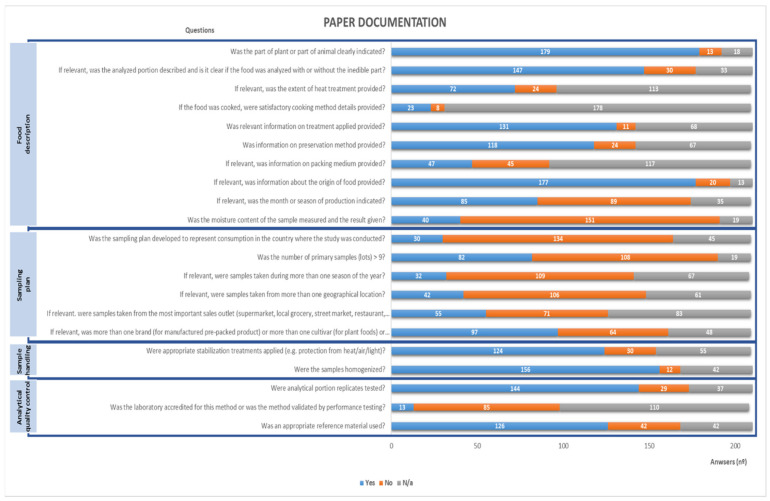
Sample analysis and the component identification obtained immediately the maximum score, which was 5. Firstly, since HPLC (or more recently UHPLC) is the method generally accepted as suitable for the analysis of individual carotenoids, only data obtained using it were considered for data collection. Additionally, only clear unequivocal identified and quantified (including units) carotenoids were included in the database. The score for the number of analytical samples was 1 due to the specification of this gathering per analytical sample. Indeed, this category was not evaluated. The results of the global evaluation of all papers are presented in Figure 1, excluding categories evaluated a priori based on the assumptions of this collection. Considering the set of papers reviewed, the average scores obtained were 4 for the Food description, 5 for the Component identification, 2 for the Sampling plan, 1 for the Number of analytical samples, 4 for the Sample handling, 5 for the Sample analysis and 3 for the Analytical quality control. Considering that seven categories were under evaluation and that each one can get a maximum score of 5, overall, a mean rating of 24 out of a possible maximum of 35 was obtained by applying strictly the method. Considering the peculiarity of this collection it could be considered that a score of 23 out of 30 was obtained. Taking into account the EuroFIR criteria for quality of food composition databases, the results reveal that the data on carotenoids, available in the scientific literature present a greater weakness in terms of the sampling plan and then in terms of analytical control. This can be attributed to the difficulty in defining representative food sampling plans, the intention of the study (for instance the representativeness of the food in the country is not usually an important aspect) or the scarcity of certified reference materials/interlaboratory tests in this area. Another important aspect is that researchers from other areas may not be aware for the importance of these quality parameters and may not describe them in their publications.
Figure 1.
Paper documentation evaluation, for food composition databases, by the EuroFIR system.
3.2.1. Factors Affecting the Level of Carotenoids
The data collected show great variations in the levels of carotenoids within and across plant foods (Tables S1–S10). All plant biosynthesize carotenoids in their photosynthetic tissues following a quite constant pattern in most cases, whereas many of them also biosynthesize a wider variety of them in structures such as fruits, several flower structures, tubers, seeds or roots [181,182]. Apart from the type of plant tissue there are manifold factors related to the accumulation of carotenoids in these foods, examples of some of which are outlined below. It is important to note that, because of the numerous factors governing the biosynthesis of carotenoids in plant foods, it is generally difficult to establish categorically the effect that a particular practice has on a given food. Thus, it is not uncommon to find in the bibliography that the effect of an agronomic practice is markedly different depending for instance on genetic factors or developmental stages.
3.2.2. Factors Related to the Plant
Genetic Factors
The carotenoid profile in qualitative terms is usually quite constant across many varieties, although there can also be spontaneous or targeted color mutations that result in varieties with a distinctive carotenoid pattern. Thus, most sweet oranges (Citrus sinensis L. Osbeck) accumulate epoxycarotenoids (typically certain isomers of violaxanthin and antheraxanthin) as major carotenoids [55], whereas a few cultivars such as Cara Cara, Shara or Hong Anliu have a reddish flesh due to the accumulation of large amounts of lycopene (absent in most orange varieties) and also of the colorless carotenoids phytoene and phytofluene [52,183]. On the other hand, the variety Pinalate has a yellowish pulp resulting from the very high accumulation of ζ-carotene, phytoene and phytofluene, which are the main carotenoids in this variety [184]. There are also tomato color mutants, for instance the R tomato, which has yellowish flesh and does not accumulate carotenoids [185] or the Delta tomato, with increased δ-carotene levels (a carotenoid not usually detected in most tomato varieties) and reduced lycopene contents [186].
In some cases, structures that do not accumulate carotenoids or that accumulate very low amounts can become good sources of these compounds as a result of spontaneous mutations. One example would be the cauliflower, where a gene mutation results in the accumulation of high levels of β-carotene in the inflorescence and other structures [187]. The accumulation of carotenoids in previously non-carotenogenic structures can also be achieved by means of genetic engineering. The biosynthesis of β-carotene in the endosperm of golden rice being an excellent example [188].
On the other hand, there can be important differences in the carotenoid levels even within the same variety under the same cultivation conditions. Thus, for instance, in a study where the accumulation of carotenoids, tocopherols and phenolic compounds in diverse tomato and wild relatives was studied it was concluded that, when ripe, the highest levels of lycopene, phytoene and phytofluene were found in two accessions of the same tomato variety (var. cerasiforme). The differences ranged between ca. 2-fold (lycopene) and ca. 6-fold (phytoene), which highlights the impact that the genotype has in the accumulation of secondary metabolites [189].
Ripening
In carotenogenic fruits, ripening is normally accompanied by the degradation of chlorophylls and the increase in the biosynthesis of carotenoids although there are some exceptions. Thus, in fruits that remain green when ripe (kiwi) or those that owe their final color to anthocyanins (strawberries and other berries and olives) the carotenoid content can decrease over ripening [182].
Part of the Tissue
The carotenoid content is not normally evenly distributed in the plant structures. Indeed, it can vary considerably both longitudinally and transversally as it can be observed with the naked eye in foods like tomato fruits or carrots. Considering this is essential when generating compositional data on carotenoids [86]. A correct sampling procedure would involve, among many other steps, to quarter longitudinally the sample and then take and combine opposite sections [190].
Location of the Fruit in the Plant
The position of a carotenogenic fruit in the plant can have an important effect in their carotenoid levels. As an example, statistically significant differences in carotenoid contents have been reported in diverse tomato genotypes as a function of the location of the tomato clusters, although a consistent pattern of changes valid for all carotenoids and varieties was not observed. The authors argued that differences in the carotenoid content depending on the location could be due to differences in exposure to radiation [141], which is a factor known to affect carotenoid biosynthesis, as discussed below.
3.2.3. Ambient Factors
Light Quality
It is well known that the spectral quality of the light that reaches the plant can have an impact in the biosynthesis of secondary metabolites. Thus, the use of shade nettings that allow for the selection of the desired spectral light is becoming an important technological concept in agronomy [191]. As an example, it has been reported that blue light can have a positive impact on the accumulation of lycopene and β-carotene in tomatoes [133]. On the other hand, short-term ultraviolet B (UV-B) irradiation on sweet basil did not lead to marked increases in the carotenoid levels of young plants and decreased them in flowering plants [192].
Light Quantity
The effect of high light exposure or intensity on the levels of not only carotenoids but also other secondary metabolites is thought to be positive in general [193]. For example, high light exposure can lead to increases in total carotenoids in the peel of apples [194] or in tomatoes [132].
Interestingly, the fine tuning dosage of blue light could be a good strategy to increase the chlorophyll and carotenoid levels of microgreens like parsley, mustard or beet, where increases in these compounds ranging from 1.2-fold to 4.3-fold have been observed [110].
Climate, Season and Geographic Site of Production
Climate is a complex phenomenon that is interrelated with factors including season or geographical location, among others.
Some studies carried out in Brazil with papayas [195], West Indian cherry [182] or mangoes [196] indicate that, in some cases, climatic factors can have a greater impact on the biosynthesis of carotenoids than cultivar differences and that greater exposure to sunlight and higher temperatures can elevate the carotenoid levels in some fruits. In some cases, 5–6-fold differences in the levels of carotenoids between different geographical regions were observed [182]. A similar conclusion was drawn from a study in which the carotenoid levels in diverse tomatoes grown in Ireland and Spain were compared [131].
Concerning seasonal effects, significant differences in the levels of both total and some individual carotenoids of kale have been reported, the total carotenoid content being higher in winter than in summer, 1.25-fold for cultivar cv. Manteiga [197] and 2-fold for galega v. acephala [32]. In a study in which the effect of regulated deficit irrigation, cluster, developmental stages and two seasons (autumn and spring) on the carotenoid levels of “Lazarino” and “Summerbrix” tomatoes was evaluated, it was concluded that, overall, lower levels of individual and total carotenoids were observed in autumn [198].
Features of climate changes, for instance the increase in temperature, the elevated atmospheric CO2 and the arrival of more UVB rays on Earth due to the decrease in the ozone layer seems to influence the content of carotenoids in food. In an experimental study the influence of temperature on total carotenoid content was evaluated and it was found that the total carotenoid content of five tomato varieties was lower at 35.4 °C than at 33.4 °C [199]. A meta-analysis conducted showed that in general an exposure of plants to high levels of CO2 decreased the content of carotenoids by 15%, with some exceptions, mainly when the plants were abiotically stressed, in which the concentration of carotenoids increased [200]. Plantains grown in the south hemisphere contained the more carotenoids provitamin A, α-carotene and β-carotene the greater the incidence of UVB rays [201].
3.2.4. Agronomic Practices
Salinity Stress
Diverse treatments leading to salinity stress have been shown to increase lycopene and other carotenoid levels (in some cases up to 3-fold) in different tomato genotypes [127,134]. Salinity stress has also been shown to lead to elevated carotenoid contents in red pepper [202] and, more markedly, in romaine lettuce [103].
Water Deficit
The availability of water is an important problem in many parts of the world, so the importance of implementing practices leading to efficient water usage in agriculture continues growing. The effect of water stress or deficit irrigation on the commercial quality or other parameters, like the levels of secondary metabolites, is therefore a timely research topic. As an example, it has been observed that a regulated deficit irrigation with a reduction of 40–50% in the leaf water potential in some tomato varieties can lead to increases in the levels of not only carotenoids but also phenolic compounds without affecting significantly their commercial quality. More specifically, ca. 1.5-fold increases in lycopene and total carotenoid levels were observed in some cases [141]. Deficit irrigation has also been reported to reduce yield, but not lycopene and fruit quality in certain watermelon varieties [203]. Interestingly, grafting of mini-watermelon under irrigation deficit led to increases of lycopene levels ca. 40% compared to the ungrafted counterparts [204]. Grafting under water stress could also lead to increases in lycopene levels in cherry tomatoes [205]. Contrastingly, regulated deficit irrigation has also been shown to reduce carotenoid levels in peaches [206]. On the other hand, water stress has been reported to significantly reduce the levels of capsanthin in red peppers, the degree of reduction being related to the intensity of the stress [207].
Use of Agrochemicals
As part of an interesting study, the carotenoid levels of kales cv. “Manteiga” grown in a farm not using agrochemicals were compared with those grown in a neighboring farm that used glyphosate (herbicide), ethyl parathion (insecticide) and a leaf fertilizer containing nitrogen, phosphorus and potassium. The results indicated that the total carotenoid contents were significantly higher (1.2-fold) in the farm not using agrochemicals [182,198]. Significantly higher lycopene contents (ranging from 1.6-fold to 2-fold) were found in Rio Red grapefruits grown conventionally (using diverse fertilizers and products for pest and weed control) relative to organically-grown counterparts in a study in which the fruits were harvest at the early, mid and late season for three consecutive years [54]. There is also evidence that fertilization with nitrogen can affect positively the accumulation of carotenoids in red pepper [208] and carrot [209] and negatively in tomatoes [210], whereas phosphorous or potassium can have a positive effect in this crop [210].
3.3. Post-Harvest Treatments, Industrial Processing, Cooking and Storage Conditions
These topics are dealt with in detail elsewhere. In general, the losses of carotenoids increase with the intensity and the time of the treatments, with important differences among matrices and carotenoid species [118,211].
It is well-known that the synthesis of carotenoids can continue after harvest if the carotenogenic material remains intact, hence the carotenogenesis can be controlled to some extent by modulating parameters such as temperature, atmosphere or light, among others. Again, different behavior due factors including matrices, carotenoid species or conditions of the treatment have been reported. For instance, freezing storage for 6 months had no significant effect on β-carotene content of peas and carrots, although frozen storage for one year led to a decrease in α- and β-carotene in processed carrots [211].
It is very important to note that very frequently; published papers report that industrial processing or cooking lead to increases of the levels of carotenoids, these results being in most cases erroneous. Thus, in most cases the treatments inactivate proteins (including of course carotenogenic enzymes) and leads to carotenoid degradation. Claims about enhanced levels of carotenoids as a result of such industrial or culinary treatments should be accompanied by supporting evidence, for instance upregulation of carotenogenic genes or downregulation of genes encoding carotenoid oxygenases, which cleave carotenoids into retinoids, apocarotenoids or other products [13].
Another reason is that, very frequently, those treatments enhance the extractability of carotenoids from treated compared to raw matrices due to the changes such as the softening of membranes or walls [182,212]. In relation to this, it is important to note that, depending on the conditions; some treatments can decrease the content of carotenoids in the food but enhance their release from it during digestion, having therefore a potential positive impact on their bioavailability. Examples of this have been reported in carrot [166], tomato [138] and orange products [37], among others. On the other hand, sometimes, an important source of error is that the changes in the weight of the processed or cooked food are not taken into account; hence, the reported retentions of carotenoids are not realistic. Several formulae for their calculation are recommended in a reference text by Rodriguez-Amaya [182]:
| % retention = (carotenoid content per g of cooked food × g of food after cooking/carotenoid content per g of raw food × g of food before cooking) × 100 | (1) |
| % retention = carotenoid content per g of cooked food (dry basis)/carotenoid content per g of raw food (dry basis) × 100 | (2) |
| % retention = carotenoid content (after cooking) per g of original raw food/carotenoid content per g of raw food × 100 | (3) |
Apart from losses of carotenoids during processing, cooking and/or storage other phenomena including geometrical isomerization [213,214] or 5,6-epoxide to 5,8-furanoid rearrangements can take place [31,197]. Given that both kind of isomerizations are accompanied by changes in the light absorption spectra [186], noticeable food color changes as a result of them could be expected in some cases.
Taken together, it can be readily inferred that the levels of carotenoids do vary considerably in any food even in set of samples from the same geographical area. As some examples of this fact in samples analyzed in the same laboratory, 18–20-fold differences have been reported in marketed orange juices and tomato fruits [31,140,215] and 60-fold differences in olive oils [216].
4. Conclusions and Research Needs
The carotenoid analytical data collected for this work, showed a good average quality index, 24/35, according to the EuroFIR classification. It has been concluded that there is the need to improve the description and/or allocation of more resources to the definition of sampling plans and to the external control of the laboratory analytical methods to increase the number of traceable and comparable results. Only a complete analytical method validation, including accuracy evaluation, enables the estimation of the uncertainty of the measurement results (topic mentioned in only two of the publications analyzed) in order to assess its impact on studies based on dietary carotenoid content and in the assessment of actual differences in carotenoid levels. The uncertainty evaluation will also enable the definition of the number of significant figures of the results, avoiding the false impression of high accuracy of the results published with too many significant figures without support.
The analytical methods used to obtain the collected data generally included several mass transfer steps and in some cases a saponification reaction, which do lead to carotenoid losses, Hence it is important to mention clearly when the saponification step is included and measures taken to correct for losses (e.g., internal standards choice). On the other hand, the complete separation of the carotenoids in the chromatographic columns is in some cases difficult and requires specialized technicians.
The investment in faster analytical methods, less prone to error and using more environmentally friendly reagents and in less quantity will be an asset for the determination of carotenoids in foods. The analytical distinction between the E and Z isomers of the different carotenoids may be important to gain further insight into differences in bioaccessibility/bioavailability, vitamin A activity and other bioactivities.
Foods contain different carotenoids in different levels, so it is advisable to consume a diversified diet to obtain appropriate levels of the major health-promoting dietary carotenoids. This approach is also advised in the case of contaminants to minimize exposure to each contaminant.
Although food carotenoid databases are very useful tools to establish recommended intakes, bioaccessibility/bioavailability studies and more integrated studies considering all diet/components/individuals interactions are also needed as the carotenoid status depend on factors depending on the matrix, other dietary components and the individual. Although there is ample evidence that a diet rich in fruits and vegetables (e.g., Mediterranean Diet) has positive effects on human health and, establishing recommendations for dietary carotenoids intakes would contribute to reinforcing the education/informed choices by the population. In addition, it could contribute to decrease the consumption of heavily processed foods, which are often rich in saturated fat, sugar and salt. Provitamin A carotenoids are particularly very important for populations with limited availability of animal foods or for those that do not eat them by choice. In line with this fact, it is important to improve their conversion into vitamin A activity, overcoming limitations of RA and RAE formulas, and to appropriately consider them in the nutritional labeling of foods.
The wide variations in the carotenoid contents in different varieties/cultivars/landraces/accessions of a certain species illustrate the importance and the need for their documentation in food composition databases. This collection includes little consumed foods with very high levels of carotenoids (for instance rosehip or sarsaparrilla). This can contribute to take better advantage of biodiversity to enhance the carotenoid intake in particular. This database can be useful to include the carotenoid content/nutritional value criterion on the choice of varieties for agricultural cultivation, in addition to others such as high production or resistance to transport and pests. In environmental terms, studies on the carbon or water footprints of foods in general are also necessary as diet and environment are essential elements for human health.
Acknowledgments
Quality technical assistance from Ana Benítez (UE) and of Milana Rosul (IFTNS) is acknowledged.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10050912/s1, Table S1: Coffee, cocoa, tea and infusions (A03GG). Table S2: Seasoning, sauces and condiments (A042N). Table S3: Composite dishes (A03VA). Table S4: Fruit and vegetable juices and nectars (including concentrates) (A039K). Table S5: Animal and vegetable fats and oils and primary derivatives thereof (A036M). Table S6: Fruit and fruit products (A01BS). Table S7: Legumes, nuts, oilseeds and spices (A011X). Table S8: Starchy roots or tubers and products thereof, sugar plants (A00ZR). Table S9 Vegetables and vegetable products (A00FJ). Table S10: Grains and grain-based products (A000J).
Appendix A
Table A1.
Root and tuber vegetables (excluding starchy- and sugar-) (A00QF) (µg/100 g).
| Food Name | Scientific Name | Origin (Country) | Purchase (Country) | Water (%) | Part Analysed | Colour | α-Carotene | β-Carotene | β-Cryptoxanthin | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Carrot | Daucus carota L. | United Kingdom | United Kingdom | peeled | 4450 ± 940 | 32,000 ± 2880 | [95] | |||
| Carrot | Daucus carota L. | Italy | Italy | 2840–4960 | 4350–8840 | [25] | ||||
| Carrot | Daucus carota L. | Germany | Germany | 91.3 | edible part | 4890 | 9020 | [53] | ||
| Carrot | Daucus carota L. | Germany | Germany | 84.3 | edible part | 3060 | 6500 | 12 | [53] | |
| Carrot | Daucus carota L. | Germany | Germany | edible part | 4120 | 4650 | 28 | [53] | ||
| Carrot | Daucus carota L. | Spain | Spain | edible part | orange | 3245 | 8162 | [24] | ||
| Carrot | Daucus carota L. | Spain | Spain | edible part | orange | 2895 | 6628 | [24] | ||
| Carrot | Daucus carota L. | Spain | Spain | edible part | orange | 3700 | 9800 | [24] | ||
| Carrot | Daucus carota L. | Turkey | Turkey | root | orange | 1344–3011 | 4160–7162 | [156] | ||
| Carrot | Daucus carota L. | Spain | Spain | edible part | 3245 | 8162 | [24] | |||
| Carrot | Daucus carota L. | Spain | Spain | edible part | 2895 | 6628 | [24] | |||
| Carrot | Daucus carota L. | Italy | Italy | all sample | L*52.2 ± 0.7a*24.1 ± 1.5b*36.1 ± 1 | 82,100 ± 1100 | 128,400 ± 800 | [111] | ||
| Carrot | Daucus carota L. | Italy | Italy | all sample | L*52.1 ± 0.8a*22.6 ± 1.1b*35.7 ± 1.9 | 85,600 ± 2400 | 101,600 ± 700 | [111] | ||
| Carrot | Daucus carota L. | Italy | Italy | all sample | L*50.2 ± 1.1a*21.4 ± 1.4b*35.3 ± 1.5 | 68,100 ± 9100 | 113,000 ± 16,700 | [111] | ||
| Carrot | Daucus carota L. | Poland | Poland | roots | 4820–9520 | [157] | ||||
| Carrot | Daucus carota L. cv Nerac | Ireland | Ireland | roots | 188,000 ± 5000 | [158] | ||||
| Carrot | Daucus carota L. cv. Nantes | Brazil | tuber | orange | 3500 | 6150 | nd | [109] | ||
| Carrot | Daucus carota L. HCM | France | France | root | dark-orange | 7583± 619 | 17,206 ± 643 | [159] | ||
| Carrot | Daucus carota L. subsp. sativus | Finland | Finland | 2200–4900 | 4600–10,300 | [86] | ||||
| Carrot | Daucus carota L. subsp. sativus | Spain | Spain | 2900 ± 300 | 6600 ± 0 | [86] | ||||
| Carrot | Daucus carota L. subsp. sativus | United Kingdom | United Kingdom | 2700–3600 | 8500–10,800 | [86] | ||||
| Carrot | Daucus carota L. subsp. sativus | USA | 3900 | 5600 | [86] | |||||
| Carrot | Daucus carota L. subsp. sativus | Egypt | 3400 | 6300 | [86] | |||||
| Carrot | Daucus carota L. subsp. sativus | Taiwan | 2800 ± 300 | 5400 ± 600 | [86] | |||||
| Carrot | Daucus carota L. subsp. sativus | Malaysia | 3400 | 6800 | [86] | |||||
| Carrot | Daucus carota L. var. Commercial French | France | France | root | orange | 2322 ± 233 | 5404 ± 305 | [159] | ||
| Carrot | Daucus carota L. var. Blanche à collet vert | France | France | root | white | nd | nd | [159] | ||
| Carrot | Daucus carota L. var. Blanche des vosges | France | France | root | white | nd | nd | [159] | ||
| Carrot | Daucus carota L. var. Carentan | France | France | root | orange | 1644 ± 50 | 5932 ± 360 | [159] | ||
| Carrot | Daucus carota L. var. Commercial French | France | France | root | orange | 1972± 183 | 5433 ± 462 | [159] | ||
| Carrot | Daucus carota L. var. Commercial French | France | France | root | orange | 3131 ± 263 | 6633 ± 564 | [159] | ||
| Carrot | Daucus carota L. var. Commercial French | France | France | root | orange | 1419 ± 99 | 4149 ± 112 | [159] | ||
| Carrot | Daucus carota L. var. Commercial French | France | France | root | orange | 2291 ± 224 | 6190 ± 403 | [159] | ||
| Carrot | Daucus carota L. var. Commercial French | France | France | root | orange | 1916 ± 138 | 4730 ± 319 | [159] | ||
| Carrot | Daucus carota L. var. Kokubu | France | France | root | orange | 1748 ± 29 | 3740 ± 25 | [159] | ||
| Carrot | Daucus carota L. var. La Merveille | France | France | root | orange | 2092 ± 36 | 5869 ± 101 | [159] | ||
| Carrot | Daucus carota L. var. Nantaise améliorée | France | France | root | orange | 1369 ± 150 | 3625 ± 329 | [159] | ||
| Carrot | Daucus carota L. var. San NaÏ | France | France | root | orange | 1333 ± 85 | 3206 ± 182 | [159] | ||
| Carrot | Daucus carota L. var. sativa, D.C. | Spain | Spain | 88 | root | orange | 2895 ± 276 | 6628 ± 45 | [24] | |
| Carrot | Daucus carota L. var. sativa, D.C. | Spain | Spain | 90 | root | orange | 3245 ± 128 | 8162 ± 364 | [24] | |
| Carrot | Daucus carota L. var. Violette jordanienne | France | France | root | purple | nd | 381 ± 24 | [159] | ||
| Carrot | Daucus carota L. var. Violette turque | France | France | root | purple | nd | 318 ± 18 | [159] | ||
| Carrot | Daucus carota L. var. yellowstone | France | France | root | yellow | nd | 332 ± 15 | [159] | ||
| Carrot | Daucus carota L., var. Bolero | Turkey | Turkey | root | 1642 ± 101 3011 ± 217 |
4826 ± 465 7162 ± 503 |
[160] | |||
| Carrot | Daucus carota L., var. Fontana | Norway | Norway | root | 5490–11,270 | 2210–5510 | [161] | |||
| Carrot | Daucus carota L., var. Maestro-F1 | Turkey | Turkey | root | 1706 ± 60 2478 ± 179 |
4160 ± 148 6706 ± 42 |
[160] | |||
| Carrot | Daucus carota L., var. Merida | Norway | Norway | root | 5550–10,200 | 1980–5220 | [161] | |||
| Carrot | Daucus carota L., var. Nanco | Turkey | Turkey | root | 1473 ± 34 2630 ± 33 |
4310 ± 118 6440 ± 146 |
[160] | |||
| Carrot | Daucus carota L., var. Nandrin | Norway | Norway | root | 5630–12,080 | 1910–4530 | [161] | |||
| Carrot | Daucus carota L., var. Nantindo | Turkey | Turkey | root | 1665 ± 113–3006 ± 66 | 4767 ± 123 6637 ± 58 |
[160] | |||
| Carrot | Daucus carota L., var. Newburg | Norway | Norway | root | 6100–10,800 | 2370–4090 | [161] | |||
| Carrot | Daucus carota L., var. Presto-F1 | Turkey | Turkey | root | 2346 ± 45 2367 ± 217 |
5185 ± 468 6198 ± 138 |
[160] | |||
| Carrot | Daucus carota L., var. PS-F1 | Turkey | Turkey | root | 2688 ± 225 2967 ± 36 |
6168 ± 302 6373 ± 476 |
[160] | |||
| Carrot | Daucus carota L., var. Tito | Turkey | Turkey | root | 1344 ± 17 1718 ± 153 |
4563 ± 311 4835 ± 473 |
[160] | |||
| Carrot | Daucus carota var. Jaune obtuse du Doubs | France | France | root | yellow | nd | 332 ± 21 | [159] | ||
| Carrot | Daucus carota var. New Kuroda | France | France | root | orange | 1635 ± 17 | 3632 ± 64 | [159] | ||
| Carrot | Daucus carota L. var. De Guérande | France | France | root | orange | 1278 ± 234 | 3354 ± 457 | [159] |
Table A2.
Spinaches and similar (A00MH) (µg/100 g).
| Food Name | Scientific Name | FoodEx2_ TermCode | Origin (Country) | Purchase (Country) | Water (%) | Part Analysed | Colour | α-Carotene | β-Carotene | Lutein | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spinach | Spinacea oleracea L. | A00MJ | Spain | Spain | edible part | green | 4626 | 6422 | [24] | ||
| Spinach | Spinacea oleracea L. | A00MJ | Spain | Spain | 92 | leaves + stalk | green | 3254 ± 330 | 4229 | [24] | |
| Spinach | Spinacea oleracea L. | A00MJ | Spain | Spain | 92 | leaves + stalk | green | 4626 ± 346 | 4229 ± 1310 | [24] | |
| Spinach | Spinacea oleracea, L. | A00MJ | Spain | Spain | edible part | green | 3254 | 6422 ± 1190 | [24] | ||
| Spinach | Spinacia oleracea L. | A00MJ | Brazil | leaves | green | nd | 4423 | 5793 | [109] | ||
| Spinach | Spinacia oleracea L. | A00MJ | Italy | Italy | nd | 3100–4810 | 5930–7900 | [25] | |||
| Spinach | Spinacia oleracea L. | A00MJ | Germany | Germany | 90.8 | edible part | 90 | 3250 | 9540 | [55] | |
| Spinach | Spinacia oleracea L. | A00MJ | Spain | Spain | edible part | 4626 | 8700 ± 100 | [28] | |||
| Spinach | Spinacia oleracea L. | A00MJ | Spain | Spain | edible part | 3254 | 218,700 ± 15,400 | [28] | |||
| Spinach | Spinacia oleracea L. | A00MJ | Italy | Italy | all sample | L*30.5 ± 1.1a*8 ± 0.6b*10 ± 0.8 | 152,100 ± 5000 | 167,000 ± 10,400 | [111] | ||
| Spinach | Spinacia oleracea L. | A00MJ | Italy | Italy | all sample | L*33.7 ± 1.1a*7.7 ± 1.1b*9.3 ± 1 | 148,100 ± 6500 | 194,200 ± 4200 | [111] | ||
| Spinach | Spinacia oleracea L. | A00MJ | Italy | Italy | all sample | L*33.1 ± 0.8a*6.9 ± 0.9b*9.2 ± 0.8 | 18,4600 ± 9300 | 6422 | [111] | ||
| Spinach | Spinacia oleracea L. | A00MJ | Slovenia | Slovenia | 90.7 | leaves | green | 4840–13,900 | [114] |
Table A3.
Mandarins and similar (A01CB) (µg/100 g).
| Food Name | Scientific Name | FoodEx2_ TermCode | Origin (Country) | Purchase (Country) | Water (%) | Part Analysed | Colour | E(v. Trans)-α-Carotene | E(v. Trans)-β-Carotene | β-Cryptoxanthin | Lutein | Phytoene | Phytofluene | Violaxanthin | Zeaxanthin | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mandarin | Citrus cv Mediterranean | A01CD | Italy | Italy | essential oil of peel fruit (free) | 610 ± 62 | [58] | |||||||||
| Mandarin | Citrus cv Mediterranean | A01CD | Italy | Italy | essential oil of peel fruit (laurate) | 1411 ± 140 | [58] | |||||||||
| Mandarin | Citrus cv Mediterranean | A01CD | Italy | Italy | essential oil of peel fruit (myristate) | 1504 ± 132 | [58] | |||||||||
| Mandarin | Citrus cv Mediterranean | A01CD | Italy | Italy | essential oil of peel fruit (palmitate) | 715 ± 74 | [58] | |||||||||
| Mandarin | Citrus deliciosa, Ten. | A01CJ | Spain | Spain | 86 | without skin | orange | 213 ± 102 | 843 ± 216 | [24] | ||||||
| Mandarin | Citrus deliciosa, Ten. | A01CJ | Spain | Spain | 85 | without skin | orange | 130 ± 10 | 1106 ± 63 | [24] | ||||||
| Mandarin | Citrus reticulata | A01CD | Spain | Spain | orange | 213 | 843 | [24] | ||||||||
| Mandarin | Citrus reticulata Blanco cv. Hansen | A01CE | France | France | juice fruit | 197 | 1500 | 163 | 446 | 500 | 467 (cis) | 128 | [43] | |||
| Mandarin | Citrus reticulata Blanco cv. Hansen | A01CE | France | France | juice fruit | 70 | 162 | 230 | 100 | 110 | 445 (cis) | 143 | [43] | |||
| Mandarin | Citrus reticulata Blanco cv. Hansen | A01CE | French Polynesia | juice fruit | 76 | 916 | 210 | 24 | 92 | 560 (cis) | 131 | [43] | ||||
| Mandarin | Citrus reticulate, L. var. Tango | A01CD | Spain | Spain | fruit | orange | 12.4 | 547 | 1331.6 | [56] |
Table A4.
Cereal grains (and cereal-like grains) (A000L) (µg/100 g).
| Food Name | Scientific Name | FoodEx2_ TermCode | Origin (Country) | Purchase (Country) | Saponification | Part Analysed | Colour | α-Carotene | (v. Trans)-α-Carotene | β-Carotene | E(v. Trans)-β-Carotene | β-Cryptoxanthin | E(V. Trans)-Lutein | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maize | Zea mays L. | A000T | Spain | Spain | edible part | yellow | 33 | 30 | [24] | |||||
| Maize | Zea mays L. | A000T | Croatia | Croatia | all sample | 117.3± 8.17 | 158.5 ± 9.5 | [174] | ||||||
| Maize | Zea mays L. | A000T#F28.A07KQ$F28.A0BA1 | USA | no | 15 | 14 | 0 | 202 | [28] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 20 ± 7 | 424 ± 6 | 157 ± 3 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 44 ± 9 | 447 ± 28 | 29 ± 2 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 58 ± 0 | 40 ± 2 | 93 ± 1 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 3 ± 1 | 246 ± 8 | 453 ± 8 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 44 ± 1 | 879 ± 28 | 37 ± 3 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 41 ± 7 | 448 ± 15 | 260 ± 10 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 17 ± 0 | 368 ± 2 | 988 ± 5 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 23 ± 2 | 56 ± 2 | 37 ± 2 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 11 ± 0 | 37 ± 0 | 41 ± 4 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 6 ± 4 | 253 ± 13 | 375 ± 15 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 16 ± 3 | 303 ± 16 | 251 ± 8 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 86 ± 6 | 277 ± 8 | 84 ± 2 | [175] | |||||
| Maize | Zea mays L. | A000T | Netherlands | Netherlands | kernels | 23 ± 1 | 305 ± 20 | 371 ± 15 | [175] |
Table A5.
Cucurbits with inedible peel (A00KD) (µg/100 g).
| Food Name | Scientific Name | FoodEx2_ TermCode | Origin (Country) | Purchase (Country) | Water (%) | Part Analysed | Colour | α-Carotene | β-Carotene | β-Cryptoxanthin | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pumpkin | Cucurbita maxima | A00KH | Spain | Spain | edible part | 490 | 60 | [24] | |||
| Pumpkin | Cucurbita maxima | A00KH | Spain | Spain | edible part | 31 | 188 | [24] | |||
| Pumpkin | Cucurbita maxima | A00KH | Spain | Spain | edible part | 53 | 692 | [24] | |||
| Pumpkin | Cucurbita maxima var. Autumn Cup | A00KH | Austria | Austria | flesh fruit | 800 | 5200 | [152] | |||
| Pumpkin | Cucurbita maxima var. Buen Gusto | A00KH | Austria | Austria | flesh fruit | 1000 | 3300 | [152] | |||
| Pumpkin | Cucurbita maxima var. Flat white Boer | A00KH | Austria | Austria | flesh fruit | 7500 | 6200 | [152] | |||
| Pumpkin | Cucurbita maxima var. Gelber Zentner | A00KH | Austria | Austria | flesh fruit | 0 | 2200 | [152] | |||
| Pumpkin | Cucurbita maxima var. Hyvita | A00KH | Austria | Austria | flesh fruit | 990 | 2500 | [152] | |||
| Pumpkin | Cucurbita maxima var. Imperial Elite | A00KH | Austria | Austria | flesh fruit | 1100 | 7400 | [152] | |||
| Pumpkin | Cucurbita maxima var. Japan 117 | A00KH | Austria | Austria | flesh fruit | 1000 | 7200 | [152] | |||
| Pumpkin | Cucurbita maxima var. Mini green Hubbard | A00KH | Austria | Austria | flesh fruit | 420 | 1400 | [152] | |||
| Pumpkin | Cucurbita maxima var. Mishti kumra | A00KH | Bangladesh | fruit | 362 ± 89.3 | [157] | |||||
| Pumpkin | Cucurbita maxima var. Snow Delite | A00KH | Austria | Austria | flesh fruit | 1500 | 6400 | [152] | |||
| Pumpkin | Cucurbita maxima var. Uchiki Kuri | A00KH | Austria | Austria | flesh fruit | 1400 | 2500 | [152] | |||
| Pumpkin | Cucurbita maxima var. Umber Cup | A00KH | Austria | Austria | flesh fruit | 790 | 3700 | [152] | |||
| Pumpkin | Cucurbita maxima var. Walfish | A00KH | Austria | Austria | flesh fruit | 900 | 4300 | [152] | |||
| Pumpkin | Cucurbita maxima x C. moschata var. Tetsuka Buto | A00KH | Austria | Austria | flesh fruit | 2400 | 3500 | [152] | |||
| Pumpkin | Cucurbita moschata Duchesne cv. Menina Brasileira and cv. Goianinha | A0DLT#F20.A07QF$F20.A07RD | Brazil | without skin and seed | orange | 2530 | 6170 | nd | [109] | ||
| Pumpkin | Cucurbita moschata var. Burpee Butterbush | A0DLT | Austria | Austria | flesh fruit | 980 | 3100 | [152] | |||
| Pumpkin | Cucurbita moschata var. Long Island Cheese | A0DLT | Austria | Austria | flesh fruit | 5900 | 7000 | [152] | |||
| Pumpkin | Cucurbita moschata var. Martinica | A0DLT | Austria | Austria | flesh fruit | 1600 | 5400 | [152] | |||
| Pumpkin | Cucurbita moschata var. Mousquée de Provence | A0DLT | Austria | Austria | flesh fruit | 2800 | 4900 | [152] | |||
| Pumpkin | Cucurbita pepo | A00KH | Italy | Italy | 490 | 60 | [25] | ||||
| Pumpkin | Cucurbita pepo L. | A00KH | Poland | Poland | 8.2 | seed and oil | yellow-green | 10–20 | 80–210 | 20–50 | [153] |
| Pumpkin | Cucurbita pepo var. Acorn Table | A00KH | Austria | Austria | flesh fruit | 150 | 2100 | [152] | |||
| Pumpkin | Cucurbita pepo var. Acorn Tay Bell | A00KH | Austria | Austria | flesh fruit | 150 | 2100 | [152] | |||
| Pumpkin | Cucurbita pepo var. Carneval di Venezia | A00KH | Austria | Austria | flesh fruit | 30 | 60 | [152] | |||
| Pumpkin | Cucurbita pepo var. Melonette Jaspée Vende | A00KH | Austria | Austria | flesh fruit | 50 | 1300 | [152] | |||
| Pumpkin | Cucurbita pepo var. Tonda padana (Americano) | A00KH | Austria | Austria | flesh fruit | 120 | 2300 | [152] | |||
| Pumpkin | Curcubita maxima | A00KH | Spain | Spain | edible part | orange | 490 | 60 | [24] | ||
| Pumpkin | Curcubita maxima | A00KH | Spain | Spain | edible part | orange | 31 | 188 | [24] | ||
| Pumpkin | Curcubita maxima | A00KH | Spain | Spain | edible part | orange | 53 | 692 | [24] |
Table A6.
Rose hips and similar (A01FR) (µg/100 g).
| Food Name | Scientific Name | FoodEx2_ TermCode | FoodEx2_ TermName | Origin (Country) | Purchase (Country) | Part Analysed | Colour | β-Carotene | E(v. Trans)-β-Carotene | Z(v. cis)-Β-carotene | E(v. Trans)-β- | Phytoene | Phytofluene | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rosehip | Rosa canina L. | A0DSS | Dog rose | Turkey | Turkey | whole plants | red and pink | 18–370 | [75] | |||||
| Rosehip | Rosa canina L. | A0DSS | Dog rose | Germany | Germany | all sample | 2495 ± 207.5 | [76] | ||||||
| Rosehip | Rosa canina L. | A0DSS | Dog rose | Germany | Germany | all sample | 500 ± 35 | [76] | ||||||
| Rosehip | Rosa canina L. | A0DSS | Dog rose | Germany | Germany | all sample | 290 ± 32.5 | [76] | ||||||
| Rosehip | Rosa canina L. | A0DSS | Dog rose | Germany | Germany | pulp | 3200 ± 200 | 500 ± 320 | 1200 ± 100 | 400 ± 100 | nd | [77] | ||
| Rosehip | Rosa canina L. | A0DSS | Dog rose | Germany | Germany | pulp | 4200 ± 200 | 900 ± 240 | 100 ± 100 | 700 ± 100 | [77] |
Table A7.
Rose hips and similar (A01FR) (µg/100 g) (continuation).
| Food Name | Scientific Name | FoodEx2_ TermCode | FoodEx2_ TermName | Origin (Country) | Purchase (Country) | Part Analysed | Colour | Lycopene | E(v. Trans)-Lycopene | Z(v. Cis)-Lycopene | Violaxanthin | E(v. Trans)-Lutein | E(v. Trans)-Zeaxanthin | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rosehip | Rosa canina L. | A0DSS | Dog rose | Germany | Germany | all sample | 23,842.5 ± 777.5 | [76] | ||||||
| Rosehip | Rosa canina L. | A0DSS | Dog rose | Germany | Germany | all sample | 3615 ± 215 | [76] | ||||||
| Rosehip | Rosa canina L. | A0DSS | Dog rose | Germany | Germany | pulp | 7900 ± 1500 | 8400 ± 1600 | 300 ± 10 | 100 ± 10 | 2700 ± 30 | [77] | ||
| Rosehip | Rosa canina L. | A0DSS | Dog rose | Germany | Germany | pulp | 7400 ± 1200 | 6300 ± 600 | 700 ± 100 | 600 ± 100 | [77] |
Table A8.
Cucurbits with inedible peel (A00KD) (µg/100 g).
| Food Name | Scientific Name | FoodEx2_ TermCode | Origin (Country) | Purchase (Country) | Water (%) | Saponi-Fication | Part Analysed | Colour | α-Carotene | β-Carotene | E(v. Trans)-β-Carotene | β-Cryptoxanthin | Lycopene | E(v. Trans)-Lycopene | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Watermelon | Citrullus lanatus (Thunb) Matsumura & Nakai | A00KJ | Italy | Italy | red | 46–546 | 959–1681 | [154] | |||||||
| Watermelon | Citrullus lanatus | A00KJ | Spain | Spain | edible part | red | 77 | 62 | 2454 | [24] | |||||
| Watermelon | Citrullus lanatus | A00KJ | USA | no | 0 | 126 | 5 | [28] | |||||||
| Watermelon | Citrullus lanatus (Thunb.) Matsum & Nakai | A00KJ | Brazil | red | nd | 365 | nd | 3550 | [109] | ||||||
| Watermelon | Citrullus vulgaris | A00KJ#F10.A0F2S | Indonesia | fruit | red | 592 (314–777) | nd | 11,389 (8731–13,523) | [59] | ||||||
| Watermelon | Citrullus vulgaris | A00KJ#F10.A0F5H | Indonesia | fruit | yellow | 140 (56–287) | 90 (59–110) | 71 (nd -109) | [59] | ||||||
| Watermelon | Citrullus vulgaris | A00KJ#F10.A0F2S | Italy | Italy | nd | 314–777 | nd | 4770–13,523 | [25] | ||||||
| Watermelon | Citrullus vulgaris | A00KJ#F10.A0F5H | Italy | Italy | nd | 56–287 | 59–110 | nd–109 | [25] | ||||||
| Watermelon | Citrullus vulgaris, Schered. | A00KJ#F20.A07QF$F20.A07RD | Spain | Spain | 92 | without rind or seeds | red | 77 ± 29 | 62 ± 20 | 2454± 319 | [24] | ||||
| Watermelon | Citrullus vulgaris, Schrad | A00KJ | Spain | Spain | edible part | red | 57.6 ± 4.8 | 1.2 ± 0.5 | [56] | ||||||
| Watermelon | Citrullus vulgaris | A00KJ | Finland | Finland | pulp | - | 3080 | [26] |
Table A9.
Cucurbits with inedible peel (A00KD) (µg/100 g).
| Food Name | Scientific Name | FoodEx2_ TermCode | Origin (Country) | Purchase (Country) | Water (%) | Saponi-Fication | Part Analysed | Colour | Lutein | E(v. Trans)-Lutein | E(v. Trans)-Zeaxanthin | Phytofluene | Phytoene | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Watermelon | Citrullus lanatus | A00KJ | Spain | Spain | edible part | red | 40 ± 13 | 1122 ± 812 | [24] | |||||
| Watermelon | Citrullus lanatus | A00KJ | USA | no | 4 | 0 | [28] | |||||||
| Watermelon | Citrullus lanatus | A00KJ | Spain | Spain | Pulp | red | 440 | 1170 | [22] |
Author Contributions
Conceptualization, M.G.D. and A.J.M.-M.; Data curation, M.G.D., G.I.A.B., K.K., A.I.M., P.M.-B., B.O.-A., A.M.P., F.R., V.T.Š., J.S., L.V.-M., J.J.V. and A.J.M.-M.; Funding acquisition, A.J.M.-M.; Methodology, M.G.D.; Writing—original draft, M.G.D., G.I.A.B., K.K., A.I.M., P.M.-B., B.O.-A., A.M.P., F.R., V.T.Š., J.S., L.V.-M., J.J.V. and A.J.M.-M.; Writing—review and editing, M.G.D., G.I.A.B., K.K., A.I.M., P.M.-B., B.O.-A., A.M.P., F.R., V.T.Š., J.S., L.V.-M., J.J.V. and A.J.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research article is based upon work from COST Action EUROCAROTEN (European network to advance carotenoid research and applications in agro-food and health, CA15136, www.eurocaroten.eu; accessed on 1 February 2021), supported by COST (European Cooperation in Science and Technology, http://www.cost.eu/; accessed on 1 February 2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dias M.G., Olmedilla-Alonso B., Hornero-Méndez D., Mercadante A.Z., Osorio C., Vargas-Murga L., Melendez-Martínez A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018;66:5055–5107. doi: 10.1021/acs.jafc.7b06148. [DOI] [PubMed] [Google Scholar]
- 2.Meléndez-Martínez A.J., Mandić A., Bantis F., Böhm V., Borge G.I., Brnčić M., Bysted A., Cano M.P., Dias M.G., Elgersma A., et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2021:1–51. doi: 10.1080/10408398.2020.1867959. [DOI] [PubMed] [Google Scholar]
- 3.Meléndez-Martínez A.J., Böhm V., Borge G.I., Cano M.P., Fikselová M., Gruskiene R., Lavelli V., Loizzo M.R., Mandić A.I., Mapelli-Brahm P., et al. Carotenoids: Considerations for Their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals, and Novel Foods in the Context of Sustainability, Circular Economy, and Climate Change. Annu. Rev. Food Sci. Technol. 2021;12:433–460. doi: 10.1146/annurev-food-062220-013218. [DOI] [PubMed] [Google Scholar]
- 4.European Commission. Scientific Committee on Food. European Food Safety Authority. Scientific Panel on Dietetic Products Nutrition and Allergies . Tolerable Upper Intake Levels for Vitamins and Minerals. European Food Safety Authority; Parma, Italy: 2006. [Google Scholar]
- 5.National Research Council (U.S.) Institute of Medicine (U.S.) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press; Washington, DC, USA: 2000. A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. [PubMed] [Google Scholar]
- 6.Buscemi S., Corleo D., Di Pace D., Petroni M.L., Satriano A., Marchesini G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients. 2018;10:1321. doi: 10.3390/nu10091321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranard K., Jeon S., Mohn E., Griffiths J., Johnson E., Erdman J. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017;56:37–42. doi: 10.1007/s00394-017-1580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regulation (EU) No 1169/2011 From: European parliament and of the council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union. 2011;L304:18–63. [Google Scholar]
- 9.IOM, Institute of Medicine of National Academies (U.S.). Panel on Micronutrients . DRI: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press; Washington, DC, USA: 2001. A Report of the Institute of Medicine; Food and Nutrition Board; Panel on Micronutrients; Subcommittee on Upper Reference Levels of Nutrients; Subcommittee of Interpretation and Uses of Dietary Reference Intakes; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Institute of Medicine. [PubMed] [Google Scholar]
- 10.IOM, Institute of Medicine of National Academies (U.S.) In: Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Otten J.J., Hellwig J.P., Meyers L.D., editors. National Academies Press; Washington, DC, USA: 2006. [Google Scholar]
- 11.FAO/INFOODS . Guidelines for Converting Units, Denominators and Expressions. FAO; Rome, Italy: 2012. Version 1.0. [Google Scholar]
- 12.Granado F., Olmedilla B., Blanco I., Gil-Martinez E., Rojas-Hidalgo E., Erdman J. Variability in the intercomparison of food carotenoid content data: A user’s point of view. Crit. Rev. Food Sci. Nutr. 1997;37:621–633. doi: 10.1080/10408399709527792. [DOI] [PubMed] [Google Scholar]
- 13.Meléndez-Martínez A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019:63. doi: 10.1002/mnfr.201801045. [DOI] [PubMed] [Google Scholar]
- 14.FAO . In: The State of the World’s Biodiversity for Food and Agriculture. Bélanger J., Pilling D., editors. FAO Commission on Genetic Resources for Food and Agriculture Assessments; Rome, Italy: 2019. [(accessed on 1 February 2021)]. 572p. Available online: http://www.fao.org/3/CA3129EN/CA3129EN.pdf. [Google Scholar]
- 15.Kapsokefalou M., Roe M., Turrini A., Costa H.S., Martinez-Victoria E., Marletta L., Berry Finglas P. Food Composition at Present: New Challenges. Nutrients. 2019;11:1714. doi: 10.3390/nu11081714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvini S., Oseredczuk M., Roe M., Møller A. Guidelines for Quality Index Attribution to Original Data from Scientific Literature or Reports for EuroFIR Data Interchange. EuroFIR AISBL; Brussels, Belgium: 2009. EuroFIR Technical Report. [Google Scholar]
- 17.Mangels A.R., Holden J.M., Beecher G.R., Forman M.R., Lanza E. Carotenoid content of fruits and vegetables: An evaluation of analytic data. J. Am. Diet. Assoc. 1993;93:284–296. doi: 10.1016/0002-8223(93)91553-3. [DOI] [PubMed] [Google Scholar]
- 18.West C.E., Poortvliet E.J. In: The Carotenoid Content of Foods with Special Reference to Developing Countries. Vitamin A Field Support Project (VITAL), editor. International Science and Technology Institute, Inc.; Arlington, VA, USA: 1993. pp. 1–207. [Google Scholar]
- 19.Loranty A., Rembiałkowska E., Rosa E.A.S., Bennett R.N. Identification, quantification and availability of carotenoids and chlorophylls in fruit, herb and medicinal teas. J. Food Compos. Anal. 2010;23:432–441. doi: 10.1016/j.jfca.2010.01.007. [DOI] [Google Scholar]
- 20.Ireland J., Shahar D., Marletta L., Oyelade O.J., Khokhar S., Henauw S. Vitamin composition of ethnic foods commonly consumed in Europe. Food Nutr. Res. 2012;56:1–7. doi: 10.3402/fnr.v56i0.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masino F., Ulrici A., Antonelli A. Extraction and quantification of main pigments in pesto sauces. Eur. Food Res. Technol. 2008;226:569–575. doi: 10.1007/s00217-007-0572-5. [DOI] [Google Scholar]
- 22.Meléndez-Martínez A.J., Mapelli-Brahm P., Benítez-González A., Stinco C.M. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch. Biochem. Biophys. 2015;572:188–200. doi: 10.1016/j.abb.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Vallverdú-Queralt A., de Alvarenga J.F.R., Estruch R., Lamuela-Raventos R.M. Bioactive compounds present in the Mediterranean sofrito. Food Chem. 2013;141:3365–3372. doi: 10.1016/j.foodchem.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 24.Beltrán B., Estévez R., Cuadrado C., Jiménez S., Olmedilla-Alonso B. Carotenoid data base to assess dietary intake of carotenes, xanthophyls and vitamin A, its use in a comparative study of vitamin a nutritional status in young adults. Nutr. Hosp. 2012;27:1334–1343. doi: 10.3305/nh.2012.27.4.5886. [DOI] [PubMed] [Google Scholar]
- 25.Maiani G., Castón M.J., Catasta G., Toti E., Cambrodón I.G., Bysted A., Granado-Lorencio F., Begoña Olmedilla-Alonso B., Knuthsen P., Valoti M., et al. Carotenoids, actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009;53(Suppl. S2):S194–S218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- 26.Pól J., Hyötyläinen T., Ranta-Aho O., Riekkola M.-L. Determination of lycopene in food by on-line SFE coupled to HPLC using a single monolithic column for trapping and separation. J. Chromatogr. A. 2004;1052:25–31. doi: 10.1016/j.chroma.2004.08.111. [DOI] [PubMed] [Google Scholar]
- 27.Seybold C., Fröhlich K., Bitsch R., Otto K., Böhm V. Changes in contents of carotenoids and vitamin E during tomato processing. J. Agric. Food Chem. 2004;52:7005–7010. doi: 10.1021/jf049169c. [DOI] [PubMed] [Google Scholar]
- 28.Perry A., Rasmussen H., Johnson E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009;22:9–15. doi: 10.1016/j.jfca.2008.07.006. [DOI] [Google Scholar]
- 29.Dhuique-Mayer C., Fanciullino A.L., Dubois C., Ollitrault P. Effect of Genotype and Environment on Citrus Juice Carotenoid Content. J. Agric. Food Chem. 2009;57:9160–9168. doi: 10.1021/jf901668d. [DOI] [PubMed] [Google Scholar]
- 30.Gama J.J.T., Sylos C.M. Major carotenoid composition of Brazilian Valencia orange juice, identification and quantification by HPLC. Food Res. Int. 2005;38:8999–9903. doi: 10.1016/j.foodres.2005.03.008. [DOI] [Google Scholar]
- 31.Meléndez-Martínez A.J., Britton G., Vicario I.M., Heredia F.J. The complex carotenoid pattern of orange juices from concentrate. Food Chem. 2008;109:546–553. doi: 10.1016/j.foodchem.2008.01.003. [DOI] [Google Scholar]
- 32.Dias M.G., Camões M.F.G.F.C., Oliveira L. Carotenoids in traditional Portuguese fruits and vegetables. Food Chem. 2009;113:808–815. doi: 10.1016/j.foodchem.2008.08.002. [DOI] [Google Scholar]
- 33.Velázquez-Estrada R.M., Hernández-Herrero M.M., Rüfer C.E., Guamis-López B., Roig-Sagués A.X. Influence of ultra high pressure homogenization processing on bioactive compounds and antioxidant activity of orange juice. Innov. Food Sci. Emerg. Technol. 2013;18:89–94. doi: 10.1016/j.ifset.2013.02.005. [DOI] [Google Scholar]
- 34.Fratianni A., Cinquanta L., Panfili G. Degradation of carotenoids in orange juice during microwave heating. LWT Food Sci. Technol. 2010;43:867–871. doi: 10.1016/j.lwt.2010.01.011. [DOI] [Google Scholar]
- 35.Melendez-Martinez A., Vicario I., Heredia F.J. A routine high-performance liquid chromatography method for carotenoid determination in ultrafrozen orange juices. J. Agric. Food Chem. 2003;51:4219–4224. doi: 10.1021/jf0342977. [DOI] [PubMed] [Google Scholar]
- 36.Salvo A., Dugo P., Giuffrida D., Salvo A., Saitta M., Dugo G. Free carotenoid and carotenoid ester composition in native orange juices of different varieties. Fruits. 2010;65:277–284. doi: 10.1051/fruits/2010023. [DOI] [Google Scholar]
- 37.Stinco M.C., Fernández-Vázquez R., Escudero-Gilete M.L., Heredia F.J., Meléndez-Martínez A.J., Vicario M.I. Effect of orange juice’s processing on the color, particle size, and bioaccessibility of carotenoids. J. Agric. Food Chem. 2012;60:1447–1455. doi: 10.1021/jf2043949. [DOI] [PubMed] [Google Scholar]
- 38.Escudero-Lopez B., Cerrillo I., Herrero-Martín G., Hornero-Méndez D., Gil Izzquierdo A., Medina S., Ferreres F., Berná G., Medina S., Martín F., et al. Fermented Orange Juice, Source of Higher Carotenoid and Flavanone Contents. J. Agric. Food Chem. 2013;61:8773–8782. doi: 10.1021/jf401240p. [DOI] [PubMed] [Google Scholar]
- 39.Delpino-Rius A., Eras J., Marsol-Vall A., Vilaró F., Balcells M., Canela-Garayoa R. Ultraperformance liquid chromatography analysis to study the changes in the carotenoid profile of commercial monovarietal fruit juices. J. Chromatogr. A. 2014;1331:90–99. doi: 10.1016/j.chroma.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 40.La Peña M.M.-D., Salvia-Trujillo L., Rojas-Graü M., Martín-Belloso O. Changes on phenolic and carotenoid composition of high intensity pulsed electric field and thermally treated fruit juice-soymilk beverages during refrigerated storage. Food Chem. 2011;129:982–990. doi: 10.1016/j.foodchem.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 41.Víctor A., Villanueva-Suárez M.J., Mateos-Aparicio I., Tenorio M.D. Colour, bioactive compounds and antioxidant capacity of mixed beverages based on fruit juices with milk or soya. J. Food Nutr. Res. 2014;53:71–80. [Google Scholar]
- 42.Andrés V., Villanueva M.J., Tenorio M.D. Simultaneous determination of tocopherols, retinol, ester derivatives and β-carotene in milk- and soy-juice based beverages by HPLC with diode-array detection. LWT Food Sci. Technol. 2014;58:557–562. doi: 10.1016/j.lwt.2014.03.025. [DOI] [Google Scholar]
- 43.Oms-Oliu G., Odriozola-Serrano I., Soliva-Fortuny R., Martín-Belloso O. Effects of high-intensity pulsed electric field processing conditions on lycopene, vitamin C and antioxidant capacity of watermelon juice. Food Chem. 2009;115:1312–1319. doi: 10.1016/j.foodchem.2009.01.049. [DOI] [Google Scholar]
- 44.Vallverdú-Queralt A., Odriozola-Serrano I., Oms-Oliu G., Lamuela-Raventós R.M., Elez-Martínez P., Martín-Belloso O. Impact of high-intensity pulsed electric fields on carotenoids profile of tomato juice made of moderate-intensity pulsed electric field-treated tomatoes. Food Chem. 2013;141:3131–3138. doi: 10.1016/j.foodchem.2013.05.150. [DOI] [PubMed] [Google Scholar]
- 45.Lazzerini C., Cifelli M., Domenici V. Pigments in extra virgin olive oils produced in different mediterranean countries in 2014, Near UV-vis spectroscopy versus HPLC-DAD. LWT Food Sci. Technol. 2017;84:586–594. doi: 10.1016/j.lwt.2017.06.025. [DOI] [Google Scholar]
- 46.Mateos R., García-Mesa J.A. Rapid and quantitative extraction method for the determination of chlorophylls and carotenoids in olive oil by high-performance liquid chromatography. Anal. Bioanal. Chem. 2006;385:1247–1249. doi: 10.1007/s00216-006-0472-8. [DOI] [PubMed] [Google Scholar]
- 47.Mapelli-Brahm P., Hernanz-Vila D., Stinco C.M., Heredia F.J., Meléndez-Martínez A.J. Isoprenoids composition and colour to differentiate virgin olive oils from a specific mill. LWT Food Sci. Technol. 2018;89:18–23. doi: 10.1016/j.lwt.2017.10.021. [DOI] [Google Scholar]
- 48.Criado M.N., Morelló J.R., Motilva M.J., MPaz Romero M.P. Effect of Growing Area on Pigment and Phenolic Fractions of Virgin Olive Oils of the Arbequina Variety in Spain. J. Am. Oil Chem. Soc. 2004;81:633. doi: 10.1007/s11746-004-954-z. [DOI] [Google Scholar]
- 49.Ernawita Wahyuono R., Hesse J., Hipler U.-C., Elsner P., Böhm V. Carotenoids of indigenous citrus species from Aceh and its in vitro antioxidant, antidiabetic and antibacterial activities. Eur. Food Res. Technol. 2016;242:1869–1881. doi: 10.1007/s00217-016-2686-0. [DOI] [Google Scholar]
- 50.Fratianni A., Di Criscio T., Mignogna R., Panfili G. Carotenoids, tocols and retinols evolution during egg pasta—Making processes. Food Chem. 2012;131:590–595. doi: 10.1016/j.foodchem.2011.09.034. [DOI] [Google Scholar]
- 51.Zheng H., Zhang Q., Quan J., Zheng Q., Xi W. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 2016;205:112–121. doi: 10.1016/j.foodchem.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Xu C.-J., Fraser P.D., Wang W.-J., Bramley P.M. Differences in the Carotenoid Content of Ordinary Citrus and Lycopene-Accumulating Mutants. J. Agric. Food Chem. 2006;54:5474–5481. doi: 10.1021/jf060702t. [DOI] [PubMed] [Google Scholar]
- 53.Müller H. Determination of the carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection/Bestimmung des Carotinoidgehaltes in ausgewaehlten Gemuese-und Obstsorten mittels HPLC und Photodiodenreihenbestimmung. Z. Fuer. Leb. Und. 1997;2:88. [Google Scholar]
- 54.Lester E.G., Manthey A.J., Buslig S.B. Organic vs. Conventionally Grown Rio Red Whole Grapefruit and Juice, Comparison of Production Inputs, Market Quality, Consumer Acceptance, and Human Health-Bioactive Compounds. J. Agric. Food Chem. 2007;55:4474–4480. doi: 10.1021/jf070901s. [DOI] [PubMed] [Google Scholar]
- 55.Stinco C.M., Escudero-Gilete M.L., Heredia F.J., Vicario I.M., Meléndez-Martínez A.J. Multivariate analyses of a wide selection of orange varieties based on carotenoid contents, color and in vitro antioxidant capacity. Food Res. Int. 2016;90:194–204. doi: 10.1016/j.foodres.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Estévez-Santiago R., Olmedilla-Alonso B., Fernández-Jalao I. Bioaccessibility of provitamin A carotenoids from fruits, application of a standardised static in vitro digestion method. Food Funct. 2016;7:1354–1366. doi: 10.1039/C5FO01242B. [DOI] [PubMed] [Google Scholar]
- 57.Dias M.G., Oliveira L., Camões M.F.G.F.C., Nunes B., Versloot P., Hulshof P.J.M. Critical assessment of three high performance liquid chromatography analytical methods for food carotenoid quantification. J. Chromatogr. A. 2010;1217:3494–3502. doi: 10.1016/j.chroma.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 58.Giuffrida D., La Torre L., Manuela S., Pellicanò T.M., Dugo G. Application of HPLC-APCI-MS with a C-30 reversed phase column for the characterization of carotenoid esters in mandarin essential oil. Flavour Fragr. J. 2006;21:319. doi: 10.1002/ffj.1601. [DOI] [Google Scholar]
- 59.Setiawan B., Sulaeman A., Giraud D.W., Driskell J.A. Carotenoid Content of Selected Indonesian Fruits. J. Food Compos. Anal. 2001;14:169. doi: 10.1006/jfca.2000.0969. [DOI] [Google Scholar]
- 60.Ruiz D., José Egea Tomás-Barberán F.A., Gil M.I. Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J. Agric. Food Chem. 2005;53:6368–6374. doi: 10.1021/jf0480703. [DOI] [PubMed] [Google Scholar]
- 61.Sass-Kiss A., Kiss J., Milotay P., Kerek M.M., Toth-Markus M. Differences in anthocyanin and carotenoid content of fruits and vegetables. Food Res. Int. 2005;38:1023–1029. doi: 10.1016/j.foodres.2005.03.014. [DOI] [Google Scholar]
- 62.Dragovic-Uzelac V., Levaj B., Mrkic V., Bursac D., Boras M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007;102:966–975. doi: 10.1016/j.foodchem.2006.04.001. [DOI] [Google Scholar]
- 63.Leccese A., Bureau S., Reich M., Renard M.G.C.C., Audergon J.M., Mennone C., Susanna Bartolini S., Viti R. Pomological and Nutraceutical Properties in Apricot Fruit, Cultivation Systems and Cold Storage Fruit Management. Plant Foods Hum. Nutr. 2010;65:112–120. doi: 10.1007/s11130-010-0158-4. [DOI] [PubMed] [Google Scholar]
- 64.De Rigal D., Gauillard F., Richard-Forget F. Changes in the carotenoid content of apricot (Prunus armeniaca, var Bergeron) during enzymatic browning, β-carotene inhibition of chlorogenic acid degradation. J. Sci. Food Agric. 2000;80:763–768. doi: 10.1002/(SICI)1097-0010(20000501)80:6<763::AID-JSFA623>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 65.Orazem P., Mikulic-Petkovsek M., Stampar F., Hudina M. Changes during the last ripening stage in pomological and biochemical parameters of the ‘Redhaven’ peach cultivar grafted on different rootstocks. Sci. Hortic. 2013;160:326–334. doi: 10.1016/j.scienta.2013.06.016. [DOI] [Google Scholar]
- 66.Lozzio M.R., Pacetti D., Lucci P., Núñez O., Menichini F., Frega N.G., Tundis R. Prunus persica var. platycarpa (Tabacchiera Peach), Bioactive Compounds and Antioxidant Activity of Pulp, Peel and Seed Ethanolic Extracts. Plant Foods Hum. Nutr. 2015;70:331–337. doi: 10.1007/s11130-015-0498-1. [DOI] [PubMed] [Google Scholar]
- 67.Crupi P., Coletta A., Antonacci D. Analysis of Carotenoids in Grapes to Predict Norisoprenoid Varietal Aroma of Wines from Apulia. J. Agric. Food Chem. 2010;58:9647–9656. doi: 10.1021/jf100564v. [DOI] [PubMed] [Google Scholar]
- 68.Mendes-Pinto M.M., Ferreira A.C.S., Caris-Veyrat C., de Pinho P.G. Carotenoid, chlorophyll, and chlorophyll-derived compounds in grapes and Port wines. J. Agric. Food Chem. 2005;53:10034–10041. doi: 10.1021/jf0503513. [DOI] [PubMed] [Google Scholar]
- 69.Giovanelli G., Brenna O.V. Evolution of some phenolic components, carotenoids and chlorophylls during ripening of three Italian grape varieties. Z. Leb. Und. Forsch. A Eur. Food Res. Technol. 2007;225:145–150. doi: 10.1007/s00217-006-0436-4. [DOI] [Google Scholar]
- 70.Leontowicz H., Leontowicz M., Latocha P., Jesion I., Park Y.S., Katrich E., Barasch D., Nemirovski A., Gorinstein S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa “Hayward” and Actinidia eriantha “Bidan”. Food Chem. 2016;196:281–291. doi: 10.1016/j.foodchem.2015.08.127. [DOI] [PubMed] [Google Scholar]
- 71.Marinova D., Ribarova F. HPLC determination of carotenoids in Bulgarian berries. J. Food Compos. Anal. 2007;20:370–374. doi: 10.1016/j.jfca.2006.09.007. [DOI] [Google Scholar]
- 72.Carvalho E., Fraser P.D., Martens S. Carotenoids and tocopherols in yellow and red raspberries. Food Chem. 2013;139:744–752. doi: 10.1016/j.foodchem.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 73.Karppinen K., Zoratti L., Sarala M., Carvalho E., Hirsimaki J., Mentula H., Martens S., Häggman H., Jaakola L. Carotenoid metabolism during bilberry (Vaccinium myrtillus L.) fruit development under different light conditions is regulated by biosynthesis and degradation. BMC Plant Biol. 2016;16:95. doi: 10.1186/s12870-016-0785-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mihalcea L., Turturică M., Ghinea I.O., Barbu V., Ioniţă E., Cotârleț M., Stănciuc N. Encapsulation of carotenoids from sea buckthorn extracted by CO2 supercritical fluids method within whey proteins isolates matrices. Innov. Food Sci. Emerg. Technol. 2017;42:120–129. doi: 10.1016/j.ifset.2017.06.008. [DOI] [Google Scholar]
- 75.Kazaz S., Baydar H., Erbas S. Variations in chemical compositions of Rosa damascena Mill. and Rosa canina L. fruits. Czech J. Food Sci. 2009;27:178–184. doi: 10.17221/5/2009-CJFS. [DOI] [Google Scholar]
- 76.Böhm V., Fröhlich K., Bitsch R. Rosehip—A “new” source of lycopene? Mol. Asp. Med. 2003;24:385–389. doi: 10.1016/S0098-2997(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 77.Al-Yafeai A., Malarski A., Böhm V. Characterization of carotenoids and vitamin E in R. rugosa and R. canina, Comparative analysis. Food Chem. 2018;242:435–442. doi: 10.1016/j.foodchem.2017.09.070. [DOI] [PubMed] [Google Scholar]
- 78.Lachowicz S., Oszmiański J., Pluta S. The composition of bioactive compounds and antioxidant activity of Saskatoon berry (Amelanchier alnifolia Nutt.) genotypes grown in central Poland. Food Chem. 2017;235:234–243. doi: 10.1016/j.foodchem.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 79.Delgado-Pelayo R., Hornero-Mendez D. Identification and Quantitative Analysis of Carotenoids and Their Esters from Sarsaparilla (Smilax aspera L.) Berries. J. Agric. Food Chem. 2012;60:8225–8232. doi: 10.1021/jf302719g. [DOI] [PubMed] [Google Scholar]
- 80.Khoo H.E., Ismail A., Mohd-Esa N., Idris S. Carotenoid Content of Underutilized Tropical Fruits. Plant Foods Hum. Nutr. 2008;63:170–175. doi: 10.1007/s11130-008-0090-z. [DOI] [PubMed] [Google Scholar]
- 81.Yemiş O., Bakkalbaşı E., Artık N. Changes in pigment profile and surface colour of fig (Ficus carica L.) during drying. Int. J. Food Sci. Technol. 2012;47:1710–1719. doi: 10.1111/j.1365-2621.2012.03025.x. [DOI] [Google Scholar]
- 82.Plaza L., Colina C., Ancos B de Sánchez-Moreno C., Pilar Cano M. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.) Food Chem. 2012;130:591–597. doi: 10.1016/j.foodchem.2011.07.080. [DOI] [Google Scholar]
- 83.Veberic R., Jurhar J., Mikulic-Petkovsek M., Stampar F., Schmitzer V. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.) Food Chem. 2010;119:477–483. doi: 10.1016/j.foodchem.2009.06.044. [DOI] [Google Scholar]
- 84.Ribeiro P.F.A., Stringheta P.C., de Oliveira E.B., Mendonca A.C., Sant’Ana H.M.P. Levels of vitamin C, beta.-carotene and minerals in camu-camu cultivated in different environments/Teor de vitamina C, beta.-caroteno e minerais em camu-camu cultivado em diferentes ambientes. Cienc. Rural. 2016;46:567–572. doi: 10.1590/0103-8478cr20150024. [DOI] [Google Scholar]
- 85.Delgado-Pelayo R., Gallardo-Guerrero L., Hornero-Méndez D. Carotenoid composition of strawberry tree (Arbutus unedo L.) fruits. Food Chem. 2016;199:165–175. doi: 10.1016/j.foodchem.2015.11.135. [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez-Amaya D.B. Some considerations in generating carotenoid data for food composition tables. J. Food Compos. Anal. 2000;13:641–647. doi: 10.1006/jfca.2000.0915. [DOI] [Google Scholar]
- 87.Mezadri T., Villaño D., Fernández-Pachón M.S., García-Parrilla M.C., Troncoso A.M. Antioxidant compounds and antioxidant activity in acerola (Malpighia emarginata DC.) fruits and derivatives. J. Food Compos. Anal. 2008;21:282–290. doi: 10.1016/j.jfca.2008.02.002. [DOI] [Google Scholar]
- 88.D’Evoli L., Moscatello S., Lucarini M., Aguzzi A., Gabrielli P., Proietti S., Battistelli A., Famiani F., Böhm V., Lombardi-Boccia G. Nutritional traits and antioxidant capacity of kiwifruit (Actinidia deliciosa Planch., cv. Hayward) grown in Italy. J. Food Compos. Anal. 2015;37:25–29. doi: 10.1016/j.jfca.2014.06.012. [DOI] [Google Scholar]
- 89.Englberger L., Wills R.B.H., Blades B., Dufficy L., Daniells J.W., Coyne T. Carotenoid content and flesh color of selected banana cultivars growing in Australia. Food Nutr. Bull. 2006;27:281–291. doi: 10.1177/156482650602700401. [DOI] [PubMed] [Google Scholar]
- 90.Ekesa B., Nabuuma D., Blomme G., van den Bergh I. Provitamin A carotenoid content of unripe and ripe banana cultivars for potential adoption in eastern Africa. J. Food Compos. Anal. 2015;43:1–6. doi: 10.1016/j.jfca.2015.04.003. [DOI] [Google Scholar]
- 91.García-Herrera P., Sánchez-Mata M.C., Cámara M., Tardío J., Olmedilla-Alonso B. Carotenoid content of wild edible young shoots traditionally consumed in Spain (Asparagus acutifolius L., Humulus lupulus L., Bryonia dioica Jacq. and Tamus communis L.) J. Sci. Food Agric. 2013;93:1692–1698. doi: 10.1002/jsfa.5952. Erratum in J. Sci. Food Agric.2014, 94, 1914–1916. [DOI] [PubMed] [Google Scholar]
- 92.Mertz C., Gancel A.-L., Gunata Z., Alter P., Dhuique-Mayer C., Vaillant F., Perez A.M., Ruales J., Brat P. Phenolic compounds, carotenoids and antioxidant activity of three tropical fruits. J. Food Compos. Anal. 2009;22:381–387. doi: 10.1016/j.jfca.2008.06.008. [DOI] [Google Scholar]
- 93.Mertz C., Brat P., Caris-Veyrat C., Gunata Z. Characterization and thermal lability of carotenoids and vitamin C of tamarillo fruit (Solanum betaceum Cav.) Food Chem. 2010;119:653–659. doi: 10.1016/j.foodchem.2009.07.009. [DOI] [Google Scholar]
- 94.Kornsteiner M., Wagner K.H., Elmadfa I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006;98:381–387. doi: 10.1016/j.foodchem.2005.07.033. [DOI] [Google Scholar]
- 95.Burns J., Fraser P.D., Bramley P.M. Identification and quantification of carotenoids, tocopherols and chlorophylls in commonly consumed fruits and vegetables. Phytochemistry. 2003;62:939–947. doi: 10.1016/S0031-9422(02)00710-0. [DOI] [PubMed] [Google Scholar]
- 96.Fattore M., Montesano D., Pagano E., Teta R., Borrelli F., Mangoni A., Seccia S., Albrizio S. Carotenoid and flavonoid profile and antioxidant activity in “Pomodorino Vesuviano” tomatoes. J. Food Compos. Anal. 2016;53:61–68. doi: 10.1016/j.jfca.2016.08.008. [DOI] [Google Scholar]
- 97.Znidarčič D., Ban D., Sircelj H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011;129:1164–1168. doi: 10.1016/j.foodchem.2011.05.097. [DOI] [PubMed] [Google Scholar]
- 98.Raju M., Varakumar S., Lakshminarayana R., Krishnakantha T.P., Baskaran V. Carotenoid composition and vitamin A activity of medicinally important green leafy vegetables. Food Chem. 2007;101:1598–1605. doi: 10.1016/j.foodchem.2006.04.015. [DOI] [Google Scholar]
- 99.Burgos G., Muñoa L., Sosa P., Bonierbale M., zum Felde T., Díaz C. In vitro bioaccessibility of lutein and zeaxanthin of yellow fleshed boiled potatoes. Plant Foods Hum. Nutr. 2013;68:385–390. doi: 10.1007/s11130-013-0381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Champagne A., Bernillon S., Moing A., Rolin D., Legendre L., Lebot V. Carotenoid profiling of tropical root crop chemotypes from Vanuatu, South Pacific. J. Food Compos. Anal. 2010;23:763–771. doi: 10.1016/j.jfca.2010.03.021. [DOI] [Google Scholar]
- 101.López A., Javier G.-A., Fenoll J., Hellín P., Flores P. Original Research Article, Chemical composition and antioxidant capacity of lettuce, Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. J. Food Compos. Anal. 2014;33:39–48. doi: 10.1016/j.jfca.2013.10.001. [DOI] [Google Scholar]
- 102.Fernández-Marín B., Míguez F., Méndez-Fernández L., Agut A., Becerril J.M., García-Plazaola J.I., Kranner I., Colville L. Seed Carotenoid and Tocochromanol Composition of Wild Fabaceae Species Is Shaped by Phylogeny and Ecological Factors. Front. Plant. Sci. 2017;8:1428. doi: 10.3389/fpls.2017.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim H.-J., Fonseca J.M., Choi J.-H., Kubota C., Kwon D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.) J. Agric. Food Chem. 2008;56:3772–3776. doi: 10.1021/jf0733719. [DOI] [PubMed] [Google Scholar]
- 104.Brazaitytė A., Sakalauskienė S., Samuolienė G., Jankauskienė J., Viršilė A., Novičkovas A., Sirtautas R., Miliauskienė J., Vaštakaitė V., Dabašinskas L., et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015;173:600–606. doi: 10.1016/j.foodchem.2014.10.077. [DOI] [PubMed] [Google Scholar]
- 105.Bengtsson A., Namutebi A., Alminger M.L., Svanberg U. Effects of various traditional processing methods on the all-trans-β-carotene content of orange-fleshed sweet potato. J. Food Compos. Anal. 2008;21:134–143. doi: 10.1016/j.jfca.2007.09.006. [DOI] [Google Scholar]
- 106.Hels O., Larsen T., Christensen L.P., Kidmose U., Hassan N., Thilsted S.H. Contents of iron, calcium, zinc and β-carotene in commonly consumed vegetables in Bangladesh. J. Food Compos. Anal. 2004;17:587–595. doi: 10.1016/j.jfca.2003.08.007. [DOI] [Google Scholar]
- 107.Fernandez-Orozco R., Gallardo-Guerrero L., Hornero-Méndez D. Carotenoid profiling in tubers of different potato (Solanum sp.) cultivars, Accumulation of carotenoids mediated by xanthophyll esterification. Food Chem. 2013;141:2864–2872. doi: 10.1016/j.foodchem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 108.Mosha T., Pace R., Adeyeye S., Laswai H., Mtebe K. Effect of traditional processing practices on the content of total carotenoid, β-carotene, α-carotene and vitamin A activity of selected Tanzanian vegetables. Plant Foods Hum. Nutr. 1997;50:189–201. doi: 10.1007/BF02436056. [DOI] [PubMed] [Google Scholar]
- 109.Vargas-Murga L., de Rosso V.V., Mercadante A.Z., Olmedilla-Alonso B. Fruits and vegetables in the Brazilian Household Budget Survey (2008–2009), carotenoid content and assessment of individual carotenoid intake. J. Food Compos. Anal. 2016;50:88–96. doi: 10.1016/j.jfca.2016.05.012. [DOI] [Google Scholar]
- 110.Samuolienė G., Viršilė A., Brazaitytė A., Jankauskienė J., Sakalauskienė S., Vaštakaitė V., Novičkovas A., Viškelienėa A., Sasnauskasa A., Duchovskis P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017;228:50–56. doi: 10.1016/j.foodchem.2017.01.144. [DOI] [PubMed] [Google Scholar]
- 111.Mazzeo T., N’Dri D., Chiavaro E., Visconti A., Fogliano V., Pellegrini N. Effect of two cooking procedures on phytochemical compounds, total antioxidant capacity and colour of selected frozen vegetables. Food Chem. 2011;128:627–633. doi: 10.1016/j.foodchem.2011.03.070. [DOI] [Google Scholar]
- 112.Duma M., Alsina I., Zeipina S., Dubova L. Leaf vegetables as source of phytochemicals; Proceedings of the 9th Baltic Conference on Food Science and Technology “Food for Consumer Well-Being” (FOODBALT 2014); Jelgava, Latvia. 8–9 May 2014. [Google Scholar]
- 113.Arnold C., Schwarzenbolz U., Bohm V. Carotenoids and chlorophylls in processed xanthophyll-rich food. LWT Food Sci. Technol. 2014;57:442. doi: 10.1016/j.lwt.2014.01.004. [DOI] [Google Scholar]
- 114.Simonovska B., Vovk I., Glavnik V., Černelič K. Effects of extraction and high-performance liquid chromatographic conditions on the determination of lutein in spinach. J. Chromatogr. A. 2013;1276:95–101. doi: 10.1016/j.chroma.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 115.Appenroth K.-J., Sree K.S., Böhm V., Hammann S., Vetter W., Leiterer M., Jahreis G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017;217:266–273. doi: 10.1016/j.foodchem.2016.08.116. [DOI] [PubMed] [Google Scholar]
- 116.Lalas S., Athanasiadis V., Karageorgou I., Batra G., Nanos G.D., Makris D.P. Nutritional Characterization of Leaves and Herbal Tea of Moringa oleifera Cultivated in Greece. J. Herbs Spices Med. Plants. 2017;23:320–333. doi: 10.1080/10496475.2017.1334163. [DOI] [Google Scholar]
- 117.Abbet C., Slacanin I., Hamburger M., Potterat O. Comprehensive analysis of Phyteuma orbiculare L., a wild Alpine food plant. Food Chem. 2013;136:595–603. doi: 10.1016/j.foodchem.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 118.Pellegrini N., Chiavaro E., Gardana C., Mazzeo T., Contino D., Gallo M., Riso P., Fogliano V., Porrini M. Effect of Different Cooking Methods on Color, Phytochemical Concentration, and Antioxidant Capacity of Raw and Frozen Brassica Vegetables. J. Agric. Food Chem. 2010;58:4310–4321. doi: 10.1021/jf904306r. [DOI] [PubMed] [Google Scholar]
- 119.Campos F.M., Chaves J.B.P., de Azeredo P.M. Handling Practices to Control Ascorbic Acid and β-Carotene Losses in Collards (Brassica oleracea) Food Sci. Technol. Int. 2010;15:445–452. doi: 10.1177/1082013209350346. [DOI] [Google Scholar]
- 120.Arnold C., Jentsch S., Dawczynski J., Bohm V. Age-related macular degeneration, Effects of a short-term intervention with an oleaginous kale extract—A pilot study. Nutrition. 2013;29:1412–1417. doi: 10.1016/j.nut.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 121.Santos J., Herrero M., Mendiola J.A., Oliva-Teles M.T., Ibáñez E., Delerue-Matos C., Oliveira M.B.P.P. Assessment of nutritional and metabolic profiles of pea shoots, the new ready-to-eat baby-leaf vegetable. Food Res. Int. 2014;58:105–111. doi: 10.1016/j.foodres.2014.01.062. [DOI] [Google Scholar]
- 122.Granado-Lorencio F., Olmedilla-Alonso B., Herrero-Barbudo C., Sánchez-Moreno C., de Ancos B., Martínez J.A., Pérez-Sacristán B., Blanco-Navarro I. Modified-atmosphere packaging (MAP) does not affect the bioavailability of tocopherols and carotenoids from broccoli in humans, A cross-over study. Food Chem. 2008;106:1070–1076. doi: 10.1016/j.foodchem.2007.07.038. [DOI] [Google Scholar]
- 123.Fernández-León M.F., Fernández-León A.M., Lozano M., Ayuso M.C., González-Gómez D. Altered commercial controlled atmosphere storage conditions for ‘Parhenon’ broccoli plants (Brassica oleracea L. var. italica). Influence on the outer quality parameters and on the health-promoting compounds. LWT Food Sci. Technol. 2013;50:665–672. doi: 10.1016/j.lwt.2012.07.028. [DOI] [Google Scholar]
- 124.Guil-Guerrero J.L., Rebolloso-Fuentes M.M. Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties. J. Food Compos. Anal. 2009;22:123–129. doi: 10.1016/j.jfca.2008.10.012. [DOI] [Google Scholar]
- 125.Dias M.G., Camões M.F.G.F.C., Oliveira L. Uncertainty estimation and in-house method validation of HPLC analysis of carotenoids for food composition data production. Food Chem. 2008;109:815–824. doi: 10.1016/j.foodchem.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 126.Krauss S., Schnitzler W.H., Grassmann J., Woitke M. The influence of different electrical conductivity values in a simplified recirculating soilless system on inner and outer fruit quality characteristics of tomato. J. Agric. Food Chem. 2006;54:441–448. doi: 10.1021/jf051930a. [DOI] [PubMed] [Google Scholar]
- 127.De Pascale S., Maggio A., Fogliano V., Ambrosino P., Ritieni A. Irrigation with saline water improves carotenoids content and antioxidant activity of tomato. J. Hortic. Sci. BioTechnol. 2001;76:447–453. doi: 10.1080/14620316.2001.11511392. [DOI] [Google Scholar]
- 128.D’Antuono L.F., Elementi S., Neri R. Genotype x environment interaction on carrot germplasm Daucus carota L., Emilia-Romagna. Italus Hortus. 2006;13:693–697. [Google Scholar]
- 129.Patanè C., Pellegrino A., Saita A., Siracusa L., Ruberto G., Barbagallo R. Mediterranean long storage tomato as a source of novel products for the agrifood industry, Nutritional and technological traits. LWT Food Sci. Technol. 2017;85:445–448. doi: 10.1016/j.lwt.2016.12.011. [DOI] [Google Scholar]
- 130.Lahoz I., Leiva-Brondo M., Martí R., Macua J.I., Campillo C., Roselló S., Cebolla-Cornejo J. Influence of high lycopene varieties and organic farming on the production and quality of processing tomato. Sci. Hortic. 2016;204:128–137. doi: 10.1016/j.scienta.2016.03.042. [DOI] [Google Scholar]
- 131.Aherne A., Jiwan M., Daly T., O’Brien N. Geographical Location has Greater Impact on Carotenoid Content and Bioaccessibility from Tomatoes than Variety. Plant Foods Hum. Nutr. 2009;64:250–256. doi: 10.1007/s11130-009-0136-x. [DOI] [PubMed] [Google Scholar]
- 132.Gautier H., Diakou-Verdin V., Bénard C., Reich M., Buret M., Bourgaud F., Poëssel J.C., Caris-Veyrat C., Génard M. How does tomato quality (sugar, acid, and nutritional quality) vary with ripening stage, temperature, and irradiance? J. Agric. Food Chem. 2008;56:1241–1250. doi: 10.1021/jf072196t. [DOI] [PubMed] [Google Scholar]
- 133.Gautier H., Rocci A., Buret M., Grasselly D., Dumas Y., Causse M. Effect of photoselective filters on the physical and chemical traits of vine-ripened tomato fruits. Can. J. Plant. Sci. 2005;85:439–446. doi: 10.4141/P03-163. [DOI] [Google Scholar]
- 134.Borghesi E., González-Miret M.L., Escudero-Gilete M.L., Malorgio F., Heredia F.J., Meléndez-Martínez A.J. Effects of Salinity Stress on Carotenoids, Anthocyanins, and Color of Diverse Tomato Genotypes. J. Agric. Food Chem. 2011;59:11676–11682. doi: 10.1021/jf2021623. [DOI] [PubMed] [Google Scholar]
- 135.Vallverdú-Queralt A., Oms-Oliu G., Odriozola-Serrano I., Lamuela-Raventós R.M., Martín-Belloso O., Elez-Martínez P. Metabolite profiling of phenolic and carotenoid contents in tomatoes after moderate-intensity pulsed electric field treatments. Food Chem. 2013;136:199–205. doi: 10.1016/j.foodchem.2012.07.108. [DOI] [PubMed] [Google Scholar]
- 136.Strati I.F., Sinanoglou V.J., Kora L., Miniadis-Meimaroglou S., Oreopoulou V. Carotenoids from Foods of Plant, Animal and Marine Origin, An Efficient HPLC-DAD Separation Method. Foods. 2012;1:52–65. doi: 10.3390/foods1010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hernández V., Hellín P., Fenoll J., Flores P. Increased temperature produces changes in the bioactive composition of tomato, depending on its developmental stage. J. Agric. Food Chem. 2015;63:2378–2382. doi: 10.1021/jf505507h. [DOI] [PubMed] [Google Scholar]
- 138.Colle I., Lemmens L., van Buggenhout S., van Loey A., Hendrickx M. Effect of Thermal Processing on the Degradation, Isomerization, and Bioaccessibility of Lycopene in Tomato Pulp. J. Food Sci. 2010;75:C753–C758. doi: 10.1111/j.1750-3841.2010.01862.x. [DOI] [PubMed] [Google Scholar]
- 139.Efthimiadou A., Katsenios N., Karkanis A., Papastylianou P., Triantafyllidis V., Travlos I., Bilalis D.M. Effects of presowing pulsed electromagnetic treatment of tomato seed on growth, yield, and lycopene content. Sci. World J. 2014 doi: 10.1155/2014/369745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stinco C.M., Rodríguez-Pulido F.J., Escudero-Gilete M.L., Gordillo B., Vicario I.M., Meléndez-Martínez A.J. Lycopene isomers in fresh and processed tomato products, correlations with instrumental color measurements by digital image analysis and spectroradiometry. Food Res. Int. 2013;50:111–120. doi: 10.1016/j.foodres.2012.10.011. [DOI] [Google Scholar]
- 141.Coyago-Cruz E., Corell M., Stinco C.M., Hernanz D., Moriana A., Meléndez-Martínez A.J. Effect of regulated deficit irrigation and cluster position on quality parameters, carotenoids and phenolics of diverse tomato varieties (Solanum lycopersicum L.) Food Res. Int. 2017;9:72–83. doi: 10.1016/j.foodres.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 142.Pugliese A., O’Callaghan Y., Tundis R., Galvin K., Menichini F., O’Brien N., Loizzo M.R. In vitro investigation of the bioaccessibility of carotenoids from raw, frozen and boiled red chili peppers (Capsicum annuum) Eur. J. Nutr. 2014;53:501–510. doi: 10.1007/s00394-013-0555-1. [DOI] [PubMed] [Google Scholar]
- 143.Giuffrida D., Dugo P., Torre G., Bignardi C., Cavazza A., Corradini C., Dugo G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013;140:794–802. doi: 10.1016/j.foodchem.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 144.Rodríguez-Burruezo A., del Carmen González-Mas M., Nuez F. Carotenoid Composition and Vitamin A Value in Ají (Capsicum baccatum L.) and Rocoto (C. pubescens R. & P.), 2 Pepper Species from the Andean Region. J. Food Sci. 2010;75:S44–S53. doi: 10.1111/j.1750-3841.2010.01795.x. [DOI] [PubMed] [Google Scholar]
- 145.Topuz A., Ozdemir F. Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. J. Food Compos. Anal. 2007;20:596–602. doi: 10.1016/j.jfca.2007.03.007. [DOI] [Google Scholar]
- 146.Molnár H., Bata-Vidács I., Baka E., Cserhalmi Z., Ferenczi S., Tömösközi-Farkas R., Adányi N., Székács A. The effect of different decontamination methods on the microbial load, bioactive components, aroma and colour of spice paprika. Food Control. 2018;83:131–140. doi: 10.1016/j.foodcont.2017.04.032. [DOI] [Google Scholar]
- 147.Simonne A.H., Simonne E.H., Eitenmiller R.R., Mills H.A., Green N.R. Ascorbic Acid and Provitamin A Contents in Unusually Colored Bell Peppers (Capsicum annuum L.) J. Food Compos. Anal. 1997;10:299–311. doi: 10.1006/jfca.1997.0544. [DOI] [Google Scholar]
- 148.Hornero-Méndez D., Costa-García J., Mínguez-Mosquera M.I. Characterization of carotenoid high-producing Capsicum annuum cultivars selected for paprika production. J. Agric. Food Chem. 2002;50:5711–5716. doi: 10.1021/jf0256236. [DOI] [PubMed] [Google Scholar]
- 149.Minguez-Mosquera M.I., Hornero-Mendez D. Separation and quantification of the carotenoid pigments in red peppers (Capsicum annuum L.), paprika, and oleoresin by reversed-phase HPLC. J. Agric. Food Chem. 1993;41:1616–1620. doi: 10.1021/jf00034a018. [DOI] [Google Scholar]
- 150.Tran X.T., Parks S.E., Roach P.D., Golding J.B., Nguyen M.H. Effects of maturity on physicochemical properties of Gac fruit (Momordica cochinchinensis Spreng) Food Sci. Nutr. 2016;4:305. doi: 10.1002/fsn3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Conti S., Villari G., Amico E., Caruso G. Effects of production system and transplanting time on yield, quality and antioxidant content of organic winter squash (Cucurbita moschata Duch.) Sci. Hortic. 2015;183:136–143. doi: 10.1016/j.scienta.2014.12.003. [DOI] [Google Scholar]
- 152.Murkovic M., Mülleder U., Neunteufl H. Carotenoid Content in Different Varieties of Pumpkins. J. Food Compos. Anal. 2002;15:633–638. doi: 10.1006/jfca.2002.1052. [DOI] [Google Scholar]
- 153.Raczyk M., Siger A., Radziejewska-Kubzdela E., Ratusz K., Rudzińska M. Roasting pumpkin seeds and changes in the composition and oxidative stability of cold-pressed oils. Acta Sci. Pol. Technol. Aliment. 2017;16:293–301. doi: 10.17306/J.AFS.0494. [DOI] [PubMed] [Google Scholar]
- 154.Bianchi G., Rizzolo A., Grassi M., Provenzi L., Lo Scalzo R. External maturity indicators, carotenoid and sugar compositions and volatile patterns in ‘Cuoredolce®’ and ‘Rugby’ mini-watermelon (Citrullus lanatus (Thunb) Matsumura & Nakai) varieties in relation of ripening degree at harvest. Postharvest Biol. Technol. 2018;136:1–11. doi: 10.1016/j.postharvbio.2017.09.009. [DOI] [Google Scholar]
- 155.Kurilich A., Juvik J. Simultaneous quantification of carotenoids and tocopherols in corn kernel extracts by HPLC. J. Liq. Chromatogr. Relat. Technol. 1999;22:2925–2934. doi: 10.1081/JLC-100102068. [DOI] [Google Scholar]
- 156.Bozalan N.K., Karadeniz F. Carotenoid Profile, Total Phenolic Content, and Antioxidant Activity of Carrots. Int. J. Food Prop. 2011;14:1060–1068. doi: 10.1080/10942910903580918. [DOI] [Google Scholar]
- 157.Borowska J.E., Zadernowski R., Szajdek A., Majewska K., Budrewicz G. Organoleptic, physical and chemical properties of some varieties of carrots suitable in juice production. Pol. J. Nat. Sci. 2005;18:173–186. [Google Scholar]
- 158.Aguiló-Aguayo I., Gangopadhyay N., Lyng J.G., Brunton N., Rai D.K. Impact of pulsed light on colour, carotenoid, polyacetylene and sugar content of carrot slices. Innov. Food Sci. Emerg. Technol. 2017;42:49–55. doi: 10.1016/j.ifset.2017.05.006. [DOI] [Google Scholar]
- 159.Nicolle C., Simon G., Rock E., Amouroux P., Rémésy C. Genetic Variability Influences Carotenoid, Vitamin Phenolic, and Mineral Content in White, Yellow, Purple, Orange, and Dark-orange Carrot Cultivars. J. Am. Soc. Hortic. Sci. 2004;129:523–529. doi: 10.21273/JASHS.129.4.0523. [DOI] [Google Scholar]
- 160.Koca N. Ph.D. Thesis. Ankara University; Ankara, Turkey: 2006. Carotenoids and Antioxidant Activity in Carrots (Daucus carota L.)/Havuçlarda (Daucus carota L.) Karotenoidler ve Antioksidan Aktivite.81p [Google Scholar]
- 161.Rosenfeld H.J., Baardseth P., Skrede G. Evaluation of carrot varieties for production of deep fried carrot chips—IV. The influence of growing environment on carrot raw material. Food Res. Int. 1997;30:611–618. doi: 10.1016/S0963-9969(98)00026-X. [DOI] [Google Scholar]
- 162.Herrero M., Jaime L., Martín-Alvarez P.J., Cifuentes A., Ibáñez E. Optimization of the extraction of antioxidants from Dunaliella salina microalga by pressurized liquids. J. Agric. Food Chem. 2006;54:5597–5603. doi: 10.1021/jf060546q. [DOI] [PubMed] [Google Scholar]
- 163.Jaeschke D.P., Menegol T., Rech R., Mercali G.D., Marczak L.D.F. Carotenoid and lipid extraction from Heterochlorella luteoviridis using moderate electric field and ethanol. Process. BioChem. 2016;51:1636–1643. doi: 10.1016/j.procbio.2016.07.016. [DOI] [Google Scholar]
- 164.Castro-Puyana M., Herrero M., Urreta I., Mendiola J.A., Cifuentes A., Ibanez E., Suárez-Alvarez S. Optimization of clean extraction methods to isolate carotenoids from the microalga Neochloris oleoabundans and subsequent chemical characterization using liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2013;13:4607–4616. doi: 10.1007/s00216-012-6687-y. [DOI] [PubMed] [Google Scholar]
- 165.Al-Duais M., Hohbein J., Werner S., Böhm V., Jetschke G. Contents of vitamin C, carotenoids, tocopherols, and tocotrienols in the subtropical plant species Cyphostemma digitatum as affected by processing. J. Agric. Food Chem. 2009;57:5420–5427. doi: 10.1021/jf9003626. [DOI] [PubMed] [Google Scholar]
- 166.Knockaert G., Lemmens L., van Buggenhout S., Hendrickx M., van Loey A. Changes in β-carotene bioaccessibility and concentration during processing of carrot puree. Food Chem. 2012;133:60–67. doi: 10.1016/j.foodchem.2011.12.066. [DOI] [Google Scholar]
- 167.Valdivielso I., Bustamante M.A., de Gordoa J.C.R., Nájera A.I., Renobales M., Barron L.J.R. Simultaneous analysis of carotenoids and tocopherols in botanical species using one step solid liquid extraction followed by high performance liquid chromatography. Food Chem. 2015;15:709–717. doi: 10.1016/j.foodchem.2014.10.090. [DOI] [PubMed] [Google Scholar]
- 168.Lenucci M.S., de Caroli M., Marrese P.P., Iurlaro A., Rescio L., Böhm V., Dalessandro G., Piro G. Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem. 2015;170:193–202. doi: 10.1016/j.foodchem.2014.08.081. [DOI] [PubMed] [Google Scholar]
- 169.Gámez M.C., Calvo M.M., Selgas M.D., García M.L., Erler K., Böhm V., Catalano A., Simone R., Palozza P. Effect of E-beam treatment on the chemistry and on the antioxidant activity of lycopene from dry tomato peel and tomato powder. J. Agric. Food Chem. 2014;62:1557–1563. doi: 10.1021/jf4048012. [DOI] [PubMed] [Google Scholar]
- 170.Paznocht L., Kotíková Z., Šulc M., Lachman J., Orsák M., Eliášová M., Martinek P. Free and esterified carotenoids in pigmented wheat, tritordeum and barley grains. Food Chem. 2018;240:670–678. doi: 10.1016/j.foodchem.2017.07.151. [DOI] [PubMed] [Google Scholar]
- 171.Brandolini A., Hidalgo A., Gabriele S., Heun M. Chemical composition of wild and feral diploid wheats and their bearing on domesticated wheats. J. Cereal Sci. 2015;63:122–127. doi: 10.1016/j.jcs.2015.03.005. [DOI] [Google Scholar]
- 172.Hidalgo A., Brandolini A. Nitrogen fertilisation effects on technological parameters and carotenoid, tocol and phenolic acid content of einkorn (Triticum monococcum L. subsp. monococcum), a two-year evaluation. J. Cereal Sci. 2017;73:18–24. doi: 10.1016/j.jcs.2016.11.002. [DOI] [Google Scholar]
- 173.Hidalgo A., Scuppa S., Brandolini A. Technological quality and chemical composition of puffed grains from einkorn (Triticum monococcum L. subsp. monococcum) and bread wheat (Triticum aestivum L. subsp. aestivum) LWT Food Sci. Technol. 2016;68:541–548. doi: 10.1016/j.lwt.2015.12.068. [DOI] [Google Scholar]
- 174.Kljak K., Grbeša D. Carotenoid content and antioxidant activity of hexane extracts from selected Croatian corn hybrids. Food Chem. 2015;167:402–408. doi: 10.1016/j.foodchem.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 175.Hulshof P.J.M., Kosmeijer-Schuil T., West C.E., Hollman P.C.H. Quick screening of maize kernels for provitamin A content. J. Food Compos. Anal. 2007;20:655–661. doi: 10.1016/j.jfca.2006.04.014. [DOI] [Google Scholar]
- 176.Pereira-Caro G., Cros G., Yokota T., Crozier A. Phytochemical Profiles of Black, Red, Brown, and White Rice from the Camargue Region of France. J. Agric. Food Chem. 2013;61:7976–7986. doi: 10.1021/jf401937b. [DOI] [PubMed] [Google Scholar]
- 177.Atienza S.G., Ballesteros J., Martín A., Hornero-Méndez D. Genetic variability of carotenoid concentration and degree of esterification among tritordeum (xTritordeum Ascherson et Graebner) and durum wheat accessions. J. Agric. Food Chem. 2007;55:4244–4251. doi: 10.1021/jf070342p. [DOI] [PubMed] [Google Scholar]
- 178.Ziegler J.U., Schweiggert R.M., Carle R. A method for the simultaneous extraction and quantitation of lipophilic antioxidants in Triticum sp. by HPLC-DAD/FLD-MSn. J. Food Compos. Anal. 2015;39:94–102. doi: 10.1016/j.jfca.2014.11.011. [DOI] [Google Scholar]
- 179.Hidalgo A., Brandolini A., Pompei C. Carotenoids evolution during pasta, bread and water biscuit preparation from wheat flours. Food Chem. 2010;121:746–751. doi: 10.1016/j.foodchem.2010.01.034. [DOI] [Google Scholar]
- 180.Alfieri M., Berardo N., Redaelli R., Hidalgo A. Carotenoid composition and heterotic effect in selected Italian maize germplasm. J. Cereal Sci. 2014;59:181–188. doi: 10.1016/j.jcs.2013.12.010. [DOI] [Google Scholar]
- 181.Britton G., Khachik F. Carotenoids in Food. In: Britton G., Liaaen-Jensen S., Pfander H., editors. Carotenoids—Volume 5: Nutrition and Health. Birkhäuser; Basel, Switzerland: Boston, MA, USA: Berlin, Germany: 2009. pp. 45–66. [Google Scholar]
- 182.Rodriguez-Amaya D. A Guide to Carotenoid Analysis in Foods. ILSI Press; Washington, DC, USA: 2001. [Google Scholar]
- 183.Alquezar B., Rodrigo M.J., Zacarías L. Regulation of carotenoid biosynthesis during fruit maturation in the red-fleshed orange mutant Cara Cara. Phytochemistry. 2008;69:1997–2007. doi: 10.1016/j.phytochem.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 184.Rodrigo M.-J. Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. J. Exp. Bot. 2003;54:727–738. doi: 10.1093/jxb/erg083. [DOI] [PubMed] [Google Scholar]
- 185.Fray R.G., Grierson D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol. Biol. 1993;22:589–602. doi: 10.1007/BF00047400. [DOI] [PubMed] [Google Scholar]
- 186.Ronen G., Cohen M., Zamir D., Hirschberg J. Regulation of carotenoid biosynthesis during tomato fruit development, expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J. 1999;17:341–351. doi: 10.1046/j.1365-313X.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- 187.Lu S., Eck J., van Zhou X., Lopez A.B., O’Halloran D.M., Cosman K.M., Conlin B.J., Dominick J., Paolillo D.J., Garvin D.F., et al. The Cauliflower or Gene Encodes a DnaJ Cysteine-Rich Domain-Containing Protein That Mediates High Levels of b-Carotene Accumulation. Plant Cell. 2006;18:3594–3605. doi: 10.1105/tpc.106.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ye X.D., Al Babili S., Kloti A., Zhang J., Lucca P., Beyer P., Potrykus I. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- 189.Meléndez-Martínez A.J., Fraser P.D., Bramley P.M. Accumulation of health promoting phytochemicals in wild relatives of tomato and their contribution to in vitro antioxidant activity. Phytochemistry. 2010;71:1104–1114. doi: 10.1016/j.phytochem.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 190.Rodriguez-Amaya D.B., Kimura M. Harvest Plus Handbook for Carotenoid Analysis. International Food Policy Research Institute (IFPRI); Washington, DC, USA: International Center for Tropical Agriculture (CIAT); Cali, Colombia: 2004. [Google Scholar]
- 191.Sivakumar D., Jifon J., Soundy P. Spectral quality of photo-selective shade nettings improves antioxidants and overall quality in selected fresh produce after postharvest. Food Rev. Int. 2017;34:290–307. doi: 10.1080/87559129.2017.1298124. [DOI] [Google Scholar]
- 192.Sakalauskaitė J., Viskelis P., Dambrauskienė E., Sakalauskienė S., Samuoliene G., Brazaitytė A., Duchovskis P., Urbonavičienė D. The effects of different UV-B radiation intensities on morphological and biochemical characteristics in Ocimum basilicum L. J. Sci. Food Agric. 2013;93:1266–1271. doi: 10.1002/jsfa.5879. [DOI] [PubMed] [Google Scholar]
- 193.Poiroux-Gonord F., Bidel L.P.R., Fanciullino A.L., Gautier H., Lauri-Lopez F., Urban L. Health benefits of vitamins and secondary metabolites of fruits and vegetables and prospects to increase their concentrations by agronomic approaches. J. Agric. Food Chem. 2010;58:12065–12082. doi: 10.1021/jf1037745. [DOI] [PubMed] [Google Scholar]
- 194.Li P., Cheng L. The shaded side of apple fruit becomes more sensitive to photoinhibition with fruit development. Physiol. Plant. 2008;134:282–292. doi: 10.1111/j.1399-3054.2008.01131.x. [DOI] [PubMed] [Google Scholar]
- 195.Kimura M., Rodriguez-Amaya D.B., Yokoyama S.M. Cultivar differences and geographic effects on the carotenoid composition and vitamin A value of papaya. Leb. Wissen Technol. 1991;24:415–418. [Google Scholar]
- 196.Mercadante A.Z., Rodriguez-Amaya D.B. Effects of Ripening, Cultivar Differences, and Processing on the Carotenoid Composition of Mango. J. Agric. Food Chem. 1998;46:128–130. doi: 10.1021/jf9702860. [DOI] [PubMed] [Google Scholar]
- 197.Mercadante A.Z., Rodriguez-Amaya D.B. Carotenoid composition of a leafy vegetable in relation to some agricultural variables. J. Agric. Food Chem. 2002;39:1094–1097. doi: 10.1021/jf00006a018. [DOI] [Google Scholar]
- 198.Coyago-Cruz E., Corell M., Moriana A., Hernanz D., Benítez-González A.M., Stinco C.M., Meléndez-Martínez A.J. Antioxidants (carotenoids and phenolics) profile of cherry tomatoes as influenced by deficit irrigation, ripening and cluster. Food Chem. 2018;240:870–878. doi: 10.1016/j.foodchem.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 199.Geetha G.A. Changes in fruit quality and carotenoid profile in tomato (Solanum lycopersicon L.) genotypes under elevated temperature. J. Hortl. Sci. 2015;10:38–43. [Google Scholar]
- 200.Loladze I., Nolan J.M., Ziska L.H., Knobbe A.R., Loladze I., Knobbe A.R. Carotenoids and Carbon Dioxide Rising Atmospheric CO2 Lowers Concentrations of Plant Carotenoids Essential to Human Health, A Meta-Analysis. Mol. Nutr. Food Res. 2019;63:e1801047. doi: 10.1002/mnfr.201801047. [DOI] [PubMed] [Google Scholar]
- 201.Dzomeku B., Wald J., Wünsche J., Nohr D., Biesalski H. Climate Change Enhanced Carotenoid Pro-Vitamin A Levels of Selected Plantain Cultivars. Plants. 2020;9:541. doi: 10.3390/plants9040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Navarro J.M., Flores P., Garrido C., Martinez V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006;96:66–73. doi: 10.1016/j.foodchem.2005.01.057. [DOI] [Google Scholar]
- 203.Bang H., Leskovar D.I., Bender D.A., Crosby K. Deficit irrigation impact on lycopene, soluble solids, firmness and yield of diploid and triploid watermelon in three distinct environments. J. Hortic. Sci. BioTechnol. 2004;79:885–890. doi: 10.1080/14620316.2004.11511861. [DOI] [Google Scholar]
- 204.Proietti S., Rouphael Y., Colla G., Cardarelli M., de Agazio M., Zacchini M., Rea E., Moscatello S., Battistelli A. Fruit quality of mini-watermelon as affected by grafting and irrigation regimes. J. Sci. Food Agric. 2008;88:1107–1114. doi: 10.1002/jsfa.3207. [DOI] [Google Scholar]
- 205.Sánchez-Rodríguez E., Leyva R., Constán-Aguilar C., Romero L., Ruiz J.M. Grafting under water stress in tomato cherry, improving the fruit yield and quality Grafting under water stress in tomato cherry, improving the fruit yield and quality. Ann. Appl. Biol. 2012;161:302–312. doi: 10.1111/j.1744-7348.2012.00574.x. [DOI] [Google Scholar]
- 206.Buendía B., Allende A., Nicolás E., Alarcón J.J., Gil M. Effect of Regulated Deficit Irrigation and Crop Load on the Antioxidant Compounds of Peaches. J. Agric. Food Chem. 2008;56:3601–3608. doi: 10.1021/jf800190f. [DOI] [PubMed] [Google Scholar]
- 207.Tian S.-L., Lu B.-Y., Gong Z.-H., Shah S.N.M. Effects of drought stress on capsanthin during fruit development and ripening in pepper (Capsicum annuum L.) Agric. Water Manag. 2014;137:46–51. doi: 10.1016/j.agwat.2014.02.007. [DOI] [Google Scholar]
- 208.Flores P., Navarro J., Garrido C., Rubio-Asensio J.S., Martínez V. Influence of Ca2+, K+ and NO3− fertilisation on nutritional quality of pepper. J. Sci. Food Agric. 2004;84:569–574. doi: 10.1002/jsfa.1694. [DOI] [Google Scholar]
- 209.Seljåsen R., Kristensen H.L., Lauridsen C., Wyss G.S., Kretzschmar U., Birlouez-Aragone I., Kahl J. Quality of carrots as affected by pre- and postharvest factors and processing. J. Sci. Food Agric. 2013;93:2611–2626. doi: 10.1002/jsfa.6189. [DOI] [PubMed] [Google Scholar]
- 210.Dorais M. Effect of cultural management on tomato fruit health qualities. Acta Hortic. 2007;744:279–294. doi: 10.17660/ActaHortic.2007.744.29. [DOI] [Google Scholar]
- 211.Jones R.B.B., Stefanelli D., Tomkins R.B.B. Pre-harvest and post-harvest factors affecting ascorbic acid and carotenoid content in fruits and vegetables. Acta Hortic. 2015;1106:31–41. doi: 10.17660/ActaHortic.2015.1106.6. [DOI] [Google Scholar]
- 212.Granado F., Olmedilla B., Blanco I., Rojas Hidalgo E. Carotenoid composition in raw and cooked Spanish vegetables. J. Agric. Food Chem. 1992;40:2135–2140. doi: 10.1021/jf00023a019. [DOI] [Google Scholar]
- 213.Knockaert G., Pulissery S.K., Colle I., van Buggenhout S., Hendrickx M., van Loey A. Lycopene degradation, isomerization and in vitro bioaccessibility in high pressure homogenized tomato puree containing oil, Effect of additional thermal and high pressure processing. Food Chem. 2012;135:1290–1297. doi: 10.1016/j.foodchem.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 214.Nguyen M., Francis D., Schwartz S. Thermal isomerisation susceptibility of carotenoids in different tomato varieties. J. Sci. Food Agric. 2001;81:910–917. doi: 10.1002/jsfa.911. [DOI] [Google Scholar]
- 215.Meléndez-Martínez A.J., Vicario I.M., Heredia F.J. Carotenoids, Color, and Ascorbic Acid Content of a Novel Frozen-Marketed Orange Juice. J. Agric. Food Chem. 2007;55:1347–1355. doi: 10.1021/jf063025b. [DOI] [PubMed] [Google Scholar]
- 216.Moyano M.J., Meléndez-Martínez A.J., Alba J., Heredia F.J. A comprehensive study on the colour of virgin olive oils and its relationship with their chlorophylls and carotenoids indexes (I), CIEXYZ non-uniform colour space. Food Res. Int. 2008;41:505–512. doi: 10.1016/j.foodres.2008.03.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.