Abstract
Laboratories worldwide find it challenging to identify enough tissues and cases for verification and validation studies of low-incidence, rare antigens. These antigens have a low frequency of occurrence in the population, or have little or no expression in normal tissues. Validation studies are essential to assure testing standardization before introducing a new instrument, product, or test into the clinical laboratory. The College of American Pathologists has published comprehensive guidelines for the verification and validation of new immunohistochemical tests introduced into the laboratory menu. Within the guidelines, varied numbers of cases are required for nonpredictive versus predictive markers. However, regarding low-incidence antigens, the laboratory medical director determines the extent of validation required. Recommended practical solutions available to clinical laboratories for low-incidence validation include developing internal resources using the laboratory information system with retrospective and prospective search(s) of archival material and purchase of tissue microarray blocks, slides, or cell lines from external resources. Utilization of homemade multitissue blocks has proved to be extremely valuable in biomarker research and demonstrated great utility in clinical immunohistochemistry laboratories. Participation in External Quality Assessment program(s) may provide insufficient numbers or the ability to calculate concordance rates. However, supplementation with in-house tissues can allow a laboratory to reach the optimal number of cases needed for verification and/or validation schemes. An alternative approach is conducting a thorough literature search and correlating staining patterns of the new test to the expected results. These solutions may be used uniquely or together to assure consistent standardized testing.
Key Words: low incidence, verification, validation, immunohistochemistry (IHC), in vitro diagnostic test (IVD), laboratory developed test (LDT), external quality assurance (EQA)
The Anatomic Pathology Patient Interest Association (APPIA) is a not-for-profit membership organization dedicated to the issues that affect laboratory practices, quality and, ultimately, the safety of the patient. In keeping with the mission of the organization, to ensure accuracy and reduce variation in test results, proper validation studies are needed at the onset of clinical test implementation. However, laboratories worldwide face challenges in gathering enough tissues and cases for antigens with rare occurrence in the population or from patients with a specific rare disease to perform required verification and validation studies for new laboratory products and immunohistochemical (IHC) tests. Procurement of biological material for these biomarkers is technology-agnostic and not unique to IHC: the same challenges exist for molecular testing. Finding and procuring biospecimens from rare cancers is also difficult. When combining a low-frequency occurrence biomarker in a rare cancer, acquiring the required number of positive samples to properly perform verification and validation seems daunting. For reference, the College of American Pathologists (CAP) currently recommends for each predictive test that a laboratory acquire 20 positive and 20 negative cases for verification and validation.1
In a recent CAP survey publication that assessed changes in IHC analytic validation practices following the publication of an evidence-based laboratory practice guideline, the 2 biggest challenges in implementing the guideline was difficulty in finding validation cases for rare antigens, and the other was time/staff resource limitations.1
For clarification, verification is defined as the process by which a laboratory determines that an IVD assay performs according to the specifications set forth by the manufacturer. Validation is defined as the process by which a laboratory confirms a laboratory developed test (LDT) or modified Food and Drug Administration (FDA)-cleared/approved test performs as intended or claimed.2
BACKGROUND
Historically, IHC laboratories have experienced difficulty in finding enough cases for verification and/or validation of new tests that use antibodies against specific markers with a low frequency of occurrence. For example, ALK+ positive lymphoma, a type of anaplastic large cell lymphoma (ALCL), is considered “rare” because it comprises only 2% to 3% of all lymphomas in adults and 10% to 30% of lymphomas in children.3 Only about half (50% to 60%) of those show positivity for the ALK fusion protein by IHC (Fig. 1). Finding a single positive case can be challenging. In addition, choice of primary antibodies and protocol parameters for the validation of ALK+ lymphomas may vary significantly than those of ALK+ lung carcinomas.
FIGURE 1.

ALK protein (+) lymphoma (ALCL) IHC. ALCL indicates anaplastic large cell lymphoma; IHC, immunohistochemical.
Lynch syndrome-associated cancers are characterized by Mismatch Repair (MMR) deficiency caused by somatic inactivation of the sole remaining wild-type MMR gene allele. The central step in diagnosing Lynch syndrome is the analysis of patient tumor tissue, usually colon or endometrial adenocarcinoma, for the presence of MMR deficiency by IHC for MLH1, PMS2, MSH1, and MSH6. Trying to find enough positive and negative cases for 1 or all 4 biomarkers to validate for MMR proteins by IHC can be difficult, as only 1 in 9 (11%) colorectal carcinomas contain the deleted MLH1 gene and only 1 in 50 (2%) contain the deleted MSH2 gene.4 Thus, a laboratory would need to test potentially ≥180 unselected cancers to identify 20 MLH1 gene deleted cases, and perhaps 1000 cancers to find 20 MSH2 gene deleted cases.
The V600E activating mutation in BRAF and its detection by IHC may or may not be rare depending on the assay application. It has been detected in ~7% of all solid tumors, including 45% of papillary thyroid carcinomas, 40% to 60% melanomas, 35% of serous ovarian carcinomas, but only 5% to 15% of colorectal adenocarcinomas, and only 1% to 3% of lung and other cancers.5
Several IHC markers are useful for the identification of spindle cell tumors and soft tissue tumors. For example, TLE-1 is a sensitive and specific marker for synovial sarcoma, yet the incidence is estimated to be only 900 new cases a year in the United States.6 The antibody INI-1 is excellent for the identification of malignant rhabdoid tumors, and Wilms tumors, typically found in young children. However, the incidence is ~0.19 per million for renal tumors, 0.89 per million for atypical teratoid/rhabdoid tumor (AT/RT), and 0.32 per million for tumors of other sites.7 Only 650 cases of Wilms tumor is diagnosed in the United States each year.
Soft tissue sarcomas (STS) comprise about 7% of all malignancies in children and adolescents under the age of 20 years and rhabdomyosarcoma (RMS) accounts for about 40% of pediatric soft tissue sarcomas.8 Antibodies used for identification of RMS, such as Myo-D1 and myogenin, are very difficult to validate because the incidence of RMS is only 4.5 cases/million children and adolescents per year.7
Each year, ~4000 to 5000 adults in the United States are diagnosed with a gastrointestinal stromal tumor (GIST). CD117 (c-kit) and DOG-1 performed by IHC have proven to be reliable and sensitive diagnostic markers for detection of GIST.9,10 However, GIST accounts for <1% of all gastrointestinal tumors.9,10 Overall, about 85% of GISTs are reported to have a protein activating mutation in KIT or PDGFRA.
Studies of PD-L1 expression in lung cancer report this biomarker to be positive in 13% to 70% of cases, partly dependent on how expression is tested for and defined.11 However, a much smaller number of “positive” cases will meet certain percent-positive cutoffs (1%, 5%, 25%, 50% etc) (Table 1).
TABLE 1.
PD-L1 Prevalence by Immunohistochemical12
| Indications | n | No. PD-L1 Positive Immune Cell (+) (>5%) Cases | % of IC Cases (>5%) | No. PD-L1 Positive Tumor Cell (+) (>5%) Cases | % of TC Cases (>5%) |
|---|---|---|---|---|---|
| Non–small cell lung Cancer | 184 | 48 | 26 | 44 | 24 |
| Renal cell cancer | 88 | 22 | 25 | 9 | 10 |
| Melanoma | 58 | 21 | 36 | 5 | 3 |
| Head/Neck SCCa | 101 | 28 | 28 | 19 | 19 |
| Gastric cancer | 141 | 25 | 18 | 7 | 5 |
| Colorectal cancer | 77 | 27 | 35 | 1 | 1 |
| Pancreatic cancer | 83 | 10 | 12 | 3 | 4 |
IC indicates immune cell; SCCa, squamous cell carcinoma; TC, tumor cell.
Patients with non–small cell lung cancer and with anaplastic lymphoma kinase (ALK) gene rearrangements constitute only 4% to 5% of all non–small cell lung cancer patients.13,14
To introduce and properly validate new tests in the clinical laboratory, the College of American Pathologists (CAP) has published comprehensive guidelines for the verification and validation of IHC tests introduced into the laboratory menu. Note that CAP recommends different numbers of cases for nonpredictive versus predictive markers; laboratory verification studies require twice as many positive and negative cases for FDA-approved predictive assays. If a laboratory chooses to develop their own predictive marker assay, such as CD117 or PD-L1, using a LDT, or chooses to alter an FDA-approved assay, even more rigorous verification analyses are required. There are specific differences in verification requirements between a laboratory developing LDTs and using an in vitro diagnostic (IVD) tests from industry, especially with predictive marker assays. Each laboratory must decide the best option, considering parameters like costs (eg, of personnel, material), development time, and skills of personnel. In some laboratories, neither of these requirements are practical or feasible, so laboratories have implemented alternative strategies in an attempt to be compliant with these guidelines. The CAP guidelines are briefly summarized in Table 2.
TABLE 2.
Guidelines for Introducing a New In Vitro Diagnostic (IVD) Antibody into the Laboratory15
| Step #1 | Optimization | Use tissues indicated for the specific intended use or clinical application. For IVD antibodies, the vendor’s package insert protocol should be used as a starting point. For other tests, optimization steps may include the testing of antigen retrieval, 1 degree Ab. titration, detection system, chromogen, amplification, counterstaining, etc |
| Step #2 | Verification | Use the optimized protocol from above: |
| For nonpredictive assays, laboratories should run a minimum of 10 positive and 10 negative cases | ||
| For FDA-approved predictive assays, laboratories should run a minimum of 20 positive and 20 negative/low-expressor cases | ||
| Labs choosing to alter FDA-approved kits or develop their own predictive marker assay(s) (LDTs), should run a minimum of 40 positive and 40 low-expressor/negative cases (0, 1+). This includes LDTs for ER, PR, and HER2 | ||
| Laboratories must verify new IHC tests before placing them into clinical service | ||
| Step #3 | Validation | After verification testing is complete, those slides are compared/correlated (validated) in at least one of the following ways: |
| Compare the new test results with a prior validated assay/test using the same tissue set | ||
| Compare the new test results with validated results from another laboratory using the same tissue set | ||
| Compare the new test with an alternate validated non-IHC test (ISH, etc) | ||
| Compare the new test with the morphology and expected results (ie, from a thorough literature search) | ||
| Testing is compared with graded results from formal Proficiency Testing challenges | ||
| For validation, every assay must achieve 90% overall concordance between the new test and the comparator test |
All tissues/cases in this process should be fixed and processed the same as cases tested clinically.
Laboratories may use whole sections, tissue microarrays, multitissue blocks as appropriate.
Tissues containing low-incidence antigens may be difficult to find.
The characteristics of the tissues or cases used for validation should be similar to those seen in the laboratory's patient population. Tissues should include relevant normal tissues, if available, and neoplasias which span a range of expression from negative to low to high.
FDA indicates Food and Drug Administration; IHC, immunohistochemical; LDT, laboratory developed test.
Estrogen receptor, progesterone receptor, and HER-2/neu testing for breast cancer have unique, specific validation guidelines.16,17 Other global quality organizations have adopted similar validation guidelines, especially with regard to overall concordance.18–21 The CAP guideline recommendations, as described, help ensure that the IHC test accurately measures the analyte of interest and that validation is appropriate for the test's intended use. Its requirements of 20 positive and 20 negative cases are descriptive of a strictly “analytic” validation process. It provides no specifications for tissues and tumors or even a range of epitope expression. However, it may not be entirely fit for purpose for validation of a predictive biomarker where a more “clinical” type of validation process, designed with tissue specimens that test the thresholds or cutoffs (eg, <1%, <10%, etc) used in clinical trials or in a comparator validated assay, is required.22
So rather than just positive and negative, this type of clinical validation tests the diagnostic sensitivity and specificity of the assay. For comparison, at least 50 positive and 50 negative samples are recommended by the CLSI EP12-A2 User Protocol for Evaluation of Qualitative Test Performance.23
Recent publications by CAP sponsored surveys for practices in IHC testing laboratories reveal that most laboratories are not using the recommended sample sizes for validation as described in Table 2. In 2 studies for initial validation, 75.4% (538 of 714) of laboratories adopted the 20-case minimum for nonpredictive markers but less than half (45.9% of the 579 responding laboratories) adopted the 40-case minimum for predictive markers as outlined in the 2014 evidence-based laboratory practice guideline.24 As reported by 714 laboratories, the median number of cases required for nonpredictive assays was 20, and the median number of cases required for predictive assays was only 25, well below the guideline recommendation of 40.1
Recent review of the FDA IHC reagent classifications, IHC validation protocols, and quality assurance (QA) procedures and documentation needed in conjunction with IHC test guidelines for Mohs Labs, IHC reagents are subject to several histologic and clinical controls to maximize the accuracy of IHC reagents, with it being at the discretion of laboratory medical director to establish these quality control procedures before offering these tests for patient diagnostics.25
In a survey of over 700 laboratories, to establish baseline parameters for IHC validation procedures and practice and to assess feasibility of implementation, Hardy and colleagues, noted that for non-FDA approved, nonpredictive IHC tests, 75% of laboratories used 21 or fewer total cases for validation. Less than 50% of laboratories used ≥25 cases to validate non–FDA-approved predictive markers (LDTs). Of those, only 47% used cases with any weak to moderate expression levels. When comparing IHC HER2/neu assays with an IHC test performed in another laboratory, only 56% of laboratories used a recommended minimum of 25 cases. Seventy-five percent of respondents had validated the most recently introduced new antibody, with a median of only 15 cases. Major challenges to meet the proper validation requirements included tissue availability (especially for cytological materials), availability of appropriate tissue controls which were highly dependent upon the particular antibody used, and time and personnel resources to find and qualify appropriate cases. Most respondents felt a minimum of 25 cases was appropriate for validation of a predictive marker and 13 cases for nonpredictive markers as predictive markers are much less likely to have morphologic clues to refute an errant stain 26
Considering another diagnostic procedure, Whole Slide Imaging (WSI), CAP has established an evidence-based guideline setting the minimum number of cases to 60 after finding that when an average of 20 cases (range, 10 to 46 cases) was used to compare WSI and glass slide, there was significantly lower accuracy (77%) and concordance (75%), but when an average of 60 cases (range, 52 to 90 cases) was used, the studies showed improved accuracy (90%) and far better concordance (95%). Thus, CAP recommended that validation studies for WSI include at least 60 routine cases per application, [eg, hematoxylin eosin (H&E)-stained sections of fixed tissue, frozen sections, cytology, hematology] that reflects the spectrum and complexity of specimen types (biopsies and resections) and diagnoses likely to be encountered during routine practice. If the laboratory intends to use its validated WSI system for another supplemental application (eg, to evaluate special stains, IHC or fluorescence stains and H&E-stained sections), then another 20 cases of the “additional” application should be validated. In addition, the study showed that the specimen preparation type (H&E, Papanicolaou, etc) was a more important performance variable than the source of the tissue or the specific analyte being assessed. Thus, a single validation study may suffice to cover a group of similar intended uses, as long as the overall process of preparation and interpretation is the same.27
Interestingly, recent CAP proficiency testing survey results on validation and implementation of Next Generation Sequencing (NGS) in clinical oncology applications showed that about half of the 58 laboratories that reported on their variant confirmation procedures are not performing variant confirmation (n =28; 48%). For those laboratories that are performing variant confirmation, they are employing various orthogonal technologies including Sanger sequencing (n=26), targeted mutation testing (n=11), pyrosequencing (n=8), or other technologies (n=5).28
SOLUTIONS
There are many IHC tests available for rare, low-incidence antigens and tissues. The increasingly demanding requirements for verifying or validating new antibody tests, while necessary, have made it difficult for laboratories wanting to perform in-house testing to identify the required number of cases and needed amount of tissue especially those tissues expressing rare antigens. CAP recognizes this fact and has addressed it in its current Anatomic Pathology accreditation checklist 29
ANP.22750—Antibody Validation
The extent of this validation is at the discretion of the medical director. If a laboratory does not use 10 positive cases and 10 negative cases for their nonpredictive marker validation, then the rationale for this needs to be documented in the validation summary. Just because a laboratory uses less than the suggested number of cases for a validation does not necessarily mean that they are out of compliance.
…the laboratory director determines that fewer validation cases are sufficient for a specific marker (e.g. a rare antigen or tissue), the rationale for that decision needs to be recorded. 29
CAP does require that ER, PR and HER2 assays employed as predictive markers on breast carcinoma have a minimum of 20 positive cases and 20 negative cases. All other predictive markers are to be validated, but the extent of that validation is, again, at the discretion of the medical director and will vary with the antibody, especially those against low-incidence antigens and rare, hard to find tissue samples. If IHC is regularly performed on specimens that are not fixed or processed in the same manner as the tissues used for validation (eg, alcohol fixed cell blocks, cytological smears, formalin post fixed tissue, or decalcified tissue), the laboratory should test a sufficient number of such tissues to ensure that assays consistently achieve expected results using the appropriate protocol for the intended use.29 Again, the laboratory director is responsible for determining the number of positive and negative cases and the number of predictive and nonpredictive markers to test.
There are several solutions available to laboratories wanting to perform verification and/or validation studies in-house for low-frequency antigens, including:
Using specimen blocks identified via retrospective and prospective LIS search/survey from “in-house” cases.
Purchase of tissue blocks, multitissue blocks (MTBs), or precut slides from qualified sources, or creating homemade MTBs.
Participation in External Quality Assessment (EQA) proficiency programs.
Correlating the new test’s results with expected results based on literature search, demonstrating same tumor morphology and antigen distribution.
Retrospective Search
A common, almost routine, activity in most academic medical center, reference, community hospital, and private laboratories is performing a keyword search(s) to identify in-house, archived cases using the laboratory information system (LIS). Most systems are able to print or save list(s) of cases that can be used to identify and/or screen cases and blocks with low-incidence antigens.
As an example, a keyword search for “Anaplastic large cell lymphoma (ALCL),” an aggressive type of non-Hodgkin T-cell lymphoma, may reveal only a few cases in the block archive because of its rarity. However, utilization of in-house, archived tissue blocks, even if rare, best fits the CAP checklist instruction that “laboratories should use validation tissues that have been processed using the same fixative and processing methods as cases that will be tested clinically.”29
Purchase
In a recent review of diagnostic IHC standardization procedures by Lin and Chen,30 tissue microarray (TMA) blocks containing a grid-like array of various tumors and/or normal tissues have proved to be extremely valuable in biomarker research and have demonstrated great utility in clinical IHC laboratories. At least 4 different prototypes of TMA blocks can be used:
TMA block containing a broad spectrum of tumors and/or normal tissues from various organs, useful for screening a new biomarker.
TMA block containing 50 to 100 tumors with a specific diagnosis, such as lung adenocarcinoma, useful for antibody validation, revalidation, estimating marker prevalence in a specific indication, and in determining the diagnostic sensitivity and specificity of a newly discovered antibody.
TMA block containing 5 to 10 cases of a specific type of tumor, useful for antibody testing and optimization.
TMA block containing 5 to 10 cases of selected, mixed tumors and/or normal tissues from various organs, useful as external positive and negative control tissues for each antibody.
Laboratories may use whole sections, TMAs, and/or MTBs in their validation sets as appropriate. Whole sections should be used if TMAs/ MTBs are not appropriate for the targeted antigen or if the laboratory medical director cannot confirm that the fixation and processing of TMAs/MTBs is similar to clinical specimens. MTBs should be employed for external positive and negative tissue controls. Use of multitissue external positive controls by IHC technologists provides consistent positive feedback because it simplifies workflow and minimizes potential error. TMA external positive controls have also demonstrated superior performance in staining consistency and reproducibility.30
Several qualified vendors offer both TMA slides and blocks for purchase in a variety of formats including formalin-fixed, paraffin-embedded tissues (Table 3). These multicore arrays, some of which contain 300+ cores, include normal, malignant, or metastatic tissues and are suitable for use in validation studies. Multicore TMAs may contain protein, RNA, or DNA and provide an ideal high throughput method for rapid analysis of IHC markers or in situ hybridization. Many companies can provide custom tissue array constructions for use in immunohistochemistry and in situ hybridization. In addition, vendors can often provide relevant preanalytic variables such as fixation times for each tissue. Cost of these products varies considerably and depends upon factors such as sample type, availability, size of sample set, and antigen presentation.
TABLE 3.
Vendors That Provide TMA Blocks and/or Slides
| Cooperative Human Tissue Network | http://chtn.sites.virginia.edu/tissue-microarrays |
| Folio Biosciences | http://www.foliobio.com/products/tissue-microarrays |
| Histocyte Laboratories | http://www.histocyte.com |
| Horizon Discovery | http://www.horizondiscovery.com |
| Invitrogen | http://www.invitrogen.com |
| Origene | http://www.origene.com/tissue/tissue_microarrays.aspx |
| Pantomics | http://www.pantomics.com |
| StatLab Medical Products | http://www.statlab.com |
| US Biolab | http://www.usbiolab.com/custom-tissue-array |
| US Biomax | http://www.biomax.us/tissue-arrays |
Several vendors are able to generate engineered cell lines with low/negative, intermediate, and high levels of protein using proprietary gene-editing platforms (Fig. 2). The raw material is then formalin-fixed, processed in a traditional manner using alcohol and xylene, and paraffin embedded. These cell buttons, which mimic cytology cell blocks, can be used as reference standards that serve as surrogates for real patient samples within an IHC testing protocol. They can also be used as a renewable and consistent point of reference, essentially run controls, when optimizing and monitoring performance of an IHC assay.31
FIGURE 2.
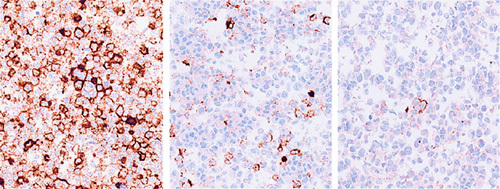
IHC staining of engineered cell lines that express PD-L1 at varying levels, from high (left) to low (right). IHC indicates immunohistochemical.
Multitissue Blocks
Homemade MTBs represent an alternative to whole sections or TMAs. MTBs containing just 8 to 12 different cases of specific tissue-types can be used to supplement existing in-house tissues for optimization and verification work, comparison and parallel testing of new antibody lots, reverification of assay modifications, and positive and negative tissue control material.
The concept involves some up-front work on the part of laboratory personnel to identify tissues and assemble the MTBs. The blocks consist mainly of normal tissue types easily found in typical pathology laboratories. The best MTBs are those put together in the user laboratory using tissues fixed and processed in that laboratory. They contain both positive and negative internal controls for a wide range of IHC tests. A single MTB paraffin block can be used for verification studies in over 100 routine IHC tests and are useful in supplementing rare antigen and hard to find tissue searches. As seen in Figure 3, for example, this simple block consists of:
2 to 3 full cross sections of appendix from different cases
2 to 3 small-medium sized pieces of liver resection, including normal liver from different cases
2 to 3 pieces of tonsil resection, to include squamous epithelium and lymphatic nodules from different cases
2 to 3 sections of normal pancreas containing islets, ducts, and acinar cells from different cases.
FIGURE 3.

Slide made from a multitissue block consisting mainly of normal tissue types. 2 to 3 full cross sections of appendix from different cases. 2 to 3 small-medium sized pieces of liver resection, including normal liver from different cases. 2 to 3 pieces of tonsil resection, to include squamous epithelium and lymphatic nodules from different cases. 2 to 3 sections of normal pancreas containing islets, ducts, and acinar cells from different cases. MTB indicates multitissue block.
The MTB contains 8 to 12 different patient tissues and can be used for a wide variety of verification and validation activities. Similarly, if sections of normal skin, melanoma, striated muscle, and brain (cerebellum) are combined in a MTB another 25 to 30 IHC markers can easily be used to verify and validate.
External Quality Assessment
Internally, if hospitals and reference laboratories find it difficult to acquire sufficient numbers of tissue samples for initial validation, some EQA programs may provide test samples that quantitatively and qualitatively support IHC test verification and validation. Participation in such programs provides appropriate support for initial and continuous revalidation of these tests as well.17
The Canadian Immunohistochemistry Quality Control (cIQc) EQA scheme regularly conducts EQA activities for rare antigen, hard-to-find tissue specimens such as (ie, ALK, IDH1, BRAF V600E, PD-L1, MMR) using medium-to-large multiple-core tissue micro-array slides (TMAs) (Fig. 4).
FIGURE 4.
BRAF V600E TMA slide from cIQc. TMA indicates tissue microarray.
Large TMAs containing multiple cores of positive and negative tissues processed by standard methods allow a laboratory to supplement in-house tissue numbers to reach the number of cases needed for verification and/or validation. They also help improve the precision and accuracy of testing. We presume that any global EQA program that provides such rare antigen samples would be acceptable for test verification and validation.
The CAP MMR survey for DNA MMR proteins provides fewer cores per slide, but 2 unique TMA slides rather than just 1 (Fig. 5). TMA slides used for EQA assessment are often available for purchase separately from the scheme as an aid to laboratories.
FIGURE 5.

MMR TMA slides from CAP. CAP indicates College of American Pathologists; MMR, Mismatch Repair; TMA, tissue microarray.
Participation in EQA programs that do not provide sufficient numbers of tissue samples may be informative and useful, but do not provide the opportunity to calculate concordance rates with adequate power unless supplemented with in-house samples, and therefore alone do not provide sufficient numbers of cases for verification or validation. Participation in EQA however, informs the laboratory of the sensitivity and specificity of their protocol and their platform choices and serves as an indicator of the need for overall optimization of the IHC assay. Incidentally, it is essential that laboratories performing Class II and/or Class III IHC testing document participation in EQA programs in the laboratory Quality Management Plan (QMP), and if suboptimal results are achieved, corrective actions are taken and documented.
Use of Expected Staining Results
Verification activities do not always have to involve stained slides being compared with previously stained slides or slides stained in another laboratory, or results being judged comparable to an alternative method (ie, FISH) to validate or revalidate a new test, especially if staining is for a low-incidence antigen. An alternative approach is a thorough literature search, guided by the pathologist(s), to reveal “both” the expected morphology of the tumor(s) under study and the correlation of staining patterns to the “expected results” with the new test.
Results from a survey of 1085 clinical laboratories, comparing and correlating the staining results of the new test with expected results, regardless of specimen size, was the “most common” validation method used among laboratories for 725 nonpredictive markers (61%) and 101 predictive markers (46.5%).22 Comparing the new test results with the same tissue set stained in another laboratory was a distant second used by only 123 (17%) of laboratories in the survey.
DISCUSSION
Many laboratories have problems finding sufficient tissues and cases to adequately validate antibodies against rare antigens with a low frequency of occurrence and little or no expression in normal tissues. There are several practical solutions available to clinical laboratories. These include developing internal resources with retrospective and prospective search(s) of archival material, purchase of TMA blocks and slides from outside resources, and participation in EQA schemes, and comparison with published literature. These solutions may be used uniquely and in combination to achieve verification and validation requirements for new and ongoing IHC tests.
Footnotes
R.L.L.: royalty payments, College of American Pathology Press; Employee, Roche Tissue Diagnostics, Tucson, AZ; and Member, CAP Histotechnology Committee. P.V.R. and E.A.S.: are employees at Roche Tissue Diagnostics, Tucson, AZ. K.A.W.: Employee, Leica Biosystems/Danaher, Inc.; Stockholder, Danaher, Inc.; and Stockholder, Novartis AG. E.E.W.: Employee and shareholder, F. Hoffmann-La Roche AG. G.K.: Employee, Sakura Finetek USA Inc., Torrance, CA. B.P.: Employee and shareholder, Agilent Technologies, Santa Clara, CA; Member, CAP Molecular Oncology Committee.
REFERENCES
- 1. Fitzgibbons PL, Goldsmith JD, Souers RJ, et al. Analytic validation of immunohistochemical assays: a comparison of laboratory practices before and after introduction of an evidence-based guideline. Arch Pathol Lab Med. 2017;141:1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. College of American Pathologists Checklist, Anatomic Pathology, v. 07-29-2013, Definition of Terms.
- 3. Vose JM. Getting the Facts: Anaplastic Large Cell Lymphoma. New York, NY: Lymphoma Research Foundation; 2009:1–2. [Google Scholar]
- 4. Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pakneshan S, Salajegheh A, Smith RA, et al. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346–356. [DOI] [PubMed] [Google Scholar]
- 6. Cure Sarcoma. Synovial sarcoma. 2019. Available at: www.curesarcoma.org/patient-resources/sarcoma-subtypes/synovial-sarcoma/. Accessed February 22, 2017.
- 7. Annual incidence of rhabdoid tumors among children <15, 2000-2008. Surveillance, Epidemiology, and End Results (SEER) Program; Bethesda, MD: 2011. Volume SEER 17. SEER*Stat version 7.0.6.
- 8. Gurney JG Young JL Roffers SD, et al. SEER Pediatric Monograph. National Cancer Institute; 2005. Soft Tissue Sarcomas.
- 9. Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. [DOI] [PubMed] [Google Scholar]
- 10. Steigen SE, Eide TJ. Gastrointestinal stromal tumors (GISTs): a review. APMIS. 2009;117:73–86. [DOI] [PubMed] [Google Scholar]
- 11. Kerr KM, Tsao MS, Nicholson AG, et al. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol. 2015;10:985–989. [DOI] [PubMed] [Google Scholar]
- 12. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chia PL, Mitchell P, Dobrovic A, et al. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin Epidemiol. 2014;6:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheung CC, Garratt J, Won J, et al. Developing ALK immunohistochemistry and in situ hybridization proficiency testing for non-small cell lung cancer in Canada: Canadian immunohistochemistry quality control challenges and successes. Appl Immunohistochem Mol Morphol. 2015;23:677–681. [DOI] [PubMed] [Google Scholar]
- 15. Fitzgibbons PL, Bradley LA, Fatheree LA, et al. Principles of analytic validation of immunohistochemical assays: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014;138:1432–1443. [DOI] [PubMed] [Google Scholar]
- 16. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolff AC, Hammond ME, Schwarz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. [DOI] [PubMed] [Google Scholar]
- 18. Hanna W, O’Malley FP, Barnes P, et al. Updated recommendations from the Canadian National Consensus Meeting on HER2/neu testing in breast cancer. Curr Oncol. 2007;14:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torlakovic EE, Riddell R, Banerjee D, et al. Canadian Association of Pathologists-Association canadienne des pathologistes National Standards Committee/Immunohistochemistry: best practice recommendations for standardization of immunohistochemistry tests. Am J Clin Pathol. 2010;133:354–365. [DOI] [PubMed] [Google Scholar]
- 20. Ellis IO, Bartlett J, Dowsett M, et al. Best Practice No 176: updated recommendations for HER2 testing in the UK. J Clin Pathol. 2004;57:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bilous M, Dowsett M, Hannah W, et al. Current perspectives on HER2 testing: a review of National Testing Guidelines. Mod Pathol. 2003;16:173–182. [DOI] [PubMed] [Google Scholar]
- 22. Torlakovic E. How to validate predictive immunohistochemistry testing in pathology? Arch Pathol Lab Med. 2019;143:907. [DOI] [PubMed] [Google Scholar]
- 23. Garrett PE, Lasky FD, Meier KL, et al. Clinical and Laboratory Standards Institute User Protocol for Evaluation of Qualitative Test Performance: Approved Guideline, 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 24. Stuart LN, Volmar KE, Nowak JA, et al. Analytic validation of immunohistochemistry assays: New Benchmark Data from a survey of 1085 laboratories. Arch Pathol Lab Med. 2017;141:1255–1261. [DOI] [PubMed] [Google Scholar]
- 25. Kinas CG, Carroll BT. General guidelines for quality assurance of immunohistochemistry in a Mohs lab. Dermatol Surg. 2017;43:507–511. [DOI] [PubMed] [Google Scholar]
- 26. Hardy LB, Fitzgibbons PL, Goldsmith JD, et al. Immunohistochemistry validation procedures and practices: a College of American Pathologists survey of 727 laboratories. Arch Pathol Lab Med. 2013;137:19–25. [DOI] [PubMed] [Google Scholar]
- 27. Pantanowitz L, Sinard JH, Hendricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137:1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagarajan R, Bartley AN, Bridge JA, et al. A window into clinical next-generation sequencing-based oncology testing practices. Arch Pathol Lab Med. 2017;141:1679–1685; 2017. [DOI] [PubMed] [Google Scholar]
- 29. College of American Pathologists, 2017 Anatomic Pathology Checklist, ANP.22750 – Antibody Validation.
- 30. Lin F, Chen Z. Standardization of diagnostic immunohistochemistry: literature review and geisinger experience. Arch Pathol Lab Med. 2014;138:1564–1577. [DOI] [PubMed] [Google Scholar]
- 31. Torlakovic E, Nielsen S, Vyberg M, et al. Getting controls under control: the time is now for immunohistochemistry. J Clin Pathol. 2015;68:879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]


